Abstract
We have shown recently that mouse Th1 cells but not Th2 cells are selectively recruited into inflamed sites of a delayed-type hypersensitivity (DTH) reaction of the skin. This migration was blocked by monoclonal antibodies (mAb) against P- and E-selectin. Here we show that Th1 cells bind to P-selectin via the P-selectin glycoprotein ligand-1 (PSGL-1). This is the only glycoprotein ligand that was detectable by affinity isolation with a P-selectin–Ig fusion protein. Binding of Th1 cells to P-selectin, as analyzed by flow cytometry and in cell adhesion assays, was completely blocked by antibodies against PSGL-1. The same antibodies blocked partially the migration of Th1 cells into cutaneous DTH reactions. This blocking activity, in combination with that of a mAb against E-selectin, was additive. PSGL-1 on Th2 cells, although expressed at similar levels as on Th1 cells, did not support binding to P-selectin. Thus, the P-selectin–binding form of PSGL-1 distinguishes Th1 cells from Th2 cells. Furthermore, PSGL-1 is relevant for the entry of Th1 cells into inflamed areas of the skin. This is the first demonstration for the importance of PSGL-1 for mouse leukocyte recruitment in vivo.
The binding of circulating leukocytes to activated endothelium is initiated by transient interactions that are mediated by the selectins (1). It is well documented in various inflammation models that blocking of the two endothelial selectins, P- and E-selectin, inhibits the entry of neutrophils into inflamed tissue (2). Less is known about the role of these adhesion molecules for T lymphocyte recruitment in inflammation. Binding to E-selectin was shown for certain subsets of human CD4+ memory T lymphocytes (3, 4) and for a large percentage of bovine γ/δ T cells (5). A small percentage of CD4+ T cells from peripheral blood (6) and also chronically activated CD4+ T cells (7) was found to bind to P-selectin. Human CD4+ T cell clones were described to bind to E- and P-selectin in static (8) as well as flow adhesion assays (9), and T cell recruitment into inflamed skin was blocked with polyclonal antibodies against P-selectin in vivo in the rat (10).
Binding of activated T cell lines to P-selectin under static conditions was partially blocked in vitro by high concentrations of an anti–human P-selectin glycoprotein ligand-1 (PSGL-1) antiserum (9, 11). PSGL-1 was originally identified on human neutrophils by affinity isolation with P-selectin (12, 13) and cloned by expression cloning (14). It was found to be the major binding site for P-selectin on human leukocytes (11, 15). Rolling of human leukocytes perfused into rat postcapillary venules was demonstrated to be blocked by a mAb against human PSGL-1 (16).
Upon activation, T helper lymphocytes polarize into Th1 and Th2 subsets, which are characterized by distinct profiles of secreted cytokines (17, 18). Th1 cells are involved in cell-mediated inflammatory reactions. Their cytokines activate cytotoxic and inflammatory functions and Th1 cells induce delayed-type hypersensitivity (DTH) reactions. Th2 cytokines support antibody production, particularly IgE responses, and in combination with their stimulatory effects on eosinophil proliferation and function, Th2 cytokines are commonly found in association with strong antibody and allergic responses. Although it is well established that Th1 cells predominate in DTH reactions, it was always unclear whether their presence is mainly due to polarized differentiation at these sites or could also be based on preferential immigration of Th1 versus Th2 cells. We have shown recently that mouse Th1 cells indeed migrate into cutaneous DTH reactions much better than Th2 cells do, and we could demonstrate that this migration is blocked by mAb against P- and E-selectin (19).
In this study, we have examined which molecules on the surface of Th1 cells would function as ligands for P-selectin during migration into cutaneous DTH reactions in the mouse. We could define the PSGL-1 as the exclusive P-selectin ligand on Th1 cells by affinity isolation experiments, FACS® analysis, and cell adhesion assays. Th2 cells carried similar amounts of PSGL-1; however, this form of PSGL-1 was unable to bind to P-selectin. Antibodies against PSGL-1 could partially block the migration of Th1 cells into cutaneous DTH reactions and showed additive effects with a mAb against E-selectin.
Materials and Methods
Cells.
