Abstract
Establishment of antigen-specific tolerance among mature T cells has been a long debated, yet poorly understood issue. In this study we have used transgenic mice bearing a class II–restricted TCR specific for the hemmagglutinin of the influenza virus in order to test the behavior of CD4+ T cells upon exposure to antigen in different forms and doses. We first studied the fate of T cells expressing the transgenic TCR (6.5) in double transgenic mice where HA was expressed as a self antigen by hemapoietic cells. In these mice, we found some mature T cells in periphery that had escaped thymic deletion and that showed signs of activation but which were anergic. Mature CD4+6.5+ cells that were transferred into antigen-containing recipients went through an initial phase of expansion after which most cells were deleted and those remaining became unresponsive, as previously described for CD8+ cells. Inducing tolerance in CD4+6.5+ cells in situ in single transgenic mice proved a difficult task: classical protocols using single doses of soluble or deaggregated antigen as well as feeding antigen all failed to induce antigen-specific unresponsiveness. It was only after decreasing cell numbers by CD4 antibody treatment and by repeatedly reintroducing antigen thereafter that unresponsiveness of 6.5+ cells was achieved and maintained. In no case could we observe the appearance of antigen-specific T cells with a Th2 cytokine profile among the remaining cells and therefore conclude that deletion and anergy represent the major mechanisms of tolerance in our studies.
Self-nonself discrimination of the immune system is in part due to an intrinsic difference in response to antigen of immature versus mature T lymphocytes: self antigens that are permanently present in the thymus delete immature T cells by apoptosis while more mature T cells that have not encountered their antigen during development can respond in different ways that range from activation to effector function to the induction of non-responsiveness. The precise mechanisms of unresponsiveness of mature T cells are still poorly understood but it has been shown that they may involve deletion (1, 2) or anergy (3–5). Other means in which regulatory T cells can interfere with certain effector functions have been proposed (6, 7) but here the evidence is more indirect and the mechanisms that may involve regulation through cytokines need to be established in detail.
Induction of tolerance in mature T cells has for years been attempted in many different ways also because of the potential applications in organ-specific autoimmune diseases and transplantation. In these studies tolerance could be achieved by injecting mice with high doses or low doses of soluble antigen, for instance with deaggregated immunoglobulin (8, 9, for review see reference 10). In other studies unresponsiveness was achieved by feeding antigen and thus was named oral tolerance (11, for review see reference 12). Some of the proceedings gave however erratic results and there is still no procedure which reproducibly induces specific tolerance to a variety of different antigens that can be used to interfere with allergy, autoimmunity or transplant rejection. This may be so because in the more ancient experiments one could not study the mechanisms of tolerance because it was difficult to follow the fate of the antigen-specific cells after the attempted tolerization. More recent studies that employed mice with transgenic T cell receptors came to the conclusion that CD8+ T cells specific for conventional antigen could either be deleted or anergized after a transient period of activation, depending on the doses of antigen (13). Studies with CD4+ cells specific for superantigens showed also that many CD4+ T cells underwent apoptotic cell death after activation by the superantigen (14). Since superantigens can bind to T cell receptors in a different way than conventional antigens without direct contact between the TCR and superantigen binding MHC molecules, it was not clear whether these results could be extrapolated to CD4+ T cells recognizing conventional antigens. In mice expressing transgenic TCRs on CD4+ T cells tolerization even with high doses of peptide failed while it appeared that, upon adoptive transfer, smaller numbers of CD4+ T cells could be tolerized by peptide (15), suggesting that the frequency of reactive T cells versus the amount of antigen could be an important parameter in the induction of certain forms of tolerance. Here we are reporting studies on TCR transgenic mice that express on CD4+ but also on some CD8+ cells a receptor specific for peptide 111-119 from influenza hemagglutinin (HA)1 presented by Ed MHC molecules (16). The transgenic receptor can be identified by a monoclonal anti-idiotypic antibody (6.5) and in the various transgenic mice the receptor is expressed on ∼7–10% of all CD4+ T cells in secondary lymphoid organs. Although this frequency of antigen-specific cells is high in comparison to the frequency of T cells specific for conventional non-MHC antigens (10−4), it is about in the same range as the frequency of T cells responding to a foreign MHC haplotype.
In these mice we analyzed various ways of antigen presentation that had the specific goal to tolerize CD4+ T cells. We first studied tolerance under conditions in which the antigen was expressed by hemopoietic cells under control of the Ig-κ promoter. Having obtained evidence that under these conditions immature as well as mature CD4+ T cells can be tolerized, we attempted to define protocols that employed soluble antigen in various forms to tolerize mature CD4+ T cells in situ. Our results indicate that CD4+ cells at a relatively high frequency are extremely difficult to tolerize in situ unless one employs strategies that reduce their number and/or responsiveness before antigen is introduced.
Materials and Methods
Mice.
TCR transgenic (6.5) mice already described (16) express a T cell receptor α/β specific for peptide 111-119 from influenza hemmagglutinin presented by I-Ed. Phenotyping of offspring was done by FACS® staining of PBLs with the clonotypic antibody. For the experimental protocols, heterozygous TCR transgenic mice between 6 and 10 wk of age were used. Mice expressing hemagglutinin (HA+) have the HA transgene under control of the Ig κ promoter and enhancer elements (Rolink A., not published) and are on Balb/c background. Transgene expression was determined by tail DNA PCR using HA primers. Balb/c mice were obtained from IFFA CREDO (France).
