Abstract
The hematologic consequences of infection with the noncytopathic lymphocytic choriomeningitis virus (LCMV) were studied in wild-type mice with inherent variations in their interferon (IFN)-α/β responder ability and in mutant mice lacking α/β (IFN-α/β R0/0) or γ IFN (IFN-γ R0/0) receptors. During the first week of infection, wild type mice demonstrated a transient pancytopenia. Within a given genetic background, the extent of the blood cell abnormalities did not correlate with the virulence of the LCMV isolate but variations were detected between different mouse strains; they were found to depend on their IFN-α/β responder phenotype. Whereas IFN-γ R0/0 mice were comparable to wild-type mice, IFN-α/β R0/0 mice exhibited unchanged peripheral blood values during acute LCMV infection. In parallel, the bone marrow (BM) cellularity, the pluripotential and committed progenitor compartments were up to 30-fold reduced in wild type and IFN-γ R0/0, but remained unchanged in IFN-α/β R0/0 mice. Viral titers in BM 3 d after LCMV infection were similar in these mice, but antigen localization was different. Viral antigen was predominantly confined to stromal BM in normal mice and IFN-γ R0/0 knockouts, whereas, in IFN-α/β R0/0 mice, LCMV was detected in >90% of megakaryocytes and 10–15% of myeloid precursors, but not in erythroblasts. Although IFN-α/β efficiently prevented viral replication in potentially susceptible hematopoietic cells, even in overwhelming LCMV infection, unlimited virus multiplication in platelet and myeloid precursors in IFN-α/β R0/0 mice did not interfere with the number of circulating blood cells. Natural killer (NK) cell expansion and activity in the BM was comparable on day 3 after infection in mutant and control mice. Adaptive immune responses did not play a major role because comparable kinetics of LCMV-induced pancytopenia and transient depletion of the pluripotential and committed progenitor compartments were observed in CD80/0 and CD40/0 mice, in mice depleted of NK cells, in lpr mice, and in perforin-deficient (P0/0) mice lacking lytic NK cells. Thus, the reversible depression of hematopoiesis during early LCMV infection was not mediated by LCMV-WE–specific cytotoxic T lymphocyte, cytolysis, or secreted IFN-γ from virally induced NK cells but was a direct effect of IFN-α/β.
Viral infections are frequently associated with a transient reduction of the number of circulating blood cells as a consequence of bone marrow (BM)1 suppression. If virusinduced dysfunction of the BM is severe, secondary bacterial invasion or bleeding may be lethal for the host (1). The majority of viruses inducing abnormalities of hematopoiesis are non- or poorly cytopathic for blood cells or have no known tropism for blood cell precursors (for review see reference 2). In vivo, few data analyzing virus and/or host mechanisms of suppressed BM function are available. In vitro, some viruses may interfere with the proliferation and maturation of hematopoietic progenitors by infecting stromal fibroblasts (e.g., cytomegalovirus [3]) or BM macrophages (e.g., human immunodeficiency virus [4]). Moreover, there is evidence suggesting a critical role of the host's own immune response in causing BM suppression in several virus infections (5, 6). Lymphocytic choriomeningitis virus (LCMV) is a non-cytopathic RNA virus for which many aspects of viral immunobiology have been studied in great detail (7, 8). Although mice are the natural host and reservoir for LCMV (9, 10), occasional transmission to laboratory workers has been followed by mild abnormalities of the cellular blood count (CBC) (11). Only very rarely, a fatal hemorrhagic disease has been observed in humans (12), but it occurs in guinea pigs (9, 10). In several other arenavirus infections (e.g., Machupo, Junin, and Tamiami viruses), hemorrhagic fever, widespread necrosis in the lymphatic tissue, and a global depression of BM are consistently found (9, 13). Few studies analyzed the mechanism of LCMV-induced hematopoietic dysfunction (14–16); some have speculated that LCMV-induced radioresistant NK cells caused the hematopoietic abnormalities (17). The respective role of the effects of soluble cytokines, NK or T cells or LCMV itself, interfering with BM function, however, were not assessed in these studies.
The control of a primary LCMV infection and successful virus elimination from infected organs depends on virus specific CTL (18). These protective CTL have also been shown to cause immunopathological disease dependent upon virus and host parameters and according to the dominant early location of the infection, i.e., choriomeningitis after intracerebral infection, hepatitis after systemic infection, and footpad swelling after local subcutaneous infection (19). Therefore, it would be expected that at least part of the BM suppression seen during LCMV infection might also be caused by T cell–mediated immunopathology. Type I IFNs (IFN-α/β) and type II IFNs (IFN-γ) play an important role in the early phase of infection. They inhibit unlimited viral replication before antiviral effector CTL are induced and thereby prevent exhaustion of virusspecific CTL (20, 21). In parallel with the IFN-α/β response, NK cells become activated, proliferate, and infiltrate infected tissue (22, 23). Effector functions of NK cells elicited during LCMV infection involve perforin-mediated cytolysis of infected target cells (24) and/or cytokines produced by NK cells, such as IFN-γ (25) or TNF-α (26). In tissue culture systems, these cytokines have been found to inhibit hematopoietic progenitor cell growth (27). In one report, minute amounts of Fas ligand on the surface of freshly isolated murine NK cells were demonstrated; however, there is still contradictory evidence for Fas-mediated cytotoxicity of NK cells against Fas-expressing targets and its functional role in vivo remains to be established (28, 29).
Therefore, murine LCMV infections offer an experimental model to analyze pathogenesis of BM suppression and virus-induced hemorrhagic fever. The present study attempts to explore the respective role of innate resistance or immune mechanisms versus that of specific immunity in the dysregulation of hematopoiesis in vivo during the acute phase of LCMV infection. By comparing wild-type with mutant mice, which had inactivated IFN-α/β receptors (IFN-α/β R0/0) or IFN-γ receptors (IFN-γ R0/0), the following questions were addressed. First, are IFNs, acting via IFN R expressed on pluripotential or on lineage-committed hematopoietic progenitors, involved in early LCMVinduced hematopoietic suppression, and what is the respective role of IFN-α/β and that of IFN-γ? Second, does the absence of IFN R expression interfere with LCMV-induced NK cell activation, and what are the consequences of possibly activated NK cells for hematopoietic cells in vivo? Third, the LCMV-induced effects on circulating blood cells and blood cell precursors were analyzed under conditions of NK cell depletion with a mAb, absent expression of Fas on hematopoietic cells, or lack of perforin produced by NK cells, or of deficiency of CD8+ or CD4+ T cells. The results show a major role for IFN-α/β but neither of IFN-γ, nor of perforin nor of Fas and neither of NK nor of T cells during the first 7 d of LCMV infection. Importantly, in absence of a functional IFN-α/β R, extensive virus replication of LCMV in megakaryocytes and, to a lesser extent, in myeloid precursors appeared not to interfere with maturation and release of differentiated blood cells, including platelets, into circulation.
Materials and Methods
Mice.
Mice with a homozygous inactivation of the IFN-α/β receptor (IFN-α/β R0/0) and those lacking the IFN-γ receptor (IFN-γ R0/0) have been described previously (21, 30). These mutant mice have a pure 129Sv/Ev (H-2b) genetic background and 129Sv/Ev mice were used as controls in all experiments. The antiviral immune response of knockout mice devoid of CD8+ (CD80/0) (31) or CD4+ (CD40/0) (32) T cells or with a disrupted perforin gene (P0/0) (24) have been reported before. CD80/0 mutants have a pure C57BL/6 (H-2b) background and CD40/0 mice have been backcrossed four times to C57BL/6 mice. CD40/0, CD80/0, and P0/0 mice were compared with normal C57BL/6 mice in all experiments. Knockout mice, C57BL/6, DBA/2, and BALB/c mice were obtained from the breeeding colony at the Institut für Versuchstierkunde (University of Zürich, Switzerland); CBA/J and C57BL/6 lpr/lpr mice were purchased from BRL (Füllinsdorf, Switzerland), and C3H/HeJ from Harlan Olac, Ltd. (Zeist, Netherlands). All mouse strains were kept under specific pathogen-free conditions. Sex-matched mice of 8–12 wk of age were used. The animal protection law of the Kanton of Zürich limits numbers of mice to be used in experiments, particularly if disease is severe; therefore, experiments generally were repeated two times with groups of 2–4 mice.
Virus.
LCMV-WE was originally obtained from Dr. F. Lehmann-Grube (Hamburg, Germany) (9) and LCMV-Docile (a variant isolated from a LCMV-WE(UBC)-carrier mouse) was obtained from Dr. C.J. Pfau (Troy, NY) (33) as a plaque-purified second passage virus. The original LCMV–Armstrong isolate was obtained from Dr. M.B.A. Oldstone (Scripps Clinic and Research Foundation, La Jolla, CA) (34). Second passage virus derived from plaque-purified isolates was propagated on BHK cells (Armstrong) or L929-fibroblast cells (WE) or MDCK cells (Docile). The viruses were titrated using an immunological focus assay, which is two to threefold less sensitive than virus titers detected by in vivo infection (35). LCMV titers of BM cells are presented as PFU per 106 nucleated BM cells.
Peripheral Blood Values and BM Cytology.
Blood samples (40 μl) were obtained from the retro bulbar plexus of ether-anesthetized mice; they were diluted 1:5 in electro-coated EDTA microtainer tubes (Becton Dickinson, Mountain View, CA) containing DMEM + 5% FCS. CBC were then determined in a hemocytometer using mouse-specific discriminator settings (TECHNICON H1®, Technicon Instruments Corp., Tarrytown, NY), and the differential white blood cell count was defined microscopically by scoring blood smears stained with May-Grünwald-Giemsa. Reticulocyte counts were quantitated by flow cytometry (SYSMEX R 3000®, Toa Medical Electronics, Kobe, Japan). BM was obtained by flushing femora and/or tibiae with a 25-gauge needle; differential counts of 200 cells were determined on MayGrünwald-Giemsa, chloracetate esterase, α-naphthyl butyrate esterase, or Sudan black B stained smears.
Pluripotential and Committed Progenitor Assays.
