Abstract
Leucine-based signals in the cytoplasmic tail of invariant chain (Ii) control targeting of newly synthesized major histocompatibility complex class II molecules to the endocytic pathway for acquisition of antigenic peptides. Some protein determinants, however, do not require Ii for effective class II presentation, although endocytic processing is still necessary. Here we demonstrate that a dileucine-based signal in the cytoplasmic tail of the class II β chain is critical for this Ii-independent presentation. Elimination or mutation of this signal reduces the rate of re-entry of mature surface class II molecules into the endocytic pathway. Antigen presentation controlled by this signal does not require newly synthesized class II molecules and appears to involve determinants requiring only limited proteolysis for exposure, whereas the opposite is true for Ii-dependent determinants. This demonstrates that related leucine-based trafficking signals in Ii and class II control the functional presentation of protein determinants with distinct processing requirements, suggesting that the peptide binding sites of newly synthesized versus mature class II molecules are made available for antigen binding in distinct endocytic compartments under the control of these homologous cytoplasmic signals. This permits capture of protein fragments produced optimally under distinct conditions of pH and proteolytic activity.
MHC class II molecules present to CD4+ T cells peptides derived from proteins entering the endosomal– phagosomal system (1, 2). Invariant chain (Ii)1 aids in assembly and transport of newly synthesized class II molecules and also protects the class II binding site from either improper folding or premature interaction with protein ligands in the secretory pathway (3). This same type II membrane protein also contains a pair of leucine-based signals in its cytoplasmic tail that promote its trafficking and that of associated newly synthesized class II molecules through early endocytic compartments to dense organelles of the endocytic pathway (4–10). Class II capture of antigenic fragments in these class II–rich organelles (MIIC) requires proteolytic removal of associated Ii (11), particularly of the class II–associated invariant peptide (CLIP) fragment (12, 13) whose replacement in the binding site by antigenic peptides is facilitated by the MHC-encoded DM molecule (14–16).
Although this general scheme of Ii-regulated class II synthesis, trafficking, and antigen capture is supported by numerous studies using transformed cell lines, transfected cells, and cells of wild-type and Ii–deficient mice, not all peptide determinants require Ii for effective presentation, although they still require processing in acidic compartments (17–21). Distinct determinants in even a single protein antigen (hen egg lysozyme, HEL) can show differential dependence on Ii for their effective presentation (19). Other studies have demonstrated that some antigenic determinants are dependent on new protein synthesis or secretory pathway export for their presentation, whereas others are not (22–25), providing a parallel to the Ii data in terms of the involvement of newly synthesized (Ii-associated) versus mature class II (Ii-free) in effective presentation of these different antigenic fragments.
These observations of Ii-independent, endosomal processing–dependent presentation of some antigenic determinants are consistent with the accumulation of class II proteins in intracellular vesicles with the characteristics of late endosomes/lysosomes in some cells lacking Ii coexpression (26–28). Functional studies have also shown that truncation of the class II β chain cytoplasmic tail affects antigen presentation to some autoreactive T cell hybridomas even by cells producing Ii (29), although other determinants remain well presented by the same mutant class II molecules (30). In experiments with intact influenza particles, truncation of the class II α or β cytoplasmic tails selectively interferes with presentation of a hemagglutinin determinant whereas a matrix protein-derived determinant is instead dependent on Ii for presentation (31). These studies revealed a correlation between hemagglutinin presentation and the involvement of the class II cytoplasmic tails in entry of mature surface class II into the endocytic pathway. Similarly, Smiley et al. have demonstrated in a mouse model system that the cytoplasmic tails of class II molecules play a key role in the presentation of some antigenic determinants (32).
These various findings suggest that two distinct pathways for class II–dependent antigen presentation exist, and imply that separate protein localization signals in the cytoplasmic tails of Ii and class II play important roles in these events. The identity of the localization signal(s) in class II chains has not been established, although Smiley et al. have hypothesized that conserved dileucines in the class II β chain tail are likely to be involved (32). In addition, the role of such localization signals in class II has not been directly compared to that of Ii signals in presenting different antigenic determinants from a single protein molecule. Finally, little information is available on what predisposes presentation of different determinants within a given protein antigen to dependence on one or the other of these pathways. In the present study, we have used the well-characterized HEL antigenic model (33) to examine these issues. We demonstrate that the dileucine-containing sequence in the class II β chain noted by Smiley et al. (32) is critical for Ii-independent presentation of two HEL determinants, whereas a third determinant in the same protein requires the tail signals in Ii for its effective presentation. In combination with earlier observations, our data support a model of class II presentation involving (a) Ii-dependent capture of buried antigenic determinants in lysosome-related, highly proteolytic compartments by newly synthesized class II molecules and (b) β chain–tail dependent antigen capture of more superficial determinants in less proteolytic (early) endocytic organelles by mature, recycling class II. These two pathways together provide a mechanism for optimal presentation of the multiple determinants often contained in proteins entering the endocytic pathway.
Materials and Methods
Reagents.
HEL (cat No. L6876, lot No. 89F8275), diethylamino-ethyl–dextran, chloroquine, and dimethyl sulfoxide were obtained from Sigma Chemical Co (St. Louis, MO). LPS was from DIFCO (3122-25-8; Detroit, MI), recombinant murine IFN-γ from PharMingen (San Diego, CA), brefeldin A (BFA) from Epicentre Technologies Corp. (Madison, WI), leupeptin from Boehringer Mannheim (Indianapolis, IN), and G418 (Geneticin) from GIBCO (Gaithersburg, MD). The peptides HEL 46-61 (NTDGSTDYGILQINSR), HEL 34-45 (FESNFNTQATNR), and HEL 116-129 (KGTDVQAWIRGCRL) were synthesized and purified by HPLC before use by the National Institute of Allergy and Infectious Diseases Peptide Synthesis Facility (Rockville, MD).
Constructs.
