Abstract
In this study, we sought to determine whether pertussis toxin (PT), an exotoxin virulence factor produced exclusively by Bordetella pertussis, is important for colonization of the respiratory tract by this pathogen by using a mouse intranasal infection model. By comparing a wild-type Tohama I strain to a mutant strain with an in-frame deletion of the ptx genes encoding PT (ΔPT), we found that the lack of PT confers a significant peak (day 7) colonization defect (1 to 2 log10 units) over a range of bacterial inoculum doses and that this defect was apparent within 1 to 2 days postinoculation. In mixed-strain infection experiments, the ΔPT strain showed no competitive disadvantage versus the wild-type strain and colonized at higher levels than in the single-strain infection experiments. To test the hypothesis that soluble PT produced by the wild-type strain in mixed infections enhanced respiratory tract colonization by ΔPT, we coadministered purified PT with the ΔPT inoculum and found that colonization was increased to wild-type levels. This effect was not observed when PT was coadministered via a systemic route. Intranasal administration of purified PT up to 14 days prior to inoculation with ΔPT significantly increased bacterial colonization, but PT administration 1 day after bacterial inoculation did not enhance colonization versus a phosphate-buffered saline control. Analysis of bronchoalveolar lavage fluid samples from mice infected with either wild-type or ΔPT strains at early times after infection revealed that neutrophil influx to the lungs 48 h postinfection was significantly greater in response to ΔPT infection, implicating neutrophil chemotaxis as a possible target of PT activity promoting B. pertussis colonization of the respiratory tract.
Bordetella pertussis is a gram-negative bacterial pathogen that colonizes the human respiratory tract, leading to a severe paroxysmal coughing disease known as whooping cough. In the absence of a human challenge model of B. pertussis infection, studies of respiratory tract colonization and disease caused by B. pertussis have been limited to animal models, of which the most well established and frequently used is the mouse intranasal inoculation model. In this model, although overt symptomatic disease is not elicited, several characteristics of the human infection are reproduced, such as multiplication and clearance of the bacteria, limitation of infection to the respiratory tract, increased severity of infection in young animals, and various systemic physiological changes (8, 25, 29, 37). Recent studies have shown that this may also be a useful model for the preclinical assessment of acellular pertussis vaccine efficacy (6, 19). However, several aspects of the pathogenic mechanisms employed by B. pertussis and the immune response to this infection remain poorly understood.
Pertussis toxin (PT) is a multisubunit exotoxin that is uniquely produced by B. pertussis and is considered one of the important virulence factors of B. pertussis. The holotoxin has an AB5 structure (33, 35) consisting of an enzymatically active A subunit (S1) that ADP-ribosylates the alpha subunits of several heterotrimeric G proteins in mammalian cells (12, 22) and a B oligomer that binds unidentified glycoconjugate receptors on cells (2, 39). PT disrupts signaling pathways in mammalian cells with a wide range of downstream effects (28, 40). When administered to experimental animals, purified PT can elicit almost all of the systemic symptoms associated with pertussis disease in humans, such as lymphocytosis, insulinemia or hypoglycemia, and histamine sensitivity (21, 23, 24), but its role in the severe coughing disease is uncertain. It is also unclear whether PT has a role in initial respiratory tract infection and colonization by B. pertussis. Early studies with Tn5 mutants of B. pertussis deficient in PT production demonstrated a reduced lethality for intranasally inoculated infant mice and a more rapid clearance from the lungs after a sublethal dose compared to the wild-type strain (5, 37, 38). Similar effects on virulence in 3- to 4-week-old mice were reported for a different pair of PT-deficient mutants of B. pertussis (13). Data from a more recent study demonstrated that a PT-deficient mutant of B. pertussis showed a similar time course profile of colonization in 4-week-old intranasally inoculated mice as the wild-type strain (1). Collectively, these data indicate a role for PT in the overall virulence of B. pertussis in respiratory tract infection but do not provide evidence of a role for PT in initial colonization. In this study, we assessed the role of PT as a colonization factor for B. pertussis in the respiratory tracts of young adult mice. From our results, we conclude that PT is a significant colonization factor that plays an early role in this host-pathogen interaction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. pertussis strains used in this study were streptomycin- and nalidixic acid-resistant derivatives of Tohama I (11), W28 (Wellcome), and 18323 (ATCC 9797). The ΔPT (mutant strain with an in-frame deletion of the ptx genes encoding PT) and PT-9K/129G (26) derivatives of these strains were constructed as described below. The ΔBVG strain contains a deletion of the bvgA and bvgS genes, with replacement of this region by a kanamycin resistance gene, and was constructed as previously described (17). B. pertussis strains were grown on Bordet-Gengou agar (Difco) plates containing 15% defibrinated sheep blood (BG-blood agar plates) and the following antibiotics at the indicated concentrations when necessary: streptomycin (400 μg ml−1), nalidixic acid (20 μg ml−1), gentamicin (10 μg ml−1), or tetracycline (15 μg ml−1). B. pertussis strains were also grown in Stainer-Scholte liquid medium (32) containing heptakis-dimethylcyclodextrin (Sigma). Escherichia coli strain DH10B (27) was used for standard cloning experiments, and E. coli strain S17.1 (30) was used for conjugation with B. pertussis; these strains were grown on Luria-Bertani agar plates containing the following antibiotics at the indicated concentrations when necessary: gentamicin (10 μg ml−1) or tetracycline (15 μg ml−1).
Plasmid and strain construction.
