Abstract
Proteases are now firmly established as major regulators of the “execution” phase of apoptosis. Here, we examine the role of proteases and their relationship to ceramide, a proposed mediator of apoptosis, in the tumor necrosis factor-α (TNF-α)–induced pathway of cell death. Ceramide induced activation of prICE, the protease that cleaves the death substrate poly(ADP-ribose) polymerase. Bcl-2 inhibited ceramide-induced death, but not ceramide generation. In contrast, Cytokine response modifier A (CrmA), a potent inhibitor of Interleukin-1β converting enzyme and related proteases, inhibited ceramide generation and prevented TNF-α–induced death. Exogenous ceramide could overcome the CrmA block to cell death, but not the Bcl-2 block. CrmA, however, did not inhibit the activation of nuclear factor (NF)-κB by TNF-α, demonstrating that other signaling functions of TNF-α remain intact and that ceramide does not play a role in the activation of NF-κB. These studies support a distinct role for proteases in the signaling/activation phase of apoptosis acting upstream of ceramide formation.
Apoptosis is an essential, highly conserved, and tightly regulated cellular process of cell death that is important for development, host defense, and suppression of oncogenesis (1). The intracellular mediators of apoptosis are poorly defined. Recently, proteases belonging to the newly described IL-1β converting enzyme (ICE)1 family emerged as central constituents of the apoptotic machinery. The evidence implicating these proteases in the induction of apoptosis stems from the following observations: (a) homology to the ced-3 gene product of the nematode Caenorhabditis elegans that is required for cell death during development (2), (b) induction of apoptosis when these proteases are overexpressed in their active form (3), (c) inhibition of apoptosis when these proteases are specifically inhibited (4–8), and (d) identification of a number of cellular protein substrates that are proteolytically cleaved through the specific action of these proteases during apoptosis including poly(ADP-ribose) polymerase (PARP) (9, 10), the 70-kD protein component of the U1 small nuclear ribonucleoprotein (11), and others (12). Current evidence implicates certain members of the ICE family, particularly CPP32/Yama/apopain and ICELAP3, in the execution phase of apoptosis (6, 7, 13, 14). Also, MACH/FLICE has been recently identified as a protease in close association with the Fas receptor (15, 16).
Another important group of cell death regulators are members of the Bcl-2 family that act as either inhibitors or promoters of apoptosis (17–19). Bcl-2 can inhibit apoptosis induced by a wide variety of stimuli (17, 20). However, the mechanism of action of Bcl-2 remains unknown.
Inhibition of apoptosis is used by some DNA viruses to attenuate host defense and increase viral progeny (21). The cowpox virus cytokine response modifier A (CrmA) protein that exhibits cross-class inhibition of both serine and cysteine proteases (22) is a potent inhibitor of apoptosis induced by serum withdrawal (23), activation of the Fas or TNF-α receptors (4, 24), or withdrawal of nerve growth factor in primary chicken neuronal cultures (25). Although CrmA has been shown to have preferential activity against ICE (26), it is also able to inhibit the proteolytic activity of other members of the ICE family implicated in apoptosis, although less potently.
Based on recent studies, the sphingolipid ceramide is emerging as a possible regulator of apoptosis. Ceramide can potently induce apoptosis in a number of different systems (27–30). TNF-α and other inducers of apoptosis (Fas ligation, serum withdrawal, some chemotherapeutic agents, γ-irradiation; 29, 31–34) have been shown to elevate cellular levels of ceramide. In turn, the addition of cell-permeable ceramide induces apoptosis in several cell types, raising the possibility that this may be a final pathway common to a variety of inducers (28–30, 32). More recently, the death signal from both Fas and TNF-α-receptor 1 was shown to converge proximally on FADD, a “death domain” containing protein that belongs to a new family of signaling molecules that associate with members of the TNF-α receptor family (35–38). Expression of a dominant negative mutant of FADD inhibited ceramide accumulation, ICE-related protease activation, and apoptosis after treatment with Fas antibody by blocking the proximal signal. Exogenous ceramide was able to bypass this block and produce apoptosis (38). Additionally, the Drososphila melanogaster protein REAPER, which is critical for the normal development of the Drosophila embryo, was recently found to activate apoptosis by the activation of an ICE-like protease associated with ceramide generation and apoptosis (39). These effects were blocked by the use of a specific peptide inhibitor of ICE-like proteases. Based on these observations, it has been suggested that ceramide may play a central role in the regulation of apoptosis (40).
Here we investigate whether the antiapoptotic molecules, CrmA and Bcl-2, interact with the ceramide pathway. We show that CrmA targets this pathway upstream of ceramide generation in response to TNF-α. In contrast, we show that Bcl-2 protects from both TNF-α and ceramide– induced cell death without interfering with ceramide generation, suggesting that it functions further downstream along the ceramide pathway. These studies identify a novel target for inhibition of apoptosis, namely, the inhibition of ceramide generation, and clarify the order by which CrmA, ceramide, and Bcl-2 interact.
Materials and Methods
Cell Lines and Cultivation.
The previously described cell lines expressing CrmA or mutant CrmA and those overexpressing Bcl-2 were derived from a TNF-α–sensitive MCF-7 parental line (4, 6, 41) (gift of Muneesh Tewari and Dr. Vishva Dixit, University of Michigan, Ann Arbor). Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 0.2% sodium bicarbonate. 500 μg/ml G418 was added to the CrmA cell line and its vector while 150 μg/ml hygromycin was added to the Bcl-2 cell line and its vector. Experiments were done in the absence of G418 or hygromycin. Cell viability was determined by the ability to exclude trypan blue.
Ceramide Measurement.
Cells were seeded at 2 × 105 cells/ml in a 10 ml volume, rested overnight, and then treated with 1.2 nM TNF-α. Lipids were collected according to the method of Bligh and Dyer (42). In brief, cells were pelleted, washed once with PBS, and then extracted with 3 ml chloroform/methanol (1:2, vol/vol) in 13 × 100 mm screw-top glass tubes. The monophase was mixed, 0.7 ml water was added, and the samples were rested for 10 min. The organic and aqueous phases were subsequently separated by addition of 1 ml chloroform and 1 ml water followed by vigorous shaking and centrifugation at 1,000 rpm. The organic phase was carefully removed and transferred to a new tube, and the samples were dried under N2. Lipids were then resuspended in 1 ml of chloroform. Ceramide levels were measured using a modified diacylglycerol kinase assay (43, 44) using external standards. In brief, 80% of the lipid sample was dried under N2. The dried lipid was solubilized in 20 μl of an octyl-β-d-glucoside/dioleoyl phophatidylglycerol micellar solution (7.5% octylβ-d-glucoside, 25 mM dioleoyl phosphatidylglycerol) by 2 cycles of sonication in a bath sonicator for 60 s followed by resting at room temperature for 15–20 min. The reaction buffer was prepared as a 2 × solution containing 100 mM imidazole HCL, pH 6.6, 100 mM LiCl, 25 mM MgCl2, 2 mM EGTA. To the lipid micelles, 50 μl of the 2 × reaction buffer were added, 0.2 μl of 1 M dithiothreitol, 5 μg of diglycerol kinase membranes, and dilution buffer (10 mM imidazole, pH 6.6, 1 mM diethylenetriaminepentaacetic acid, pH 7) to a final volume of 90 μl. The reaction was started by adding 10 μl 2.5 mM [γ-32P]ATP solution (specific activity of 75,000– 200,000 cpm/nmol). The reaction was allowed to proceed at 25°C for 30 min. Lipids were extracted as described above and a 1.5-ml aliquot of the organic phase was dried under N2. Lipids were then resuspended in a volume of 100 μl methanol/chloroform (1:20, vol/vol) and 20 μl was spotted on a 20-cm silica gel thin layer chromatography plate. Plates were developed with chloroform/acetone/methanol/acetic acid/H2O (50:20:15:10:5), air dried, and then subjected to autoradiography. The radioactive spots corresponding to phosphatidic acid and ceramide phosphate, and the phosphorylated products of diacylglycerol, and ceramide, respectively, were scraped into a scintillation vial containing 4 ml scintillation fluid and counted on a scintillation counter. Linear curves of phosphorylation were produced over a concentration range of 0–640 pM of external standards (dioleoyl glycerol and CIII ceramide; Sigma Chemical Co., St. Louis, MO). Diacyglycerol and ceramide levels were always normalized to lipid phosphate which was measured according to the method of Rouser et al. (45). In brief, 20% of the lipid sample was dried down under N2 and oxidized with 150 μl of 70% perchloric acid on a heating block at 160°C for 45 min. The tubes were allowed to cool, and then 830 μl of H20 was added, followed by 170 μl of 2.5% ammonium molybdate, and 170 μl of 10% ascorbic acid with vortexing after each addition. The tubes were then incubated at 50°C for 15 min, allowed to cool, and absorbance read at 820 nm and compared to standard.