The cDNA constructs coding for the wild-type α or β chain of Ak in the plasmid CDM8 (34) and the cDNA coding for murine Ii31 in the plasmid pcEXV3 have been previously described (35). A construct coding for the β chain of Ak with an 18 amino acid deletion at the COOH-terminus was made by placing a stop codon after the codon encoding isoleucine 220, as described elsewhere (29). The construct was placed into the expression plasmid vector pCDL-SR (36), provided by Dr. J. Bonifacino (National Institute of Child Health and Development, National Institutes of Health). The β chain with this COOH-terminal 18 amino acid deletion is designated as βCT18. A construct coding for a β chain with the cytoplasmic leucines at positions 236 and 237 each changed to alanine was made by oligonucleotide-directed mutagenesis using PCR, and cloned into the pCDL-SR vector. The 5′ primer was 5′ GTC AGA GCT CGA GTG TGC CTT AGA GAT GGC TC 3′ and the 3′ primer was 5′ GAG CAC CAG ATC TCA CTG AGC TGC CCC TGC TGG AGG AGG GCC ACG AGG TCC 3′; mutagenesis was performed as described (37). The resultant mutant β chain was designated as βCTAA. The sequences of all constructs were confirmed as correct using an automated DNA sequencer.
Transient Transfection.
Cos 7.2 cells were transiently transfected with DNA of the cDNA constructs coding for the α and β chain of Ak alone (3 μg each) or together with DNA for the cDNA coding for murine Ii31 (10 mg), using a diethyl-amino-ethyl– dextran method as previously reported (38).
Stable Transfectants.
Rat basophil leukemia cells (RBL)-5 were stably transfected by electroporation with cDNA constructs coding for the following combinations: α/β, α/βCT18, and α/βCTAA, with or without DNA for the construct encoding mIi31, using a modified procedure from Engel et al. (36). 18 μg of DNA encoding each chain of class II and 2 μg of pfneo (39) conferring resistance to G418 were used for each electroporation. In the case of Ii cotransfection, an additional 54 μg of DNA encoding Ii was added. Transfected cells expressing the desired proteins were cloned by limiting dilution.
FACS® Staining.
The Ak expression level on transfectants was measured by staining the surface using a saturating amount of H116.32 antibody (40) on ice for 30 min. A FITC-conjugated rabbit anti–mouse IgG (Jackson Laboratory, Bar Harbor, ME) was used to detect the bound H116.32. Ii expression by the transfectants was measured by intracellular staining with IN-1 (41) and an FITC-conjugated goat anti–rat IgG (Jackson Laboratory). The fluorescence intensity was measured on a FACScan® flow cytometer (Becton-Dickinson, Mountain View, CA). Transfectants with comparable levels of surface class II and intracellular Ii in appropriate combinations were chosen for use in functional experiments.
Antigen Presentation Using B Cells and MΦs as APCs.
Single cell suspensions from the spleens of CBA mice (Jackson Laboratory; 8–10 wk old, female) were made and red blood cells removed using ammonium chloride-potassium lysing buffer (Biofluids Inc., Rockville, MD). The splenocytes were cultured for 2 d in the presence of 10 μg/ml LPS in RPMI medium containing 10% FCS. The B cell blasts were then harvested and used as APCs. For MΦ preparation, CBA mice were injected intraperitoneally with 4 ml thioglycollate (National Institutes of Health Media Unit, Bethesda, MD) and 4 d later, the exudate MΦs were harvested by washing the peritoneal cavity with ice-cold PBS. The exudate cells were incubated in the wells of 96-well plates for 2 d in complete RPMI 1640 medium containing 20 U/ml of murine IFN-γ. The IFN-γ activated MΦs (IFN-γ MΦs) were then used without harvesting as APCs. In the antigen presentation assay, the B cell blast or MΦ APCs were pulsed with various concentrations of intact HEL antigen for 6 h, and then fixed with 1% paraformaldehyde for 20 min followed by neutralization with 0.1 M glycine. The fixed APCs pulsed with antigen (5 × 104 cells/well) were mixed with antigen-specific T cell hybridomas (5 × 104 cells/well) in a total volume of 200 μl in complete RPMI medium containing 2-ME. The cultures were incubated for 18–24 h and IL-2 production in the supernatant was measured by ELISA. The hybridoma 3A9.1 specific for HEL epitope 46-61 was from Dr. Paul Allen (Washington University, St. Louis, MD), and hybridomas 3B11.1 and 1B9.1 specific for HEL 34-45 and 116-129 respectively were provided by Dr. L. Adorini (Roche Milano Ricerche, Milan, Italy). For inhibition of antigen presentation, APCs were pretreated with various concentrations of BFA for 15 min, chloroquine for 30 min, or leupeptin for 2 h before HEL was added. All APCs were then pulsed with 50 μM of HEL for 6 h in the continued presence of inhibitor before fixation and addition to T cells. Presentation of peptide was also assessed in parallel by adding the indicated concentration of peptide to the cells for the same 6-h period before washing and fixing.
Antigen Presentation Using Transfectants.
COS or RBL transfectants were seeded onto 96-well plates at 5 × 104 cells/well in complete RPMI medium. HEL was added to the cells at various concentrations followed by T cell hybridomas (5 × 104/well). The cultures were incubated at 37°C for 18 to 24 h and IL-2 measured in the supernatants. No fixation was used for presentation assays with these transfectants.
IL-2 ELISA.
The IL-2 level in culture supernatants was measured by sandwich ELISA performed as previously described (42). Under these conditions, the assay is linear to an OD of ∼3. Absolute levels of IL-2 produced in the various experiments were generally between 10 and 100 U/ml.
Immunofluorescence Microscopy and Class II Internalization Assay.
To evaluate internalization of surface class II, a modified version of the procedure described by Roche et al. (43) was used. Cells were grown on coverslips, washed twice with cold, complete DMEM, and reacted with saturating amounts of 10-2.16 antibody for 2 h on ice to ensure monovalent binding. Cells were then washed extensively to remove unbound antibody. Washed cells were either left at 4°C or warmed to 37°C for various periods of time extending up to 150 min. After the appropriate time interval, the cells were fixed with 10% paraformaldehyde for 10 min at room temperature, permeabilized with 0.2% saponin for 30 min, and then blocked with 2% BSA at 4°C overnight. Samples were then washed with PBS and stained using rabbit anti–mouse IgG antibodies conjugated with FITC (Jackson Laboratory). To exclude the possible class II–independent uptake of antibody into endocytic structures, untransfected RBLs were treated in the same manner. Coverslips bearing stained cells were mounted and analyzed using a confocal microscope (MRC1024; Bio Rad Labs., Hercules, CA).
Results
The Presentation of Three Distinct Peptide Determinants Within HEL That Require Active Endocytic Processing Shows Differential Dependence on Newly Synthesized Proteins.