An in-frame deletion of the ptx genes (Fig. 1) was derived by PCR amplification of two fragments from the ptx region, an upstream fragment (containing sequence encoding the first 9 amino acids of the S1 subunit signal peptide) (amplified by using oligonucleotide primers 947 [5′-GATAGAATTCGGAACCGACCCCAAGATAA-3′] and 948 [5′-GATTGGATCCTTGGCGAATTGCCCGAGT-3′]) and a downstream fragment (containing sequence encoding the last 25 amino acids of the S3 subunit) (amplified by using oligonucleotide primers 949 [5′-GATAGGATCCGACTACGAGGACGCCACA-3′] and 950 [5′-GATATCTAGAGGGTGCAGCCCTTGAAAA-3′]). The fragments were doubly digested with EcoRI/BamHI and BamHI/XbaI, respectively, and ligated with EcoRI/XbaI-digested pJHC1 (15) to derive the plasmid pJ-ΔPT. The correct deletion and flanking sequences were confirmed by restriction enzyme digestion and DNA sequencing. The plasmid was transformed into E. coli S17.1 and then introduced into the B. pertussis chromosome by conjugation and allelic exchange as described previously (34). Exconjugants were screened by PCR amplification using oligonucleotides 105 (5′-GCGAATTCAGCCCTCCAACGCGC-3′) and 763 (5′-CAGCAGATAACGAGCGAT-3′) to identify strains with the ΔPT deletion. Plasmid pJ-PT-9K/129G was obtained by subcloning a 2.3-kb EcoRI/BsrGI fragment (containing the ptxA and ptxB genes) from pS-PT-9K/129G (a 4.6-kb EcoRI fragment containing all the ptx genes inserted into the allelic exchange vector plasmid pSS1129) (4) into EcoRI/Acc65I-digested pJHC1. The PT-9K/129G mutation (encoding inactivating replacements of Lys for Arg9 and Gly for Glu129 in S1) (26) was then introduced into the B. pertussis chromosome by conjugation and allelic exchange, and exconjugants with the mutation were identified by PCR amplification using oligonucleotides 953 (5′-CGCCACCGTATACAAG-3′) and 954 (5′-GGTGTGCCAGATACC-3′).
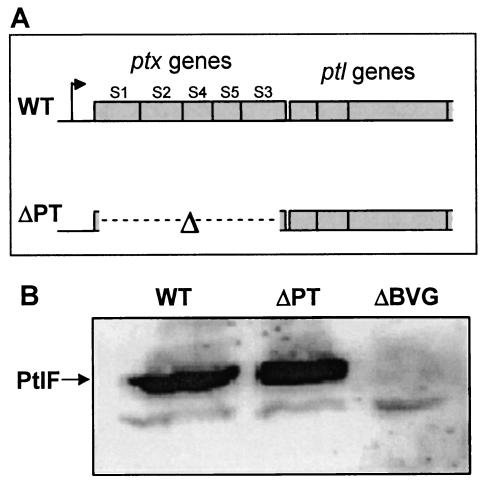
FIG. 1.
(A) The ptx-ptl gene region. The intact ptx genes in B. pertussis strain WT encode the subunits (S1 to S5) of PT, and the downstream ptl genes are in the same operon (the promoter is indicated by the arrow upstream of the ptx genes). The B. pertussis ΔPT strain was constructed by introducing an in-frame deletion of the ptx genes (indicated by the Δ symbol and broken line) into the chromosome of B. pertussis strain Tohama I. (B) Western blot showing PtlF expression in strains WT, ΔPT, and ΔBVG.
Colony hybridization.
B. pertussis colonies were patched onto nitrocellulose disks (Millipore) on BG-blood agar plates. After the colonies were grown for 2 days at 37°C, the disks were removed, and colony hybridization was performed as previously described (3), using 32P-labeled oligonucleotide 1048 (5′-GTAGTCGGATCCTTGGCGA-3′) (which spans the deleted sequence of the ΔPT construct and therefore hybridizes only with ΔPT colonies).
Western blotting.
Trichloroacetic acid precipitates of B. pertussis culture supernatants were prepared as described previously (4) and analyzed by Western blotting with S1-specific monoclonal antibody 1C7 to confirm the lack of PT production by ΔPT strains. For PtlF expression, trichloroacetic acid precipitates of B. pertussis suspensions were prepared as described previously (9) and analyzed by Western blotting with a PtlF-specific mouse polyclonal antibody (generously supplied by Drusilla Burns).
PT preparation.
PT and PT-9K/129G were prepared from B. pertussis culture supernatants by the fetuin affinity method of Kimura et al. (14), resuspended in phosphate-buffered saline (PBS), and stored at −80°C until use. The protein concentration was determined by the bicinchoninic acid (BCA) assay (Pierce), and the presence or absence of toxin activity was assessed by a CHO cell clustering assay (10).
Mouse infection.
Six-week-old female BALB/c, C57BL6, or SCID (BALB/c background) mice (Charles River Laboratories or Harlan) were used for infection experiments. Inocula were prepared by plating B. pertussis strains from frozen culture and growing for 3 days at 37°C on BG-blood agar plates with streptomycin. The strains were then transferred to new plates and grown for two additional days. Bacterial cells were taken from the plates and resuspended, and appropriate dilutions were made in sterile PBS. Mice were lightly anesthetized with Metofane (Medical Developments Australia) and inoculated intranasally with 20 μl of the inoculum, which was also diluted and plated on BG-blood agar plates with streptomycin to determine the viable count. At the indicated time points, mice were sacrificed by carbon dioxide inhalation, the respiratory tract (trachea and lungs) was removed and homogenized in 2 ml of PBS, and dilutions were plated on BG-blood agar plates with streptomycin. Four days later, the number of CFU per respiratory tract was determined and normalized to take into account small differences in viable counts of the inocula. Statistical analysis was performed using a t test of the normalized data. For PT administration experiments, intranasal administration was performed as described above, while systemic administration was performed on anesthetized mice by injection of 2 ng of PT (in 100 μl of PBS) in the left rear leg muscle (intramuscular), in the peritoneal cavity (intraperitoneal), or under the skin in the scruff of the neck (subcutaneous).
BAL.
Mice were sacrificed by carbon dioxide inhalation, and the respiratory tract was exposed by dissection. A small incision was made near the top of the trachea, and a blunt-ended 20-gauge needle was inserted and tied in place with surgical thread around the trachea. Bronchoalveolar lavage (BAL) fluid was obtained by filling the lungs with 0.8 ml of PBS and withdrawing as much of the liquid as possible (this procedure was performed two times). BAL fluid samples were centrifuged to pellet the cells, and the supernatant was stored at −80°C. BAL cells were resuspended in 1 ml of medium, and aliquots were removed for counting on a hemocytometer and for cytospin centrifugation onto a microscope slide, followed by staining with modified Wright stain to identify the cell type. To determine the percentage of neutrophils in these samples, 100 cells from several microscopy fields were identified.