Measurement of Ceramide Uptake.
Cells were seeded at 2.5 × 105/well of a 6-well plate in a 2-ml volume of RPMI with 2% FBS and treated with 14C6ceramide at 20 μM. Cells were harvested by scraping at the indicated time points, washed twice with PBS, and the radioactivity retained in the pellet was counted in a scintillation counter.
Preparation of Cell Lysates.
For experiments analyzing PARP proteolysis, cells were seeded and treated as described for the ceramide measurement experiments. At the indicated time points, cells were harvested by scraping in media followed by centrifugation at 4°C and washed once with ice-cold PBS. The cell pellet was then resuspended in 50 μl PBS and lysed with sample buffer (30 mM Tris-HCL [pH 6.8], 10% glycerol, 6% β-mercaptoethanol, 4% SDS) and boiled for 10 min. Protein concentrations were determined using the Bio-Rad assay.
Western Blotting.
Equal amounts of protein, usually 100 μg, were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. PARP and its cleaved fragment were detected using a rabbit polyclonal antiserum at a dilution of 1:2,000, and a goat anti–rabbit secondary antibody at a dilution of 1:5,000. The signal was visualized by enhanced chemiluminescence (Amersham Intl., Buckinghamshire, UK).
Nuclear Factor-κB Assay.
Nuclear extracts were prepared as described previously (46, 47). In brief, 2 × 106 cells were washed once in PBS. The cell pellet was rapidly frozen in dry ice and isopropanol, and then thawed by adding 100 μl of ice-cold buffer A (10 mM Hepes, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol) resulting in 100% cell lysis. The nuclei were pelleted by microcentrifugation at 3,500 rpm for 10 min at 4°C. The supernatant was discarded and the nuclei suspended in 15 μl of buffer C (20 mM Hepes, pH 7.9, 0.4 M NaCl, 1.5 mM MgCl2, 25% vol/vol glycerol, 0.2 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). The suspension was mixed gently for 20 min at 4°C, and then microcentrifuged at 14,000 rpm for 20 min at 4°C. The supernatant was diluted with 50–70 μl of buffer D (20 mM Hepes, pH 7.9, 50 mM KCl, 20% vol/vol glycerol, 0.2 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride) and aliquots stored at −80°C. Protein concentrations were determined using the Bio-Rad assay. Electrophoretic mobility-shift assay reactions were performed in 20 μl volume, using 8–10 μg of nuclear extract in the presence of 1 μg of poly(d[I-C]) 1 μg of pd(N)6, and 10 μg of BSA. Incubations were in the presence of HDKE buffer with the following final concentrations: 20 mM Hepes, pH 7.9, 50 mM KCl, 1 mM EDTA, and 5 mM dithiothreitol. Reactions were started by the addition of 10,000–50,000 cpm radiolabeled nuclear factor (NF)- κB oligonucleotide probe (Promega Corp., Madison, WI). After incubation for 10 min, the reactions were terminated by adding 6 μl of 15% ficoll solution containing indicator dyes. Equal amounts of the reactions were loaded on 5% nondenaturing polyacrylamide gels in 1 × Tris/Borate/EDTA and run at 200 volts. Specificity of the NF-κB bands was determined by competition with excess unlabeled probe and lack of competition with 100fold excess of a probe containing a one basepair mutation in the NF-κB binding sequence.
Results
Ceramide Accumulation Is Delayed, but Precedes Early Apoptotic Signs.
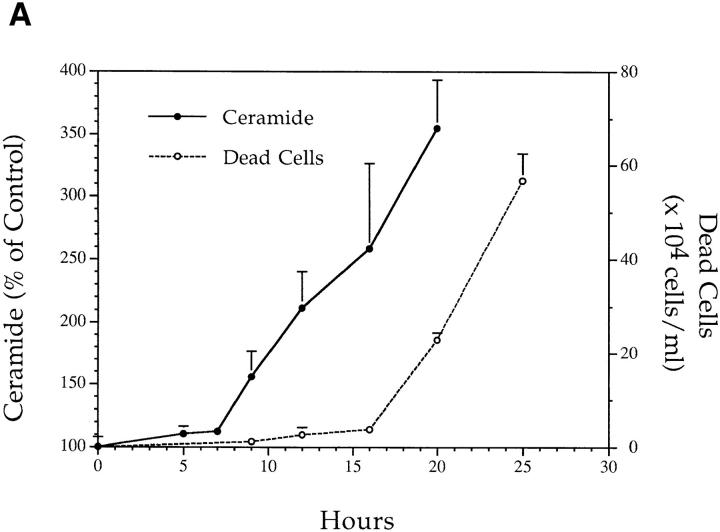
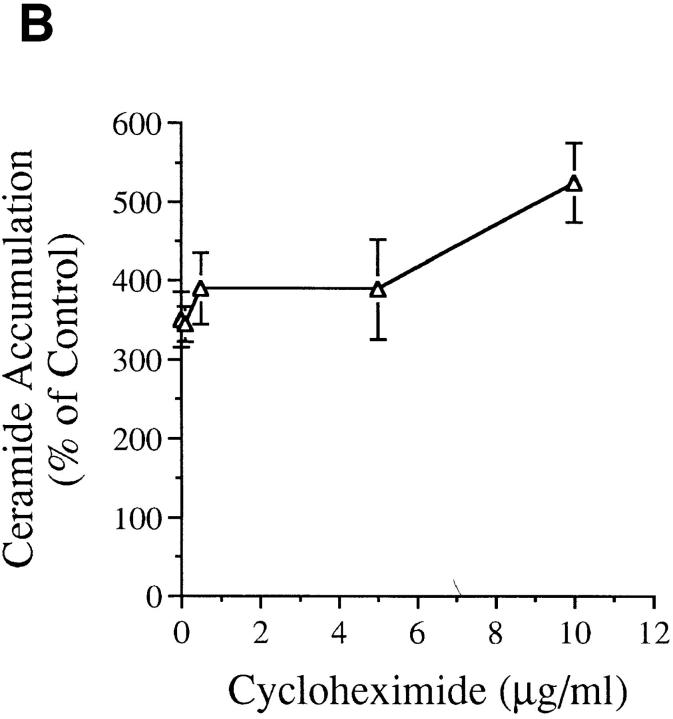
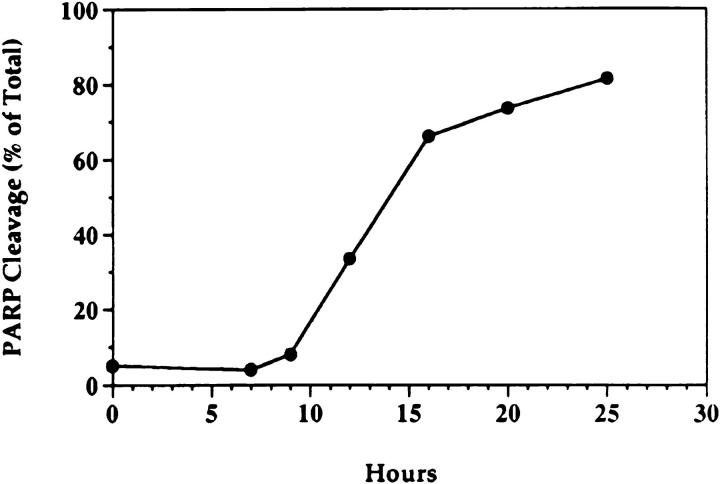
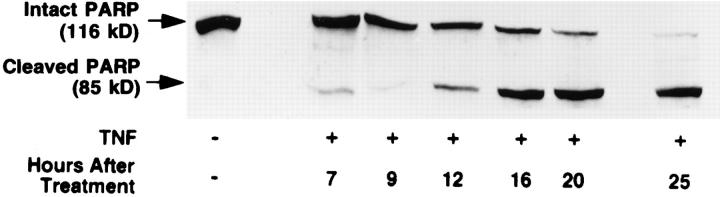
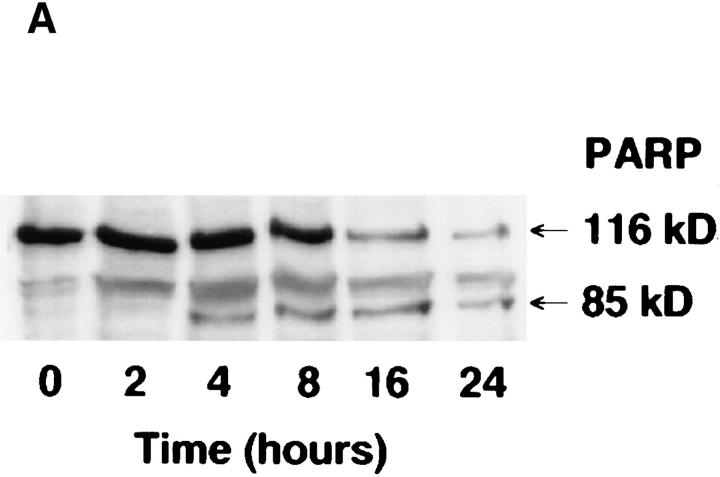