The pluripotential hematopoietic progenitor pool (CFU-S) of LCMV-infected mice was enumerated as described before (36). Recipient mice were immunized with 102 PFU LCMV-WE. 2–3 wk later these mice were lethally irradiated (9 Gy), and within 3 h were reconstituted intravenously with 104 or 105 syngeneic sex-matched BM cells from acutely infected or uninfected control mice. The rationale in preimmunizing recipient mice was to prevent growth inhibition of the transplanted CFU-S by cotransferred virus from infected mice, which activates innate effector mechanisms in the naive unprimed recipient and thus would alter the CFU-S content in question (14). 8 d after irradiation, the recipient mice were killed and the spleens fixed in Tellesnicky's fixative. Individual colonies visible on the surface of the spleen were counted under a dissecting microscope and the numbers of CFU-S per femur were calculated.
Cell suspensions from BM in isotonic balanced salt solution (BSS) were counted and assessed for viability by Trypan blue exclusion before testing in vitro for colony formation. The culture mixture was prepared in IMDM supplemented with 5 × 10−5 mol/l 2-ME, penicillin–streptomycin, 0.9% methylcellulose, 30% FCS, 1% BSA in the presence of optimized concentrations of growth factors (mIL-3 [30 U/ml] and mGM-CSF [30 U/ml] for CFUGM; mIL-3 [30 U/ml] and hEpo [5 U/ml] for BFU-E; mIL-3 [30 U/ml] and mTPO [500 ng/ml] for CFU-Meg). Recombinant murine IL-3 and GM-CSF were obtained from PharMingen, Inc. (San Diego, CA), recombinant human erythropoietin was purchased from Cilag AG (Schaffhausen, Switzerland), and recombinant murine thrombopoietin (mTPO) was a gift from Genentech, Inc. (South San Francisco, CA). Total BM cells were plated at a concentration of 5 × 104 cells/ml in duplicate plates. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 5–7 d (CFU-Meg), 7 d (CFU-GM), or 8 d (BFU-E) and individual colonies were scored by morphology. Colonies were defined as containing at least three large refractile cells for CFU-Meg (37) and >50 cells for CFU-GM. For the microscopic identification of the cell type present, colonies were periodically picked, spread on glass slides, and stained with MayGrünwald-Giemsa or acetylcholinesterase (megakaryocytes) for confirmation. Each experiment was performed using individual unpooled BM cells and results from four to eight culture plates were combined for each determination. The total number of colonies per femur was calculated using the cellularity obtained for the individual BM.
Immunocytochemistry Procedures.
BM smears on slides were fixed in acetone for 10 min. The rat anti-LCMV mAb VL-4 was used as primary Ab and detected by an indirect immunoenzymatic staining procedure using peroxidase-labeled goat anti–rat Ig (Tago; diluted 1:30), followed by alkaline phosphatase–labeled donkey anti–goat Ig (Jackson Immunoresearch Laboratories, Inc., West Grove, PA; diluted 1:30). Smears were counterstained with Meyer's hemalum (Kantonsapotheke, Zürich, Switzerland) for 2 min. Percentages of infected BM cells were quantitated microscopically by counting at least 200 cells from one or more smears per individual mouse. The infected cell type was readily defined by the cytomorphology after counterstaining.
Antibody and Poly I/C Treatment.
Mice were treated intraperitoneally with 1 mg of the IL-2Rβ-specific mAb TM-β1 (rat IgG2b) (38) or control normal rat IgG (Sigma Chemical Co., St. Louis, MO) in 500 μl of PBS. One single dose of Ab was injected 2 d before virus challenge. Poly I/C (Serva, Heidelberg, Germany) was administered intravenously at a concentration of 200 μg/mouse 20 h before the cytotoxicity assay.
Cytotoxicity Assays.
NK cell activity of spleen and BM cells was determined by a 51Cr-release assay as described previously (20). In brief, mice were infected intravenously 3 d previously with LCMV. Doses and virus strains used are indicated in the results. BM cells (pooled cells from both femora) were resuspended at 7 × 106/ml in MEM plus 2% FCS. YAC-1 target cells were labeled with 1 μCi of 51Cr sodium chromate for 1 h at 37° C, washed twice, and resuspended at 105/ml. Threefold dilutions of spleen cells were incubated with 100 μl of targets in 96-well microtiter round-bottomed plates for 5 h. A total of 70 μl of the supernatant was assayed for released 51Cr. The percent-specific release of 51Cr was calculated as ([experimental release − spontaneous release] × 100/[total release − spontaneous release]). LU, a measure of relative cytolytic activity, was determined according to established methods to be the number of lymphocytes necessary to lyse 33% of the standard number of target cells during the standard test duration. LUs per femur were computed by dividing the total number of lymphocytes per organ by LU.
Flow Cytometry.
Single-cell suspensions of spleen or BM were preparated at 4°C in BSS containing 2% FCS and 0.2% NaN3. Cell surface markers were quantified by staining with mAbs conjugated with fluorochromes; the antibodies used were the following: anti-CD4 and anti-CD8 (Becton Dickinson), anti-NK 1.1 (PharMingen), anti-TER 119 (PharMingen). To analyze NK expansion, cells were triple stained with FITC-conjugated anti-CD8, FITC-conjugated anti-CD4, PE-conjugated NK 1.1, a biotinylated anti-erythroid (TER 119) Ab, and a Tricolor–streptavidin conjugate. Multiparameter analysis was performed with a FACScan® (Becton Dickinson) using logarythmic scales. Viable cells were gated by forward and side scatter of light.
Statistics.
Hematologic data are presented as means ± SD unless stated otherwise. Virus titers are expressed as log10 of PFU per organ. Comparisons were made using the two-tailed unpaired Student's t test, the analysis of variance (ANOVA), and the Scheffe's post hoc procedure as appropriate. A P value of less than 0.05 was regarded as significant.
Results
Abnormalities of the CBC in Acute LCMV Infection: Dependence upon Virus Isolate, Dose, and Genetic Background of Mice.
The transient depression of the peripheral CBC during the first week of LCMV infection varied according to the virus dose and the different genetic backgrounds of the murine host. For all virus isolates tested, maximally decreased blood values were observed between days 3 and 5 after infection and were most prominent in the reticulocyte, granulocyte, and platelet compartments (in decreasing order of suppression). There was some variation in the absolute CBC of uninfected control mice between the different mouse strains (e.g., mean reticulocyte count: 182 × 106/ml in C57BL/6; 247 × 106/ml in BALB/c mice); therefore, the relative decrease from baseline values (percent) was compared (as shown for reticulocytes; Table 1). Within one mouse strain, the extent of suppression of blood cell numbers did weakly correlate with the LCMV isolate, i.e., all blood cell parameters were comparably reduced on days 3–5 after infection in Armstrong-, WE-, or Docileinfected mice. However, between different mouse strains the CBC varied after infection with low LCMV doses (2 × 102 PFU): C57BL/6, CBA/J, and C3H/HeJ had lower relative reticulocyte (Table 1) and granulocyte counts than DBA/2 and BALB/c mice; less stringently, the same pattern was observed in the platelet numbers (e.g., CBA/J, 83%; C3H/ HeJ, 49%; and C57BL/6, 37% below baseline after LCMVWE 2 × 102 PFU). The magnitude of these variations between different mouse strains was small but significant and correlated with the IFN-α/β responder status (i.e., IFN-α/β high responders had a lower CBC than IFN-α/β low responders) as reported after infection with LCMV (39, 40). In contrast, infection with a high dose of LCMV (2 × 106 PFU) induced an extensive decrease of all blood cell parameters, to an extent that was comparable for all the mouse strains tested (Table 1, granulocytes 90–95%, platelets 78– 95% below baseline). After infection with all three LCMV isolates, reductions in RBC counts were small. If the halflife of the mouse RBCs is taken into account (around 50 d), it is probable that decreases from baseline were mainly due to repetitive individual blood sampling. Thus, high doses of LCMV (2 × 106 PFU) induced a drastic transient pancytopenia with minimal values 3–5 d after infection, independently of the virus isolate or the genetic background of mice. To avoid variations, high doses of LCMV-WE therefore were used for all subsequent experiments.
Table 1.
Influence of LCMV Isolate, Virus Dose, and IFN Responder Status on Circulating Reticulocytes 3 d after LCMV Infection
| Mouse strain | H-2 | IFN peak titer (LCMV)‡ | Percent reduction of reticulocyte numbers in blood | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low dose (2 × 102 PFU)* | High dose (2 × 106 PFU)* | |||||||||||||||
| Armstrong | WE | Docile | Armstrong | WE | Docile | |||||||||||
| C57BL/6 | b | h | 50 ± 4 | 60 ± 7 | 52 ± 8 | 96 ± 1 | 96 ± 1 | ND | ||||||||
| DBA/2 | d | l | 20 ± 9 | 26 ± 9 | 20 ± 11 | 95 ± 2 | 95 ± 1 | ND | ||||||||
| BALB/c | d | l | 17 ± 2 | 21 ± 8 | 16 ± 2 | 84 ± 3 | 89 ± 3 | ND | ||||||||
| CBA/J | k | h | 64 ± 1 | 74 ± 1 | 60 ± 1 | 96 ± 1 | 96 ± 1 | ND | ||||||||
| C3H/HeJ | k | h | 61 ± 7 | 69 ± 7 | 52 ± 4 | ND | ND | ND | ||||||||
Mice were infected i.v. with the indicated doses of LCMV. Reticulocyte numbers were determined cytofluorometrically in blood collected from the retrobulbar plexus on day 3 after infection; values are presented as percent below baseline values, as determined in uninfected control mice. Indicated are means ± SD of three mice.
Peripheral Blood Values of IFN R-deficient Mice after Acute Infection with LCMV.
Because the responder phenotype of mice seemed to influence the severity of LCMV-induced blood cell abnormalities, peripheral hematologic parameters after LCMV infection were studied in mice with inactive receptors for IFN-α/β (IFN-α/β R0/0) or for IFN-γ (IFN-γ R0/0). The hematologic status of uninfected receptor-deficient and wild-type mice maintained under specific pathogen-free conditions was identical (data not shown). Infection of 129Sv/Ev wild-type mice with a high dose of LCMV-WE (2 × 106 PFU) resulted in the same kinetics of peripheral pancytopenia as seen in infected mice of other genetic backgrounds (Table 2). Starting on day 1 after infection, maximal depression of blood cell values was seen on days 3–5 with predominant abnormalities in the circulating reticulocyte (97% below baseline), platelet (65% below baseline), and neutrophil (72% below baseline) compartments. All peripheral parameters returned near baseline values on day 7. A similar time course was observed for infected IFN-γ R0/0 mice. In contrast, LCMV-WE–infected IFN-α/β R0/0 mice showed stable hematologic parameters comparable to that of uninfected controls during the entire study period. When compared with infected wild-type or IFN-γ R0/0 mice they had a 180–200-fold higher reticulocyte, a three- to fivefold increased platelet, and a two- to threefold increased neutrophil count on day 3 after infection (Table 2).
