Abstract
The interleukin-2 receptor β chain (IL-2Rβ) is expressed on a variety of hematopoietic cell types, including natural killer (NK) cells and nonconventional T lymphocyte subsets such as intestinal intraepithelial lymphocytes (IEL). However, the importance of IL-2Rβ-mediated signaling in the growth and development of these cells has yet to be clearly established. We have investigated IEL and NK cells in mice deficient for IL-2Rβ and describe here striking defects in the development of these cells. IL-2Rβ−/− mice exhibited an abnormal IEL cell population, characterized by a dramatic reduction in T cell receptor αβ CD8αα and T cell receptor γδ lymphocytes. This selective decrease indicates that IEL can be classified into those whose development and/or differentiation is dependent on IL-2Rβ function and those for which IL-2Rβ–mediated signaling is not essential. NK cell development was also found to be disrupted in IL-2Rβ–deficient mice, characterized by a reduction in NK1.1+CD3− cells in the peripheral circulation and an absence of NK cytotoxic activity in vitro. The dependence of NK cells and certain subclasses of IEL cells on IL-2Rβ expression points to an essential role for signaling through this receptor, presumably by IL-2 and/or IL-15, in the development of lymphocyte subsets of extrathymic origin.
The cytokine interleukin-2 (IL-2) and its receptor (IL2R) have long been known to play a role of prime importance in the activation and proliferation of T lymphocytes (reviewed in reference 1). However, it is unclear whether signaling via the IL-2R is required for the normal development and differentiation of other lymphoid or myeloid cells. The IL-2R is expressed on T lymphocytes (1), B cells (2), NK cells (3), neutrophils (4), and monocytes (5). The high affinity IL-2R is composed of three subunits, the α, β, and γ chains, whereas the intermediate affinity form contains only the β and γ chains (6, 7). Components of the IL-2R have been identified in other cytokine receptors: the IL-2Rγ chain (γc, or common γ chain) is common to the IL-2, IL-4, IL-7, IL-9, and IL-15 receptors, whereas the IL-2Rβ chain is shared between the IL-2 and IL-15 receptors (8).
The importance of individual IL-2R chains in signal transduction has been clarified recently by the engineered deletion of the gene product for each of the three known receptor subunits. Given the diverse nature of the cytokine receptors that contain the β and γ chains, and the wide range of cell types that express components of the IL-2R, the disruption of multiple immunomodulatory roles might be expected in mice deficient for any one of the three subunits. Mice deficient in IL-2Rα exhibit polyclonal T and B cell expansion (9), which correlates with a defect in activation-induced cell death in T cells, and the development of autoimmune disorders and inflammatory bowel disease. Similarly, in IL-2Rβ–deficient mice, T cells are spontaneously activated, resulting in plasma cell accumulation and high levels of autoantibodies (10). IL-2Rγ–deficient mice also show a defect in mature T and B cell development, but in contrast with the lymphoproliferation seen in IL-2Rα– and IL-2Rβ–deficient mice, a 10-fold reduction in absolute lymphocyte numbers is observed (11). In addition, NK cells are completely absent in IL-2Rγ–deficient mice, and the number of intestinal intraepithelial cells (IEL)1 cells is severely diminished (11). In humans, loss of the γc function leads to X-linked severe combined immunodeficiency (SCID) (12), a disease characterized by multiple defects in T, NK, and B cell development, as might be expected from the impairment of several cytokine receptors (13). Thus, signaling via components of the IL-2R plays an essential regulatory role in homeostasis and autoimmunity.
The importance of IL-2 and IL-15 in NK cell activation and proliferation has been well documented (1, 8, 14). Because both cytokines signal via receptors containing the β and γ chains, and because IL-2Rγ–deficient mice exhibit profound defects in IEL and NK cell development, it was of interest to examine the role of IL-2Rβ in these lymphocyte populations. We report here that expression of IL-2Rβ is crucial for the normal development of both TCRγδ and TCRαβ CD8αα IEL and for NK cells. These results suggest that certain cell populations that undergo extrathymic development and differentiation have an important developmental requirement for either IL-15 and/or IL-2.