We treated MCF-7 breast carcinoma cells with TNF-α and measured ceramide levels and cell death concomitantly at several time points (Fig. 1 A). Ceramide levels did not change appreciably in the first 5 h (data not shown) but were significantly increased between 7 and 9 h and continued to increase with time, up to 400% by 20 h. This accumulation was not dependent on new protein synthesis since the addition of cycloheximide to the cells before treatment with TNF-α enhanced the accumulation of ceramide (Fig. 1 B). Although these are delayed and persistent changes in ceramide, it is becoming increasingly apparent that this is the pattern most closely related to the apoptotic responses. Although this raises the concern that ceramide accumulation is a consequence of death, studies with Bcl-2 (see below) negate this possibility. Cell death, as measured by the inability to exclude trypan blue, was not seen until 20 h, occurring several hours after the increase in ceramide levels, indicating that ceramide accumulation occurs long before loss of membrane integrity. To verify that cell death was occurring through induction of apoptosis, we assayed for cleavage of the 116-kD PARP polypeptide to a specific 85-kD apoptotic fragment (9, 10). This proteolytic cleavage has been shown to occur in apoptosis and to be mediated by Yama/CPP32/apopain or related proteases. As shown previously (6), treatment of MCF-7 cells with TNF-α resulted in specific cleavage of PARP to the 85-kD fragment (Fig. 2). Significant PARP cleavage did not occur until 12 h after treatment with TNF-α and was maximal by 25 h. These results indicate that ceramide accumulation precedes one of the early signs of apoptosis, i.e., PARP cleavage, by at least 3–4 h. Moreover, loss of cell membrane integrity, as determined by trypan blue uptake, is unlikely to contribute to ceramide accumulation since it occurs several hours after the dramatic increase in endogenous ceramide levels.
Figure 1.
(A) Effects of TNF-α on cell death and ceramide levels in MCF-7 cells. MCF-7 breast carcinoma cells were treated with TNF-α at 1.2 nM. At the indicated time points, adherent and floating cells were harvested and the number of dead cells was determined by their inability to exclude trypan blue (open circles). Concomitantly, lipids were collected and ceramide levels were measured (filled circles) and compared to time-matched controls. Levels of ceramide in control cells ranged between 4–6 pmole/ nmole of lipid phosphate. Results are averages of three experiments. Standard deviation for all points is indicated. (B) Effect of cycloheximide on ceramide accumulation after TNF-α. MCF-7 cells were treated with TNF-α at 1.2 nM as in A in the presence of increasing concentrations of cycloheximide as indicated. Cells were harvested at 18 h after TNF-α treatment, and ceramide levels were measured in the lipid extracts.
Figure 2.
Kinetics of PARP cleavage after TNF-α treatment. MCF-7 cells were seeded at 2 × 106 cells/10-cm plate, rested overnight, and then treated as in Fig. 1. At the indicated time points, cells were harvested by scraping in media to insure inclusion of the floating cells at later time points. Cell lysis and Western blotting were performed as described in Materials and Methods. Densitometric analysis of the two resulting bands was performed. The cleaved PARP fragment is represented as a percent of the total of both fragments. A representative experiment is shown (out of three).