Antigen Administration Protocols.
The peptide (SVSSFERFEIFPK) was synthesized in the Basel Institute for Immunology. The chimeric immunoglobulin HA-Ig contains the peptide 111119 of HA in the CDR3 region of the heavy chain as already described (17), and was prepared from the hybridoma supernatant cultured in complete IMDM + 3% FCS previously run over a protein G column (Fast Flow; Pharmacia LKB, Upsala, Sweden).
The antigen was administered by oral route by gastric intubation with a gauge needle. Intravenous injections were done by the lateral tail-vein. All the reagents were diluted in sterile PBS before administration.
In the protocols using the CD4 antibody plus the HA-Ig protein, one first dose (50 μg) of YTA3.1.2 was injected i.v. the first day and the same dose of YTA3.1.2 plus 0.1 mg of HA-Ig were injected the second day.
Antibodies and FACS® Analysis.
Hybridoma supernatants containing mAbs YTA3.1.2 (18) or 6.5 (anti-clonotypic antibody described before [15]) were purified by protein G (Fast Flow) affinity chromatography. 6.5 mAb was labeled using fluorescein succinyl ester (FLUOS) (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions.
The following mAbs were used: FLUOS-labeled 6.5 mAb, anti CD8-red613 (GIBCO BRL, Gaithersburg, MD), anti-CD4PE (Becton Dickinson and Co., Mountain View, CA), biotin-conjugated anti-CD69 (PharMingen, San Diego, CA), biotin-conjugated anti-CD25 (PharMingen), biotin-conjugated anti-CD45RB (PharMingen), anti-CD62L (Mel 14, kind gift of F. Lepault), SA-PE (Southern Biotechnology Associates Inc., Birmingham, AL) and mouse anti-rat κ-PE (Sigma Chem. Co., St. Louis, MO). Two- and three-color flow cytometry was performed on FACScan® (Becton Dickinson and Co.). Stainings were done in 96-well plates (5 × 105 cells per well) in 10-20 μl of mAb at optimal dilution in PBS + 5% FCS + 0.1% azide. Between first and second step reagents cells were washed in 250 μl of PBS + 5% FCS + 0.1% azide as was done after the last step. Data (5 × 104 events) were stored and analyzed with the LYSIS II software (Becton Dickinson and Co.).
T Cell Proliferation and Cytokine Assays.
All in vitro assays were performed in complete IMDM (β-mercaptoethanol [5 × 10−5M] penicillin [100 UI/ml]) supplemented with 10% FCS. Responder cells were isolated from the spleen and lymph nodes and depleted of B cells with the help of a sheep anti–mouse IgG (H+L) (Biosys, France) pre-coated on a petri dish at 10 μg/ml. T cells (2 × 105/ well) were then cultured with 5 × 105 irradiated (2,200 rads) Balb/c splenocytes in the presence of 10 μg/ml, 1 μg/ml of peptide, coated 6.5 mAb (100 μg/ml), or medium alone. Exogenous IL2 was added in some cases, at 30 UI/ml. 3H-incorporation was measured over the last 18 h of a 66 h culture. Proliferation assays were done for both spleen T cells and lymph node T cells and since they gave similar results, only the former are shown. The supernatant of a 66 h culture in the same conditions as for the proliferation but containing 4 × 105 T spleen cells, was collected and used for ELISA assay. The standards used permitted the detection in the supernatant of a minimum concentration of 150 pg/ml for IL4 and of 600 pg/ml for IFN-γ. The antibodies used were mAb AN-18.17 and biotin-conjugated R4-6A2 for IFN-γ (ATCC HB-170), and 11B11 and biotin-conjugated BVD6-24G2 for IL4 (PharMingen). Horseradish peroxidase streptavidin (VECTOR Laboratories, Inc. Burlingame, CA) activity was revealed by O-phenylenediamine dihydrochloride substrate (Sigma Chem. Co.). Plates were read at 490 nm.
Transfer Experiments.
For the transfer of 6.5+ cells into HA+ transgenic recipients, lymph node cells from 6.5+/+RAG2−/− mice were washed and resuspended in serum-free medium. 2.5 × 106 cells were injected into previously irradiated (500 rads) HA+ transgenic mice or HA− littermates. Recipients were killed and analyzed 4, 8, 14, and 30 d after the transfer.
Results
Tolerance of CD4+ Cells in Mice that Express the Antigen on Hemopoietic Cells.