Materials and Methods
Mice.
Mice deficient in IL-2Rβ were generated as previously described (10) and maintained under specific pathogen-free conditions in our animal facility. Littermate controls were used within experiments. Because IL-2Rβ–deficient mice develop severe autoimmune disorders with age (10), all assays were performed on mice 3–5 wk of age.
Preparation of IEL.
Whole small intestine was removed from mice and the lumen flushed with MEM medium. The intestine was incised to expose the epithelial layer, and pieces ∼1 cm in length were shaken in 35 ml of MEM at room temperature for 30 min to displace IEL. Cells were passed through gauze to remove intestinal debris and centrifuged at 1,000 rpm (250 g) for 5 min. Cells were resuspended in 8 ml of 44% Percoll (Sigma Chemical Co., St. Louis, MO), overlayed onto 67.5% Percoll, and centrifuged at 2,100 rpm (600 g) for 20 min. The interface was recovered, washed, and resuspended in αMEM containing 10% FCS and Hepes (CM [complete medium]). The total number of cells isolated at this step was counted as the total IEL cell number.
Induction of NK Activity In Vivo.
Mice were injected intraperitoneally with 0.1 μg of polyinosinic:polycytidilic acid (poly[I]: poly[C], Pharmacia, Upsala, Sweden) in 0.2 ml of PBS on day 0 and day 1. Spleens were removed on day 2, and mononuclear cells (MNC) were isolated by density gradient centrifugation using Lympholite M (Cedarlane, Hornby, Ontario).
Induction of NK Activity In Vitro.
Spleen MNC isolated by density gradient centrifugation were incubated with 10 ng/ml murine recombinant IL-12 (Genzyme, Cambridge, MA) for 18 h in CM. After two washes in CM, viable MNC were counted and cytolytic activity was assessed.
NK Cytotoxic Assay.
MNC obtained after in vivo or in vitro induction of NK activity were added to V-bottomed plates (Microwell; Nunc, Raskilde, Denmark) in complete medium. The NKsensitive Moloney leukemia virus–induced mouse lymphoma cell line YAC-1 was grown in CM, labeled with Na2 51CrO4 (NEN, Boston, MA) (35 μCi/106 cells), and added to wells containing effector cells. Target cell lysis was determined after a 4-h incubation by measurement of 51Cr release using the following formula: percentage specific lysis = (a − b / c − b) × 100%, where a = test release, b = spontaneous release, and c = release in the presence of 1% SDS. Spontaneous release was always <10%.
mAbs.
mAbs used in this study were as follows: anti-mouse CD3ε (FITC-conjugated, clone 145-2C11), anti-mouse Thy-1.2 (biotin-conjugated, clone 53-2.1), anti-mouse CD4 (FITC-conjugated, clone RM4-5), anti-mouse CD8α (biotin-conjugated, clone 53-6.7), anti-mouse CD8β (PE-conjugated, clone 53-5.8), anti-mouse TCRαβ (PE-conjugated, clone H57-597), anti-mouse TCRγδ (FITC-conjugated, clone GL3), anti-mouse NK1.1 (PE-conjugated, clone PK136), and anti-mouse Ly-49A (PEconjugated, clone A1). All mAbs were from PharMingen (San Diego, CA).
Flow Cytometry.
IEL cells, peripheral blood (PB) leukocytes, or spleen MNC from poly(I):poly(C)–treated or untreated mice were resuspended in 0.1 ml of PBS containing 1% BSA and 0.1% sodium azide, and incubated with 2 μl of anti–CD32/CD16 (Fc block; PharMingen) for 10 min at room temperature. Cells were subsequently incubated on ice with mAbs for 30 min and analyzed by flow cytometry (FACScalibur®; Becton Dickinson, San Jose, CA) using CELLQuestTM software (Becton Dickinson). Viable lymphocytes were gated on the basis of forward and side scatter characteristics. A total of 10,000 gated events was analyzed for each sample.
Evaluation of Cell Numbers in PB and Spleen.