The mouse neutrophilic cell line 32Dcl3 was cultured as described (20). Th1 and Th2 cells were generated from lymph node lymphocytes of SPF-reared BALB/c mice. CD4+ T cells were derived by panning of isolated lymphocytes with mAb against CD8 (53-672), CD25 (PC/6), Fc-Receptor II/III (2.4G2), Mac-1 (M1/70), and I-Ad (17/227). Of the resulting cells, 98– 99% were positive for CD4 staining. These cells (106/well) were incubated either in the presence of IL-12 (1,000 U/ml) and IFN-γ (200 U/ml) (for generation of Th1 cells) or in the presence of IL-2 (50 U/ml) and IL-4 (10 ng/ml) (for the generation of Th2 cells) in 24-well plates coated with mAb 145-2C11 against CD3. After 2 d, cells were transferred to noncoated plates without changing medium and cultured for another 3 or 4 d as described (21). Under these conditions, the differentiated effector cells have returned into a resting state (21). Purity of Th1/Th2 subsets were verified for cytokine production by intracellular FACS® analysis (22). The two generated T cell populations contained routinely >50% cells of the desired phenotype and were contaminated by <1% with cells of the opposite phenotype.
In Vivo Studies.
Th1 cells harvested 6 d after initial activation were subjected to density gradient centrifugation (17.1% isotonic Nycodenz; Nyegaard, Oslo, Norway) to remove dead cells. 107 cells per ml were incubated for 1 h at 37°C in RPMI with 10% FCS and 20 μCi 51Cr (Amersham Corp., Arlington, IL). Dead cells were removed by a second density gradient step, and recovered cells were washed twice with PBS. 106 cells in 400 μl PBS (with or without antibodies) were injected into the tail vein of BALB/c mice (7–10 wk; breeding facilities UKE Hamburg) which had been sensitized to 2.4-dinitrofluorobenzene (DNFB) by skin painting with a solution of 0.5% DNFB in acetone–olive oil (4:1) at day −21, −20, and challenged with 0.3% DNFB at day −1. After 1 h, mice were killed, and distribution of radioactivity in different organs and the remaining body was measured in a γ-scintillation counter.
Flow Cytometric Analysis.
Th1 and Th2 cells, harvested 5 d after initial activation, were separated from dead cells by density gradient centrifugation, incubated with 25 μg/ml selectin-IgG chimera or 10 μg/ml affinity-purified rabbit antibodies in HBSS (Biochrom, Berlin, Germany) with 3% FCS, and stained with PElabeled F(ab′)2 donkey anti–human IgG (Dianova, Hamburg, Germany) or FITC labeled goat anti–rabbit IgG (Sigma). Analysis was performed on a Becton Dickinson FACSCalibur® and CellQuest analysis software.
Antibodies and Selectin-IgG Chimera.
The mAb UZ4 (rat IgM) against mouse E-selectin (23) and the mAb RB40.34 (rat IgG 1) against mouse P-selectin (24) were produced in and purified from protein-free hybridoma medium (Gibco/BRL, Karlsruhe, Germany). In analogy to Yang et al. (25), we raised the rabbit anti– mouse PSGL-1 antiserum 124 against a peptide covering amino acids 42–60 of the sequence of murine PSGL-1. This sequence starts after the cleavage site of the propeptide, and the analogous region of human PSGL-1 is known to be necessary for P-selectin binding (26) and to harbor the binding epitope of the adhesion blocking mAb PL-1 (27). Specific antibodies were purified by affinity isolation on the BSA-conjugated PSGL-1 peptide linked to CNBr-Sepharose as described (28). The FACS® signal which was obtained with these antibodies on 32Dcl3 cells was abolished after pretreatment of the cells with O-sialoglycoprotease (treatment done as described in 29). For the preparation of Fab-fragments, 2.5 mg of affinity-purified anti–PSGL-1 antibodies were incubated in 500 μl of 20 mM sodium phosphate, pH 7.0, 10 mM EDTA, 10 mM cysteine with 100 μl of washed papain Sepharose beads (Pierce Chemical Co., Rockford, IL) for 4 h at 37°C. The supernatant was dialyzed against PBS, and Fc fragments and undigested antibodies were removed with protein A–Sepharose (Pharmacia LKB, Piscataway, NJ). The P-selectin–IgG fusion protein was produced as described (28).
Immunoprecipitation.