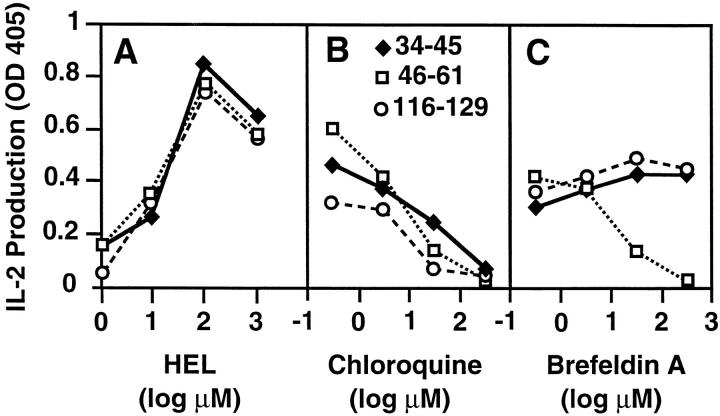
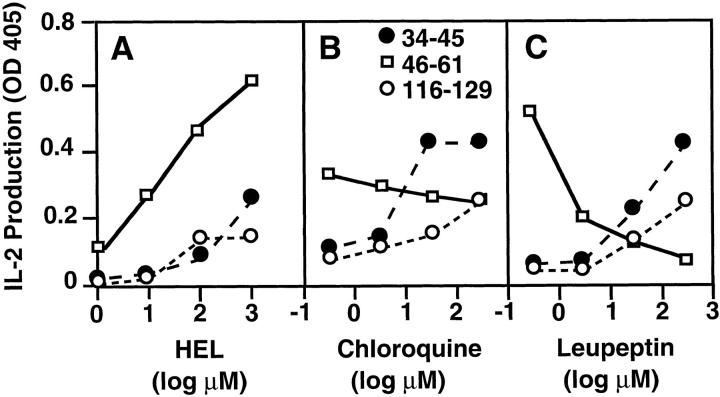
Many previous studies have shown varying sensitivities of class II antigen presentation to metabolic inhibitors, or distinct requirements for particular cell types, for new protein synthesis, or for Ii, suggesting the existence of multiple distinct pathways for antigen processing and presentation by class II molecules. Most of these, however, have involved comparisons between different proteins or distinct MHC class II alleles. To more systematically explore this issue, we have turned to a well-studied model antigen. HEL contains three distinct determinants (34-45, 46-61, and 116-129) that bind to the same class II molecule, AαkAβk, with a different dependence on the coexpression of Ii (19) or lysosome-dependent disulfide bond reduction and fragmentation of the HEL protein (44, 45). We first verified that all three determinants require pH -sensitive intracellular processing (Fig. 1). The lysosomotropic amine chloroquine inhibits the presentation of all three determinants by normal B cell blasts (Fig. 1 B). Furthermore, if these B cell APCs are chemically fixed before antigen exposure, they present synthetic peptides and denatured HEL, but not intact, nondenatured HEL (data not shown). These results indicate that T cell recognition of all three determinants requires active intracellular processing of the native protein. Despite this, when BFA is used to block export of newly synthesized proteins from the endoplasmic reticulum, only the presentation of HEL 46-61 is strongly inhibited (Fig. 1 C). This suggests that presentation of HEL 34-45 and 116-129 may involve a pool of mature class II molecules.
Figure 1.
The 34-45, 46-61, and 116-129 determinants in HEL all require endocytic processing for presentation, but only 46-61 requires newly synthesized proteins. B lymphoblasts were incubated with the indicated concentrations of HEL in standard medium for 6 h or with 50 μM HEL for 6 h in the presence of the indicated concentrations of drug. The B cells were then fixed and used as APCs with T hybridomas specific for each determinant. IL-2 production at 24 h is presented as OD obtained using an IL-2–specific capture ELISA. The data represent one of three independent experiments.
The Role of Ii in the Presentation of HEL Determinants.
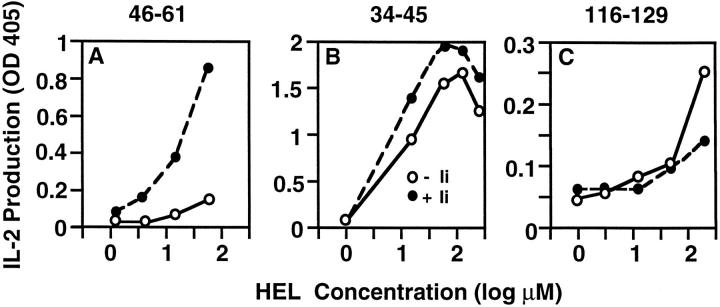
We next reexamined the Ii dependence of presentation of the three determinants. The lack of a requirement for newly synthesized proteins suggested that Ii-associated class II molecules would not be required for presentation of 34-45 or 116-129, in contrast to 46-61. COS cells were transiently transfected with plasmid constructs encoding I-Ak α and β chains or I-Ak plus mouse Ii 31, and the transfectants were monitored for class II surface expression levels by FACS®. All transfectant pools used in these experiments expressed equivalent levels of Ak as detected with mAb H116.32 (data not shown), in agreement with our earlier data showing that in COS, Ii coexpression does not noticeably increase surface levels of Ak (38). COS transfectants expressing Ak alone efficiently present 34-45 and 116-129, but not 46-61. Cotransfection with Ii dramatically increases the presentation of 46-61 (Fig. 2 A), but has little effect on presentation of 34-45 and 116-129 (Fig. 2, B and C). Again, these data are consistent with studies using rat-2 transfectants and the differential requirement for protein export from the secretory pathway for presentation of the three determinants.
Figure 2.
Only the 46-61 determinant is highly dependent on Ii coexpression with Ak for its effective presentation. COS cells transfected with cDNA expression constructs encoding either Ak or Ak and the p31 form of mouse Ii were used as APCs with the indicated concentrations of HEL for stimulation of T hybridomas specific for the indicated determinants of HEL. IL-2 production at 24 h is presented as OD obtained using an IL-2–specific capture ELISA. The data represent one of three independent experiments.
Role of the Cytoplasmic Tails of Aαk and Aβk in Presentation of the Three HEL Fragments.