Table 2.
Kinetics of Peripheral Blood Values of Wild-type, IFN-α/β R0/0, and IFN-γ R0/0 Mice after Acute Infection with LCMV-WE
| Genotype | Days after infection* | RBC‡ | Reticulocytes | Platelets | WBC | Neutrophils | Eosinophils | Lymphocytes | Monocytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | ||||||||||||||||||
| (Sv129/Ev) | 1 | 9.1 ± 0.5§ | 110.2 ± 9.2 | 635 ± 44 | 3.3 ± 1.0 | 0.9 ± 0.2 | <0.1 | 2.4 ± 0.7 | <0.1 | |||||||||
| 3 | 9.0 ± 0.5 | 5.2 ± 1.6 | 216 ± 28 | 2.7 ± 0.4 | 0.7 ± 0.1 | <0.1 | 2.0 ± 0.3 | <0.1 | ||||||||||
| 7 | 8.0 ± 0.2 | 106.5 ± 21.0 | 381 ± 42 | 15.1 ± 3.6 | 7.1 ± 1.7 | <0.1 | 7.6 ± 1.8 | 0.3 ± 0.1 | ||||||||||
| IFN-α/β R0/0 | 1 | 9.4 ± 0.7 | 173.7 ± 18.9 | 650 ± 33 | 9.3 ± 1.1 | 2.2 ± 0.4 | 0.2 ± 0.02 | 6.5 ± 0.6 | 0.4 ± 0.03 | |||||||||
| 3 | 8.9 ± 0.4 | 183.8 ± 23.3‖ | 530 ± 26¶ | 7.1 ± 0.2 | 2.4 ± 0.7** | 0.1 ± 0.01 | 4.3 ± 0.2 | 0.2 ± 0.01 | ||||||||||
| 7 | 8.6 ± 0.3 | 217.2 ± 24.9 | 580 ± 14 | 10.2 ± 1.2 | 3.5 ± 0.4 | 0.2 ± 0.03 | 6.2 ± 0.8 | 0.3 ± 0.04 | ||||||||||
| IFN-γ R0/0 | 1 | 9.1 ± 0.2 | 124.6 ± 25.1 | 508 ± 34 | 1.5 ± 0.3 | 0.4 ± 0.1 | <0.1 | 1.1 ± 0.2 | <0.1 | |||||||||
| 3 | 9.1 ± 0.3 | 1.1 ± 1.0‖ | 124 ± 11¶ | 4.4 ± 0.6 | 1.2 ± 0.2** | <0.1 | 3.1 ± 0.5 | <0.1 | ||||||||||
| 7 | 8.1 ± 0.4 | 130.5 ± 29.5 | 374 ± 50 | 13.7 ± 1.6 | 2.5 ± 0.4 | <0.1 | 10.5 ± 1.3 | 0.73 ± 0.15 |
Sex-matched 8–12-wk-old mice of the indicated genotype (129Sv/Ev background, H-2b) were infected i.v. with LCMV-WE (2 × 106 PFU per mouse; four mice per group). Blood was collected from the retrobulbar plexus of individual mice at the indicated timepoints. Cellular blood values were quantified in hemocytometer and differential blood counts were determined microscopically from blood smears.
RBC, total erythrocytes (×109/ml); WBC, total leucocytes (×106/ml); reticulocytes, platelets, neutrophils, eosinophils, lymphocytes, and monocytes (×106/ml).
Data represent means ± SD. Student's t-tests for differences between LCMV-WE–infected IFN-α/β R0/0 vs IFN-γ R0/0 mice on day 3 after infection:
P <0.0001;
P <0.0001;
P <0.01.
BM Analysis of LCMV-infected, IFN R-deficient, and Wildtype Mice.
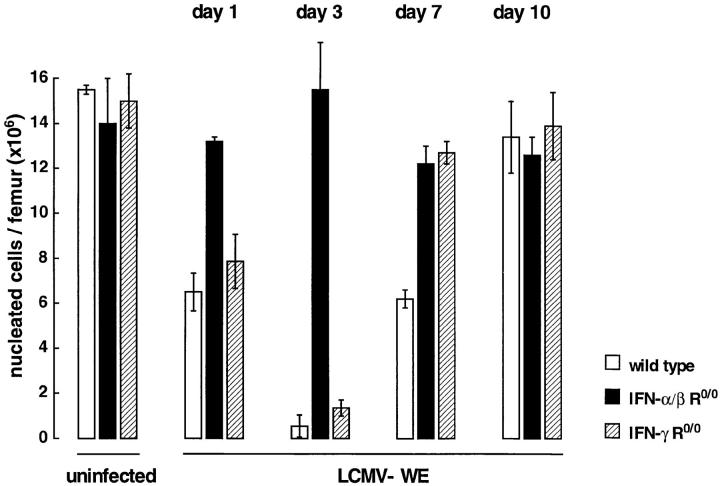
BM function was evaluated in wild-type and IFN R mutants after acute infection with LCMV–WE (2 × 106 PFU). Femoral cellularity of wild-type and IFN-γ R0/0 mice was reduced by half on day 1 after infection and reduced to a minimum of 7% in wild-type mice and 11% of baseline values in IFN-γ R0/0 mice on day 3, respectively (Fig. 1). Thereafter, total cellularity returned to normal values in IFN-γ R0/0 mice by day 7 and in wild-type mice by day 10. In contrast, IFN-α/β R0/0 mice showed minimal changes in femoral cell content during the entire study period. In parallel, splenic cellularity decreased considerably until day 3 in wild-type and IFN-γ R0/0 mice but remained unchanged in IFN-α/β R0/0 mice (data not shown). Cytological analysis of BM preparations on day 3 after infection revealed a marked deficiency of the nonmitotic mature cell population in wild-type and IFN-γ R0/0 mice but not in IFN-α/β R0/0 mice (Table 3). In wild-type and IFN-γ R0/0 mice, LCMV-WE infection resulted in a 2–2.4-fold reduction of erythroid elements, which was more apparent when absolute cell numbers per femur were compared (wild type 0.4 × 106; IFN-γ R0/0, 0.7 × 106; IFN-α/β R0/0, 4.5 × 106). Polymorphnuclear granulocytes of wildtype and IFN-γ R0/0 mice were reduced 3–3.4-fold and the proportion of early myeloid precursors (blasts, promyelocytes) was significantly increased by a factor of 2.5 when compared with IFN-α/β R0/0 mice. The relative increase of marrow lymphocytes in wild-type and IFN-γ R0/0 mice on day 3 after infection had disappeared by day 7; monocytes were slightly increased in both mutants and wild-type mice 7 d after LCMV-WE infection. The amount of megakaryocytes was determined semiquantitatively and was two- to fourfold reduced in wild-type and IFN-γ R0/0 mice, but unchanged in IFN-α/β R0/0 mice during the entire study period.
Figure 1.
Kinetics of BM cellularity after LCMV-WE infection. Mice of wild type genotype (open columns) or mutant mice with inactivated α/β IFN (closed columns) or γ IFN (hatched columns) receptors were infected intravenously with LCMV-WE (2 × 106 PFU). At the indicated timepoints, mice were killed and the absolute number of nucleated BM cells per femur was determined. The data represent mean ± SD of 3–4 mice per group.
Table 3.
BM Cytology of Wild type, IFN-α/β R0/0, and IFN-γ R0/0 Mice Infected with LCMV-WE
| Genotype | Days after infection* | Megakaryocyte§ | Cell type (%)‡ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erythroblast | Myeloblast promyelocyte | Myelocyte metamyelocyte band | Polymorph | Monocyte | Lymphocyte | |||||||||||
| Wild type | Uninfected | 17–25 | 35.5 ± 1 | 9.5 ± 1.8 | 36.5 ± 2.8 | 7.7 ± 1.5 | 0.8 ± 0.3 | 10 ± 1.4 | ||||||||
| Wild type | 3 | 4–11 | 15.5 ± 1.4 | 28.5 ± 1.3 | 37.5 ± 2.5 | 4.0 ± 1 | <0.5 | 14.5 ± 2.3 | ||||||||
| IFN-α/β R0/0 | 3 | 21–26 | 37.5 ± 1.5 | 10.5 ± 0.5 | 33.5 ± 2.6 | 12.0 ± 2 | 1.0 ± 0.4 | 5.5 ± 0.1 | ||||||||
| IFN-γ R0/0 | 3 | 8–13 | 17.0 ± 1.8 | 28.5 ± 1.8 | 39.5 ± 3.2 | 3.5 ± 0.5 | <0.5 | 11.5 ± 0.3 | ||||||||
Sex-matched mice (8–12 wk) of different genotypes (three mice per group) were infected with LCMV-WE (2 × 106 PFU). At the time points indicated, BM smears were stained with May-Günwald/Giemsa, chloracetate esterase, α-naphthyl butyrate esterase, and Sudan black B. Differential counts were obtained microscopically by counting 200 or more cells.
Data are means ± SD of the relative number of total cells per lineage. Eosinophils were included in granulocyte counts.
Numbers represent megakaryocytes per visual field at a magnification of ×80. They were determined semiquantitatively by counting at least four visual fields.