We first analyzed the fate of class II restricted cells in double transgenic mice where the antigen would be constantly present at relatively high concentrations both in the thymus and in the periphery. For that purpose, 6.5+ TCR transgenic mice were crossed to mice transgenic for the HA gene under control of the immunoglobulin κ promoter. In the latter mice the antigen is expressed on hemopoietic cells and their spleen cells stimulate a good response of unprimed T cells bearing the transgenic TCR. In double transgenic mice (HA+6.5+), the absolute number of thymocytes was about half that of HA−6.5+ littermates and, as shown in Fig. 1, the percentage of 6.5+ thymocytes among the CD4+CD8− and CD4−CD8+ subpopulations was drastically decreased. Note that in peripheral lymphoid tissue 6.5+ cells express less of the transgenic receptor than thymocytes. This is in part due to the fact that the peripheral T cells in these mice express in general slightly lower TCR levels as judged by staining with the TCR-β antibodies and analysis of RAG−/−6.5+ mice and in part due to the fact that 6.5+ cells in the transgenic mice express endogenous TCR-α chains (not shown). The percentages of double-negative, double-positive, and singlepositive thymocytes were not very different from that of HA transgene negative littermates in part because other receptors generated by endogenous TCR α rearrangements were selected. Likewise, the absolute number of peripheral T cells was not greatly affected in HA+6.5+ mice and the percentage of B and T cells in the spleen was similar to that observed in 6.5+HA− littermates (data not shown). Surprisingly, however, 6.5+ cells could be detected in the periphery of the double transgenic mice and the frequency of splenic 6.5+ cells was comparable to that of 6.5+HA− mice (Fig. 1). There was no evidence for TCR or coreceptor downregulation among peripheral 6.5+ cells from double transgenic mice that had escaped deletion, but these cells differed from T cells from 6.5+HA− mice in that they had upregulated CD69 and downregulated CD62-L and CD45RB levels (not shown). When splenic or lymph node T cells from the double transgenic mice were stimulated in vitro either with antigen at different concentrations or with the clonotypic antibody, the 6.5+ cells were unresponsive unlike those from 6.5+HA− mice which proliferated well without prior immunization (Fig. 2, A and B). There was no response when cells from normal, non-transgenic BALB/c mice were stimulated either by antigen or the clonotypic antibody indicating that 6.5+ cells were essential for a proliferative response as well as cytokine production (see below). Addition of IL2 did not restore the response. These data demonstrate that tolerance in class II restricted cells can be induced by an antigen expressed in hemopoietic cells and that the mechanisms may involve deletion of immature cells as well as anergy of peripheral lymphocytes. The anergy may have in fact been induced after thymic emigration of the 6.5+ cells since at birth we cannot detect in the thymus HA that can stimulate 6.5+ cells in vitro (not shown).
Figure 1.
6.5+ cells in the thymus, lymph nodes and spleen of HA−6.5+ and HA+6.5+ mice. Total cell numbers (×107) for the single and double transgenic mice were 6.7 and 3.8 in the thymus, 6.0 and 12.0 in the lymph nodes and 13.0 and 8.0 in the spleen, respectively. Single cell suspensions were used for three-color staining with CD4, CD8, and 6.5 antibodies and 5 ×104 events were stored and analyzed. In this and all experiments, the marker for the 6.5high expressing cells in periphery was established according to the level of 6.5 expression by the CD4+thymocytes of single TCR transgenicmice.
Figure 2.

Proliferative response of lymph node (A) and spleen (B) cells of double versus single transgenic mice upon antigenic stimulation in vitro. 2 ×105 sIg−cells were cultured with 5 ×105 irradiated Balb/c splenocytes and stimulated with different doses of peptide (10 and 1 μg/ml), with coated 6.5 mAb (100 μg/ml) or with medium alone. 3H was added to the culture 48 h afterwards and left for an additional 18 h. The number of 6.5+ cells per well was determined and proliferation values shown correspond to cpm/ 103 6.5+ cells. Values correspond to one set of experiments that included two HA+6.5+ mice and one HA−6.5+ mouse. Similar data were obtained in separate experiments.
CD4+ Cells Can Be Rendered Tolerant upon Transfer into Antigen-bearing Recipients.
We next addressed the question whether mature naive CD4+ cells, when exposed to high doses of antigen, would follow the same fate as previously described for CD8+ cells (2, 13) . For this purpose, lymph node cells from RAG−/−6.5+/+ mice were injected i.v. into x-irradiated HA+ mice or HA negative littermates and recipients were analyzed at different time points after transfer. As can be seen in Fig. 3, during the first 4 d 6.5+ cells expanded vigorously upon transfer into antigen-positive recipients and cell numbers declined thereafter. In contrast, when 6.5+ cells were transferred into HA− recipients, there was no significant expansion of these cells during the period of observation though about five percent of the injected cells could be recovered from these recipients. 6.5+ cells recovered at day 4 after transfer into HA-containing recipients were capable of responding to antigenic stimulation in vitro, though their response was much reduced when compared to 6.5+ naive cells. Curiously, contrary to 6.5+ cells from single TCR transgenic mice, the recovered cells proliferated better upon stimulation with the 6.5+ mAb than upon stimulation with the peptide. At 6 or 8 d after transfer, the remaining 6.5+ cells gave an even lower response than on day 4 to either the peptide or the clonotypic antibody (Table 1) and their anergic state could not be reversed by exogenous IL-2. Cells recovered from HA+ recipients at day 4 after transfer were still capable of expanding in vivo when retransferred into another HA+ recipient, while cells recovered 21 d after transfer were no longer capable of doing so (data not shown). Cytokine production by the 6.5+ cells recovered at different timepoints after transfer into antigen-positive recipients was also studied: IFN-γ and IL-4 were produced at significant levels when cells were stimulated in vitro at early timepoints after transfer. At day 30 after transfer, no proliferation and no IFN-γ secretion by remaining cells could be detected (data not shown).