CrmA Prevents Ceramide Accumulation
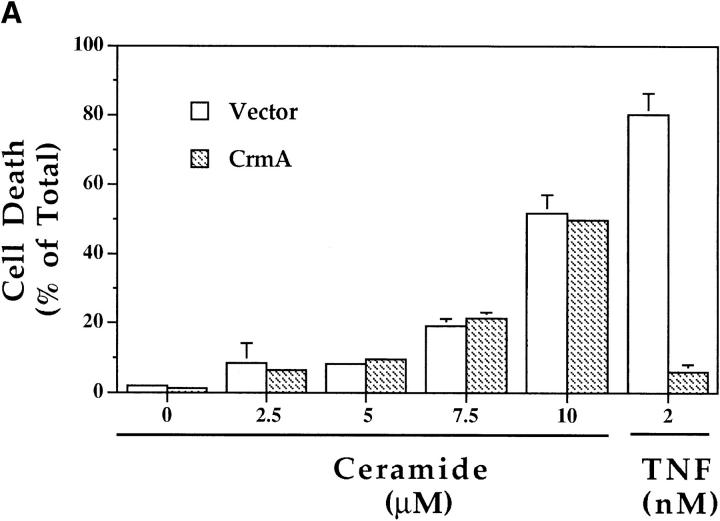
The ability of CrmA to inhibit, with variable efficiency, the ICE family of cysteine proteases (6, 7, 22, 26) was used to examine the relationship of these proteases to ceramide along the death pathway. MCF-7 cells stably expressing CrmA were treated with 2 nM TNF-α or increasing concentrations of C6-ceramide, a cell-permeable ceramide analogue, and compared with control (vector) cells. As shown previously (4), CrmA offered almost complete protection from TNF-α–induced cytotoxicity (Fig. 3 A). However, CrmA offered no protection from the cytotoxic effects of ceramide so that cells died equally in the presence or absence of CrmA (Fig. 3 A). Comparison of another pair of vector and CrmA-expressing MCF-7 clones described previously (4) yielded similar results (data not shown). Therefore, CrmA appeared not to interfere with the downstream effects of ceramide.
Figure 3.
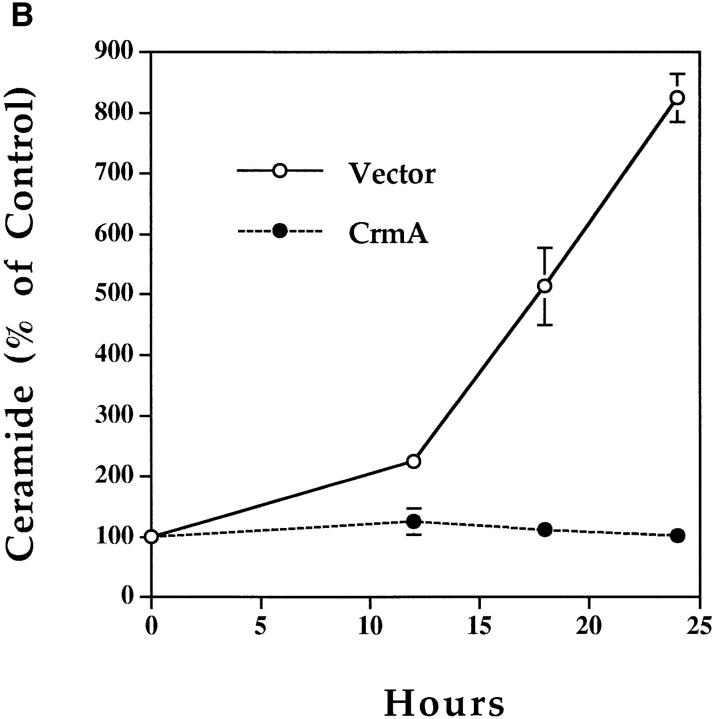
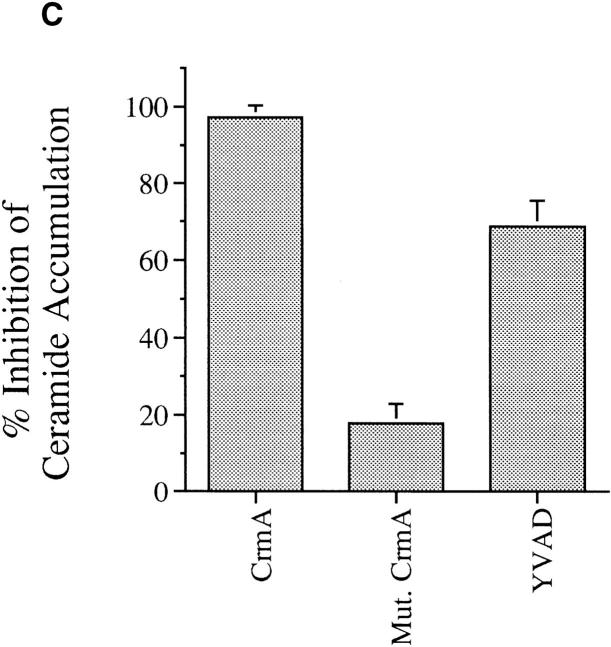
Effects of CrmA on the TNF-α–activated ceramide pathway. (A) CrmA protects from TNF-α–induced, but not ceramide-induced, cell death. TNF-α–sensitive MCF-7 cells transfected with either pcDNA3 vector (open bars) or pcDNA3/crmA (filled bars) were seeded in 24-well plates at 5 × 104 cells/well in 2% FBS, rested overnight, and then treated with the indicated concentrations of C6-ceramide or 2 nM TNF-α. Cell death, determined by the inability to exclude trypan blue, was evaluated at 48 h. (B) CrmA inhibits ceramide generation after TNF-α stimulation. The CrmA-expressing MCF-7 cells (filled circles) and their vector control cells (open circles) were treated with TNF-α as in Fig. 1. Ceramide levels were measured at the indicated time points as in Fig. 1. Results are the average of three experiments with the standard deviation indicated. (C) Antiproteolytic activity of CrmA is important in inhibiting ceramide accumulation. Cells expressing CrmA, point-mutant CrmA, or wild-type MCF-7 cells pretreated 1 h with 50 μm Ac-YVAD-CHO (Bachem, King of Prussia, PA) were treated, along with their respective controls, with 1.2 nM TNF-α for 18 h. Ceramide was then measured as in Fig. 1. Percent inhibition was calculated by comparison with the results from TNF-α– treated controls. The average of three experiments is shown.
Since endogenous ceramide elevation may drive the cell to die, we next examined whether CrmA interferes with ceramide generation. We measured endogenous ceramide levels in response to TNF-α in control and in CrmAexpressing MCF-7 cells. Ceramide levels increased dramatically in vector cells at 18 and 24 h (Fig. 3 B). However, CrmA-expressing cells treated similarly with TNF-α showed no increase in ceramide levels. To determine whether the inhibition of ceramide accumulation was dependent on the ability of CrmA to inhibit proteases, we used two approaches. First, we used a point-mutant of CrmA that has no antiproteolytic activity due to the substitution of Arg for Thr at amino acid 291 in the reactive site loop (6). In MCF-7 cells expressing this mutant, there was no significant inhibition of ceramide accumulation (Fig. 3 C) and no protection from TNF-α–induced apoptosis (6). Second, we used the synthetic peptide Ac-YVAD-CHO that, like CrmA, is a potent competitive inhibitor of ICE (7). Pretreatment of cells with this peptide, resulted in significant inhibition of ceramide accumulation after TNF-α treatment (Fig. 3 C). In contrast, other protease inhibitors not known to inhibit the ICE family of proteases, including N-tosyl-l-phenylalanine chloromethyl ketone and leupeptin, were unable to inhibit ceramide accumulation after TNF-α treatment (data not shown). Therefore, CrmA inhibits TNF-α–induced apoptosis by acting at a point upstream of the generation of ceramide, and this inhibition is dependent on its antiproteolytic activity.
Bcl-2 Inhibition of Apoptosis Is Downstream of Ceramide Generation.