RESULTS
Construction and analysis of a PT deletion strain.
An in-frame deletion of the ptxA to -E genes (encoding the five PT subunits) was constructed in the Tohama I background (Fig. 1A). The resulting strain (ΔPT) was compared to a parental strain (called WT) emerging from the same conjugation (which was used as the wild-type strain for mouse infection experiments) for growth characteristics in vitro, and no differences were observed (data not shown). Western blotting and CHO cell clustering assays were used to confirm the lack of PT production by ΔPT (data not shown). Two approaches were taken to confirm that the deletion in ΔPT did not affect expression of the downstream ptl genes (within the same operon as the ptx genes and encoding the type IV secretion system for PT secretion by B. pertussis) (16). First, secretion of PT was restored by introducing plasmid pL-PT (ptxA to -E genes subcloned in pNMD603) (15) into ΔPT (data not shown). Second, Western blotting revealed similar expression of PtlF (one of the proteins encoded by the downstream ptl genes) in ΔPT and WT (Fig. 1B), while no PtlF expression was detected in ΔBVG, a mutant strain with a deletion of the virulence regulatory locus bvg (confirming that expression of the protein detected in this Western blot is regulated by Bvg). Therefore, ΔPT differs from WT putatively only by the lack of PT production.
Respiratory tract colonization of mice by WT and ΔPT strains.
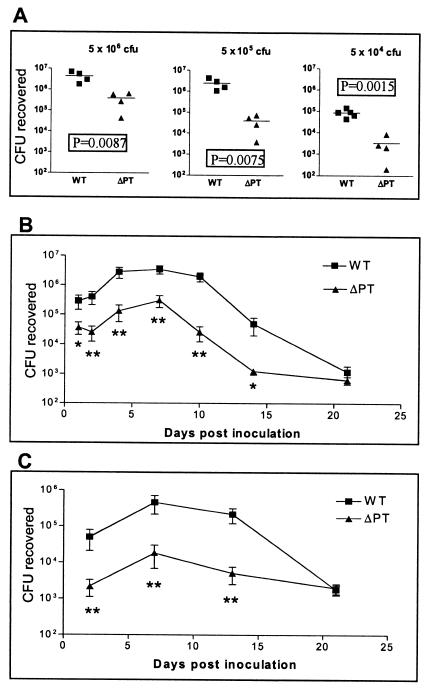
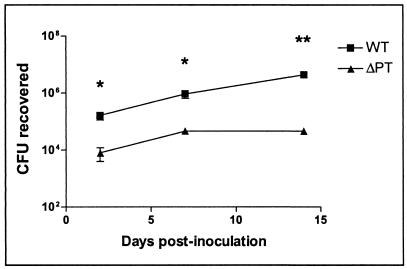
Six-week-old female BALB/c mice were intranasally inoculated with a range of doses of either WT or ΔPT, and respiratory tract colonization was assessed 7 days later. ΔPT showed a significant defect in colonization (reduction of 1 to 2 log10 units) at all doses tested (Fig. 2A). The time course of colonization by these two strains in BALB/c mice was monitored after inoculation with 5 × 105 CFU. WT showed a characteristic increase in colonization with a peak at day 7 postinoculation, followed by gradual clearance of the infection over the next 2 weeks (Fig. 2B). In comparison, ΔPT showed a defect in colonization at all time points and a more rapid clearance (Fig. 2B), although an increase to a peak at day 7 postinoculation was still observed. This experiment was repeated (with fewer time points) in C57BL6 mice, and a similar profile of colonization for each strain was observed, with ΔPT once again showing a colonization defect until clearance at 21 days postinoculation (Fig. 2C). This demonstrates that the colonization defect associated with ΔPT is not restricted to a particular mouse genetic background. Of particular interest was the observation that the defect in colonization by ΔPT was apparent early in the time course, by day 1 to 2 postinoculation (Fig. 2B and C). Overall, these data demonstrate that PT plays a role in the host-pathogen interaction that allows optimal colonization of the mouse respiratory tract by B. pertussis. In support of this conclusion, by using this infection model, we have obtained additional data (not shown) demonstrating a significant defect in the peak colonization of ΔPT strains in W28 and 18323 B. pertussis backgrounds and also of strain Tohama I PT-9K/129G, which produces an enzymatically inactive form of PT.
FIG. 2.
(A) Groups of four or five BALB/c mice were inoculated with the indicated doses of either B. pertussis WT (squares) or ΔPT (triangles), and colonization levels were assessed after 7 days (values from individual mice are shown, with the bar indicating the mean). Results show a significant defect (P values by the t test are shown) in colonization by ΔPT at all doses. (B) Comparison of the time course of respiratory tract colonization by WT or ΔPT after inoculation of BALB/c mice with 5 × 105 CFU of either strain. Each value is the mean ± standard deviation (error bar) for a group of four or five mice. Values that are significantly different from the values for the WT strain are indicated as follows: **, P < 0.05; *, P < 0.07. (C) Comparison of the time course of respiratory tract colonization by WT or ΔPT after inoculation of C57BL6 mice with 5 × 105 CFU of either strain. Each value is the mean ± standard deviation of a group of four or five mice. Values that are significantly different (P < 0.05) from the values for the WT strain are indicated (**).
Mixed infection with WT and ΔPT strains.