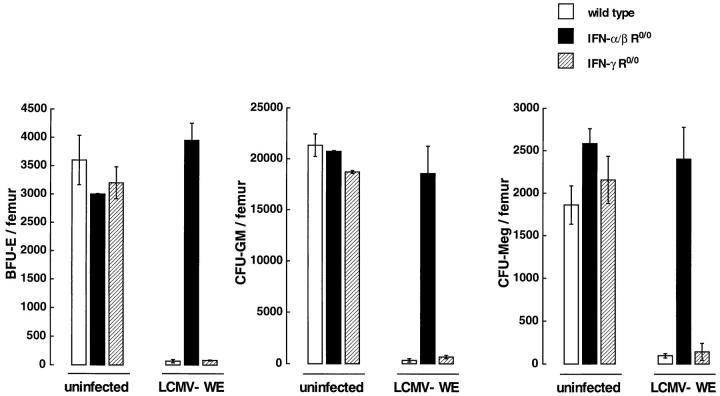
Analysis of committed hematopoietic progenitors 3 d after infection with LCMV-WE showed a distinct effect on different lineages in the BM of wild-type and IFN-γ R0/0 mice: the absolute numbers of BFU-E and CFU-GM per femur were 30-fold below baseline levels and, to some lesser extent, also the numbers of CFU-Meg were reduced (20-fold) (Fig. 2). This was in contrast with IFN-α/β R0/0 mice, which, when compared with uninfected control mice, had normal or slightly increased numbers of all lineagecommitted progenitors 3 d after LCMV-WE infection. Infection with LCMV of wild-type and of IFN-γ R0/0 mice resulted in much smaller sizes of colonies and the relative frequencies of all CFUs per plated BM cells were at least 10-fold reduced as compared with IFN-α/β R0/0 mice.
Figure 2.
Lineage-committed precursor cells in wild-type (open columns) or mutant (closed columns, IFN-α/β R0/0; hatched columns, IFN-γ R0/0) mice 3 d after LCMV-WE (2 × 106 PFU) infection. Results are presented as the mean number (± SD) of BFU-E, CFU-GM, or CFUMeg for duplicate methylcellulose cultures of total BM cells per femur. Pooled data from two independent experiments with 2–3 individual mice per group are shown.
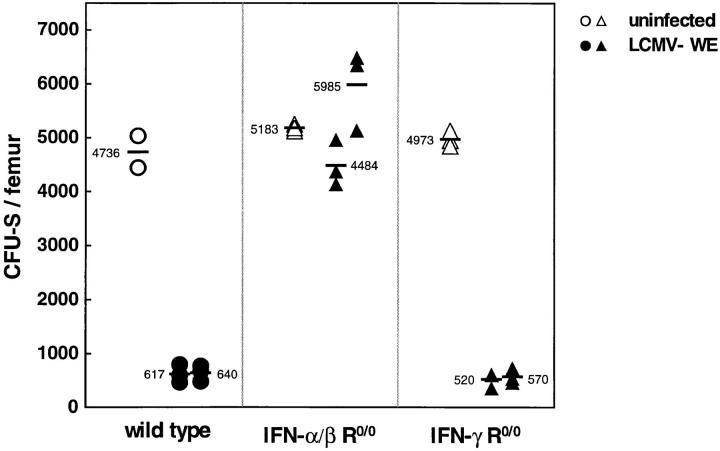
We then examined the effect of LCMV-WE infection on the number of primitive clonal progenitors (CFU-S) depending on their expression of IFN R. Syngeneic lethally irradiated LCMV-WE immune mice of the 129Sv/Ev wildtype strain were used as recipients in these experiments. The absolute number of CFU-S per femur 3 d after infection with LCMV-WE was ∼10-fold reduced in wildtype and IFN-γ R0/0 mice as compared with uninfected control mice of the same genotype (Fig. 3). In contrast, IFN-α/β R0/0 mice had a normal or even higher frequency of CFU-S after LCMV-WE infection. Obvious differences in the mean size of the CFU-S obtained were observed, i.e., spleen colonies of uninfected wild-type and LCMV-WE-infected IFN-α/β R0/0 mice had a three- to fourfold increase in diameter as compared with infected wild-type and IFN-γ R0/0 mice.
Figure 3.
Number of pluripotential hematopoietic progenitors in the BM 3 d after infection with LCMV-WE (2 × 106 PFU). 105 syngeneic BM cells of uninfected (open symbols) or infected (closed symbols) wild-type (129Sv/Ev, H-2b) or mutant (IFN-α/β R0/0 or IFN-γ R0/0, H-2b) donor mice were injected intravenously into lethally irradiated LCMV-immune recipient wild-type mice (129Sv/Ev, H-2b). CFU-S in the spleens were determined 8 d later. Each dot shows the number of colonies of an individual recipient. The horizontal lines represent the mean number of colonies per femur transferred from one individual donor mouse. The mean CFU-S of two individual donor mice per group are shown.
These results indicate that the abnormalities of circulating blood cells observed early in LCMV-WE infection were caused by a marked deficiency of all functional compartments of the BM. The reduction of the frequency of the pluripotential and the lineage-committed progenitors was much greater than that of the cytomorphologically differentiated population. This transient, LCMV-induced suppression of BM was abrogated in mice lacking a functional IFN-α/β R, but not in mice deficient of the IFN-γ R.
Replication of LCMV-WE in Hematopoietic Cells Depending upon IFN R Expression.
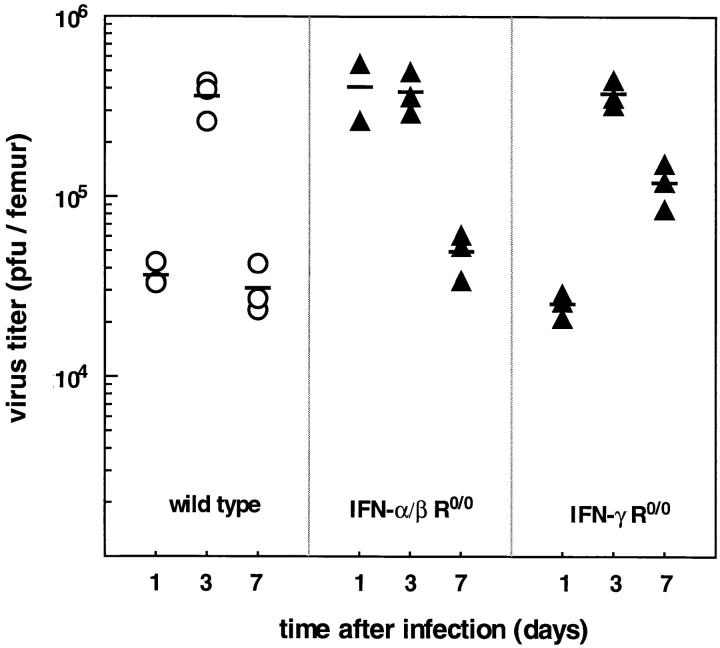
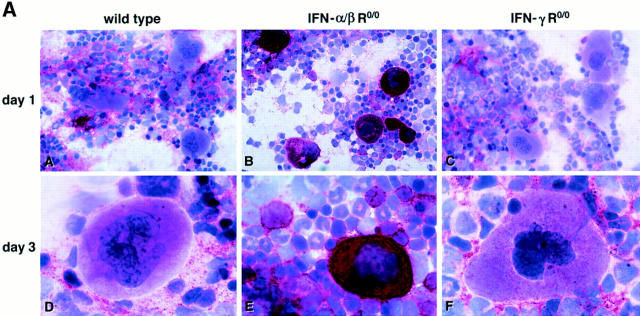
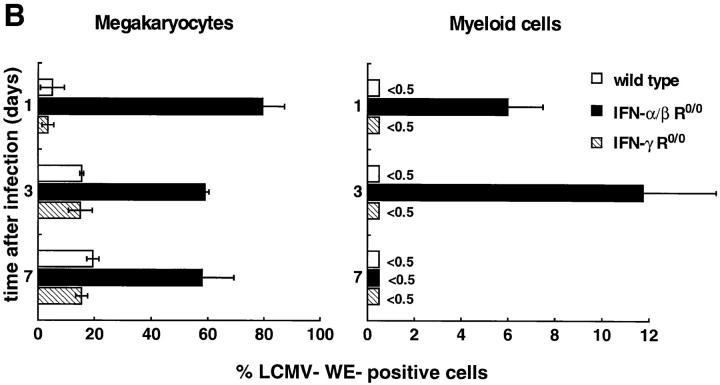
In vivo susceptibility to LCMV infection of wild-type and IFN R-deficient hematopoietic BM cells was evaluated for a high-dose (2 × 106 PFU) challenge with LCMV-WE. Wild-type and IFN-γ R0/0 mice showed a similar kinetics of LCMV replication with slightly higher titers in IFN-γ R0/0 mice 7 d after infection (Fig. 4). In contrast, IFN-α/β R0/0 mice exhibited an enhanced initial replication, i.e., the highest titer in the BM was reached already on day 1. All three groups showed a comparable maximal viral load of ∼5.6 log10 PFU/femur 3 d after virus inoculation. Thus, under high-dose conditions, absence of the IFN-α/β R favored uncontrolled viral replication maximally in the initial phase of infection, but overall maximal viral loads in BM were not affected by the expression of IFN receptors (note that IFN-α/β R0/0 mice become persistently infected with LCMV but titers are 1–2 log10 lower in the chronic state than the peak virus titer measured on day 3 [21, 41]). The kind of BM cell permissive for LCMV replication was characterized in wild-type, IFN-α/β R0/0, and IFN-γ R0/0 mice at different timepoints after infection (Fig. 5 A). Viral antigen was detected by an LCMV-WE–specific mAb on BM smears; in parallel, the type of infected hematopoietic cell was identified microscopically by cytomorphologic criteria by counterstaining with Meyer's hemalum. The prevalence of LCMVWE–positive morphologically differentiated hematopoietic cells in wild-type and IFN-γ R0/0 mice was low and did not exceed 20% of megakaryocytes and 0.5% of granulocytes at any timepoint of infection (Fig. 5 B). Virtually all detectable LCMV-specific antigen in wild-type and IFN-γ R0/0 mice was confined to stromal BM cells also on day 3, at times when highest LCMV titers were recovered. In contrast, in IFN-α/β R0/0 mice >90% of megakaryocytes were positive for viral antigen 1 d after virus inoculation, ∼60% stained positively on day 3 and ⩽50% of all megakaryocytes remained infected during the following week. In addition, ∼12% predominantly immature myeloid precursors (promyelocytes, myelocytes) of IFN-α/β R0/0 BM exhibited viral antigen peaking on day 3 after LCMV-WE infection (Fig. 5, A and B). In comparison to wild-type and IFN-γ R0/0 mice, stromal BM cells of IFN-α/β R0/0 mice were infected to a comparable extent. Interestingly, erythroblasts were resistant to LCMV-WE infection in all three mouse strains tested, i.e., the frequency of LCMV-positive erythroblasts in IFN-α/β R0/0 mice was less than 1:800.