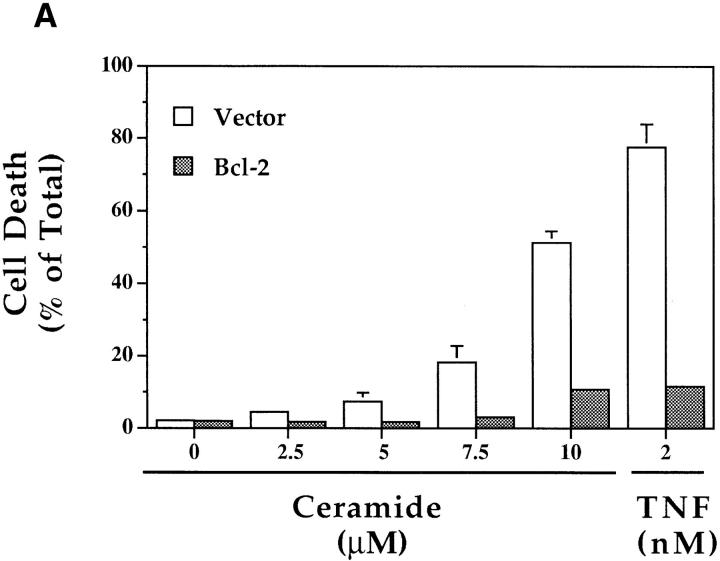
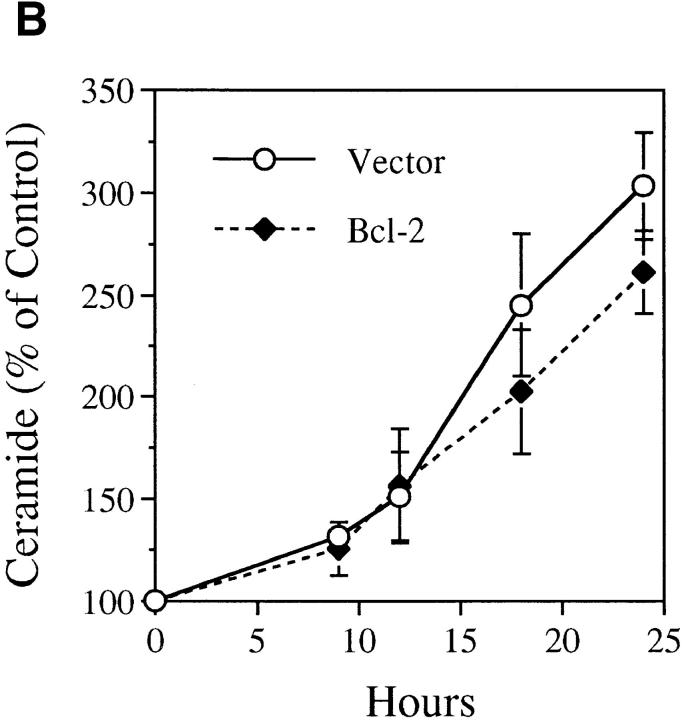
As ceramide generation occurred in cells destined to die due to treatment with TNF-α and not in the protected, CrmA-expressing cells, it became important to verify that this increase in ceramide levels, as well as the suppression of this increase by CrmA, were specific events correlating with cell death or survival, respectively. We used Bcl-2, another antiapoptotic molecule that has recently been shown to protect from ceramide-induced apoptosis (48, 49). MCF-7 cells expressing Bcl-2 or vector controls were treated with TNF-α or increasing concentrations of C6-ceramide. Control cells died in response to both treatments, but Bcl-2–expressing cells displayed resistance to TNFα–induced cell death as seen in cells expressing CrmA (Fig. 4 A). However, unlike CrmA-expressing cells, Bcl-2–expressing cells were resistant to ceramide-induced cell death. Additionally, generation of endogenous ceramide was nearly equal in both vector and Bcl-2 cells in response to TNF-α, indicating that Bcl-2 does not interfere with ceramide generation (Fig. 4 B). Therefore, Bcl-2 functions at a point downstream of ceramide to inhibit apoptosis. More importantly, this delayed accumulation of ceramide is not a consequence of cell death since it is still observed in the viable Bcl-2–expressing cells. Thus, these data demonstrate that the elevation in ceramide is proximal to the biochemical and morphological changes of cell death.
Figure 4.
Effects of Bcl-2 overexpression on the TNF-α–activated ceramide pathway. (A) Bcl-2 prevents both TNF-α– and ceramide–induced cell death. TNF-α– and Fas–sensitive MCF-7 cells overexpressing pEBS7 (open bars) or pEBS7/Bcl-2 (filled bars) were treated as in Fig. 3 A and evaluated for cell death by trypan blue at 48 h. (B) Bcl-2 does not prevent the TNF-α–induced elevation in ceramide levels. The Bcl2–overexpressing MCF-7 cells (filled diamonds) and control vector cells (open circles) were treated as in Fig. 3 B, and ceramide levels were measured at the indicated time points.
Ceramide-Induced PARP Cleavage Is Inhibited by Bcl-2, but Not CrmA.
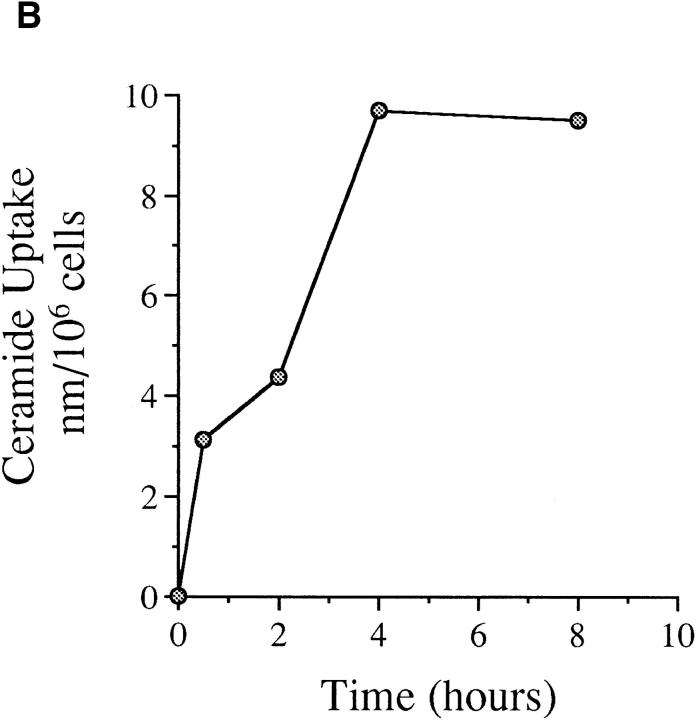
To determine the relationship between TNF-α, ceramide, and subsequent PARP cleavage and apoptosis, it became imperative to study the effects and kinetics of ceramide on PARP cleavage. Consistent with results in Molt 4 cells (50), treatment of MCF-7 cells with ceramide resulted in cleavage of PARP (Fig. 5 A), and PARP cleavage after 4 h of ceramide treatment was equivalent to that caused by 12–16 h of TNF-α treatment (compare with Fig. 2). By using 14C-labeled ceramide and evaluating the kinetics of its uptake by MCF-7 cells at several time points, we found that ceramide was taken up slowly by these cells with maximal uptake reached at only 4 h (Fig. 5 B). Therefore, the delay in PARP cleavage after ceramide treatment can be attributed to the delay in uptake of exogenous ceramide. Hence, these experiments support the hypothesis that ceramide accumulation following TNF-α treatment may represent a trigger for PARP cleavage and apoptosis.