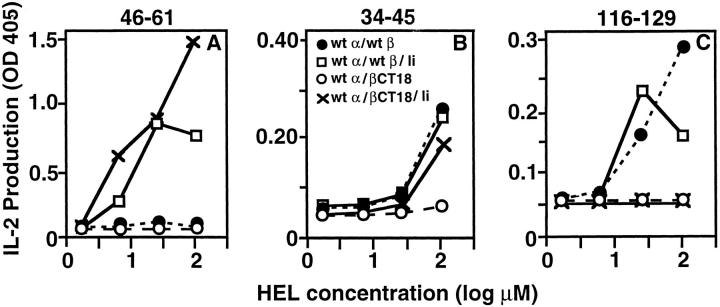
In certain cells, leucine-based targeting signals in the Ii cytoplasmic tail are important for the endocytic accumulation of newly synthesized class II molecules (4–9). However, this is not true in all cells, and the localization of class II to late endocytic structures has been observed in several cell expressing class II without Ii (26–28). Pinet et al. have reported that presentation of a hemagglutinin determinant by DR is independent of Ii, but requires uncharacterized internalization signals in the cytoplasmic tails of the class II α and β chains (31). Smiley et al. (32) have also shown a role for class II cytoplasmic tails in antigen presentation and suggested that a dileucinebased motif in the β chain may be involved in this function. To assess whether signals in the cytoplasmic tails of the Ak molecule itself are involved in the presentation of 34-45 or 116-129, RBLs were stably transfected with constructs expressing various combinations of wild-type Ak or Ak tail deletion mutants with or without Ii, and clones with comparable levels of surface class II and intracellular Ii selected. As with COS and rat-2 cells, efficient presentation of 46-61, but not 34-45 and 116-129, by wild-type Ak expressed by RBLs depends on Ii (Fig. 3). Although cells expressing wild-type Ak alone can effectively stimulate 34-45– and 116-129–specific T cells after HEL exposure, truncation of the β chain tail prevents this presentation. Examination of presentation of the three determinants by cells with the β tail deleted Ak coexpressed with Ii revealed that each determinant has a distinctive combination of requirements for either Ii or the β chain tail. The 46-61 and 34-45 determinants are both presented well by cells expressing I-Ak with a truncated β chain tail together with Ii, but Ii expression does not rescue the ability of β tail deleted class II molecules to present 116-129. Therefore, the efficient presentation of 46-61 requires Ii irrespective of the presence or absence of the class II β chain tail, presentation of 34-45 requires either intact class II tail or Ii and the class II β tail is required for the presentation of 116-129 and this function cannot be replaced by Ii. Similar data were obtained using transiently transfected COS cells (data not shown).
Figure 3.
Differential requirements for Ii coexpression and for the cytoplasmic tail of the class II Aβ chain in the presentation of distinct determinants of HEL. RBLs stably transfected with constructs encoding the indicated Aαk and Aβk proteins with or without Ii p31 were used as APCs for mouse T hybridomas specific for the indicated determinants. IL-2 production at 24 h is presented as OD obtained using an IL-2–specific capture ELISA. The data represent one of three independent experiments.
The Dileucine Region Within the Cytoplasmic Tail of the Class II β Chain Is Required for Effective Ii-independent Antigen Presentation.
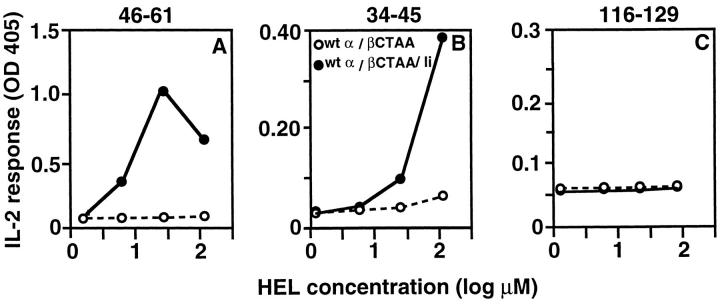
In discussing their evidence that the class II β chain tail contributes to effective antigen presentation, Smiley et al. have noted the existence of a conserved dileucine in this segment of the protein, and suggested that it may be part of a targeting motif (32). We have independently compared the sequences of multiple class II β chains from various species, looking for highly conserved sequences and also for sequences resembling either the known leucine- or tyrosine-containing signals mediating endocytic protein targeting. As shown in Table 1, like Smiley et al., we identified the same conserved dileucine near the COOH terminus of the β chain, preceded by an invariant glycine, giving rise to the sequence GLL that closely resembles one of the two localization signals in Ii (GLI in the mouse). This sequence in class II is also spaced only one residue further from the end of the transmembrane domain as compared to the comparable Ii sequence; previous studies have shown that the distance of a signal from the membrane can play an important role in its traffic control function (46). To test directly whether these dileucines are a critical part of a functional targeting motif in the class II tail, a mutant cDNA was prepared that encodes a complete Aβk chain except for substitution of the two cytoplasmic leucines with alanines (βCTAA). This construct was stably transfected into RBLs along with plasmids encoding wild-type Aαk chains, with or without the construct for Ii. The ability of the transfected cells to present the three HEL determinants was compared to the wild-type Ak transfectants (Fig. 4). Like deletion of the entire β chain tail, substitution of the two leucines with alanine prevents the presentation of 34-45 and 116-129 in cells lacking Ii, and Ii coexpression with class II containing βCTAA can rescue presentation of 34-45 but not 116-129.
Table 1.
Leucine-Based Motif in the Class II β Chain Cytoplasmic Tail
| Species/protein | Cytoplasmic tail sequence | |
|---|---|---|
| Human DR | RNQKGHSGLQPTGFLS | |
| Human DQ | RSQKGPQGPPPAGLLH | |
| Chimp DR | RNQKGHSGLQPTGFLS | |
| Mouse I-A | RSQKGPRGPPPAGLLQ | |
| Mouse I-E | RNQKGQSGLQPTGLLS | |
| Rat I-A | R-QKGPQGPPPAGLLQ | |
| Rat I-E | RSQKGNSGLQPTGLLN | |
| Dog DR | RNQKGHSGLQPTGLLS | |
| Chicken | RGQKGRPVAAAPGMLN | |
| ↕ | ||
| Mouse I-A | RSQKGPRGPPPAGLLQ | |
| Mouse Ii | RSCREPERPRNGLIPLQEHNSILDRQDDM | |
| Alignment of the sequences in the cytoplasmic tails of MHC class II β chains of various species (63), and of mouse class II Aβ and mouse Ii. For Ii, the sequence is shown C→ N from left to right because this is a type II membrane protein shown in its proper orientation relative to the membrane and the adjacent class II sequence. The double-headed arrow points to an invariant glycine residue that precedes the conserved leucine-containing pair of amino acids. | ||
Figure 4.