Total numbers of leukocytes in EDTA-anticoagulated blood and density gradient (Lympholite M) purified spleen MNC were assessed by staining with Turk's stain.
Results and Discussion
IEL cells, a unique subset of lymphocytes located between the epithelial cells of the gut mucosa, have been postulated to play a key role in mucosal immunity (15). IEL constitute the most abundant extrathymic T lymphocyte pool and exist as a heterogeneous population (16). In the mouse, approximately half of IEL cells resemble peripheral T cells in that they are Thy-1+ and TCRαβ+. While most of these IEL are CD8αβ+, some CD4+ and a few doublepositive cells are also present. In contrast, the remaining IEL population expresses the CD8αα homodimer and may be either Thy-1+ or Thy-1−, and TCRαβ+ or TCRγδ+. Although it is generally believed that TCRγδ IEL develop in situ within the intestine, some controversy exists regarding the origin of the various IEL subsets expressing TCRαβ (17). The evidence does, however, point to an extrathymic origin for TCRαβ IEL expressing CD8αα (18). This heterogeneity within the IEL compartment has made the exact determination of IEL function and development difficult, and little is known concerning the requirements for the normal differentiation of these lymphocyte populations.
IEL have been found to express the intermediate affinity form of the IL-2R (IL-2Rβγ) (19), and examination of IL-2Rβ expression has shown that IEL with high and low levels of IL-2Rβ can be identified (20). To address the role of IL-2Rβ in IEL cell development, flow cytometric analyses of IEL obtained from IL-2Rβ−/− and IL-2Rβ+/− mice were performed. Despite an equivalent number of total IEL cells, a distinctly different population profile was observed in IL-2Rβ−/− mice compared with that in their IL-2R+/− or wild-type littermates. As shown in both Fig. 1 and Table 1, IEL from normal mice comprise heterogeneous populations of TCRαβ+ and TCRγδ+ cells. However, IEL from IL2Rβ–deficient mice showed a preponderance of TCRαβ+ cells and a dramatic reduction in TCRγδ+ cells. The normal ratio of αβ:γδ cells in IEL (shown here in IL-2Rβ+/− mice) is ∼2:1, but this ratio was dramatically increased to 9:1 in IL-2Rβ−/− mice. The number of Thy-1− IEL cells was also profoundly decreased in the absence of IL-2Rβ. In IL-2Rβ+/− mice used as controls, more than half of both TCRαβ+ and TCRγδ+ IEL were negative for Thy-1. However, Thy-1− cells represented <5% of both αβ+ and γδ+ IEL in IL-2Rβ–deficient mice. Immunohistological analysis revealed cells that stained positively with antiTCRαβ mAb, but not with anti-TCRγδ mAb (data not shown), confirming the reduced number of TCRγδ+ IEL observed using FACS® analysis. Similar results have been reported for other cytokine receptor mutants. In IL-7Rα knockout mice, γδ T cells (including γδ IEL) failed to develop, whereas αβ T cells (including αβ IEL) were present in slightly reduced numbers, and NK cells were normal (21, 22; H. Kiyono, personal communication). The determination of γδ expression in spleen or lymph node cells has proven to be problematic in IL-2Rβ–deficient mice because of the increased αβ T cell and granulocyte populations occurring in these mutants. Because αβ cells normally constitute only a small proportion of overall spleen and lymph node cells, at this time we cannot distinguish between a relative and real decrease in γδ cell numbers in the knockout mouse as a whole.
Figure 1.