Figure 3.
Recovery of 6.5+ cells after transfer into antigen-positive and antigen-negative recipients. 2.5 ×106 LN cells from a 6.5+/+RAG−/− mouse were injected i.v. into sublethally irradiated HA+ or HA− littermates. Recipients were killed at different timepoints and single cell suspensions from lymph nodes and spleen were used for three-color staining. The absolute number of 6.5+ cells recovered from the periphery (lymph nodes plus spleen) was determined. Values correspond to one set of experiments that included two antigen-positive mice and one antigen-negative control analyzed at each timepoint. Separate transfer experiments gave similar results.
Table 1.
Prolifeeration and Cytokine Production by 6.5+ Cells Recovered from the Spleen of HA+ Recipients
| Days after transfer | Proliferation 10 μg/ml peptide | Proliferation 6.5 mAb | IFN-γ production | IL4 production | ||||
|---|---|---|---|---|---|---|---|---|
| cpm | cpm | ng/ml | pg/ml | |||||
| 0 | 2000 ± 100 | 1200 ± 94 | 3.6 | 0 | ||||
| 4 | 17 ± 5 | 120 ± 4 | 0.8 | 5.6 | ||||
| 6 ± 2 | 59 ± 1 | 1.0 | 3.9 | |||||
| 6 | 20 ± 2 | 17 ± 4 | 0.6 | 0.8 | ||||
| 25 ± 2 | 14 ± 1 | 1.9 | 0.7 | |||||
| 8 | 13 ± 2 | 23 ± 3 | 1.6 | 0 | ||||
| 10 ± 1 | 18 ± 1 | 2.0 | 0.8 | |||||
| 14 | 2 | 2 | ND | ND |
Values correspond to two recipients for each timepoint, except for day 14, with one recipient. All values were calculated to correspond to the response of 103 6.5+ cells. Proliferation values for unstimulated cells were lower than 2 cpm per 103 6.5+ cells. IFN-γ and IL4 values were determined from the supernatants of the cells stimulated with the 6.5 mAb.
These data thus indicate that class II restricted mature T cells can undergo deletion or become anergic when exposed to high doses of antigen for longer timepoints. We next determined whether any of the classical protocols of inducing tolerance would induce a similarly unresponsive state of 6.5+ cells in situ.
Single Doses of Antigen Applied by Different Routes Do Not Induce Tolerance.
Single doses of oral administration of either high or low doses of antigen were reported to induce tolerance by deletion and anergy, respectively (19, 20, 21). We therefore fed 6.5+ mice with high (1 mg of peptide) or with low (0.1 mg of HA-Ig) doses of antigen in order to assess the effects of oral feeding on the 6.5 cells. We used low doses of HA-Ig rather than peptide since in the original protocols protein antigen rather than peptides were used. The fed mice were analyzed at different timepoints and numbers of antigen-specific cells as well as their proliferative capacity and cytokine secretion were studied. Feeding with both high and low doses of antigen decreased cell numbers in thymus and periphery to a certain extent (to one half), indicating that the orally administered antigen reached the antigen-specific T cells. Nevertheless, no significant effect on cell proliferation or cytokine release could be observed at any timepoint analyzed. It has also been described that oral feeding of antigen shifts the cytokine production pattern among the antigen specific cells towards IL-4, TGF-β, and IL-10 production (12). However, we observed no decrease in IFN-γ production and did not detect significant IL-4 production by 6.5+ cells from orally fed mice when stimulated with antigen in vitro. The ELISA system that was used can reproducibly detect up to 150 pg/ ml of IL-4 meaning that any shift towards IL4 production was below that level.
Also administration of soluble antigen given intravenously or intraperitoneally has been reported to induce antigen-specific tolerance (8, 10, 22, 23). We analyzed therefore the effect of the similar doses of antigen given by these routes. Since the injection of 1 mg of peptide was reproducibly lethal at 20–24 h after injection (the same quantity of peptide injected into non-transgenic mice had no such effect), we used 0.75 mg of peptide as the highest dose and 0.1 mg of HA-Ig as a lower dose of antigen. Again, we analyzed the numbers of 6.5+ cells, as well as their capacity to proliferate and to produce cytokines. The data shown are for lymph nodes and were similar with spleen cells. Administration of both peptide at high doses and HA-Ig at low doses had a marked effect on numbers of 6.5+ thymocytes at day 3 and on peripheral 6.5+ cells at day 6 after injection (data not shown). The in vitro proliferative response of the antigen-specific cells at day 6 after injection was reduced by one half but this was due in most part to the lower percentage of 6.5+ cells per well. Thus, the functional capacity of the remaining 6.5+ cells was not significantly affected even 6 d after antigen administration, at a point in time when the number of peripheral antigen-specific cells was decreased the most.
We also injected 6.5+ mice with a single dose (0.1 mg) of deaggregated HA-Ig since it was reported that a single injection of deaggregated Ig could induce long lasting unresponsiveness (9). In the transgenic mice, however, such protocol had no measurable effect.