Figure 5.
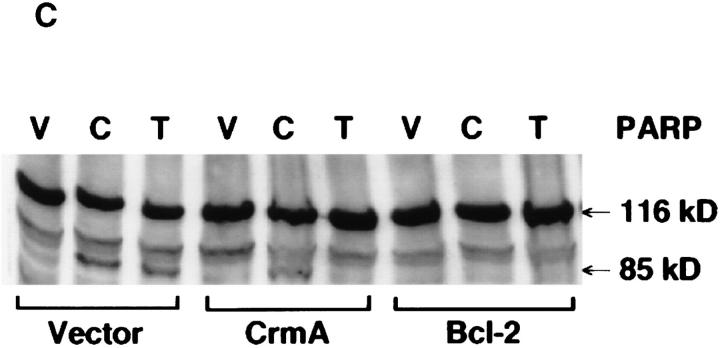
(A) Kinetics of PARP cleavage after ceramide treatment. MCF-7 cells were seeded and treated with ceramide, and then harvested at the indicated time points. PARP cleavage was assayed as in Fig. 2. Intact PARP (116 kD) and its cleaved product (85 kD) are indicated. (B) Kinetics of exogenous ceramide uptake. MCF-7 cells were seeded and treated with 14C6-ceramide (specific activity of 1.5 × 1013 cpm/mole) at a similar concentration. At the indicated time points, cells were harvested, washed twice with PBS, and the radioactivity retained in the pellet was counted and presented as a percent of total radioactivity delivered. (C) Effects of CrmA and Bcl-2 on ceramide-induced PARP cleavage. Vector, CrmA-expressing, or Bcl-2–overexpressing cells were seeded at 2.5 × 105 cells/well of a 6-well plate. The cells were rested overnight then treated with vehicle (V) or ceramide (C) for 8 h or TNF-α (T) for 16 h. The final concentration of ceramide was 0.32 pmole/cell, and 1.2 nM for TNF-α. Cells from a total of six wells for each treatment were then harvested, combined, and PARP cleavage was assayed as in Fig. 2.
Next, it became important to determine the effect of CrmA or Bcl-2 expression on ceramide-induced PARP cleavage in MCF-7 cells. Although both Bcl-2 and CrmA inhibited TNF-α–induced PARP cleavage, only Bcl-2 expression provided protection from ceramide-induced cleavage. PARP was cleaved after exogenous ceramide treatment despite high levels of expression of CrmA, indicating that ceramide bypasses the CrmA target and activates a downstream protease capable of cleaving PARP (i.e., prICE) (Fig. 5 C). Densitometric quantitation of PARP cleavage showed 17.7 and 20.4% cleavage in the ceramide– or TNF-α–treated vector cells, respectively, whereas CrmAexpressing cells treated with ceramide or TNF-α had 16.6 and 0% cleavage, respectively. Therefore, in MCF-7 cells, CrmA expression does not significantly interfere with activation of PARP cleavage by ceramide.
CrmA Does Not Protect from a Ceramide-Independent Pathway of Apoptosis.
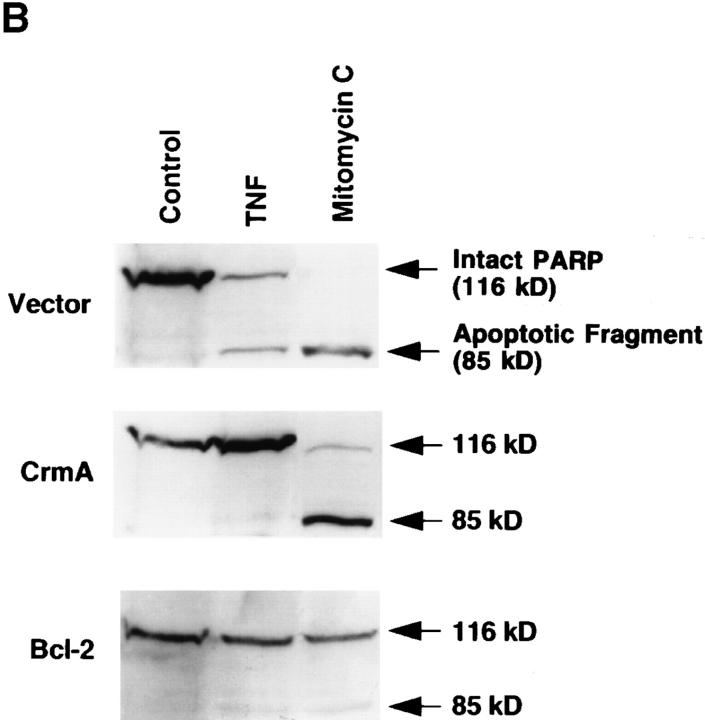
To further dissociate the site of action of CrmA from that of Bcl-2, we employed the alkylating agent mitomycin C. Mitomycin C did not cause any significant change in endogenous ceramide levels (data not shown). We next compared the effects of TNF-α, C6-ceramide, and mitomycin C on the survival of CrmA– or Bcl-2–expressing MCF-7 cells. Whereas the CrmA-expressing cells were protected from TNF-α–induced apoptosis, they were equally susceptible to mitomycin C (Fig. 6 A) as were vector cells. However, cells expressing Bcl-2 were protected from the cytotoxic effects of mitomycin C as well as TNF-α and ceramide. Biochemically, expression of CrmA prevented PARP cleavage after activation of the ceramide-dependent TNF-α pathway (Fig. 6 B). However, PARP cleavage was almost complete, despite expression of CrmA, after treatment with mitomycin C that activates a ceramide-independent apoptotic pathway. In contrast, Bcl-2 overexpression prevented PARP cleavage induced by both the TNF-α (ceramidedependent) and mitomycin C (ceramide-independent) pathways. These results provide additional evidence that CrmA functions distinctly from Bcl-2. Specifically, CrmA appears to inhibit an event proximal to ceramide accumulation in ceramide-dependent pathways whereas Bcl-2 works by inhibiting a more distal target that is common to both apoptotic pathways.
Figure 6.
Differential protection from cell death by CrmA and Bcl-2. (A) Vector, CrmA-expressing, and Bcl-2–overexpressing MCF-7 cells were treated as in Fig. 3 A with C6-ceramide (10 μM), TNF-α (2 nM), or mitomycin C (2.5 μg/ml). Cell death was evaluated at 48 h as in Fig. 2 A. Results are the average of three experiments. (B) Vector, CrmA-expressing, and Bcl-2–overexpressing MCF-7 cells were seeded as in Fig. 2 and treated with PBS vehicle (lane 1), 1.2 nM TNF-α (lane 2), or 10 μg/ml mitomycin C (lane 3). Cells were harvested after 20 h of treatment and PARP cleavage was assayed as in Fig. 2. The bands representing intact or cleaved (apoptotic) PARP are shown. One out of three different experiments is shown.
CrmA-Induced Block of Ceramide Accumulation Does Not Affect NF-κB Activation.