Th1 and Th2 cells harvested 5 d after initial activation were subjected to density gradient centrifugation, washed three times in PBS, and surface biotinylated in 1 ml of PBS containing 0.5 mg/ml Sulfo-NHS-biotin (Pierce) for 30 min on ice. The reaction was blocked by incubating the cells for 10 min in 1 ml of DMEM (Gibco/BRL) without FCS on ice. Cells were washed with PBS twice and subjected to immunoprecipitations performed as described (29). Immunoprecipitated proteins were separated by electrophoresis on 6% SDS-PAGE and transferred to nitrocellulose (Schleicher & Schuell, Keene, NH). Filters were analyzed for biotinylated proteins with peroxidase-conjugated streptavidin (Dianova, Hamburg, Germany) and the ECLsystem (Amersham Corp., Arlington Heights, IL).
Cell Adhesion Assay.
Adhesion assays in rotating 96-well microtiter plates coated with selectin–Ig fusion proteins were performed as described (29), except for evaluating the number of bound cells, which was done by computer-aided image analysis using the NIH Image 1.55 software. For each value, three wells were analyzed, counting three randomly chosen areas per well.
Results and Discussion
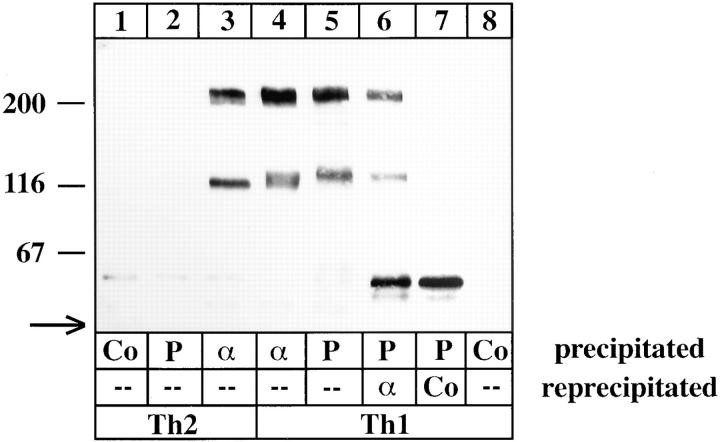
We have recently shown that Th1 cells but not Th2 cells efficiently enter sites of acute inflammation in the skin (19). This migration was blocked by a combination of antibodies against P- and E-selectin (19). Based on these findings, we have now examined which molecules on Th1 cells would function as ligands for P-selectin in this process. We first analyzed which glycoproteins on the surface of Th1 cells bind to P-selectin. To this end, equal numbers of Th1 and Th2 cells were surface biotinylated and subjected to affinity isolation experiments with a P-selectin–Ig fusion protein. As shown in Fig. 1 (lane 5), two proteins of 230 and 130 kD were isolated with P-selectin–Ig that could be reprecipitated with affinity-purified antibodies of the rabbit antiserum 124 against an amino-terminal peptide of mouse PSGL-1 (lane 6). The 230-kD form is the not completely reduced dimeric form of the 130-kD PSGL-1. No selectin ligands could be isolated with the P-selectin probe from Th2 cells (lane 2), although the PSGL-1 protein was detectable with antibodies (lane 3) at similar levels as on Th1 cells (lanes 3 and 4). Interestingly, the 130-kD monomeric form of PSGL-1 on Th2 cells was slightly smaller than the one on Th1 cells (compare lane 3 with 5). Whereas antibodies against PSGL-1 precipitated a broader band from Th1 cells (lane 4), including a slightly smaller molecular species of the size as in Th2 cells, P-selectin–Ig only precipitated the higher molecular weight species. We conclude that only Th1 cells express a modified form of PSGL-1 that is capable of binding to P-selectin. Furthermore, PSGL-1 was the only glycoprotein ligand on Th1 cells that could be detected by this technique.
Figure 1.
PSGL-1 from Th1 cells but not from Th2 cells can be affinity isolated by P-selectin–IgG. Equal numbers of Th1 or Th2 cells were surface biotinylated, and detergent extracts were incubated with either immobilized human Ig as control (Co), P-selectin–IgG (P), or affinity-purified antibodies against PSGL-1 (α). Specifically bound proteins were either directly electrophoresed (lanes 1–5 and 8) or subjected to a reprecipitation (as indicated) with affinity-purified anti–PSGL-1 rabbit antibodies (lane 6) or immobilized nonimmune rabbit antibodies (lane 7). Isolated proteins were separated on 6% polyacrylamide gels under reducing conditions. The material in lanes 6 and 7 corresponds to twice as many cells as used for lanes 1–5 and 8. The front of the gel is marked by an arrow on the left. Molecular mass markers (in kD) are indicated on the left.