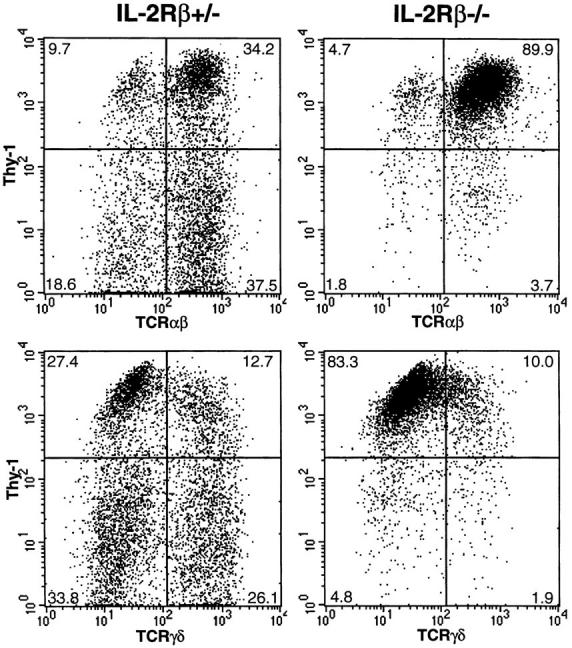
Flow cytometric analysis of Thy-1, TCRαβ, and TCRγδ expression on IEL from heterozygous (IL-2Rβ+/−) and homozygous (IL2Rβ−/−) IL-2Rβ–deficient mice. IEL isolated from IL-2Rβ+/− or IL2Rβ−/− mice were stained with anti–Thy-1 mAb, and anti-TCRαβ or anti-TCRγδ mAb, and viable cells were analyzed by flow cytometry. Numbers represent the percentage of total cells found in each quadrant.
Table 1.
Expression of TCR, Thy-1, CD8, and CD4 in IEL of IL-2Rβ–deficient Mice
| CD3+ | CD3− (n = 4) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRαβ+ (n = 4) | TCRαβ+ (n = 3) | TCRγδ+ (n = 3) | ||||||||||||||
| CD8αα+ | CD8αβ+ | CD4+ | Thy-1+ | Thy-1− | Thy-1+ | Thy-1− | ||||||||||
| IL-2Rβ+/− | 33.0 ± 5.0* | 38.2 ± 8.9 | 4.5 ± 1.0 | 36.3 ± 7.8 | 24.0 ± 12.2 | 15.3 ± 4.0 | 23.3 ± 3.1 | 31.6 ± 13.7 | ||||||||
| IL-2Rβ−/− | 2.8 ± 2.1 | 56.0 ± 2.8 | 28.3 ± 1.0 | 91.3 ± 1.5 | 3.0 ± 1.0 | 7.5 ± 3.5 | 1.7 ± 3.5 | 34.4 ± 10.2 | ||||||||
Numbers are mean ± SEM percentage of CD3+ cells (CD3+) or CD3− lymphocytes (CD3−) found in each quadrant as determined by FACS® analysis.
When TCRαβ+ IEL were analyzed for the expression of CD8α and CD8β, a significant reduction in CD8α+β− (CD8αα) cells was observed in IL-2Rβ−/− IEL compared with IL-2Rβ+/− IEL (Fig. 2). Thus, IEL in IL-2Rβ–deficient mice can be characterized as lacking TCRγδ+ and TCRαβ+ CD8αα cells. Similar total numbers of IEL were recovered after density gradient centrifugation of intestinal cells in both IL-2Rβ−/− mice (0.8 ± 0.2 × 106, n = 4) and IL-2Rβ+/− mice (1.1 ± 0.2 × 106, n = 5). Only TCRαβ+ CD8αβ+ or CD4+ cells were not reduced in IL-2Rβ−/− mice (Table 1). In fact, as previously reported (10), the ratio of CD4:CD8 cells was elevated in IL-2Rβ−/− mice. These results clearly show that two subpopulations of IEL exist: an IL-2Rβ–dependent population, characterized by expression of TCRαβ CD8αα and TCRγδ, and an IL2Rβ–independent population, characterized by TCRαβ CD8αβ expression. Two distinct populations of IEL cells have been previously described (23), from which it was proposed that those IEL undergoing thymus-dependent development display the CD8β+ marker and those that develop independently of the thymus express the CD8αα homodimer. It has been postulated that this thymus-independent population differentiates from CD3− precursors present in the gut wall (24), and that expression of CD8αα on these cells is induced by the immediate gut microenvironment. The presence of similar numbers of CD3− cells in the IEL populations isolated from both IL-2Rβ+/− and IL2Rβ−/− mice suggests that the reduction in CD8αα+ IEL observed in IL-2Rβ–deficient animals is not the result of a lack of CD3− precursors in the gut wall, but rather due to a lack of differentiation of these cells into CD8αα+ cells, possibly caused by a defect in gut microenvironment–IEL interaction. Therefore, we hypothesize that the establishment of a gut microenvironment that is permissive for the development of lymphocyte subsets of extrathymic origin is dependent on IL-15 and/or IL-2 through signaling via the IL-2Rβ chain.