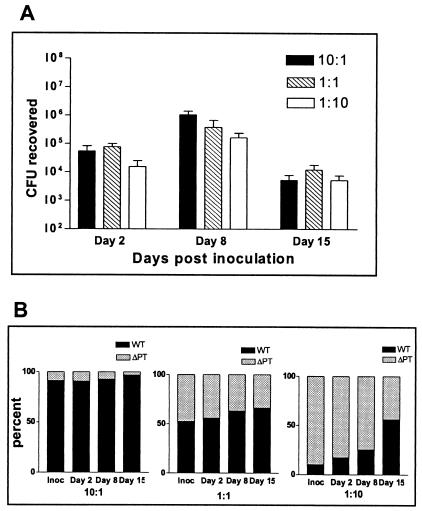
To determine whether ΔPT is also at a competitive disadvantage when coinfecting mice with WT, we performed mouse infection experiments with inocula consisting of WT and ΔPT mixed together in different ratios. For a preliminary control, we mixed the two strains in the same ratios, grew them in Stainer-Scholte medium, plated aliquots at various time points, and determined the ratio of the strains by colony hybridization. The ratios remained equivalent to the starting ratio throughout the growth of the cultures (data not shown), demonstrating that ΔPT has no competitive disadvantage versus WT during in vitro growth. Six-week-old female BALB/c mice were intranasally inoculated with 5 × 105 CFU of mixtures of WT and ΔPT at ratios of 10:1, 1:1, and 1:10, and respiratory tract colonization and the ratio of the colonizing strains were determined on days 2, 8, and 15 postinoculation. As shown in Fig. 3A, the time course of colonization followed the normal pattern for each mixture, with the peak of colonization at day 8 postinoculation. When the ratio of the two strains recovered from each infection was determined by colony hybridization, results showed that the starting ratios did not change significantly throughout the course of infection (Fig. 3B). Even with a starting WT/ΔPT ratio of 10:1, the mutant strain was not significantly outcompeted at the peak of infection. In each case, there was a slight increase in the proportion of WT over the time course of infection, most notably at the 1:10 starting ratio, though the relatively high proportion was largely due to the exclusively WT colonies emerging from one mouse.
FIG. 3.
Groups of four BALB/c mice were inoculated with 5 × 105 CFU of a mixture of B. pertussis strains WT and ΔPT at the indicated ratios. (A) Colonization levels from each infection at days 2, 8, and 15 postinoculation. The means ± standard deviations (error bars) are shown. (B) Ratios of the two strains in the inoculum (Inoc) or the CFU recovered from the infections at days 2, 8, and 15 expressed as a percentage. Ratios were determined by colony hybridization.
Another important result from these experiments was the observation that ΔPT colonized at higher levels in the mixed-strain infection than in the single-strain infections shown previously (Fig. 2). For example, in the 10:1 ratio inoculum, the number of ΔPT bacteria was 5 × 104 CFU, a dose that previously gave a mean peak (day 7) colonization of 3.3 × 103 CFU (Fig. 2A). However, in the mixed infection at this ratio, the number of colonizing ΔPT bacteria at the peak of infection was approximately 105 CFU. The increase in ΔPT colonization was also apparent in the 1:1 ratio experiment, but not in the 1:10 ratio experiment, where the proportion of WT was low and the overall colonization level was lower than in the other mixed infections. We repeated this experiment with B. pertussis W28 WT and ΔPT strains and observed essentially the same results (data not shown), demonstrating that this result is not peculiar to the Tohama I strain. These results demonstrate that ΔPT is not at a significant competitive disadvantage versus WT in a mixed infection and instead suggest that ΔPT gains an advantage for colonization from the presence of WT in the same infection. Although this is not definitive evidence identifying the role of PT in B. pertussis colonization, the data are more consistent with the idea that PT is a soluble factor that can benefit all B. pertussis bacteria present in an infection than its previously reported role as a bacterial adhesin (36).
Effect of coadministration of purified PT on ΔPT colonization.
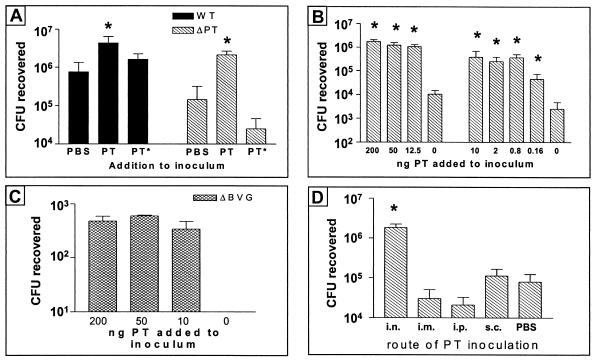
To test further the idea that PT can act as a soluble factor to promote ΔPT colonization, we performed mouse infection experiments in which we coadministered purified PT or the inactive PT-9K/129G mixed with the bacterial inoculum. Six-week-old female BALB/c mice were intranasally inoculated with a mixture of approximately 4 × 105 CFU of either WT or ΔPT and 200 ng of purified PT or PT-9K/129G (or an equivalent volume of PBS for a control), and respiratory tract colonization was assessed 4 days postinoculation. The results showed that coadministration of PT, but not PT-9K/129G, significantly increased the colonization level of both WT (P < 0.01) and ΔPT (P < 0.005) versus the PBS control (Fig. 4A). Indeed, coadministration of this amount of PT increased the colonization of ΔPT to a level significantly higher than that of the WT and PBS control (P < 0.01), demonstrating that the presence of soluble PT could more than compensate for the lack of PT production by ΔPT to promote respiratory tract colonization. Coadministration of PT-9K/129G did not significantly decrease the colonization level of WT (Fig. 4A), indicating that there is no blocking or steric hindrance of the PT produced by WT.
FIG. 4.
(A) BALB/c mice (three or four per group) were inoculated with approximately 5 × 105 CFU of B. pertussis strain ΔPT coadministered with 200 ng of purified PT or PT-9K/129G (PT*) or PBS, and colonization levels at day 4 postinoculation are shown. The means ± standard deviations (error bars) are shown in all four panels. Values that are significantly different (P < 0.05) from the values for the control (PBS) or PT* strain are indicated by asterisks. (B) BALB/c mice (three or four per group) were inoculated with approximately 5 × 105 CFU of ΔPT with coadministration of the indicated amount of purified PT, and colonization levels at day 4 postinoculation (results from two different experiments) are shown. Values that are significantly different (P < 0.05) from the values when no PT was added to the inoculum are indicated by asterisks. (C) BALB/c mice (three per group) were inoculated with approximately 5 × 106 CFU of B. pertussis strain ΔBVG with coadministration of the indicated amount of purified PT, and colonization levels at day 4 postinoculation are shown. (D) BALB/c mice (four per group) were inoculated intranasally with approximately 5 × 105 CFU of ΔPT, with either intranasal (i.n.), intramuscular (i.m.), intraperitoneal (i.p.), or subcutaneous (s.c.) coadministration of 2 ng of PT (or intranasal coadministration of PBS), and colonization levels at day 4 postinoculation are shown. The value that is significantly different (P < 0.05) from the value when no PT was added to the inoculum is indicated by an asterisk.