Activation of the transcription factor NF-κB is one of the major functions of TNF-α. For activation to occur, NF-κB has to dissociate from its cytoplasmic inhibitory protein I-κB so that it can translocate to the nucleus (51). This is accomplished through site-specific serine phosphorylation followed by proteolytic degradation of I-κB (52, 53). The mediators of this activation have not been clarified, although the “death domain”-containing protein TRADD, but not FADD, has been shown to be important for its activation (35, 36). Recently, ceramide generated through the action of an acidic sphingomyelinase was implicated in the activation of NF-κB (54). The antiproteolytic activity of CrmA, as well as its ability to inhibit ceramide accumulation after TNF-α treatment led us to examine the effect of CrmA expression on NF-κB activation. We used electrophoretic mobility shift assays which rely on the ability of activated NF-κB to bind to its specific DNA sequence resulting in retardation of the protein– DNA complex on nondenaturing polyacrylamide gels. Treatment of MCF-7 cells expressing CrmA, Bcl-2, or their corresponding vector with 2 nM TNF-α resulted in equal activation of NF-κB (Fig. 7, A and B). Therefore, CrmA does not interfere with the signaling pathway leading to activation of NF-κB, despite its demonstrated ability to completely inhibit ceramide accumulation. This suggests that a physiologic role for ceramide in NF-κB activation is unlikely.
Figure 7.
Activation of NF-κB is not inhibited by CrmA (A) or Bcl-2 (B). Electrophoretic mobility shift assay (EMSA) for the transcription factor NF-κB in MCF-7 cells expressing CrmA, Bcl-2, or vector is shown. Cells were seeded at 2 × 106 cells/10-cm dish in RPMI media containing 10% FBS and rested overnight. Treatment with 2 nM TNF-α proceeded for 30 min after which the cells were trypsinized, nuclear extracts prepared, and EMSA performed using 10 μg of nuclear protein and a 32P-labeled NF-κB oligonucleotide probe as described in Materials and Methods. Bands representing the specific NF-κB-DNA complex, a nonspecific band (n.s.), and the free probe are indicated.
Discussion
The results from this study show that ceramide accumulation observed after treatment of MCF-7 cells with TNF-α is completely inhibited by CrmA (Fig. 3 B). Exogenous ceramide can bypass this block and produce apoptosis by activation of downstream proteases (Fig. 3 A and 5 A). Similar results were obtained with BJAB cells after ligation of the Fas receptor (data not shown). These data suggest that CrmA targets a protease acting upstream of ceramide generation. Since CrmA did not inhibit activation of prICE by ceramide or mitomycin C, these results raise the possibility that CrmA may target proteases that act upstream of the PARP protease.
The antiapoptotic activity of CrmA has been thought to be due to its ability to inhibit members of the ICE family of cysteine proteases that are homologues of the C. elegans cell death protease CED-3 (2, 4, 25). However, the potency by which CrmA inhibits the various members of this family varies significantly. Whereas picomolar concentrations of CrmA can potently inhibit ICE, concentrations that are over 1,000-fold higher are required to achieve comparable inhibition of Yama/CPP32/apopain, a putative PARP cleaving protease (7). Similarly, CrmA is a poor inhibitor of ICH-1 (23) and CED-3 (3, 55), indicating that it preferentially targets only a subset of ICE family members. This may explain why CrmA is not a universal inhibitor of apoptosis. Based on our findings, the more physiologically relevant target of CrmA appears to be a protease that is activated by TNF-α and is involved in the activation phase, rather than the execution phase, of apoptosis. The recently described Fas– and TNF-α receptor–associated ICE protease FLICE/MACH, is a likely candidate since it was demonstrated that apoptosis induced by its overexpression can be inhibited by CrmA (16). Other apoptotic stimuli such as mitomycin or sodium azide (data not shown) that use a ceramide-independent pathway to activate the death proteases are not inhibited by CrmA (Fig. 8).
Figure 8.
Schematic presentation of the proposed sites of inhibition of the ceramide pathway by CrmA and Bcl-2. Activation of sphingomyelinases requires several stages of proteolytic processing (63). The inhibition of ceramide accumulation by CrmA is hypothesized to be due to its ability to inhibit cysteine or serine proteases probably involved in the processing of sphingomyelinase(s). Bcl-2 functions further downstream by inhibiting effector molecules involved in the execution of the death order without interfering with the generation of ceramide. The specific target of Bcl-2 is not yet known. The diagram illustrates the possibility that either proteases or regulatory molecules are targeted by Bcl-2.
Our findings define a novel site of CrmA-induced protection from apoptosis that is clearly distinct from that of Bcl-2. Whereas CrmA interferes with ceramide accumulation, Bcl-2 does not modulate this event and appears to work downstream by inhibiting the execution machinery of apoptosis (Fig. 8). This is additionally supported by the ability of both CrmA and Bcl-2 to protect from cell death when induced with TNF-α while only Bcl-2 is capable of protecting cells treated with C6-ceramide or mitomycin C. These findings implicate Bcl-2 as a more generalized inhibitor of apoptosis acting on a target common to different apoptotic pathways whereas CrmA may specifically inhibit pathways that lead to a sustained ceramide signal such as that stimulated by TNF-α or Fas.
These studies also define distinct pathways that lead to NF-κB activation and apoptosis. The activation of the ceramide pathway has been implicated by some groups to be a necessary step for activation of NF-κB (54, 56, 57). Others have been unable to reproduce these results (47, 58–61). The ability of CrmA to inhibit the generation of ceramide provided a new tool to help resolve these contradictions. Our finding that CrmA does not inhibit NF-κB activation by TNF-α despite a complete shutdown of ceramide generation makes a role for ceramide in NF-κB activation highly questionable. Rather, ceramide accumulation appears to be more intimately related to apoptosis and growth suppression. These results are consistent with published studies showing that CrmA does not interfere with NF-κB activation stimulated by TRADD overexpression, but blocks TRADD-driven apoptosis (36), and that the dominant negative FADD mutant can potently suppress FADD-dependent apoptosis without interfering with NF-κB activation (38). Additionally, in cells undergoing Fas-induced apoptosis which is accompanied by massive accumulation of ceramide, NF-κB activation is strikingly absent (Gamard, C., G. Dbaibo, L. Obeid, and Y. Hannun, manuscript submitted). These studies clearly support the previous findings that ceramide is neither necessary nor sufficient for NF-κB activation.
Taken together, these findings are consistent with an important role for ceramide in TNF-α–induced apoptosis. The sustained elevations in endogenous ceramide levels observed over several hours that are inhibited by CrmA are more closely related to apoptosis than the transient, but more modest, increases in ceramide observed within minutes in other cell lines and implicated in mediating early signaling events (27). Indeed, these sustained levels of ceramide are more compatible with cellular levels achieved with 1–20 μM of exogenous ceramide. Accumulation of ceramide with delayed kinetics has been implicated as a stress response that drives the cell to either undergo apoptosis (29, 34) or cell cycle arrest (29, 62). Indeed, these delayed kinetics of ceramide accumulation are more commensurate with the observed delay in the onset of apoptotic morphology and subsequent cell death after TNF-α. Our findings that this accumulation occurs before PARP cleavage and the ability of Bcl-2 to prevent ceramide-induced death support this emerging role for ceramide. The results obtained using MCF-7 cells suggest that, in the apoptotic response of these cells, ceramide functions much more as a downstream sensor and integrator rather than as an upstream acute signaling switch.