Mutation of the conserved LL sequence at the COOH terminus of the Aβ chain cytoplasmic tail eliminates the ability of this protein to mediate Ii-independent presentation of either the 34-45 or 116-129 determinants of HEL. RBLs stably transfected with constructs encoding the indicated Aαk and Aβk proteins with or without Ii p31 were used as APCs for mouse T hybridomas specific for the indicated determinants. IL-2 production at 24 h is presented as OD obtained using an IL-2–specific capture ELISA. The data represent one of three independent experiments.
Replacement of the β Chain Cytoplasmic Leucines with Alanines Delays, but does Not Prevent, Ak Internalization into Endosomes.
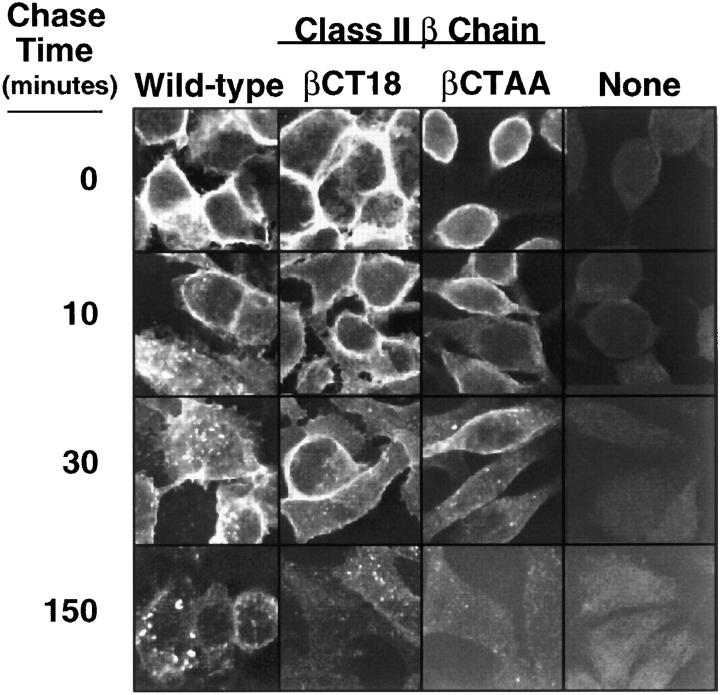
Based on the results of Pinet et al. showing that deletion of either the class II α or β tails precluded internalization of surface DR (31), we expected that the βCTAAcontaining Ak molecules would fail to accumulate in the endocytic pathway, thus accounting for the lack of effective presentation of the 34-45 determinant in the absence of Ii. However, confocal immunofluorescence microscopy unexpectedly revealed the steady state localization of class II in a variety of endocytic structures, including lamp 1+ compartments, in cells expressing mutant molecules with either the β tail deletion or βCTAA (data not shown). This suggested that either a recycling model for presentation of these determinants was incorrect, or that the loss of presenting function due to these mutations resulted from a more subtle change in the trafficking of class II than revealed by such staining studies. We therefore examined the entry of mature surface class II molecules into endosomes using surface-bound antibody as a probe. Cells expressing wild-type Ak with or without Ii rapidly internalize a substantial fraction of antibody-bound surface class II; within 5 to 10 min of warming to 37°C, distinct staining of small vesicles is observed and accumulation of signal in larger vesicular structures is readily apparent by 15 to 30 min (Fig. 5). In contrast, cells expressing Ak containing either the taildeleted or double alanine mutant β chain show little evidence of internalized class II at 10 min and only modest staining of very small vesicles at 30 min. Cells expressing class II molecules lacking both the β and α chain cytoplasmic regions show no detectable intracellular class II accumulation using this method at up to 150 min of incubation (data not shown), suggesting that the residual internalization seen with the βCTAA–wild-type α combination may depend on a separate, as yet uncharacterized, signal in the α chain tail.
Figure 5.
Deletion or mutation of the Aβ cytoplasmic tail results in a delayed rate of internalization of mature surface class II into the endocytic pathway. RBL stable transfectants expressing Aαk along with the indicated Aβ chains were bound to anti–class II mAbs in the cold, then warmed to 37°C for the indicated times. Cells were then permeabilized and stained for mouse immunoglobulin and analyzed by confocal immunofluorescence microscopy.
Differential Presentation of 34-45 and 116-129 Versus 4661 by Macrophages and B Cell Blasts, and Their Relationship to Proteolytic Activity.
The 34-45, 46-61, and 116-129 determinants of HEL lie in very different locations in the folded structure of the intact HEL protein, with 116-129 being most superficial, 34-45 being partially buried, and 46-61 lying at the core of the protein in a structure maintained by disulfide bonds. Presentation of the 46-61 determinant of HEL requires the reducing environment found in lysosomes (44, 45) and this correlates with where newly synthesized class II–Ii complexes accumulate before Ii digestion and CLIP removal (10, 47–51). On the other hand, the inability of Ii to rescue presentation of 116-129 in cells expressing tail-mutated class II suggests that this determinant may not be available in such highly acidic, dense, endocytic organelles. In this regard, Frosch et al. have reported that IFN-γ activated MΦ fail to present a particular insulin determinant (52), although B cells from the same mouse are fully competent to do so. This difference appears to be related to the more rapid and complete digestion of insulin by the MΦ, which prevents effective capture by class II. Given the different locations of the three HEL determinants in the native protein, one might expect that 116-129 and perhaps 34-45, but not 46-61, to be overdigested in activated macrophages, as compared to the B cell blasts used in the studies shown in Fig. 1. As shown in Fig. 6, this is the case. IFN-γ–activated macrophages are quite effective in presenting the 46-61 determinant, but inefficient in presenting either the 34-45 or 116-129 determinants. If chloroquine or leupeptin is added to HEL-exposed macrophages before fixation, there is a paradoxical dose-dependent gain in presentation of the 34-45 and 116-129 determinants, but a decline in the presentation of 46-61.
Figure 6.
Differential presentation of the three HEL determinants by IFN-γ–activated macrophages and the effects of inhibitors of protein degradation on this presentation. Peritoneal macrophages were cultured in IFN-γ for 48 h, and then exposed to the indicated concentrations of HEL for 6 h or 50 μM HEL for 6 h with or without the indicated drugs. Cells were then washed, fixed, and used as APCs for the appropriate T hybridomas. IL-2 production at 24 h is presented as OD obtained using an IL-2–specific capture ELISA. The data represent one of the three independent experiments.