To determine the minimal level of purified PT that could enhance ΔPT colonization, we performed dose-response experiments comparing the effect of coadministration of smaller amounts of PT on ΔPT colonization (4 days postinoculation with approximately 5 × 105 CFU). The results showed that as little as 800 pg of PT could increase ΔPT colonization to WT levels (Fig. 4B), and even 160 pg of PT significantly increased ΔPT colonization versus the PBS control. Remarkably, coadministration of as little as 10 ng of PT increased the colonization (4 days postinoculation with approximately 5 × 106 CFU) of an avirulent B. pertussis mutant strain (ΔBVG, which lacks expression of all known virulence factors) to detectable levels (Fig. 4C), whereas no bacteria were recovered from the control mice given bacteria mixed with PBS (nor have we ever previously recovered any bacteria from mice infected with this strain). Overall, these data demonstrate the powerful colonization-enhancing effect that PT possesses for B. pertussis infection of the respiratory tract.
Effects of alternative routes of PT coadministration on ΔPT colonization.
Since some of the effects of PT activity are observed systemically during pertussis infection, we asked whether coadministration of PT by systemic routes could also enhance respiratory tract colonization by ΔPT. One group of 6-week-old female BALB/c mice was intranasally inoculated with a mixture of approximately 5 × 105 CFU of ΔPT and 2 ng of purified PT. Another group was intranasally inoculated with the same number of bacteria mixed with PBS. The other groups of mice were intranasally inoculated with the same dose of ΔPT, and 2 ng of purified PT was immediately injected via the intramuscular, intraperitoneal, or subcutaneous route. Respiratory tract colonization was assessed 4 days postinoculation, and the results showed that systemic administration of PT by any route did not significantly enhance colonization by ΔPT versus the intranasal PBS control (Fig. 4D), whereas intranasal coadministration of PT once again significantly increased ΔPT colonization. These results suggest that PT plays a role locally at the site of infection in promoting B. pertussis respiratory tract colonization and that its systemic activities may not be important for this aspect of the host-pathogen interaction.
Effect of prior or delayed intranasal administration of purified PT on ΔPT colonization.
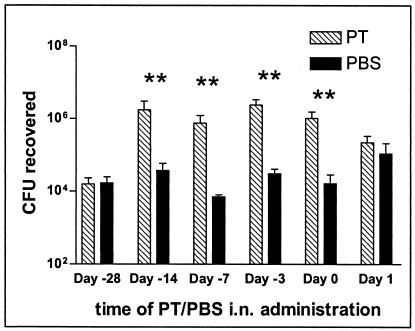
We next addressed the question of whether the timing of PT administration relative to bacterial inoculation of mice influenced the ability of PT to enhance colonization by ΔPT. Two nanograms of purified PT, or an equivalent volume of PBS for a control, was administered intranasally to 6-week-old female BALB/c mice, and groups of mice were intranasally inoculated with approximately 2 × 105 CFU of ΔPT 3, 7, 14, and 28 days later. Another group of mice (day 0) was inoculated with a mixture of the same number of bacteria plus 2 ng of purified PT (as in previous experiments), while the last group (day +1) was administered 2 ng of purified PT intranasally 24 h after the bacterial inoculation. Colonization levels were assessed 4 days postinoculation for all groups. Remarkably, administration of PT up to 14 days prior to bacterial inoculation significantly enhanced colonization by ΔPT, to the same extent as the simultaneous (day 0) coadministration of PT (Fig. 5). This enhancing effect had disappeared by day 28. In marked contrast, there was no enhancement of ΔPT colonization versus the PBS control when PT was administered just 1 day after the bacteria. The numbers of colonizing bacteria in these day +1 groups were slightly higher than in mice given PBS in other groups, suggesting that intranasal instillation of liquid 1 day after bacterial inoculation may enhance colonization, although since the level was no higher in the PT-administered group than in the PBS-administered group, this effect cannot be assigned to PT activity. Therefore, we conclude that PT plays an early role in the host-pathogen interaction to promote B. pertussis colonization, with a crucial activity within the first 24 h of interaction. In addition, the effect of PT activity on the host that promotes subsequent bacterial colonization is relatively long-lived, maintaining its full effect for at least 2 weeks.
FIG. 5.
BALB/c mice (three or four per group) were inoculated with approximately 5 × 105 CFU of B. pertussis strain ΔPT. Mice were given either 2 ng of PT or PBS intranasally at the indicated time relative to bacterial inoculation (from 28 days before [−28] to 1 day after), and colonization levels at day 4 postinoculation are shown. Means ± standard deviations (error bars) are shown. Values that are significantly different (P < 0.05) from the values for the control (PBS given intranasally [i.n.]) are indicated (**).
Neutrophil influx into the lungs of WT- and ΔPT-infected mice.