Responsiveness Is Reduced More Efficiently by HA-Ig than by Peptide when Given Repeatedly.
Because it had been demonstrated that tolerance induction in CD8+ cells is dependent on antigen persistence (24, 25), we injected 6.5+ mice i.v. with 0.1 mg of HA-Ig or peptide five times every other day and analyzed them 6 days after the last injection. The numbers of 6.5+ cells in the thymus and periphery are shown in Fig. 4 and were markedly decreased in the thymus and in the lymph nodes of treated animals. The decrease in lymph nodes was antigen-specific because the proportion of 6.5+ cells among CD4+ and CD8+ cells was likewise decreased. Despite the fact that, in terms of molarity, 0.1 mg of the peptide represents at least 2 × 104 more peptide molecules than 0.1 mg of the HA-Ig, no difference was observed in their capacity to induce partial deletion or to decrease the proliferative response of antigen-specific cells. This could mean that peptide may be very rapidly cleared upon injection when compared with proteins. Furthermore, the fact that the proteic antigen that contains the peptide sequence has an Ig-Fc part may facilitate its uptake and presentation by Fc receptor–bearing APCs. For these reasons, in all protocols tested hereafter we used the HA-Ig and not the peptide as source of soluble antigen.
Figure 4.
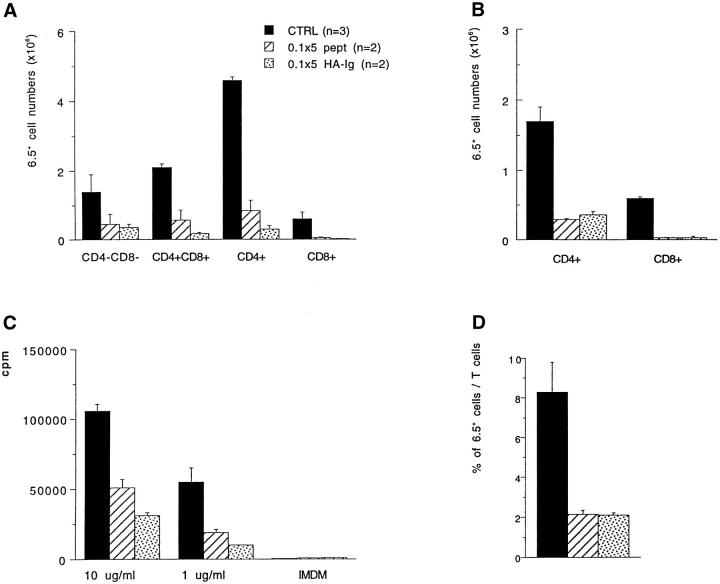
Effect of repeated doses of antigen on the number and function of 6.5+ cells. 6.5+mice were injected i.v. with PBS, 0.1 mg of peptide or 0.1 mg of HA-Ig five times every other day and were killed 6 d after the last injection. The absolute numbers of 6.5+ cells in the thymus (A) and lymph nodes (B) are shown as well as the proliferation values of lymph node cells upon antigenic stimulation in vitro with different peptide doses (C). Percentages of 6.5+ cells among total T cells are also shown (D).
In spite of the prolonged presence of antigen, the remaining antigen-specific cells in the periphery were still responsive to antigenic stimulation in vitro (Fig. 4 c). In addition, mice treated with the HA-Ig had very big spleens that contained higher numbers of 6.5+ cells when compared to the control mice or peptide-treated mice. This represents certainly an undesired effect of protocols aiming to induce tolerance in mature T cells.
The Combination of CD4 Antibodies and HA-Ig Can Induce a Transient State of Unresponsiveness.