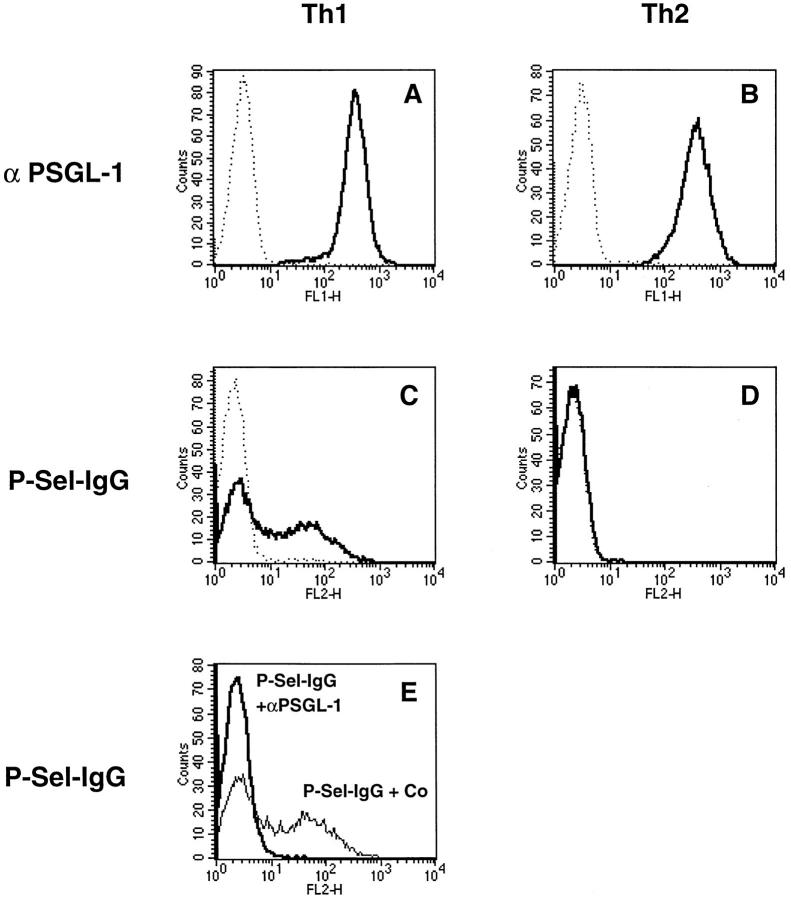
In line with the immunoprecipitation results, a comparison of Th1 and Th2 cells by FACS® analysis revealed that both cell types expressed similar levels of PSGL-1 (Fig. 2, A and B). However, 45% of the Th1 cells, but none of the Th2 cells, could be stained with P-selectin–Ig (Fig. 2, C and D). Staining with P-selectin–Ig was completely blocked by preincubating the cells with affinity-purified antibodies against PSGL-1, indicating that P-selectin binding to Th1 cells is mediated exclusively by PSGL-1 (Fig. 2 E).
Figure 2.
FACS® analysis of Th1 and Th2 cells with P-selectin–Ig and antibodies against PSGL-1. Th1 and Th2 cells (as indicated) were analyzed by flow cytometry either with affinitypurified rabbit antibodies against mouse PSGL-1 (A and B, solid lines) or with P-selectin–Ig (C and D, solid lines). Dotted lines show negative control staining either with nonimmune rabbit IgG (A and B) or with human IgG (C and D). E shows the staining of Th1 cells with P-selectin–IgG after preincubation of the cells either with nonimmune rabbit IgG (faint line) or with affinitypurified rabbit anti–PSGL-1 antibodies (bold line). P-selectin–Ig was detected with PE-conjugated F(ab′)2 donkey anti–human IgG, and rabbit antibodies were detected with FITC-conjugated goat anti–rabbit IgG.