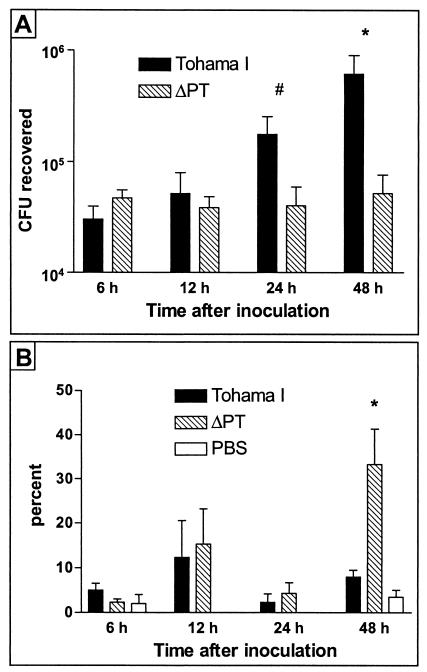
Since the effect of PT as a colonization factor appears to be important early in the host-pathogen interaction, we hypothesized that a possible role for PT is to combat innate antibacterial immune functions of the host, which are mechanisms of immunity elicited early after initial infection. One such mechanism for protection against respiratory tract infection is the recruitment to the airways of neutrophils, which can phagocytose and kill bacteria (20). To test whether PT may affect neutrophil influx into the lungs in the early course of respiratory tract infection by B. pertussis, we performed BAL experiments on mice infected with either WT or ΔPT. Six-week-old female BALB/c mice were intranasally inoculated with approximately 5 × 105 CFU of either WT or ΔPT (or PBS as a control), and groups of mice were sacrificed at 6, 12, 24, and 48 h postinoculation either for assessment of bacterial colonization or for BAL. The results showed that in this experiment WT colonization was once again superior to that of ΔPT by 24 h postinoculation (Fig. 6A), with the level of ΔPT colonization failing to increase over the 48-h time course. Analysis of the cell content of the BAL fluid samples (Fig. 6B) revealed that the level of neutrophil influx into the lungs of these mice was relatively low for the first 24 h postinoculation, but at 48 h postinoculation, there was a significantly higher level of neutrophils in the lungs of ΔPT-infected mice than in WT-infected mice (in which the level remained low and not significantly different than the level in the PBS control group). The total numbers of white blood cells in BAL fluid samples did not differ significantly between groups (data not shown). From these data, we conclude that at least one of the roles of PT as a B. pertussis colonization factor may be the disruption of neutrophil recruitment to the site of infection.
FIG. 6.
BALB/c mice were inoculated with approximately 5 × 105 CFU of B. pertussis Tohama I or ΔPT (or PBS), and at the indicated times after inoculation, groups of mice were sacrificed and assayed. (A) Colonization levels of B. pertussis strains. Means ± standard deviations (error bars) of the values for four mice are shown. (B) Neutrophil numbers in BAL fluid samples (expressed as a percentage of total white blood cells). Means ± standard deviations (error bars) for three mice are shown. Values that are significantly different from the values for Tohama I strain are indicated as follows: #, P = 0.08; *, P < 0.05.
Respiratory tract colonization of SCID mice by WT and ΔPT.
We have recently found that serum antibody responses to several B. pertussis antigens were greater in ΔPT-infected mice than in WT-infected mice (G. Artamonova and N. Carbonetti, unpublished results), despite the lower colonization by ΔPT, suggesting that PT may suppress an overall antibody response during B. pertussis infection. Therefore, adaptive immunity may be a target for the colonization-enhancing activity of PT. SCID mice lack T and B lymphocytes and are therefore deficient in adaptive immunity, but they retain a largely intact innate immune system. If the major colonization-enhancing role of PT were to combat innate immunity mechanisms rather than adaptive immunity, then the defect in colonization by ΔPT seen in healthy mice should also be apparent in SCID mice. To test this prediction, 6-week-old female SCID mice (BALB/c background) were intranasally inoculated with approximately 5 × 105 CFU of either WT or ΔPT, and groups of mice were sacrificed on days 2, 7, and 14 postinoculation for assessment of bacterial colonization of the respiratory tract. The results (Fig. 7) showed that ΔPT had a significant colonization defect (reduction of 1 to 2 log10 units) at all time points, consistent with the hypothesis that innate immunity mechanisms are less effective against the WT strain than the PT-deficient mutant. As predicted, the SCID mice failed to show any clearance of either infection by day 14 postinoculation, indicating a role for adaptive immunity in this clearance. However, since the WT strain continued to multiply in the mice after day 7, whereas ΔPT apparently did not, PT may have additional roles in promoting B. pertussis infection beyond the disruption of innate immunity.
FIG. 7.
SCID mice (BALB/c background) were inoculated with approximately 5 × 105 CFU of B. pertussis strain WT or ΔPT, and colonization levels were assessed at days 2, 7, and 14 postinoculation. Means ± standard deviations (error bars) of the values for four mice are shown. Values that are significantly different from the values for the WT strain are indicated as follows: *, P < 0.05; **, P < 0.001.
DISCUSSION
PT has been ascribed a dominant role in the overall virulence of B. pertussis (5, 23, 24), but its specific functions in promoting infection and disease have not been established. It is difficult to identify clues as to the relevant activities of PT for B. pertussis infection from the substantial literature on the numerous and wide-ranging effects of purified PT on cultured cells and experimental animals. This task is further complicated by the widely different doses of PT used and, in most cases, the absence of an inactive PT control (such as PT-9K/129G) to determine whether any effects seen can be ascribed to the ADP-ribosylation activity of PT.
We have chosen to investigate the role of PT in B. pertussis infection by using isogenic strains differing only in the presence or absence of PT production in combination with a young adult mouse intranasal infection model. In this way, other bacterial factors that may influence any PT activities are present in this interaction. Using this approach, we have demonstrated that PT plays a significant role in colonization of the respiratory tract. Our Tohama I strain with an in-frame deletion of the ptx genes (ΔPT), with no apparent defect in downstream gene expression (and therefore putatively different from WT only in the lack of PT production), showed significantly reduced peak colonization at a range of doses and at nearly all time points postinoculation. In less extensive studies, we saw very similar results (not shown) when we compared WT and ΔPT strains in B. pertussis W28 and 18323 backgrounds and when we compared a B. pertussis Tohama I strain expressing the inactive PT-9K/129G to WT. Together these data demonstrate that PT is an important colonization factor for B. pertussis and that the enzymatic activity of PT (rather than just its cell-binding capacity) is necessary for full colonization. It is not clear why a role for PT in B. pertussis colonization has not been observed previously in similar experiments using mouse models of infection (1, 5, 13). The nature of the specific mutation leading to loss of PT production is different in the different strains used. This led us to confirm that our in-frame ptx deletion did not alter expression of downstream genes and that other in vitro properties of the ΔPT strain did not differ from the corresponding WT strain. Another possible explanation of the discrepant results is that mice of different ages and strains were used in the studies—the previous studies were performed using either infant or 3- to 4-week-old mice of various different strains. Subtle differences in bacterial dose, preparation of inocula, or handling of mice may also account for the different observed results.