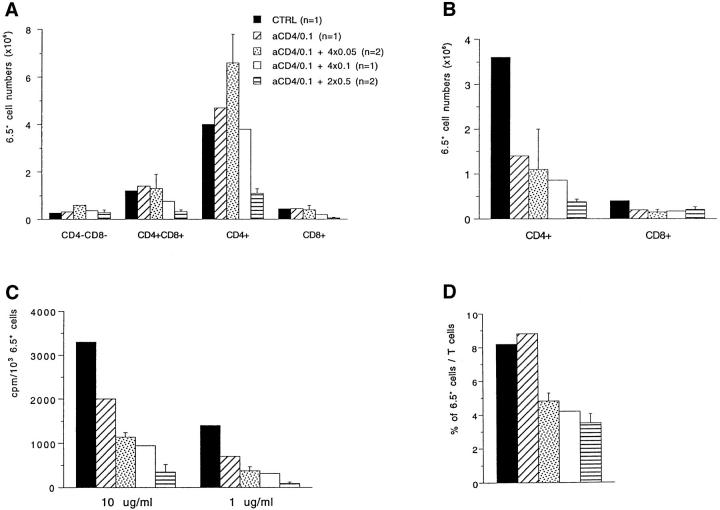
It has been shown by several groups that administration of CD4 and/or CD8 antibodies can be helpful in the establishment of peripheral tolerance (26, 27, for review see reference 28). Again, the precise mechanisms are not known. To study the effect of CD4 antibodies on responsiveness to HA we adopted a protocol described by Bushell et al. (29) with the aim of (a) decreasing CD4 cell numbers and (b) potentially providing negative signals that may favor the induction of unresponsiveness. The CD4 antibody (YTA3.1.2) depleted 60–70% of peripheral CD4 cells at day 7, as previously described (18, 29). Mice were injected i.v. with 50 μg of the antibody YTA3.1.2 and 1 d later with 50 μg of YTA3.1.2 plus 0.1 mg of HA-Ig. Control mice received either only antigen or only CD4 antibody. Mice were killed at days 3, 7, and 14 and the number and functional capacity of the 6.5+ cells was determined. As can be seen in Fig. 5, combining anti-CD4 and antigen considerably decreased cell numbers and induced non-responsiveness in terms of proliferation as well as of IFN-γ production when analyzed 7 d later. At day 14, the cells began to regain responsiveness. This could have been due to the fact that newly produced 6.5 cells, which had not been in contact with antigen, were entering secondary lymphoid tissues from the thymus. This seemed, however, not to be the case since 6.5+ cells from mice thymectomized before treatment and analyzed 21 d after treatment also recovered responsiveness. Therefore, the protocol proved to be efficient in induction, but not in maintenance of tolerance. It is also clear that anti-CD4 on its own had an effect on the proliferative capacity of 6.5+ cells, suggesting that part of this anergy at that point in time was not antigen specific. No effect of anti-CD4 alone was observed in terms of IFN-γ production, however, suggesting that the decrease in cytokine production observed at day 7 in anti-CD4 plus antigen treated mice depended on antigen. Such a decrease in IFN-γ was not due to a Th1 to Th2 shift among the antigen-specific cells since no IL4 production was detected. The lack of an effect of anti-CD4 on IFN-γ production and the decreased IFN-γ production after anti-CD4 plus antigen may be due to the fact that antigen is required to stop IFN-γ production by the TCR expressing CD8+ T cells.
Figure 5.
Co-administration of CD4 antibody plus HA-Ig antigen. 6.5+ mice received i.v. 50 μg of YTA3.1.2 one day and 50 μg of YTA plus 0.1 mg of HAIg or PBS the next day. Other mice received antigen (0.1 mg HA-Ig) alone. Between two and four mice per group were analyzed at different timepoints after treatment. The absolute numbers of CD4+6.5+ cells in the periphery is shown (CD8+6.5+ cells remained relatively constant). 3H incorporation is shown for LN cells stimulated in vitro with 1 μg/ml of peptide (B). The response to higher doses of peptide (10 μg/ml) was similarly decreased in treated animals. Proliferation values for day 21 were obtained from thymectomized mice (Tx) treated with PBS, anti-CD4 or anti-CD4/0.1 mg HA-Ig. Percentages of 6.5+ cells among total T cells were determined for each mouse (D). IFN-γ values were determined by ELISA from the supernatant of spleen T cells stimulated in vitro with 10 μg/ml of peptide (C).
The Administration of Anti-CD4/HA-Ig Followed by Repeated Antigen Administration Can Induce a Long-lasting Antigen-specific Tolerance.
Since the above protocol was efficient in inducing unresponsiveness but not in maintaining it, we reasoned that administering antigen after such treatment in a manner that would favor its persistence could help to maintain the tolerant state and would render the treatment more antigen-specific. Therefore, mice treated 7 d earlier with anti-CD4 plus 0.1 mg of HA-Ig received further injections of antigen. Different doses of HA-Ig (ranging from 50 to 500 μg) were injected seven days after treatment with CD4 antibody and 0.1 mg HA-Ig in 3-d intervals. All mice were analyzed 7 d after the last injection of antigen. As show in Fig. 6 there was a further reduction in number of 6.5 cells in an antigen-dose dependent manner in mice that received antigen in addition to the initial CD4 treatment. Significantly lowering the numbers of CD4+ cells by the CD4 antibody avoided the splenomegaly that was otherwise seen with repeated doses of HA-Ig. Likewise, the proliferative capacity of CD4+ cells to HA was reduced in a dose-dependent manner and this reduction lasted for long periods of time (30 d) in thymectomized mice (data not shown). There was an increase in the proportion of 6.5+CD69+ and of 6.5+ CD62-L− cells that correlated with antigen doses (Fig. 7). The 6.5+ cells recovered from the mice that repeatedly received either 0.5 or 0.1 mg of HA-Ig secreted very low levels of IFN-γ (less than one-tenth of what was produced by the control mice) and produced no IL4 (data not shown). It is interesting to note that while IL4 production was detected after transfer of 6.5 cells into antigen-bearing recipients (Table 1), it could not be detected after any of the protocols aiming at tolerance induction in situ. This difference may be due to the fact that, upon transfer into antigen-containing recipients, cells were exposed to much larger doses of antigen and for a longer period of time.
Figure 6.