Figure 2.
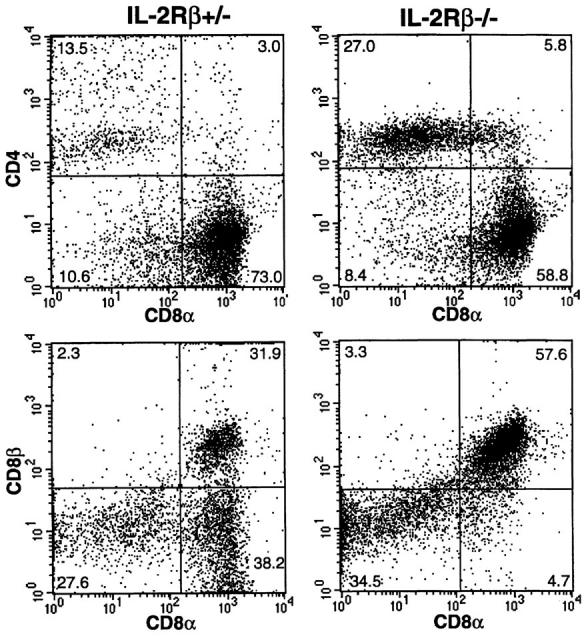
Flow cytometric analysis of CD8αβ expression on TCRαβ+ IEL from IL-2Rβ–deficient mice. IEL isolated from IL-2Rβ+/− or IL2Rβ−/− mice were stained with anti-CD4, anti-CD8α, and anti-CD8β mAb. Cells gated on TCRαβ+ cells were analyzed for their expression of CD8α and CD8β. Numbers represent the percentage of total cells found in each quadrant.
The role of IL-2Rβ in NK cell development and function was also investigated in this study. NK cells are lymphocytes capable of non-MHC-restricted cytotoxicity against virally infected and tumor cells (25), and activated NK cells are known to express both the high and intermediate affinity IL-2 receptors (26). Because both forms of the IL-2R contain the β chain, which is expressed at a high level in resting NK cells (3), and anti–IL-2Rβ mAb treatment leads to long-term elimination of NK cells in vivo (27), it was thought that the IL-2R β chain might play a role in NK cell function and development.
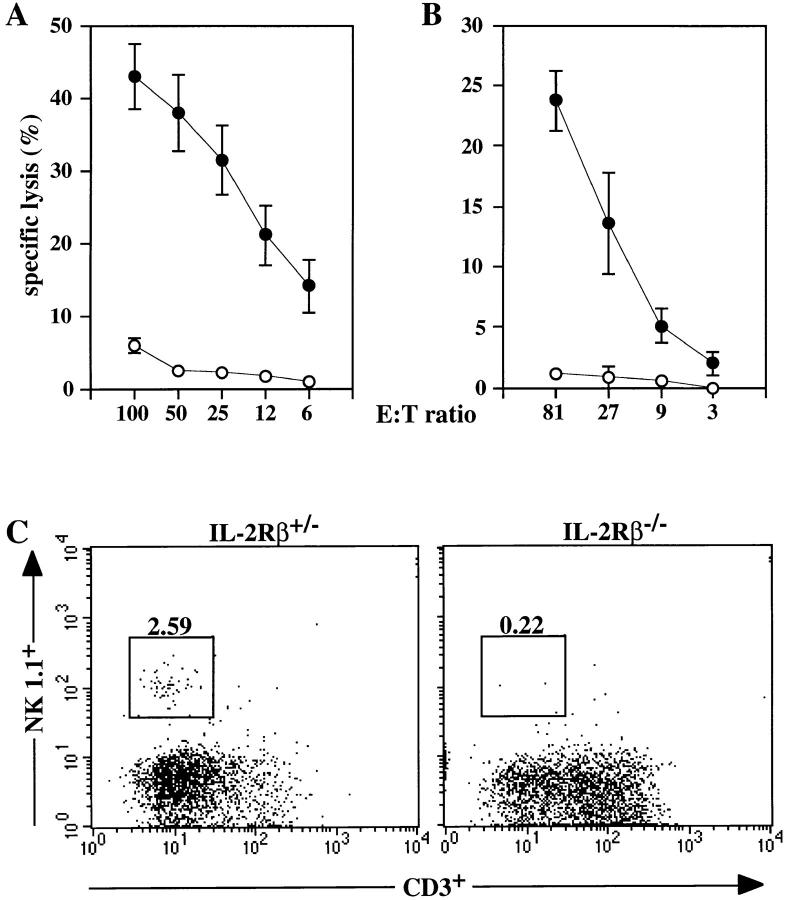
NK cell–mediated cytotoxicity was evaluated using spleen MNC as a source of NK cells and the prototypic NK target cell line YAC-1. No cytotoxic activity was apparent in unstimulated spleen MNC of 3–5-wk-old wild-type or mutant mice (data not shown). To study in vivo induction of NK activity, mice were treated with the type I interferon inducer poly(I):poly(C). While substantial NK cell–mediated cytotoxic activity was induced in IL-2Rβ+/− mice, no cytotoxic activity was apparent in spleen NK cells derived from IL-2Rβ−/− mice (Fig. 3 A). To examine the activation of NK activity in vitro, spleen MNC from IL-2Rβ+/− and IL-2Rβ−/− mice were incubated overnight with IL-12. IL-12 induced significant cytolytic activity in MNC of IL2Rβ+/− mice but was completely ineffective in inducing detectable NK cytolytic activity in