Experiments using mixed inocula consisting of different ratios of ΔPT and WT bacteria indicated that ΔPT is not significantly outcompeted by WT at any ratio and furthermore that ΔPT colonization is enhanced by this mixed infection versus a single-strain inoculation. Although there is more than one possible explanation for this observation, it is most consistent with the hypothesis that PT produced by the WT bacteria benefits all bacteria in the infection, presumably as a soluble factor interacting with host cells. These data do not rule out the possibility that PT can act as a “bridging” adhesin by binding simultaneously to both host cells and bacteria, but this property would presumably be maintained by the strain expressing the mutant PT-9K/129G (whose colonization capacity is reduced to the same level as ΔPT), making this a less likely explanation. Further support for the idea that soluble PT can enhance ΔPT colonization was obtained from our experiments with coadministration of purified PT with the ΔPT inoculum, in which low levels (submicrogram) of PT, but not PT-9K/129G, could restore a wild-type level of colonization to ΔPT. This was true if PT was coadministered at the site of bacterial infection (i.e., the respiratory tract), but not when PT was coadministered at systemic sites, demonstrating that the colonization-enhancing property of PT acts at or near the respiratory tract. This may not seem surprising, but systemic symptoms due to PT activity are a well-recognized part of pertussis infection (in both humans and experimental animals); therefore, we sought to rule out the possibility that these systemic effects contributed to B. pertussis colonization of the respiratory tract. Particularly interesting from this series of experiments was the observation that the colonization-enhancing property of PT was relatively long-lived, with PT administration up to 2 weeks prior to bacterial inoculation giving the same increase in ΔPT colonization as simultaneous coadministration. This suggests that ADP-ribosylation of target host cells or some downstream effect of this modification is a relatively stable phenomenon. If this is the case, this may be a contributing factor to the longevity of disease in pertussis patients, in which coughing episodes can persist for months.
At least two lines of evidence from our study suggest that PT activity is important for B. pertussis colonization very early after the initial host-pathogen interaction. First, comparison of the time course of infection between the ΔPT and WT strains revealed that the defect in colonization was apparent even at the earliest time points (24 to 48 h postinoculation). Second, intranasal administration of purified PT just 24 h after bacterial inoculation failed to enhance ΔPT colonization (versus the PBS control). These observations led us to hypothesize that a possible target of PT activity is innate immunity, the antibacterial mechanisms of which are active early in the host-pathogen encounter. One such immune mechanism relevant to the respiratory tract is the influx of neutrophils into the airways of the lungs, which can directly kill bacteria. In support of our hypothesis, we found that neutrophil influx into the lungs of mice 48 h after infection with ΔPT was significantly greater than that in the lungs of mice infected with WT, potentially identifying a specific mechanism of antibacterial innate immunity that may be a target for PT activity. It will be interesting to determine whether the neutrophils are directly modified by PT (a distinct possibility, since neutrophil chemotaxis to sites of infection is known to be regulated by PT-sensitive G proteins [20, 31]) or whether other mechanisms inducing neutrophil migration, such as chemokine production, may be altered by PT activity. If the former possibility is true, then PT must be able to diffuse rapidly from the primary site of infection to the underlying tissues and blood vessels in order to interact directly with neutrophils before they have migrated to the airway. If the latter possibility is true, PT may act directly on lung macrophages and/or epithelial cells to alter their production of neutrophil-attracting chemokines. These mechanisms are not mutually exclusive, and both may be significant targets of PT activity contributing to bacterial colonization. Since ΔPT does however achieve some level of colonization, with bacterial numbers increasing between days 2 and 7 postinoculation, other B. pertussis factors presumably also contribute to defense against innate immunity. A previous report demonstrated a role for adenylate cyclase toxin in combating neutrophils during Bordetella bronchiseptica infection of the mouse respiratory tract (7). Since this toxin is also produced by B. pertussis and is a significant colonization factor (5, 13), it will be interesting to determine whether this role also holds true for B. pertussis infection.
Since SCID mice exhibit deficient adaptive immunity but possess relatively normal innate immunity, a prediction of the hypothesis that innate immunity is a target for PT is that defective colonization by ΔPT would also be manifested in SCID mice. Indeed, we found this to be the case, and the lack of clearance of bacteria in these mice at the later time point confirmed that adaptive immunity is responsible for this clearance. However, this does not mean that adaptive immunity mechanisms may not be additional targets for PT activity in promoting overall B. pertussis virulence. A previous report showed that mice infected with a PT-deficient B. pertussis strain had a greater serum antibody response to filamentous hemagglutinin (a surface adherence factor) than mice infected with the wild-type strain (18). In addition, we have recently found that, despite inferior colonization by ΔPT, serum antibody responses to several B. pertussis antigens, including Bvg-independent proteins, were greater in ΔPT-infected mice than in WT-infected mice (Artamonova and Carbonetti, unpublished), suggesting that PT may suppress antibody responses during B. pertussis infection. Therefore, PT may play multiple roles in combating host immune defenses to promote bacterial colonization, in addition to possible roles in eliciting disease associated with pertussis infection.
Acknowledgments
This work was supported in part by PHS grants AI50022 and AI45732.
We thank Drusilla Burns for antibodies and technical advice, Jeff Hasday and Ju-Ren He for help with the BAL procedure, and Jim Nataro for critiquing the manuscript.