Figure 4.
Time kinetics of virus titers in the BM after infection with LCMV. Mice were infected intravenously with LCMV-WE (2 × 106 PFU) and virus titers were determined in 106 nucleated BM cells at the timepoints indicated. Data points are values for individual mice and the horizontal lines represent the mean of the indicated group.
Figure 5.
(A) Infection of stromal cells in wild-type and IFN-γ R0/0 mice versus blood precursor cells in IFN-α/β R0/0 mice. Viral protein in differentiated BM cells was detected by immunocytochemical staining 1 d (A–C) and 3 d (D–F) after infection with 2 × 106 PFU LCMV-WE. BM smears were stained with a LCMV-specific mAb followed by a peroxidase-labeled goat anti–rat Ig and by an alkaline phosphatase–labeled donkey anti–goat Ig. LCMV-WE–infected cells are colored red and uninfected cells appear blue due to counterstaining with Meyer's hemalum. Note the intense staining of viral antigen in megakaryoctes and, to a lesser extent, in myeloid precursors and stromal cells in IFN-α/β R0/0 mice (B, E). In wild-type and IFN-γ R0/0 mice, LCMV-WE is detected in substantial amounts exclusively in stromal fibroblast and endothelial cells (A, D, C, F). Original magnification, (A–C) ×500; (D–F) ×1,000. (B) Differential quantification of LCMV-infected BM cells. Fixed BM smears of mice of the indicated genotypes were stained with a LCMV-specific mAb at different timepoints after infection with LCMV-WE. The frequency of infected cells was determined microscopically by counting 200 cells per individual mouse. Bars represent the mean ± SD of the percentage of WE-positive BM cells of 2–3 individual mice per group.
NK Activity During Acute Infection with LCMV in IFN R-deficient Mice versus Wild-type Mice.
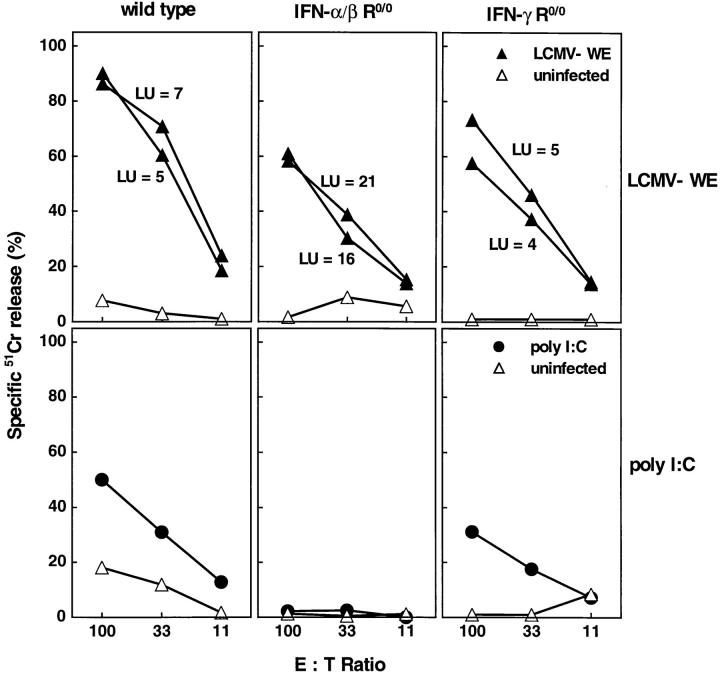
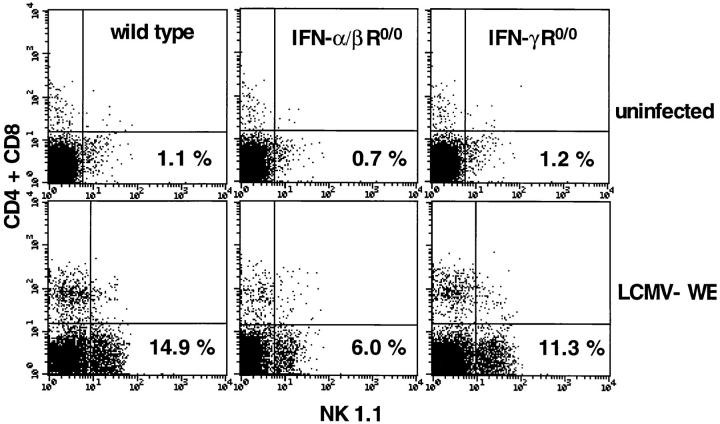
Because IFN-α/β plays an important role in NK responses and because NK cells might be involved in regulation of differentiation of stem cells, probably dependent upon MHC expression, the various mice used in this study were tested for NK activation in the BM. Earlier studies had shown that NK activity in spleens generated in IFN-α/β R0/0 mice was drastically reduced when compared with wild-type or IFN-γ R0/0 mice (21). Primary ex vivo NK-mediated cytolysis was measured on YAC-1 targets. When compared at maximal effector to target ratios, a vigorous cytolytic NK activity was observed in the wild-type mice and, to some lesser extent, in the IFNα/β R0/0 and IFN-γ R0/0 mice after infection with 2 × 106 PFU LCMV-WE (Fig. 6). Given the three- to fourfold reduction of marrow cellularity of wild-type and IFN-γ R0/0 mice, the absolute number of cytolytic NK cells in the BM (expressed as LU per femur) was two- to threefold higher in IFN-α/β R0/0 mice. Interestingly, a comparable NK response was seen in BM after infection with a low dose of LCMV-WE (2 × 102 PFU; data not shown) in both mutants and the wild-type strain, but not in IFN-α/β R0/0 mice treated with poly I/C. Next, we examined the LCMV-WE–induced NK proliferation in the BM by flow cytometry. A triple staining of total marrow cells using an erythroid-specific mAb (TER 119), a NK cell–specific mAb (NK 1.1), and a mixture of anti-CD8 and anti-CD4 was performed 3 d after infection with 2 × 106 PFU LCMV-WE. To increase sensitivity and detection levels of NK cells, subset analysis was performed with cells gated negatively on TER 119; this eliminated the high number of erythroid cells in uninfected mice. Baseline NK and CD4/CD8 percentages were comparable for uninfected wild-type and IFN R-deficient mice (<2%). After infection, the percentage of NK 1.1-positive cells in the BM increased ninefold in wild-type mice, four- to fivefold in IFN-α/β R0/0 mice, and six- to sevenfold in IFN-γ R0/0 mice (Fig. 7). To a lesser extent, an expansion of the CD4/ CD8+ population was observed in the BM of wild-type and IFN-γ R0/0 mice, whereas in the IFN-α/β R0/0 mutant mice no significant increase in the percentage of CD4/ CD8+ cells was detected on day 3 after infection. Thus, there was no correlation between NK activity in BM and susceptibility to LCMV-induced BM suppression.
Figure 6.
NK activity in the BM of acutely infected (day 3) IFN R-deficient/competent mice. Effector cells of LCMV-WE–infected (closed triangle) or uninfected (open triangle) or poly I/C-treated (closed circle) mice were incubated for 5 h with 51Cr-labeled NK-sensitive YAC-1 cells. LUs were determined as the number of lymphocytes necessary to lyse 33% of the target cells during the standard test duration. The indicated number represents the LU per BM of the lower extremities and was computed by dividing the total number of BM cells by LU. Spontaneous 51Cr release was <25%.
Figure 7.
Expansion of NK cells in the BM in response to LCMV studied by FACS® analysis 3 d after infection with LCMV-WE (2 × 106 PFU). NK cells in the BM were detected by positive expression of the NK 1.1 marker and absence of CD8 and CD4. CD4/CD8 (FITC) and NK 1.1 (PE) staining is shown for cells electronically gated on the erythroid (TER 119 Tricolor) negative BM population. Percentages are given for dot plot quadrants. Plots are repesentative for three mice per group.
Hematologic Effects in Absence of Perforin- or FAS-mediated Cytolytic Effector Functions of NK Cells or Virus-specific CTL after LCMV-WE Infection.
The correlation between LCMVWE–associated changes of blood cells and NK activity was evaluated in infected C57BL/6 mice depleted of NK cells with TM-β1 (38), in P0/0 mice, which show no cytolytic T cell or NK acitvity on susceptible target cells in vitro (24), and in lpr mutants with no functional Fas–Fas ligand interaction. LCMV-WE–infected (2 × 106 PFU) mice of all groups showed the same kinetics of blood cell depression as observed for wild-type C57BL/6 mice. On day 3 after viral inoculation, all groups had clearly reduced reticulocyte counts (82–98% below baseline), thrombocytopenia (75– 82% below baseline), two- to fivefold reduction of neutrophils, and some lymphopenia (Table 4). Mutant mice deficient for the CD8 or CD4 molecule were included in this experiment, because LCMV-specific CTL responses in these mice are either absent (CD80/0) (31) or partially (CD40/0) (32) reduced. Both of these LCMV-WE–infected mutant mouse strains showed a comparable pancytopenia on day 3, as did lpr, P0/0, normal rat IgG–treated mice, and TM-β1–treated mice. TM-β1 treatment 2 d before LCMV-WE infection efficiently eliminated NK-mediated cytolytic activity on sensitive YAC-1 targets when compared with normal rat IgG–treated controls (Table 4).
Table 4.