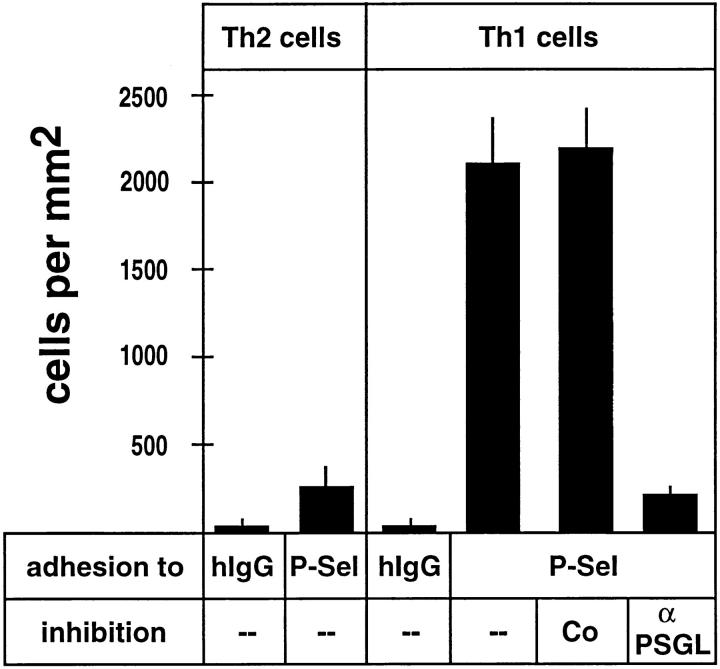
Using nonstatic (rotation) adhesion assays, we analyzed the binding of Th1 and Th2 cells to P-selectin–Ig coated onto plastic. In agreement with the results obtained by FACS® analysis, Th1 cells bound efficiently to P-selectin– Ig and, this binding was blocked by 89% with affinity-purified antibodies against PSGL-1 (Fig. 3). Binding of Th2 cells to P-selectin was low and comparable to the residual binding of anti–PSGL-1–blocked Th1 cells. Thus, PSGL-1 almost exclusively mediates P-selectin binding to Th1 cells in FACS® analysis as well as in adhesion assays and is the only glycoprotein ligand that can be detected by affinity isolation with a P-selectin probe from these cells.
Figure 3.
Adhesion of Th1 and Th2 cells to immobilized P-selectin–Ig. Cell adhesion assays were performed with Th2 or Th1 cells (as indicated) in 96-well microtiter plates coated with human IgG (hIgG) or P-selectin– IgG (P-Sel). Before the assay, cells were incubated with HBSS(--), HBSS with 50 μg/ml rabbit nonimmune antibodies (Co), or HBSS with 50 μg/ ml affinity-purified antibodies against PSGL-1 (α PSGL). Bound cells were counted by computer-aided image analysis in three randomly chosen areas of defined size (per well) in three different wells for each determination. The depicted experiment represents one of three similar experiments.
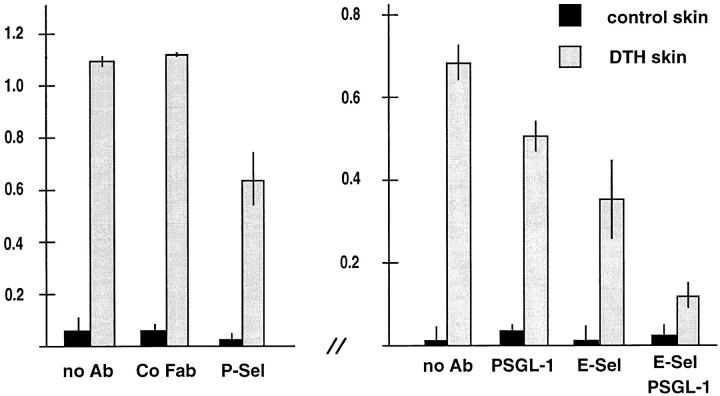
To determine whether PSGL-1 would be involved in the P-selectin–mediated step during migration of Th1 cells into inflamed sites of the skin, we examined the effect of affinitypurified antibodies against PSGL-1 on Th1 cell homing in a DNFB-elicited cutaneous DTH reaction. 51Cr-labeled cells were preincubated with the antibodies for 15 min and injected into mice intravenously. Organs were taken 1 h later and analyzed for the presence of infiltrated lymphocytes by measuring radioactivity. Affinity-purified, complete rabbit antibodies against PSGL-1 blocked T cell migration into inflamed skin by 43% but also caused some trapping of these cells in the lung (not shown). Therefore, Fab fragments were generated. As shown in Fig. 4, anti–PSGL-1 Fab fragments reduced the entry of Th1 cells into the inflamed skin area by 26% (± 5.2%), while no effect was seen with Fab fragments from preimmune serum. Migration of Th1 cells into noninflamed control skin of the same mice was low. No effect of the Fab fragments on Th1 cell accumulation in other organs was observed (not shown). The anti–P-selectin antibody RB40.34 and the anti–E-selectin mAb UZ4 blocked Th1 cell entry into inflamed skin by 42% (± 9.8%) or 48% (± 14%), respectively, when injected together with the cells. The effect of anti–PSGL-1 Fab fragments and the anti–E-selectin mAb were additive, resulting in 83% (± 5.2%) reduction of Th1 cell entry into inflamed skin. This is in agreement with the additive inhibitory effect of 92% that we observed when the anti–P- and anti–E-selectin mAbs were simultaneously injected (19).