Repeated antigen administration in mice previously treated with anti-CD4 plus antigen. Mice having received 50 μg of YTA3.1.2 plus 0.1 mg of HAIg 7 d earlier were repeatedly injected i.v. with 0.05, 0.1, or 0.5 mg of HA-Ig. For the first two doses, four injections were given every third day. For the highest dose, two injections were given, also every third day. Mice were killed 7 d after the last injection. Control mice received either PBS or anti-CD4 plus antigen only. Absolute numbers of 6.5+ cells in the thymus (A) and in the periphery (B) are shown. Proliferation values of sIg− splenic cells upon in vitro antigenic stimulation with peptide were calculated to correspond to the response of 103 6.5+ cells (C). Percentages of 6.5+ cells among total T cells are also shown (D).
Figure 7.
CD69 and CD62-L expression in mice that received repeated antigen doses after anti-CD4 plus antigen treatment. A two-color staining was performed on single lymph node cell suspensions. Staining of spleen cells gave similar results. Histograms represent live cells gated for 6.5int+high expression. Values of CD69+ or CD62-L− cells are given for both mice that received 2 × 0.5 mg of HA-Ig after anti-CD4/0.1 treatment and are compared to those for mice that received PBS or only the anti-CD4/0.1 treatment.
Discussion
This study was initiated with the aim to analyze the effect of various protocols that have previously been used to induce tolerance on CD4+ HA-specific T cells with a transgenic TCR that can be recognized by a monoclonal antibody. In initial studies, HA antigen was expressed as a self antigen in the hemopoietic system in HA+6.5+ double transgenic mice. 6.5+ mature thymocytes were greatly diminished presumably because of the deletion of immature thymocytes. The compartment of CD4+8+ cells was however not significantly affected much like it has been observed with superantigen (30) or with TCR transgenic mice specific for the C5 protein (31). In fact, CD4+CD8+ cells expressing higher levels of the transgenic TCR that were present in HA−6.5+ mice were absent in 6.5+HA+ mice (Fig. 1). Surprisingly however, there were a few cells with the transgenic TCR in the lymph nodes of HA+6.5+ double transgenic mice and a highly significant number was found in the spleen of these mice. These cells showed signs of activation but could not be induced by either antigen or receptor antibody to proliferate in vitro even in the presence of exogenous IL2. These cells may represent migrants from the thymus that escaped deletion there and were anergized in secondary lymphoid organs. The function of these cells differs from that of CD8lo cells that were detected in the spleen of mice with a class I MHC–restricted TCR (2, 32) by the fact that these CD4+ cells could not be activated by receptor antibodies. The existence of these autoreactive but anergic T cells is of interest and their origin and migration needs to be addressed in future studies. In this context it is worthwhile pointing out that in a recent study, autoreactive T cells were likewise detected in the periphery of TCR transgenic mice with a class II MHC– restricted TCR and were causing arthritis in those mice (33). In the 6.5+ HA+ mice we have, however, not seen any obvious signs of disease.
When mature HA-specific T cells encountered the HA antigen expressed by hemopoietic cells for the first time after transfer from 6.5+ mice into HA+ recipients, they behaved much like mature CD8+ T cells that were similarly exposed to a class I MHC–presented antigen: after an initial phase of rapid expansion, cells were deleted and the remainder became anergic. The induction of unresponsiveness in terms of proliferation and IFN-γ production took several days.
The experiments with mice that express HA-antigen on hemopoietic cells are relevant with regard to self tolerance and the transfer experiments with regard to graft versus host disease for instance after transplantation of allogeneic bone marrow that contains mature T cells. It would appear that in the latter case T cells specific for antigens that are ubiquitously expressed on hemopoietic cells become tolerant much like the CD4+ cells with the transgenic TCR when transferred into HA+ recipients, and thus one might expect that cells which cause late or chronic graft versus host disease are directed against different antigens. On the other hand, the depletion of hemopoietic cells before bone marrow transplantation may interfere with the tolerization process that is observed in our experiments.
The experiments in mice expressing HA on hemopoietic cells are obviously of limited relevance for procedures that aim at the tolerization of T cells in situ with the goal to prevent or interfere with graft rejection, autoimmunity or allergy. We therefore tried to develop procedures that would reliably interfere with the response of a relatively high frequency of T cells roughly equivalent to that of cells responding to a foreign MHC haplotype. It may be primarily for this reason that the traditional experimental protocols that were often but not always successful in establishing tolerance to non-MHC antigens failed here. Whether this is solely due to the relatively high frequency of antigen-specific T cells or to the absence of regulatory T cells in our TCR transgenic mice is not known. It should, however, be noted that in our mice the majority of CD4+ and CD8+ T cells do not express the transgenic TCR. One would assume that some of these cells are autoreactive, and thus, if regulatory T cells have an important role in self tolerance they would have to be operative in these TCR transgenic mice. One may wonder why tolerization failed even when repeated doses of antigen were given. This could be due to the fact that cells once exposed to antigen in a dose insufficient to delete become more resistant to antigen-mediated deletion. Whatever the reason for the failure of these traditional protocols, we noted that decreasing the number of antigen-specific T cells by reducing the total number of CD4+ T cells by CD4 antibodies was helpful. It was under these conditions that the addition of antigen caused unresponsiveness that could be maintained for long periods of time if antigen was given repeatedly. Although we cannot rule out any negative signal delivered by the CD4 antibodies, the data are certainly consistent with the notion that the frequency of reactive T cells and the dose and persistence of antigen are an important parameter determining tolerance. This would be in agreement with a study by Kearney et al. (15) where tolerance induction of cells expressing a class II–restricted transgenic TCR was only achieved after transfer of a small quantity of such cells into syngeneic non-transgenic recipients even though it is not clear what influence the transfer procedure had on these cells. Similarly, a study of Förster et al. (34) indicated that the ratio of the number of responsive T cells over the amount of antigen represented a crucial parameter in tolerance induction although in their study the antigen may have been expressed in the thymus.