Peripheral Blood Values of Mice of Different Genotypes and NK-depleted C57BL/6 Mice after Infection with LCMV-WE
| Genotype or treatment with* | RBC‡ | Reticulocytes | Platelets | WBC | Neutrophils | Eosinophils | Lymphocytes | Monocytes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uninfected (P0/0) | 9.1 ± 0.3§ | 226.1 ± 36.0 | 1289 ± 124 | 8.8 ± 1.4 | 2.0 ± 0.6 | 0.13 ± 0.06 | 4.1 ± 0.8 | <0.1 | ||||||||
| CD40/0 | 9.6 ± 0.5 | 7.5 ± 2.5 | 255 ± 65‖ | 2.8 ± 0.5 | 0.9 ± 0.3 | <0.1 | 1.5 ± 0.3 | <0.1 | ||||||||
| CD80/0 | 9.2 ± 0.5 | 1.6 ± 0.5 | 272 ± 24‖ | 2.0 ± 0.5 | 0.5 ± 0.2 | <0.1 | 1.3 ± 0.3 | <0.1 | ||||||||
| P0/0 | 9.0 ± 0.4 | 8.9 ± 2.4 | 318 ± 24‖ | 2.4 ± 0.4 | 0.7 ± 0.1 | <0.1 | 1.6 ± 0.2 | <0.1 | ||||||||
| lpr | 8.8 ± 0.5 | 18.0 ± 4.5 | 228 ± 65‖ | 1.2 ± 0.3 | 0.4 ± 0.05 | <0.1 | 0.8 ± 0.1 | <0.1 | ||||||||
| ¶TM-β1 | 10.0 ± 0.7 | 10.7 ± 4.8 | 268 ± 61‖ | 3.3 ± 0.7 | 0.9 ± 0.4 | <0.1 | 2.5 ± 0.4 | <0.1 | ||||||||
| normal rat IgG | 9.3 ± 0.4 | 11.9 ± 5.9 | 235 ± 18‖ | 2.7 ± 0.6 | 0.8 ± 0.05 | <0.1 | 1.7 ± 0.3 | <0.1 |
Sex-/age-matched C57BL/6 mice of the indicated genotypes or antibody treatment (3–4 mice per group) were infected with LCMV-WE (2 × 106 PFU). Blood values 3 d after infection are shown.
RBC, total erythrocytes (×109/ml); WBC, total leucocytes (×106/ml); reticulocytes, platelets, neutrophils, eosinophils, lymphocytes, and monocytes (×106/ml).
Data represent means ± SD. One way analysis of variance (ANOVA),
P = 0.16, NS.
Mice were treated i.p. with 1 mg TM-β1 or normal rat IgG and infected 2 d later with LCMV-WE (2 × 106 PFU). Splenic NK activity, determined 3 d later by percent specific lysis of 51Cr-labeled YAC-1 target cells was 7, 7, 2, and 0 at E/T ratios of 90:1, 45:1, 22.5:1, and 11:1 in TM-β1treated mice; percent specific lysis at the corresponding E/T ratios was 72, 76, 63, and 47 after treatment with normal rat IgG.
To assess potential effects of perforin- or Fas-dependent early induced CTL on pluripotential and lineage-committed progenitors, a quantitative analysis of hematopoietic precursors was performed in CD40/0, CD80/0, P0/0, and lpr mice infected with 2 × 106 PFU of LCMV-WE (Table 5). The absolute numbers of BFU-E, CFU-GM, and CFU-Meg per femur did not differ significantly between the mutant mouse strains tested on day 3 of infection and all values were in the range of 72–85% below baseline levels. BFU-E and CFU-GM of these four mouse strains were less affected by LCMV when compared with infected wild-type 129Sv/ Ev and IFN-γ R0/0 mice. This finding suggested that non– MHC-linked genes were important for quantitative differences, because CD80/0, P0/0, and lpr mice are of pure C57BL/6 origin (H-2b) and CD40/0 mice are four times backcrossed to C57BL/6, whereas IFN-γ R0/0 mutants are of pure 129Sv/Ev background (H-2b). Accordingly, lethally irradiated LCMV immune C57BL/6 mice were used as recipients to determine CFU-S numbers of LCMVWE–infected CD40/0, CD80/0, P0/0, and lpr mice. The absolute numbers of CFU-S per femur on day 3 after LCMV infection were in the same range in these mutants and 10fold lower as compared with uninfected controls (see Table 5). Thus, CTL or NK effectors are apparently not influencing suppression of hematopoiesis in early LCMV infection.
Table 5.
Frequency of Pluripotential and Committed Progenitors in Mice of Different Genotypes after Infection with LCMV-WE
| Genotype* | Virus | Cellularity‡ | Colonies/femur§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFU-S | BFU-E | CFU-GM | CFU-Meg | |||||||||
| CD40/0 | None | 13.3 ± 1.4 | 5,978 ± 345 | 3,306 ± 500 | 33,672 ± 690 | 2,085 ± 237 | ||||||
| WE | 4.4 ± 1.1 (33%)‖ | 639 ± 163 (11%) | 566 ± 30 (17%) | 7,509 ± 899 (22%) | 312 ± 26 (15%) | |||||||
| CD80/0 | None | 13.8 ± 0.6 | 6,700 ± 379 | 3,956 ± 379 | 35,376 ± 3,790 | 2,756 ± 152 | ||||||
| WE | 3.7 ± 1.3 (27%) | 569 ± 148 (8%) | 888 ± 140 (22%) | 9,562 ± 984 (27%) | 634 ± 15 (23%) | |||||||
| P0/0 | None | 15.3 ± 1.3 | 7,452 ± 916 | 3,263 ± 33 | 27,356 ± 1,498 | 2,825 ± 162 | ||||||
| WE | 3.7 ± 0.5 (24%) | 667 ± 224 (9%) | 796 ± 111 (24%) | 7,712 ± 316 (28%) | 565 ± 56 (20%) | |||||||
| lpr | None | 19.5 ± 2.6 | ND | 3,728 ± 247 | 28,302 ± 2,860 | 2,527 ± 188 | ||||||
| WE | 7.0 ± 1.5 (35%) | ND | 559 ± 28 (15%) | 5,377 ± 524 (19%) | 430 ± 37 (17%) | |||||||
Sex-matched 10–12-wk-old C57BL/6 mice of the indicated genotypes were infected with LCMV-WE (2 × 106 PFU) or left untreated.
BM cellularity ×106/femur.
BM cells were harvested 3 d after infection and colony-forming cells per femur were determined in methylcellulose cultures containing IL-3 and erythropoietin (BFU-E), IL-3 and GM-CSF (CFU-GM), or IL-3 and thrombopoietin (CFU-Meg). CFU-S were counted on the spleen surface of lethally irradiated LCMV-immune C57BL/6 mice reconstituted with BM from infected or control mice. Shown are combined data from duplicate cultures of three mice tested individually.
Data represent means ± SD, values in brackets are relative numbers of cells/colonies compared with uninfected control mice of the same genotype.
Discussion
The current study implicates endogenously produced IFN-α/β in causing depletion of the pluripotential and lineage-committed hematopoietic progenitors during the first week of LCMV infection. LCMV-induced abnormalities of peripheral blood parameters correlated positively with the IFN responder phenotype of mice. The key finding was that in IFN-α/β R0/0 mice, peripheral hematologic parameters and the frequency of blood cell precursors in the BM were comparable in LCMV-infected and uninfected control mice. Conversely, the decrease in peripheral blood values after infection with LCMV in wild-type, IFN-γ R0/0, CD40/0, CD80/0, P0/0, or lpr mice was associated with a substantial reduction of the numbers of pluripotential progenitor cells and lineage-committed precursors and, to a lesser extent, of the morphologically recognizable compartment in the BM. The depletion of the blood cell progenitors was attributable to a direct effect of IFN-α/β and was independent of contact-dependent cytotoxicity or secreted factors from NK cells. This is suggested by the NK activity in BM of wild-type mice and in mice lacking either the IFN-α/β R or the IFN-γ R. Additionally, virus specific effector T cells generated within the first 7 d of infection apparently played no critical role in LCMV-induced transient BM aplasia, because knockout mice either lacking peripheral CD8+ or CD4+ T cells were similarly depleted of pluripotential and committed progenitors. A summary of the relevant findings in wild type and in the different mutant mouse strains is presented in Table 6.
Table 6.
Summary of the Correlation Between Immune Status and BM Function in the Acute Phase of LCMV Infection
| Genotype | LCMV-WE (day + 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Transient BM aplasia | Virus Replication in BM cells | NK activity in BM | LCMV-specific CTL (references) | |||||
| Wild type | ++++ | ± | +++ | + (21) | ||||
| IFN-γ R0/0 | +++ | ± | +++ | + (21, 30) | ||||
| IFN-α/β R0/0 | − | +++ | ++ | − (21) | ||||
| CD40/0 | ++++ | ND | ND | ± (32) | ||||
| CD80/0 | ++++ | ND | ND | − (31) | ||||
| P0/0 | ++++ | ± | − | − (24) | ||||
| lpr | ++++ | ND | +++ | + | ||||
The viral and host parameters defining the abnormalities of peripheral blood cells in the acute phase were influenced to a minor extent by the LCMV isolate; however, they correlated with the IFN-α/β responder phenotype of the genetic background of the host. This is documented by the fact that within a given mouse strain, the extent of the pancytopenia in the acute phase of infection was not determined by the divergent replication kinetics of a rapidly (Docile), intermediate (WE), or slowly (Armstrong) replicating LCMV isolate. This observation is in accordance with earlier findings, demonstrating comparable maximal serum levels of IFN-α/β induced by several LCMV isolates in mice of the same genetic background (40). Between the various mouse strains marked differences in the amount of IFN-α/β production have been measured, yet in adult mice no correlation between serum levels of IFN-α/β and susceptibility to lethal choriomeningitis has been demonstrated (40). Evidence for a link between IFN-α/β induction and disease, however, was established in newborn mice infected with LCMV (39). In these studies, the severity of the delay in organ maturation and liver necrosis as well as the incidence of death was directly dependent on the extent of the endogenous IFN-α/β response of the different mouse strains infected with LCMV. Moreover, the concept of a causal correlation between the amount of endogenously induced IFN-α/β by LCMV and the severity of BM suppression is in line with in vitro findings, demonstrating IFN-α/β to be a potent inhibitor of proliferation/ maturation of normal BM-derived hematopoietic progenitors (42), as well as of certain leukemic cells in vivo (e.g., hairy cell leukemia [43], and CML [44]).
Interestingly, expansion and functional activation of NK cells in LCMV-infected BM of IFN-α/β R0/0 mice was comparable to that in IFN-γ R0/0 and wild-type mice, especially when absolute numbers of NK cells were considered. NK cells are cytolytically activated and proliferate after stimulation with IFN-α/β, IFN-γ, or IL-2 in vitro and in vivo (45, 46) and the peak of NK activity after LCMV infection parallels the maximal serum concentration of IFN-α/β (22, 23). Apparently, in our experimental model, NK cells from IFN-α/β R0/0 mice, which cannot be stimulated by IFN-α/β signaling via the IFN-α/β R, were comparably activated. This result may hypothetically signal an IFN-α/β effect that is not mediated via the classic IFN-α/β R deleted in these mice. An alternative (i.e., not IFN-α/β R-dependent) activation pathway of NK cells in LCMV-infected IFN-α/β R0/0 mice is documented further by the finding, that poly I/C, which is a synthetic double-stranded RNA and a potent inducer of the IFN-α/β system in vivo (47), did not stimulate NK cells in the BM. More likely, NK activity in LCMV-infected IFN-α/β R0/0 mice could be due to stimulation by other cytokines (e.g., IL-2, IL-12) induced by the overwhelming replication of LCMV in IFN-α/β R0/0 mice.