Figure 4.
Partial inhibition of Th1 cell immigration into inflamed skin by antibodies against PSGL-1. Radiolabeled Th1 cells were injected together with PBS (no Ab) or the same buffer containing 100 μg of nonimmune rabbit IgG Fab fragments (Co Fab), 100 μg of affinity-purified anti–PSGL-1 Fab fragments (PSGL-1), 200 μg of mAb RB40 against mouse P-selectin (P-Sel), 200 μg of mAb UZ4 against mouse E-selectin (E-Sel). Immigration of cells into the noninflamed control skin region of the same mice is depicted as solid bars. For each determination, four mice were analyzed. Experiments shown by the left graph were performed with a different preparation of Th1 cells than the experiments depicted by the right graph. Numbers on the left refer to the percentage of injected cells that were found in the analyzed skin area of 2.5 cm2.
Our data demonstrate that PSGL-1 is the major P-selectin ligand on Th1 cells and that it is relevant for the entry of these cells into inflamed skin. The fact that anti–PSGL-1 Fab fragments inhibited Th1 cell migration less efficiently than the anti–P-selectin mAb is probably due to the lower blocking efficiency of the Fab fragments as compared with complete antibodies. In in vitro adhesion assays, 50 μg/ml anti–PSGL-1 Fab inhibited adhesion of mouse 32Dcl3 neutrophilic cells to P-selectin–Ig by only 35%, while 20 μg/ ml complete anti–PSGL-1 antibodies blocked adhesion by 70% (not shown).
Although the cell surface expression level of PSGL-1 on Th1 and Th2 cells is similar, only PSGL-1 on Th1 cells is able to mediate binding to P-selectin. It is well known that PSGL-1 requires certain posttranslational modifications for binding to P-selectin, such as fucosylation (14), tyrosinesulfation (30–32), and branched carbohydrate side chains generated by the core-2 enzyme (33–35). It is likely that one or several of these modifications are upregulated in the course of the differentiation process that leads to the acquisition of the Th1 cell phenotype. Indeed, careful analysis of the apparent molecular weight of PSGL-1 on Th1 and Th2 cells revealed that the P-selectin–binding form of PSGL-1 on Th1 cells has a slightly larger apparent molecular weight than the nonfunctional form on Th2 cells (Fig. 1, lanes 3 and 5). Besides the different repertoire of cytokines, the functional form of PSGL-1 is a cell surface molecule that distinguishes Th1 cells from Th2 cells. The expression of active PSGL-1 is relevant for the migration of Th1 cells into inflamed skin.
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft to D. Vestweber, R. Hallmann, and A. Hamann and from the Mildred Scheel Stiftung to A. Hamann.