Discussion
The various organelles comprising the endosomal/lysosomal pathway have distinct pHs, protease contents, and capacities to promote protein unfolding (53). These properties and the rate of luminal content transfer among these organelles also vary for cells of distinct tissue origin or state of activation. These differences all affect the function of the class II antigen presentation system by influencing the availability of specific sequences within any given protein for binding to MHC class II molecules in the same compartment. Likewise, heterogeneity in protein structure and binding site occupancy also modify the capacity of class II molecules to capture protein/peptide substrates in these organelles. In part, ensuring the presentation of diverse antigenic ligands is promoted by the simultaneous expression of multiple class II proteins in the cells of a single individual. However, because the different proteins of pathogens may vary from those that are highly sensitive to protease attack to some requiring extremely harsh conditions for digestion, it also makes sense that the immune system would evolve the means to enable these polymorphic class II proteins to sample several endocytic organelles for suitable ligands. The data presented in this paper suggest that two different cohorts of MHC class II molecules sample endocytic content in an overlapping pattern that permits ligand capture from both early and late endocytic compartments. The control of protein trafficking necessary for the operation of these two presentation pathways lies in related cytoplasmic leucine-based signals, one of which is characterized in this study. However, it is the timing of binding site availability that appears to actually distinguish where class II molecules in each pathway most effectively acquire antigen.
Previous studies have shown that leucine-based motifs within the cytoplasmic tail of Ii are essential for effective localization of newly synthesized class II–Ii complexes to late endocytic/lysosomal organelles (7–9). These leucine-containing signals are similar to others found in proteins such as the TCR CD3γ and δ chains (54) and GLUT4 (55) that also promote trafficking of the respective proteins to endocytic organelles. Ii proteolysis and removal is minimal during passage through early endosomes, modest in late endosomes, and extensive in a lysosome-like fraction (10) that corresponds to the MIIC defined by immunoelectron microscopy (6). Class II molecules cannot bind a new ligand until Ii is degraded and CLIP is removed or dissociated from the class II binding site, in large part through the action of DM that is concentrated in the MIIC by a distinct tyrosinecontaining motif (56). Therefore, Ii-associated, newly synthesized class II molecules are most likely to participate primarily in the presentation of determinants surviving until, or first exposed in, this highly proteolytic, acidic environment. In accord with this, the 46-61 determinant of HEL, which is located in the core of this globular protein and protected by disulfide bonds, shows a strong dependence on Ii for its presentation. This agrees with studies indicating that the best presentation of this determinant occurs when lysosome-like organelles with strong reducing capacity are accessed by HEL (44, 45) and showing that this peptide has a pH optimum of 5 for binding to Ak (57).
In contrast, two other determinants within the same HEL molecule, at residues 34-45 and 116-129, show a very different behavior. Neither is well presented by activated macrophages, and partial inhibition of protease activity improves presentation of these determinants in such macrophages, but not in less proteolytically active B cell blasts. Inhibition of the trafficking of newly synthesized proteins had at best modest effects on the presentation of these determinants and neither required Ii for their presentation. Taken together, these data argue that both of these determinants are available to a cohort of class II molecules distinct from that associated with Ii. Because control experiments showed that endocytic processing was involved, these data suggested that exposure and capture of these regions of HEL occurs in early endocytic compartments and involves mature class II molecules. This in turn led to an attempt to understand how a surface pool of mature class II molecules could gain access to such endocytic organelles. Sequence alignment revealed a highly conserved GLL motif in the tail of class II β chains which, when deleted or altered to GAA, resulted in class II molecules unable to present the 34-45 or 116-129 determinants in the absence of Ii. The variable effect of mutation at these leucines on presentation of distinct antigenic determinants is consistent with the earlier suggestion by Smiley et al. (32) that a signal containing these residues could play a role in differential antigen presentation by tailless versus wild-type class II molecules.
The steady state distribution of class II in transfectants with wild-type or signal-minus class II molecules did not differ grossly. Internalization experiments, however, revealed a clear effect of the mutations, namely a significant delay in the rate of internalization of surface class II into the endocytic pathway. Previous results have shown that class II binding sites lose their function rapidly at physiological temperature in the absence of ligand engagement (3, 58, 59). If the major pool of class II sites involved in acquisition of determinants such as HEL 116-129 become empty on the cell surface before internalization, delayed uptake would result in such molecules becoming incapable of peptide binding by the time they reached the site of antigen availability. Alternatively, the class II molecules involved in HEL 116-129 presentation may only shed their original peptide upon exposure to the mildly acidic pH of early endosomes, whereupon they would be immediately available to bind new ligand. Substantially decreasing the pool of such molecules reaching the endocytic pathway at any point in time could then markedly reduce antigen presentation. Both of these effects may in fact contribute to the functional defect observed with the mutant class II chains lacking the GLL motif. This model also can account for the recent findings of Smiley et al. (60), who demonstrated an increased density of CLIP–class II complexes on cells expressing class II molecules with a β chain whose cytoplasmic tail had been truncated. Because CLIP release is facilitated at acidic pH (61), decreased internalization due to the β mutation would allow such complexes to accumulate to a higher level on the plasma membrane, rather than dissociating upon internalization and recycling. The decrease we observed in class II internalization rate without a loss of steady-state localization in endocytic organelles following mutation of a dileucine signal is also very similar to results obtained in studies of the GLUT4 glucose transporter (55).
The timing of binding site availability appears to play a predominant role in dictating which set of determinants is presented by which cohort of class II molecules. The binding sites of Ii-associated class II become available mainly in the MIIC via the action of cathepsins and DM, and this biases these dimers for capture of those determinants able to survive transport through earlier endocytic compartments. These same class II molecules are ineffective in the capture of determinants present only in early endosomes because they do not have an accessible binding site in that location. Conversely, mature class II molecules, using a very homologous targeting signal to traffic to the same or a similar set of early and late endocytic organelles, primarily bind those ligands available in early endocytic compartments because the binding site is accessible there. Some determinants such as 34-45 appear to be available in more than one location, permitting capture by class II via either pathway.