There is conflicting data on the role of NK cells inhibiting hematopoiesis in vitro. Under some conditions, human NK cells suppress myelopoietic (48, 49) and erythropoietic (50) colony formation. In murine systems, NK cells have been reported to inhibit growth of syngeneic hematopoietic progenitors (51) or the more primitive CFU-S (52), but other studies have demonstrated normal hematopoiesis in vitro in the presence of NK cells (53). Moreover, it has been suggested that both the antiviral properties (25, 26) as well as the suppressive effect on in vitro colony formation of NK cells function via the secretion of soluble factors, such as IFN-γ or TNF-α (27, 54). As for acute LCMV infection, one report proposed syngeneic rejection of stem cells by activated NK cells, because treatment with antiasialo GM1 (a rabbit antiserum depleting NK cells but also partially LCMV-specific CTL nonspecifically [55]) partially restored the marrow repopulating ability of stem cells from uninfected syngeneic controls transferred into irradiated LCMV-infected mice (17). In our experimental system, there was no evidence of NK-mediated suppression of hematopoiesis, either via contact-dependent cytotoxicity or secreted cytokines, because (a) at the timepoint of maximal NK activity we found normal precursor and differentiated blood cell frequencies in the BM of IFN-α/β R0/0, but a drastic reduction of all hematopoietic compartments in IFN-γ R0/0, lpr, and P0/0 mice, in which functionally inactive NK cells proliferate after LCMV infection (24), and (b) TM-β1, which is a mAb specific for the IL-2 R β chain and which induces a long-lasting selective depletion of NK cells, but considerably less of T cells (38, 56), did not abrogate LCMV-induced suppression of peripheral blood parameters. A subtle effect of IFN-γ could be observed 7 d after LCMV infection, at a timepoint when bone marrow cellularity was restored in IFN-γ R0/0 mice, but not in wild-type mice (Fig. 1). When compared with IFN-α/β, the efficiency of LCMV-induced endogenous IFN-γ in suppressing BM function seems to be much weaker, however, because the CBC and BM cellularity of IFN-α/β R0/0 mice were not altered at that stage of LCMV infection.
The finding of megakaryocytes and myeloid precursors expressing LCMV antigen in high amounts in the presence of activated NK cells, and yet lack of NK-mediated immunopathology in the BM of IFN-α/β R0/0 mice, was surprising. One possible explanation might be that LCMV by itself, in contrast with some other viruses, such as murine cytomegalovirus, does not alter class I MHC expression nor does it inhibit the ability of IFN-γ to upregulate MHC class I in virus-infected cells (57). Therefore, LCMV-infected MHC class I–expressing hematopoietic precursors could be protected from NK cell–mediated lysis because cells expressing high levels of MHC class I are more resistant to NK cells than those lacking expression of MHC class I (58). Although there is evidence for Fas ligand expression on the cell surface of freshly isolated murine NK cells (29), the role of the Fas pathway in NK killing in vivo is still poorly understood. In vitro studies have demonstrated some Fas expression on human erythroid and myeloid colonies in the presence of TNF-α and/or IFN-γ (59), as well as upregulation of the Fas antigen by influenza virus–elicited IFN-α/β on HeLa cells (60). The theoretical possibility, that Fas-mediated cytotoxicity of NK cells against Fas-expressing BM cells could be involved in LCMV-induced BM aplasia, is not supported here because NK cells were not critical in the first place. Furthermore, lpr mice exhibited a comparable suppression of BM and blood values in the presence of highly activated NK cells (data not shown) 3 d after LCMV infection; this documents a minor significance of the Fas receptor–Fas ligand system of NK cells, because a nonoperational apoptotic signaling in lpr mice did not revert LCMV-induced transient depression of hematopoiesis.
Mutant IFN-α/β R0/0 mice exhibited an intense viral replication in megakaryocytes and, to a lesser extent, in myeloid precursors. Interestingly, LCMV by itself did not interfere with maturation and the release of platelets or granulocytes into the circulation, because the number of these blood cells remained unchanged in acutely infected IFN-α/β R0/0 mice. We do not not know at which stage of maturation megakaryocytes are infected in IFN-α/β R0/0 mice, but it is conceivable that LCMV might infect morphologically unidentifiable progenitors of the megakaryocytic lineage. A similar finding has been described for HIV patients, for which it has been proposed that HIV infection of megakaryocytic or myeloid progenitors by itself does not inhibit their proliferation/differentiation potential in vivo unless antibodies suppressing the growth of these progenitors are present in the serum (61, 62). We had no evidence of infection of pluripotential hematopoietic progenitors, because erythroblasts did not express viral Ag in serial BM analysis during the first week of LCMV infection. LCMV seemed to persist for a longer time period in megakaryocytes than in myeloid precursors, because immature granulocytes were virus free on day 7 of LCMV infection, whereas a considerable proportion of megakaryocytes still stained positively for viral Ag. This finding may be due to differences in the cellular tropism of LCMV but could simply reflect the fact that the turnover of myeloid cells is much higher and, therefore, infected myeloid precursors were released into circulation very efficiently.
Although IFN-α/β R0/0 mice are able to mount normal Th and CTL responses against cytopathic viruses like vaccinia or vesicular stomatitis virus, LCMV specific CTL activity is lacking due to exhaustive activation of virus-specific CTL (21). Therefore, the excessive viral multiplication in megakaryocytes and, to a lesser extent, in myeloid precursors was not associated with a CTL-mediated immunopathology, documented by the normal range of these cells in the BM and normal values of circulating blood cells. In addition, LCMV-activated CD4+ or CD8+ T cells or their secreted products (e.g., TNF-β, IFN-γ) could not account for BM suppression in acute LCMV infection, since CD80/0 and CD40/0 mutant mice, which have no CD8+ or CD4+ T cells in the periphery (31, 32), respectively, had comparably reduced hematopoietic progenitors and blood cells. It should be pointed out, however, that the present study analyzed the acute phase of LCMV infection, in which innate immune mechanisms dominate and the frequency of LCMVspecific CTL is still increasing. Therefore, the pathogenesis of hematologic abnormalities at later timepoints of infection might be distinct from the one described here and may involve other immunopathologic mechanisms, e.g., cytolytic activity or secreted cytokines from CTL, such as TNF-β or IFN-γ. The presented results and concepts may be generalizable and offer explanations for mechanisms of hematopoietic disease in noncytopathic viral infections and hemorrhagic fevers caused not only by infection with arenaviruses such as LCMV or Junin virus but also by Dengue fever virus. For these viruses, high concentrations of IFN-α/β have been measured in the serum, and the magnitude and the duration of circulating IFN-α/β correlated directly with the severity and the evolution of the disease (63–65).
Acknowledgments
We are grateful to A. Althage, E. Horvath, and R. Rüegg for excellent technical assistance and to N. Wey for processing the microphotographs. We thank M. Aguet, P. Aichele, M. van den Broek, S. Ehl, Paul Klenerman, and K. Maloy for helpful discussions and critical reading of the manuscript.
Footnotes
Recombinant mTPO was kindly provided as a gift by Genentech, Inc. (South San Francisco, CA).
This work was supported by the Union Bank of Switzerland, the Swiss National Science Foundation, and the Kanton of Zürich.
1 Abbrevations used in this paper: BFU-E, burst-forming unit-erythroid; BM, bone marrow; CBC, cellular blood count; CFU-GM, colony-forming unit-granulocyte/macrophage; CFU-Meg, colony-forming unit-megakaryocyte; CFU-S, colony-forming unit-spleen; hEpo, human erythropoietin; LCMV, lymphocytic choriomeningitis virus; mTPO, murine thrombopoietin.
References
- 1.Rosenfeld S, Young NS. Viruses and bone marrow failure. Blood Rev. 1991;5:71–77. doi: 10.1016/0268-960x(91)90037-d. [DOI] [PubMed] [Google Scholar]
- 2.Young, N.S., M. Yoshida, and W. Sugden. 1992. Viral pathogenesis of hematologic disorders. In Molecular Basis of Blood Diseases. G. Stamatoyanapoulos, A.S. Nienhuis, P. Leder, P. Majerus and H. Varmus, editors. WB Saunders Company, Philadelphia.
- 3.Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, Gordon M, Goldman JM. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989;17:38–45. [PubMed] [Google Scholar]
- 4.Molina J-M, Scadden DT, Sakaguchi M, Fuller B, Woon A, Groopman JE. Lack of evidence for infection of or effect on growth of hematopoietic progenitor cells after in vivo or in vitro exposure to human immunodeficiency virus. Blood. 1990;76:2476–2482. [PubMed] [Google Scholar]
- 5.Shadduck RK, Winkelstein A, Ziegler Z, Lichter J, Goldstein M, Michaels M, Rabin B. Aplastic anemia following infectious mononucleosis: possible immune etiology. Exp Hematol. 1979;7:264–271. [PubMed] [Google Scholar]
- 6.Liang DC, Lin KH, Lin DT, Yang CP, Hung KL, Lin KS. Post-hepatitis aplastic anemia in children in Taiwan, a hepatitis prevalent area. Br J Haematol. 1990;74:487–491. doi: 10.1111/j.1365-2141.1990.tb06339.x. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MBA. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 8.Oldstone MBA. The arenaviruses—an introduction. Curr Topics Microbiol Immun. 1987;133:1–4. [PubMed] [Google Scholar]
- 9.Lehmann-Grube F. Lymphocytic choriomeningitis virus. Monogr Virol. 1971;10:1–173. [Google Scholar]
- 10.Hotchin JE. Persistent and slow virus infections. Monogr Virol. 1971;3:1–211. [Google Scholar]
- 11.Hinmann AR, Fraser DW, Douglas RG, Bowen GS, Kraus AL, Winkler WG, Rhodes WW. Outbreak of lymphocytic choriomeningitis virus infections in medical center personnel. Am J Epidemiol. 1975;101:103–110. doi: 10.1093/oxfordjournals.aje.a112076. [DOI] [PubMed] [Google Scholar]
- 12.Smadel, J.E., R.H. Green, R.M. Paltauf, and T.A. Gonzales. 1942. Lymphocytic choriomeningitis: two human fatalities following an unusual febrile illness. Proc. Soc. Exp. Biol., N.Y. 49:683–686.