Editor: A. D. O'Brien
REFERENCES
- 1.Alonso, S., K. Pethe, N. Mielcarek, D. Raze, and C. Locht. 2001. Role of ADP-ribosyltransferase activity of pertussis toxin in toxin-adhesin redundancy with filamentous hemagglutinin during Bordetella pertussis infection. Infect. Immun. 69:6038-6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan, M. J., J. L. David, J. G. Kenimer, and C. R. Manclark. 1988. Lectin-like binding of pertussis toxin to a 165 kilodalton Chinese hamster ovary cell glycoprotein. J. Biol. Chem. 263:4895-4899. [PubMed] [Google Scholar]
- 3.Carbonetti, N. H., and P. F. Sparling. 1987. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 84:9084-9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonetti, N. H., T. J. Irish, C. H. Chen, C. B. O'Connell, G. A. Hadley, U. McNamara, R. G. Tuskan, and G. K. Lewis. 1999. Intracellular delivery of a cytolytic T-lymphocyte epitope peptide by pertussis toxin to major histocompatibility complex class I without involvement of the cytosolic class I antigen processing pathway. Infect. Immun. 67:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiso, N., C. Capiau, G. Carletti, J. Poolman, and P. Hauser. 1999. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine 17:2366-2376. [DOI] [PubMed] [Google Scholar]
- 7.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hausman, S. Z., J. D. Cherry, U. Heininger, C. H. Wirsing von Konig, and D. L. Burns. 1996. Analysis of proteins encoded by the ptx and ptl genes of Bordetella bronchiseptica and Bordetella parapertussis. Infect. Immun. 64:4020-4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch. Exp. Med. 27:57-62. [PubMed] [Google Scholar]
- 12.Katada, T., M. Tamura, and M. Ui. 1983. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 224:290-298. [DOI] [PubMed] [Google Scholar]
- 13.Khelef, N., H. Sakamoto, and N. Guiso. 1992. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb. Pathog. 12:227-235. [DOI] [PubMed] [Google Scholar]
- 14.Kimura, A., K. T. Mountzouros, P. A. Schad, W. Cieplak, and J. L. Cowell. 1990. Pertussis toxin analog with reduced enzymatic and biological activities is a protective immunogen. Infect. Immun. 58:3337-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinnear, S. M., P. E. Boucher, S. Stibitz, and N. H. Carbonetti. 1999. Analysis of BvgA activation of the pertactin gene promoter in Bordetella pertussis. J. Bacteriol. 181:5234-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotob, S. I., S. Z. Hausman, and D. L. Burns. 1995. Localization of the promoter for the ptl genes of Bordetella pertussis, which encode proteins essential for secretion of pertussis toxin. Infect. Immun. 63:3227-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques, R. R., and N. H. Carbonetti. 1997. Genetic analysis of pertussis toxin promoter activation in Bordetella pertussis. Mol. Microbiol. 24:1215-1224. [DOI] [PubMed] [Google Scholar]
- 18.Mielcarek, N., G. Riveau, F. Remoue, R. Antoine, A. Capron, and C. Locht. 1998. Homologous and heterologous protection after single intranasal administration of live attenuated recombinant Bordetella pertussis. Nat. Biotechnol. 16:454-457. [DOI] [PubMed] [Google Scholar]
- 19.Mills, K. H. G., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizgerd, J. P. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin. Immunol. 14:123-132. [DOI] [PubMed] [Google Scholar]
- 21.Morse, S. I., and J. H. Morse. 1976. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J. Exp. Med. 143:1483-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss, J., S. J. Stanley, D. L. Burns, J. A. Hsia, D. A. Yost, G. A. Myers, and E. L. Hewlett. 1983. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J. Biol. Chem. 258:11879-11882. [PubMed] [Google Scholar]
- 23.Munoz, J. J., H. Arai, R. K. Bergman, and P. L. Sadowski. 1981. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect. Immun. 33:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittman, M. 1979. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev. Infect. Dis. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 25.Pittman, M., B. L. Furman, and A. C. Wardlaw. 1980. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J. Infect. Dis. 142:56-66. [DOI] [PubMed] [Google Scholar]
- 26.Pizza, M., A. Covacci, A. Bartoloni, M. Perugini, L. Nencioni, M. T. DeMagistris, L. Villa, D. Nucci, R. Manetti, M. Bugnoli, F. Giovannoni, R. Olivieri, J. T. Barbieri, H. Sato, and R. Rappuoli. 1989. Mutants of pertussis toxin suitable for vaccine development. Science 246:497-500. [DOI] [PubMed] [Google Scholar]
- 27.Raleigh, E. A., N. E. Murray, H. Revel, R. M. Blumenthal, D. Westaway, A. D. Reith, P. W. Rigby, J. Elhai, and D. Hanahan. 1988. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 16:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisine, T. 1990. Pertussis toxin in the analysis of receptor mechanisms. Biochem. Pharmacol. 39:1499-1504. [DOI] [PubMed] [Google Scholar]
- 29.Sato, Y., K. Izuyima, H. Sato, J. L. Cowell, and C. R. Manclark. 1980. Aerosol infection of mice with Bordetella pertussis. Infect. Immun. 29:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 31.Spangrude, G. J., F. Sacchi, H. R. Hill, D. E. Van Epps, and R. A. Daynes. 1985. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J. Immunol. 135:4135-4143. [PubMed] [Google Scholar]
- 32.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 33.Stein, P. E., A. Boodhoo, G. D. Armstrong, S. A. Cockle, M. H. Klein, and R. J. Read. 1994. The crystal structure of pertussis toxin. Structure 2:45-57. [DOI] [PubMed] [Google Scholar]
- 34.Stibitz, S., W. Black, and S. Falkow. 1986. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene 50:133-140. [DOI] [PubMed] [Google Scholar]
- 35.Tamura, M., L. Nogimori, S. Murai, M. Yajima, K. Itio, T. Katada, M. Ui, and S. Ishii. 1982. Subunit structure of the islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry 21:5516-5522. [DOI] [PubMed] [Google Scholar]
- 36.Tuomanen, E., and A. A. Weiss. 1985. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J. Infect. Dis. 152:118-125. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, A. A., and M. S. Goodwin. 1989. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect. Immun. 57:3757-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 39.Witvliet, M. H., D. L. Burns, M. J. Brennan, J. T. Poolman, and C. R. Manclark. 1989. Binding of pertussis toxin to eukaryotic cells and glycoproteins. Infect. Immun. 57:3324-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong, W. S. F., and P. M. Rosoff. 1996. Pharmacology of pertussis toxin B-oligomer. Can. J. Physiol. Pharmacol. 74:559-564. [DOI] [PubMed] [Google Scholar]