The added breadth of determinants presented to CD4+ T cells through use of a recycling pathway has obvious advantages for the immune system. Viruses enter cells by fusing with or disrupting either plasma or early endosomal membranes. The relevant viral proteins often undergo structural changes and remain in these membranes when the viral genome enters the cytosol (62). Such refolded proteins may be highly susceptible to proteolysis shortly after viral entry, and the presentation of determinants derived from these proteins would help elicit a T cell response at the earliest time after viral infection. Data consistent with this suggestion come from the work of Pinet et al. (31), who showed that a determinant from influenza hemagglutinin was presented in an Ii-independent, class II tail-dependent manner. Another rationale besides sensitivity to proteolysis for such a pathway is the capture of ligands unable to bind tightly to class II at the highly acidic pH of the MIIC.
The results presented here, together with the recent study of Pinet et al. (31) help rationalize a contradictory literature concerning the role of newly synthesized versus mature class II molecules and of Ii in class II presentation to T cells. They also emphasize the important differences in processing and presentation of the same protein by different cell types, as evidenced by the inability of activated macrophages to present 34-45 or 116-129 without addition of an inhibitor of lysosomal function. The demonstration that cytoplasmic signals in the class II β chain tail control the rate of class II entry into the endocytic pathway for capture of only a subset of antigenic determinants provides an explanation for earlier reports of a variable influence of class II tail deletion on antigen presentation (29, 30). Our evidence that a leucine-based motif is involved in this endocytic targeting emphasizes the importance of this motif in immunological function, especially as concerns T cell antigen recognition. On the other hand, the rather modest effect of elimination of the class II tail motif on in vivo immunity to a range of pathogens (32) raises some question as to the physiologic significance of this Ii-independent pathway. However, the organisms studied in this report were primarily those resident in the endocytic compartment itself, and not viruses, for which we argue the recycling pathway may have a special importance. It is also the case that the studies conducted here have not been performed in typical hematopoietic antigen presenting cells. This clearly leaves to future studies a fuller determination of the roles of the two class II pathways defined here in the protective functions of the immune system.
Footnotes
The authors wish to thank members of the Lymphocyte Biology Section for many helpful discussions and suggestions throughout the course of this work. We also thank Drs. Jack Bennink and Jon Yewdell for their assistance with confocal microscopy, Luciano Adorini for providing critical T hybridomas, and Eric Long, Flora Castellino, and Caetano Reis e Sousa for comments on the manuscript. We also thank an anonymous reviewer for very useful suggestions for improving the original submitted manuscript.
1 Abbreviations used in this paper: BFA, brefeldin A; CLIP, class II–associated invariant chain peptide; HEL, hen egg lysozyme; Ii, invariant chain; MIIC, class II–rich organelles; RBL, rat basophil leukemia cell.
References
- 1.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Germain RN. Binding domain regulation of MHC class II molecule assembly, trafficking, fate, and function. Semin Immunol. 1995;7:361–372. doi: 10.1006/smim.1995.0041. [DOI] [PubMed] [Google Scholar]
- 4.Bakke O, Dobberstein B. MHC class II–associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 5.Teyton L, O'Sullivan D, Dickson PW, Lotteau V, Sette A, Fink P, Peterson PA. Invariant chain distinguishes between the exogenous and endogenous antigen presentation pathways. Nature (Lond) 1990;348:39–44. doi: 10.1038/348039a0. [DOI] [PubMed] [Google Scholar]
- 6.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature (Lond) 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 7.Pieters J, Bakke O, Dobberstein B. The MHC class II–associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993;106:831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 8.Bremnes B, Madsen T, Gedde–Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107:2021–2032. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- 9.Odorizzi CG, Trowbridge IS, Xue L, Hopkins CR, Davis CD, Collawn JF. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994;126:317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellino F, Germain RN. Extensive trafficking of MHC class II–invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;1:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 11.Cresswell P, Avva RR, Davis JE, Lamb CA, Riberdy JM, Roche PA. Intracellular transport and peptide binding properties of HLA class II glycoproteins. Semin Immunol. 1990;2:273–280. [PubMed] [Google Scholar]
- 12.Riberdy JM, Newcomb JR, Surman MJ, Barbosa JA, Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature (Lond) 1992;360:474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli P, Germain RN. The CLIP region of invariant chain plays a critical role in regulating MHC class II folding, transport, and peptide occupancy. J Exp Med. 1994;180:1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature (Lond) 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 15.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II αb dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 16.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain–derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 17.Stockinger B, Pessara U, Lin RH, Habicht J, Grez M, Koch N. A role of Ia-associated invariant chains in antigen processing and presentation. Cell. 1989;56:683–689. doi: 10.1016/0092-8674(89)90590-4. [DOI] [PubMed] [Google Scholar]
- 18.Peterson M, Miller J. Antigen presentation enhanced by the alternatively spliced invariant chain gene product p41. Nature (Lond) 1992;357:596–598. doi: 10.1038/357596a0. [DOI] [PubMed] [Google Scholar]
- 19.Momburg F, Fuchs S, Drexler J, Busch R, Post M, Hämmerling GJ, Adorini L. Epitope-specific enhancement of antigen presentation by invariant chain. J Exp Med. 1993;178:1453–1458. doi: 10.1084/jem.178.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II–associated invariant chain. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 21.Bodmer H, Viville S, Benoist C, Mathis D. Diversity of endogenous epitopes bound to MHC class II molecules limited by invariant chain. Science (Wash DC) 1994;263:1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 22.Harding CV, Unanue ER. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989;142:12–19. [PubMed] [Google Scholar]
- 23.St. Pierre Y, Watts TH. MHC class II–restricted presentation of native protein antigen by B cells is inhibitable by cycloheximide and brefeldin A. J Immunol. 1990;145:812–818. [PubMed] [Google Scholar]
- 24.Kakiuchi T, Watanabe M, Hozumi N, Nariuchi H. Differential sensitivity of specific and nonspecific antigen-presentation by B cells to a protein synthesis inhibitor. J Immunol. 1990;145:1653–1658. [PubMed] [Google Scholar]