In conclusion, ceramide accumulation is emerging as an important component in a TNF-α–induceable pathway of apoptosis. This pathway can be inhibited by CrmA that blocks ceramide generation or Bcl-2 that interferes with the function of effector molecules downstream of ceramide (Fig. 8). Although both Bcl-2 and CrmA appear to inhibit activation of proteases, these results show that they interfere with the death pathway at two different points. These results will need to be evaluated in other cell lines and further exploration of this pathway will be essential for the understanding of this complex aspect of cell regulation and may identify more specific targets for inhibition of apoptosis.
Acknowledgments
We thank Muneesh Tewari and Dr. Vishva M. Dixit for providing us with different MCF-7 cell lines and for helpful discussions and advice, Alicja Bielawska for C6-ceramide and 14C6-ceramide synthesis, Milton Campbell and William E. Kraus for help with densitometry, and Marsha Mangum for expert secretarial assistance.
Footnotes
This work was supported by a National Institute of Child Health and Human Development grant 5P30HD28828-03 to G. Dbaibo, National Institutes of Health grant GM-43825 to Y. Hannun, and Department of the Army Medical Division grant 17-94-J4301 to Y. Hannun.
1 Abbreviations used in this paper: CrmA, cytokine response modifier A; FBS, fetal bovine serum; ICE, IL-1β converting enzyme; NF, nuclear factor; PARP, poly(ADP-ribose) polymerase.
References
- 1.Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. eleganscell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 3.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1β converting enzyme, a mammalian homolog of the C. eleganscell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 4.Tewari M, Dixit VM. Fas- and tumor necrosis factor–induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 5.Los M, Van de Craen M, Penning LC, Schenk H, Westendorp M, Baeuerle PA, Droge W, Krammer PH, Fiers W, Schulze-Osthoff K. Requirement of an ICE/ CED-3 protease for Fas/APO-1–mediated apoptosis. Nature (Lond) 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 6.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature (Lond) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin T, Nicholson DW. CPP32/Apopain is a key Interleukin 1 β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 10.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature (Lond) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 11.Casciola-Rosen LA, Miller DK, Anhalt GJ, Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–30760. [PubMed] [Google Scholar]
- 12.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? . Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis eleganscell death protein Ced-3 and mammalian interleukin-1 β-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 14.Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He W, Dixit VM. ICE-LAP3, a novel mammalian homolog of the Caenorhabditis eleganscell death protein CED-3 is activated during Fas– and tumor necrosis factor–induced apoptosis. J Biol Chem. 1996;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 15.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO1– and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 16.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/ CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 17.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature (Lond) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 19.Nunez G, Merino R, Grillot D, Gonzalez-Garcia M. Bcl-2 and Bcl-x: regulatory switches for lymphoid death and survival. Immunol Today. 1994;15:582–588. doi: 10.1016/0167-5699(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner MO, Horvitz HR. C. eleganscell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 21.Razvi ES, Welsh RM. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 22.Komiyama T, Ray CA, Pickup DJ, Howard AD, Thornberry NA, Peterson EP, Salvesen G. Inhibition of interleukin-1 β converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J Biol Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- 23.Wang L, Miura M, Bergeron L, Zhu H, Yuan J. Ich-1, an Ice/ced-3–related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 24.Miura M, Friedlander RM, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagliardini V, Fernandez PA, Lee RK, Drexler HC, Rotello RJ, Fishman MC, Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science (Wash DC) 1994;263:826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- 26.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 27.Hannun YA. The Sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 28.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science (Wash DC) 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 29.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva MYU, Obeid LM, Hannun YA. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270:2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gewirtz DA, Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strum JC, Small GW, Pauig SB, Daniel LW. 1-β-D-arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. J Biol Chem. 1994;269:15493–15497. [PubMed] [Google Scholar]
- 32.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, Kolesnick RN. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tepper CG, Jayadev S, Liu B, Bielawska A, Wolff R, Yonehara S, Hannun YA, Seldin MF. Role of ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 36.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 37.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 38.Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD 95 (Fas/APO-1) and tumor necrosis factor receptorinduced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 39.Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science (Wash DC) 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 40.Pushkareva M, Obeid LM, Hannun YA. Ceramide: an endogenous regulator of apoptosis and growth suppression. Immunol Today. 1995;16:294–297. doi: 10.1016/0167-5699(95)80184-7. [DOI] [PubMed] [Google Scholar]
- 41.Jaattela M, Benedict M, Tewari M, Shayman JA, Dixit VM. Bcl-x and Bcl-2 inhibit TNF and Fasinduced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 42.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 43.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2diacylglycerols present in platelets, hepatocytes, and ras- and sis- transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- 44.Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1α,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 45.Rouser GAN, Fleischer S, Yamamoto Y. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 46.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dbaibo GS, Obeid LM, Hannun YA. TNF-α signal transduction through ceramide: dissociation of growth inhibitory effects of TNF-α from activation of NF-κB. J Biol Chem. 1993;268:17762–17766. [PubMed] [Google Scholar]
- 48.Martin SJ, Takayama S, McGahon AJ, Miyashita T, Corbeil J, Kolesnick RN, Reed JC, Green DR. Inhibition of ceramide-induced apoptosis by Bcl-2. Cell Death Differ. 1995;2:253–257. [PubMed] [Google Scholar]
- 49.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of bcl-2. Biochem J. 1996;316:25–28. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 52.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBa: a mechanism for NF-κB activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henkel T, Machleidt T, Alkalay I, Kronke M, BenNeriah Y, Baeuerle P. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 54.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 55.Xue D, Horvitz HR. Inhibition of the Caenorhabditis eleganscell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature (Lond) 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 56.Machleidt T, Wiegmann K, Henkel T, Schütze S, Baeuerle P, Krönke M. Sphingomyelinase activates proteolytic IκB-α degradation in a cell-free system. J Biol Chem. 1994;269:13760–13765. [PubMed] [Google Scholar]
- 57.Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor κB translocation in intact HL-60 cells. J Biol Chem. 1993;268:20520–20523. [PubMed] [Google Scholar]
- 58.Johns LD, Sarr T, Ranges GE. Inhibition of ceramide pathway does not affect ability of TNF-α to activate nuclear factor-κB. J Immunol. 1994;152:5877–5882. [PubMed] [Google Scholar]
- 59.Betts JC, Agranoff AB, Nabel GJ, Shayman JA. Dissociation of endogenous cellular ceramide from NF-κB activation. J Biol Chem. 1994;269:8455–8458. [PubMed] [Google Scholar]
- 60.Kuno K, Sukegawa K, Ishikawa Y, Orii T, Matsushima K. Acid sphingomyelinase is not essential for the IL-1 and tumor necrosis factor receptor signaling pathway leading to NFκB activation. Int Immunol. 1994;6:1269–1272. doi: 10.1093/intimm/6.8.1269. [DOI] [PubMed] [Google Scholar]
- 61.Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 62.Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci USA. 1995;92:1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurwitz R, Ferlinz K, Vielhaber G, Moczall H, Sandhoff K. Processing of human acid sphingomyelinase in normal and I-cell fibroblasts. J Biol Chem. 1994;269:5440–5445. [PubMed] [Google Scholar]