References
- 1.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 3.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature (Lond) 1991;349:796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y, Shaw S, Graber N, Gopal TV, Horgan KJ, Van Seventer GA, Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature (Lond) 1991;349:799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- 5.Walcheck B, Watts G, Jutila MA. Bovine γ/δ T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J Exp Med. 1993;78:853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luscinskas FW, Ding H, Lichtman AH. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+T lymphocytes on tumor necrosis factor α-activated vascular endothelium under flow. J Exp Med. 1995;181:1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damle NK, Klussman K, Dietsch MT, Mohagheghpour N, Aruffo A. GMP-140 (P-selectin/CD62) binds to chronically stimulated but not resting CD4+T lymphocytes and regulates their production of proinflammatory cytokines. Eur J Immunol. 1992;22:1789–1793. doi: 10.1002/eji.1830220718. [DOI] [PubMed] [Google Scholar]
- 8.Rossiter H, van Reijsen F, Mudde GC, Kalthoff F, Bruijnzeel-Koomen CAFM, Picker LJ, Kupper TS. Skin disease-related T cells bind to endothelial selectins: expression of cutaneous lymphocyte antigen (CLA) predicts E-selectin but not P-selectin binding. Eur J Immunol. 1994;24:205–210. doi: 10.1002/eji.1830240132. [DOI] [PubMed] [Google Scholar]
- 9.Alon R, Rossiter H, Wang X, Springer TA, Kupper TS. Distinct cell surface ligands mediate T lymphocyte attachment and rolling on P and E selectin under physiological flow. J Cell Biol. 1994;127:1485–1495. doi: 10.1083/jcb.127.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipping PG, Huang XR, Berndt MC, Holdsworth SR. P-selectin directs T lymphocyte-mediated injury in delayed-type hypersensitivity responses: studies in glomerulonephritis and cutaneous delayed-type hypersensitivity. Eur J Immunol. 1996;26:454–460. doi: 10.1002/eji.1830260228. [DOI] [PubMed] [Google Scholar]
- 11.Vachino G, Chang XJ, Veldman GM, Kumar R, Sako D, Fouser LA, Berndt MC, Cumming DA. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J Biol Chem. 1995;270:21966–21974. doi: 10.1074/jbc.270.37.21966. [DOI] [PubMed] [Google Scholar]
- 12.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norgard KE, Moore KL, Diaz S, Stults NL, Ushiyama S, McEver RP, Cummings RD, Varki A. Characterization of a specific ligand for P-selectin on myeloid cells: A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993;268:12764–12774. [PubMed] [Google Scholar]
- 14.Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 15.Moore KL, Patel KD, Bruehl RE, Fugang L, Johnson DA, Lichenstein HS, Cummings RD, Bainton DF, McEver RP. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- 17.Mosmann TR, Coffmann RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–176. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 19.Austrup F, Vestweber D, Borges E, Löhning M, Bräuer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T helper 1 but not of T helper 2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 20.Levinovitz A, Mühlhof J, Isenmann S, Vestweber D. Identification of a glycoprotein ligand for E-selectin on mouse myeloid cells. J Cell Biol. 1993;121:449–459. doi: 10.1083/jcb.121.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austrup F, Rebstock S, Kilshaw PJ, Hamann A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa specific homing. Eur J Immunol. 1995;25:1487–1491. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 22.Assenmacher M, Schmitz JA, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-β and in interleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 23.Hallmann R, Zimmermann U, Sorokin LM, Needham L, Von-der-Mark K. Adhesion of leukocytes to the inflamed endothelium. Scand J Rheumatol Suppl. 1995;101:107–109. doi: 10.3109/03009749509100909. [DOI] [PubMed] [Google Scholar]
- 24.Bosse R, Vestweber D. Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur J Immunol. 1994;24:3019–3024. doi: 10.1002/eji.1830241215. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Galipeau J, Kozak CA, Furie BC, Furie B. Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood. 1996;87:4176–4186. [PubMed] [Google Scholar]
- 26.DeLuca M, Dunlop LC, Andrews RK, Flannery JV, Ettling R, Cumming DA, Veldman GM, Berndt MC. A novel cobra venom metalloproteinase, mocarhagin, cleaves a 10-amino acid peptide from the mature N terminus of P-selectin glycoprotein ligand receptor, PSGL-1, and abolishes P-selectin binding. J Biol Chem. 1995;270:26734–26737. doi: 10.1074/jbc.270.45.26734. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 28.Hahne M, Jäger U, Isenmann S, Hallmann R, Vestweber D. Five tumor necrosis factor-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J Cell Biol. 1993;121:655–664. doi: 10.1083/jcb.121.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E-and P-selectin on myeloid cells. J Cell Biol. 1994;125:471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 31.Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins PP, Moore KL, McEver RP, Cummings RD. Tyrosine sulfation of P-selectin glycoprotein ligand-1 is required for high affinity binding to P-selectin. J Biol Chem. 1995;270:22677–22680. doi: 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]
- 33.Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked Poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- 34.Li F, Wilkens PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 35.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]