- 25.Pinet V, Malnati MS, Long EO. Two processing pathways for the MHC class II–restricted presentation of exogenous influenza virus antigen. J Immunol. 1994;152:4852–4860. [PubMed] [Google Scholar]
- 26.Humbert M, Raposo G, Cosson P, Reggio H, Davoust J, Salamero J. The invariant chain induces compact forms of class II molecules localized in late endosomal compartments. Eur J Immunol. 1993;23:3158–3166. doi: 10.1002/eji.1830231218. [DOI] [PubMed] [Google Scholar]
- 27.Salamero J, Humbert M, Cosson P, Davoust J. Mouse B lymphocyte specific endocytosis and recycling of MHC class II molecules. EMBO (Eur Mol Biol Organ) J. 1990;9:3489–3496. doi: 10.1002/j.1460-2075.1990.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonsen A, Momburg F, Drexler J, Hämmerling GJ, Bakke O. Intracellular distribution of the MHC class II molecules and the associated invariant chain (Ii) in different cell lines. Int Immunol. 1993;5:903–917. doi: 10.1093/intimm/5.8.903. [DOI] [PubMed] [Google Scholar]
- 29.Nabavi N, Ghogawala Z, Myer A, Griffith IJ, Wade WF, Chen ZZ, McKean DJ, Glimcher LH. Antigen presentation abrogated in cells expressing truncated Ia molecules. J Immunol. 1989;142:1444–1447. [PubMed] [Google Scholar]
- 30.Wade WF, Chen ZZ, Maki R, McKercher S, Palmer E, Cambier JC, Freed JH. Altered I-A proteinmediated transmembrane signaling in B cells that express truncated I-Ak protein. Proc Natl Acad Sci USA. 1989;86:6297–6301. doi: 10.1073/pnas.86.16.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinet V, Vergelli M, Martin R, Bakke O, Long EO. Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature (Lond) 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 32.Smiley ST, Laufer TM, Lo D, Glimcher LH, Grusby MJ. Transgenic mice expressing MHC class II molecules with truncated Aβ cytoplasmic domains reveal signaling-independent defects in antigen presentation. Int Immunol. 1995;7:665–677. doi: 10.1093/intimm/7.4.665. [DOI] [PubMed] [Google Scholar]
- 33.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 34.Bonnerot C, Marks MS, Cosson P, Robertson EJ, Bikoff EK, Germain RN, Bonifacino JS. Association with BiP and aggregation of class II molecules synthesized in the absence of invariant chain. EMBO (Eur Mol Biol Organ) J. 1994;13:934–944. doi: 10.1002/j.1460-2075.1994.tb06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller J, Germain RN. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986;164:1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel I, Ottenhoff THM, Klausner RD. Highefficiency expression and solubilization of functional T cell antigen receptor heterodimers. Science (Wash DC) 1992;256:1318–1321. doi: 10.1126/science.1598575. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi, R. 1989. Using PCR to engineer DNA. In PCR Technology. H. Erlich, editor. Stockton Press, New York.
- 38.Layet C, Germain RN. Invariant chain promotes egress of poorly expressed, haplotype-mismatched class II major histocompatibility complex AαAβ dimers from the endoplasmic reticulum/cis-Golgi compartment. Proc Natl Acad Sci USA. 1991;88:2346–2350. doi: 10.1073/pnas.88.6.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn WC, Menzin E, Saito T, Germain RN, Bierer BE. The complete sequences of plasmids pFNeo and pMH-Neo: convenient expression vectors for high-level expression of eukaryotic genes in hematopoietic cell lines. Gene (Amst) 1993;127:267–268. doi: 10.1016/0378-1119(93)90731-h. [DOI] [PubMed] [Google Scholar]
- 40.Hämmerling GJ, Lemke H, Hämmerling U, Hohmann C, Wallich R, Rajewsky K. Monoclonal antibodies against murine cell surface antigens: anti-H-2, anti-Ia and anti-T cell antibodies. Curr Top Microbiol Immunol. 1978;81:100–106. doi: 10.1007/978-3-642-67448-8_15. [DOI] [PubMed] [Google Scholar]
- 41.Koch N, Koch S, Hämmerling GJ. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature (Lond) 1982;299:644–645. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- 42.Reis e Sousa C, Germain RN. MHC class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–852. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roche PA, Teletski CL, Stang E, Bakke O, Long EO. Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA. 1993;90:8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 45.Collins DS, Unanue ER, Harding CV. Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol. 1991;147:4054–4059. [PubMed] [Google Scholar]
- 46.Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romagnoli P, Layet C, Yewdell J, Bakke O, Germain RN. Relationship between invariant chain expression and MHC Class II transport into early and late endocytic compartments. J Exp Med. 1993;177:583–596. doi: 10.1084/jem.177.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harding CV, Geuze HJ. Immunogenic peptides bind to class II MHC molecules in an early lysosomal compartment. J Immunol. 1993;151:3988–3998. [PubMed] [Google Scholar]
- 49.Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature (Lond) 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 50.West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide–loading in human B-lymphoblastoid cells. Nature (Lond) 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- 51.Qiu Y, Xu X, Wandinger-Ness A, Dalke DP, Pierce SK. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994;125:595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frosch S, Bonifas U, Reske-Kunz AB. The capacity of bone marrow–derived macrohpages to process bovine insulin is regulated by lymphokines. Int Immunol. 1993;5:1551–1558. doi: 10.1093/intimm/5.12.1551. [DOI] [PubMed] [Google Scholar]
- 53.Mellman I. Endocytosis and antigen processing. Semin Immunol. 1990;2:229–237. [PubMed] [Google Scholar]
- 54.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 55.Verhey KJ, Yeh JI, Birnbaum MJ. Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J Cell Biol. 1995;130:1071–1079. doi: 10.1083/jcb.130.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harding CV, Roof RW, Allen PM, Unanue ER. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1991;88:2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadegh-Nasseri S, Stern LJ, Wiley DC, Germain RN. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature (Lond) 1994;370:647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- 59.Mason K, Denney DJ, McConnell HM. Myelin basic protein peptide complexes with the class II MHC molecules I-Au and I-Akform and dissociate rapidly at neutral pH . J Immunol. 1995;154:5216–5227. [PubMed] [Google Scholar]
- 60.Smiley ST, Rudensky AY, Glimcher LH, Grusby MJ. Truncation of the class II β-chain cytoplasmic domain influences the level of class II/invariant chain-derived peptide complexes. Proc Natl Acad Sci USA. 1996;93:241–244. doi: 10.1073/pnas.93.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urban RG, Chicz RM, Strominger JL. Selective release of some invariant chain-derived peptides from HLA-DR1 molecules at endosomal pH. J Exp Med. 1994;180:751–755. doi: 10.1084/jem.180.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doms RW. Protein conformational changes in viruscell fusion. Methods Enzymol. 1993;221:61–72. doi: 10.1016/0076-6879(93)21007-u. [DOI] [PubMed] [Google Scholar]
- 63.Kabat, E.A., T.T. Wu, H.M. Perry, K.S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest. U.S. Department of Health and Human Services.