It is interesting to note that with none of the employed protocols, be it peptide injection or injection of soluble and deaggregated protein, feeding of antigen, or giving the antigen together with CD4 antibodies, did we observe either a complete deletion of antigen specific T cells or a qualitative change in the lymphokine secretion profile of the remaining cells in terms of shift from IFN-γ secretion to the production of IL4 as suggested by Zhang et al. (35). So far we have consistently only been successful with one type of tolerization protocol, namely shifting the balance between the frequency of reactive T cells and the dose of antigen in favor of the latter and observing deletion of cells as well as induction of anergy.
Acknowledgments
We thank B. Rocha for helpful advice.
Footnotes
This work was supported by funds from the Institut National de la Santé et de la Recherche Médicale. H. von Boehmer is supported by the Human Frontier Science Program.
1 Abbreviations used in this paper: FLUOS, fluorescein succinyl ester; HA, hemagglutinin.
References
- 1.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 2.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science (Wash DC) 1991;251:1225–1227. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 3.Rammensee HR, Kroschewski R, Frangoulis B. Clonal anergy induced in mature Vb6+T lymphocytes on immunizing Mls-1b with Mls-1a expressing cells. Nature (Lond) 1989;339:541–543. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 4.Kawabe Y, Ochi A. Selective anergy of Vb8+ CD4+cells in staphylococcus enterotoxin B-primed mice. J Exp Med. 1990;172:1069–1078. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–310. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 6.Gershon RK. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 7.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. Infectious transplantation tolerance. Science (Wash DC) 1993;259:974–976. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 8.Chiller JM, Habicht GS, Wiegle WO. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science (Wash DC) 1971;171:813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- 9.Romball CG, Weigle WO. In vivo induction of tolerance in murine CD4+cell subsets. J Exp Med. 1993;178:1637–1644. doi: 10.1084/jem.178.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondino A, Khoruts A, Jenkins MK. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA. 1996;93:2245–2252. doi: 10.1073/pnas.93.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells H. Studies on the chemistry of anaphylaxis. III. Experiments with isolated proteins, especially those of the hen's egg. J Infect Dis. 1911;9:147–151. [Google Scholar]
- 12.Weiner HL, Friedman A, Miller A, Khoury SJ, AlSabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trenthan DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 13.Rocha B, Grandien A, Freitas A. Anergy and exhaustion are independent mechanisms of peripheral tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald HR, Baschieri S, Lees RK. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963–1966. doi: 10.1002/eji.1830210827. [DOI] [PubMed] [Google Scholar]
- 15.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 16.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaghouani H, Steinman R, Nonacs R, Shah H, Gerhard W, Bona C. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science (Wash DC) 1993;259:224–227. doi: 10.1126/science.7678469. [DOI] [PubMed] [Google Scholar]
- 18.Qin S, Cobbold S, Tighe H, Benjamin R, Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987;17:1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- 19.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 20.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Nature (Lond) 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo V, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature (Lond) 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 22.Dresser DW. Efectiveness of lipid and lipidophilic substances as adjuvants. Nature (Lond) 1961;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 23.Mitchison NA. Proc Roy Soc. 1964;161:275–290. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- 24.Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistance of antigen. Science (Wash DC) 1992;257:1130–1132. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 25.Rocha B, Tanchot C, von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin S, Cobbold S, Benjamin R, Waldmann H. Induction of classical transplantation tolerance in the adult. J Exp Med. 1989;169:779–794. doi: 10.1084/jem.169.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood KJ. Transplantation tolerance with monoclonal antibodies. Semin Immunol. 1990;2:389–399. [PubMed] [Google Scholar]
- 28.Cobbold SP, Qin S, Leong LY, Martin G, Waldmann H. Reprogramming the immune system for peripheral tolerance with CD4 and CD8 monoclonal antibodies. Immunol Rev. 1992;129:165–201. doi: 10.1111/j.1600-065x.1992.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 29.Bushell A, Morris PJ, Wood KJ. Transplantation tolerance induced by antigen pretreatment and depleting anti-CD4 antibody depends on CD4+ T cell regulation during the induction phase of the response. Eur J Immunol. 1995;25:2643–2649. doi: 10.1002/eji.1830250936. [DOI] [PubMed] [Google Scholar]
- 30.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 31.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. JExp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 33.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoreactivity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 34.Förster I, Hirose R, Arbeit JM, Clausen BE, Hanahan D. Limited capacity for tolerization of CD4+ T cells specific for a pancreatic beta cell neoantigen. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Miller RG, Zhang J. Characterization of apoptosis-resistant antigen-specific T cells in vivo. J Exp Med. 1996;183:2065–2073. doi: 10.1084/jem.183.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]