- 13.Martinez Peralta, L.A., C.E. Coto, and M.C. Weissenbacher. 1993. The Tacaribe complex: the close relationship between a pathogenic (Junin) and a nonpathogenic (Tacaribe) arenavirus. In The Arenaviridae. M.S. Salvato, editor. Plenum Press, New York.
- 14.Bro-Jørgensen K. The interplay between lymphocytic choriomeningitis virus, immune function, and hemopoiesis in mice. Adv Virus Res. 1978;22:327–369. doi: 10.1016/s0065-3527(08)60777-0. [DOI] [PubMed] [Google Scholar]
- 15.Broomhall KS, Morin M, Pevear DC, Pfau CJ. Severe and transient pancytopenia associated with a chronic arenavirus infection. J Exp Pathol. 1987;3:259–269. [PubMed] [Google Scholar]
- 16.Silberman SL, Jacobs RP, Cole GA. Mechanisms of hemopoietic and immunological dysfunction induced by lymphocytic choriomeningitis virus. Infect Immun. 1978;19:533–539. doi: 10.1128/iai.19.2.533-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen AR, Pisa P, Bro-Jørgensen K, Kiessling R. Mechanisms of lymphocytic choriomeningitis virusinduced hemopoietic dysfunction. J Virol. 1986;59:428–433. doi: 10.1128/jvi.59.2.428-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinkernagel RM, Welsh RM. H-2 compatibility requirement for virus-specific T cell–mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976;117:1495–1502. [PubMed] [Google Scholar]
- 19.Hotchin J. The biology of lymphocytic choriomeningitis infection: Virus induced immune disease. Cold Spring Harbor Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 20.Moskophidis D, Battegay M, Bruendler MA, Laine E, Gresser I, Zinkernagel RM. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science (Wash DC) 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 22.Biron CA, Welsh RM. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982;129:2788–2795. [PubMed] [Google Scholar]
- 23.Welsh RM. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 25.Karupiah G, Blanden RV, Ramshaw IA. Interferon-γ is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990;172:1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paya CV, Kenmotsu RA, Schoon RA, Leibson PJ. Tumor necrosis factor and lymphotoxin secretion by human natural killer cells leads to antiviral cytotoxicity. J Immunol. 1988;141:1989–1995. [PubMed] [Google Scholar]
- 27.Murphy M, Loudon R, Kobayashi M, Trinchieri G. Gamma-interferon and lymphotoxin released by activated T cells synergize to inhibit granulocyte/monocyte colony formation. J Exp Med. 1986;164:263–279. doi: 10.1084/jem.164.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Broek MF, Kägi D, Zinkernagel RM, Hengartner H. Perforin dependence of natural killer cell– mediated tumor control in vivo. Eur J Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- 29.Hisashi A, Noriko A, Takashi S. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science (Wash DC) 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 31.Fung-Leung WP, Kündig TM, Zinkernagel RM, Mak TW. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahemtulla A, Fung-Leung WP, Schilham MW, Kündig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but a markedly decreased helper cell activity in mice lacking CD4. Nature (Lond) 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 33.Pfau CJ, Valenti JK, Pevear DC, Hunt KD. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J Exp Med. 1982;156:79–89. doi: 10.1084/jem.156.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutko FJ, Oldstone MBA. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 35.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 or 96 well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 36.Bro-Jørgensen K, Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 2. The viral effect upon the function of colony forming stem cells. Acta Pathol Microbiol Scand (B) 1972;80:853–862. [PubMed] [Google Scholar]
- 37.Broudy VC, Nancy LL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- 38.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long-term elimination of natural killer cells in vivo by an anti-interleukin 2 receptor β chain monoclonal antibody in mice. J Exp Med. 1993;178:1103–1107. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riviere Y, Gresser I, Guillon JC, Bandu MT, Ronco P, Morel-Maroger L, Verroust P. Severity of lymphocytic choriomeningitis virus disease in different strains of suckling mice correlates with increasing amounts of endogenous interferon. J Exp Med. 1980;152:633–640. doi: 10.1084/jem.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leist TP, Aguet M, Hassig M, Pevear DC, Pfau CJ, Zinkernagel RM. Lack of correlation between serum titres of interferon alpha, beta, natural killer cell activity and clinical susceptibility in mice infected with two isolates of lymphocytic choriomeningitis virus. J Gen Virol. 1987;68:2213–2218. doi: 10.1099/0022-1317-68-8-2213. [DOI] [PubMed] [Google Scholar]
- 41.van den Broek MF, Müller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 42.Raefsky E, Platanias L, Zoumbos N, Young NS. Studies of interferon as a regulator of hematopoietic proliferation. J Immunol. 1985;135:2507–2512. [PubMed] [Google Scholar]
- 43.Quesada JR, Reuben J, Manning JT, Hersh EM, Gutterman JU. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984;310:15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- 44.Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med. 1986;314:1065–1069. doi: 10.1056/NEJM198604243141701. [DOI] [PubMed] [Google Scholar]
- 45.Biron CA, Young HA, Kasaian MT. Interleukin 2-induced proliferation of murine natural killer cells in vivo. J Exp Med. 1990;171:173–188. doi: 10.1084/jem.171.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biron CA. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 47.Hansson M, Kiessling RW, Andersson B, Welsh RM. Effect of interferon and interferon inducers on the NK sensitivity of normal mouse thymocytes. J Immunol. 1980;125:2225–2231. [PubMed] [Google Scholar]
- 48.Hansson M, Beran M, Andersson B, Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982;129:126–132. [PubMed] [Google Scholar]
- 49.Degliantoni G, Perussia B, Mangoni L, Trinchieri G. Inhibition of bone marrow colony formation by human natural killer cells and by natural killer cell–derived colony-inhibiting activity. J Exp Med. 1985;161:1152–1168. doi: 10.1084/jem.161.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrmann F, Schmidt RE, Ritz J, Griffin JD. In vitro regulation of human hematopoiesis by natural killer cells: Analysis at a clonal level. Blood. 1987;69:246–254. [PubMed] [Google Scholar]
- 51.Holmberg LA, Miller BA, Ault KA. The effect of natural killer cells on the development of syngeneic hematopoietic progenitors. J Immunol. 1984;133:2933–2939. [PubMed] [Google Scholar]
- 52.Barlozzari T, Herberman RB, Reynolds CW. Inhibition of pluripotent hematopoietic stem cells of bone marrow by large granular lymphocytes. Proc Natl Acad Sci USA. 1987;84:7691–7695. doi: 10.1073/pnas.84.21.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemeyer CM, Sieff CA, Smith BR, Ault KA, Nathan DG. Hematopoiesis in vitro coexists with natural killer lymphocytes. Blood. 1989;74:2376–2382. [PubMed] [Google Scholar]
- 54.Cannistra SA, Groshek P, Griffin JD. Monocytes enhance gamma-interferon-induced inhibition of myeloid progenitor cell growth through secretion of tumor necrosis factor. Exp Hematol. 1988;16:865–870. [PubMed] [Google Scholar]
- 55.Stitz L, Baenziger J, Pircher HP, Hengartner H, Zinkernagel RM. Effect of rabbit anti-asialo GM1 treatment in vivo or with anti-asialo GM1 plus complement in vitro on cytotoxic T cell activities. J Immunol. 1986;136:4674–4680. [PubMed] [Google Scholar]
- 56.Ehl S, Nuesch R, Tanaka T, Myasaka M, Hengartner H, Zinkernagel RM. A comparison of efficacy and specificity of three NK depleting antibodies. J Immunol Methods. 1996;199:149–153. doi: 10.1016/s0022-1759(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 57.Bukowski JF, Welsh RM. Inability of interferon to protect virus-infected cells against lysis by natural killer (NK) cells correlates with NK cell–mediated antiviral effects in vivo. J Immunol. 1985;135:3537–3541. [PubMed] [Google Scholar]
- 58.Karre K. Express yourself or die: peptides, MHC molecules, and NK cells. Science (Wash DC) 1995;267:978–979. doi: 10.1126/science.7863341. [DOI] [PubMed] [Google Scholar]
- 59.Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by IFN γ and tumor necrosis factor α and potentiates cytokine-mediated hematopoetic suppression in vitro. Blood. 1995;11:3183–3190. [PubMed] [Google Scholar]
- 60.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigenencoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–296. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 61.Zucker-Franklin D, Cao Y. Megakaryocytes of human immunodeficiency virus–infected individuals express viral RNA. Proc Natl Acad Sci USA. 1989;86:5595–5599. doi: 10.1073/pnas.86.14.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donahue RE, Johnson MM, Zon LI, Groopman LI. Suppression of in vitro haematopoiesis following human immunodeficiency virus infection. Nature (Lond) 1987;326:200–203. doi: 10.1038/326200a0. [DOI] [PubMed] [Google Scholar]
- 63.Levis SC, Saavedra MC, Ceccoli C, Feuillade MR, Enria DA, Maiztegui JI, Falcoff R. Correlation between endogenous interferon and the clinical evolution of patients with Argentine hemorrhagic fever. J Interferon Res. 1985;5:383–389. doi: 10.1089/jir.1985.5.383. [DOI] [PubMed] [Google Scholar]
- 64.Levis SC, Saavedra MC, Ceccoli C, Falcoff E, Feuillade MR, Enria DA, Maiztegui JI, Falcoff R. Endogenous interferon in Argentine hemorrhagic fever. J Infect Dis. 1984;149:428–433. doi: 10.1093/infdis/149.3.428. [DOI] [PubMed] [Google Scholar]
- 65.Kurane I, Innis BL, Nimmannitya S. High levels of interferon alpha in the sera of children with dengue virus infection. Am J Trop Med Hyg. 1993;48:222–229. doi: 10.4269/ajtmh.1993.48.222. [DOI] [PubMed] [Google Scholar]