IL-2Rβ−/− MNC (Fig. 3 B). Analysis of IFN-γ production (by ELISA [Intertest γ; Genzyme, Cambridge, MA]) in culture supernatants of spleen MNC incubated with IL-12 revealed a 270-fold increase in IFN-γ production over unstimulated controls in IL-2Rβ+/− cells, but only a 2-fold increase in IL-2Rβ−/− cells (data not shown). These results strongly indicate an absence of functional NK cells in IL-2Rβ–deficient mice.
Figure 3.
NK cell cytolytic activity and development in IL-2Rβ–deficient mice. Top panels show lysis of YAC-1 target cells by (A) spleen MNC from poly(I):(C )–treated animals or (B) spleen MNC incubated in vitro with IL-12. IL-2Rβ+/− MNC are represented by closed symbols; IL-2Rβ−/− MNC are represented by open symbols. Data are shown as mean ± SEM for (A) five IL-2Rβ+/− and five IL-2Rβ/- mice and for (B) four IL-2Rβ+/− and two IL-2Rβ−/− mice, analyzed in two separate experiments. (C) Flow cytometric analysis of NK1.1 and CD3 expression on NK cells in IL-2Rβ–deficient mice. PB cells from IL-2Rβ+/− and IL2Rβ−/− mice were stained with anti-NK1.1 and anti-CD3, and 10,000 viable lymphocytes were gated. Numbers represent the percentage of NK1.1+ cells in the gated population for a representative individual from each group.
To determine whether the defect in IL-2Rβ−/− NK cell cytotoxic activity was due to a functional inactivation of NK cells or to a block in NK cell development, NK cell numbers were evaluated using flow cytometry (as judged by the presence of NK1.1+/CD3− cells). Absolute numbers of PB leukocytes and spleen MNC in IL-2Rβ–deficient mice were reduced compared with heterozygous controls (Table 2). Flow cytometric analysis of lymphocytes from PB (Fig. 3 C; Table 2) and spleen MNC (Table 2) in untreated mice revealed a fivefold decrease in NK1.1+/ CD3− cells in IL-2Rβ–deficient mice. Similar results were obtained for PBL and spleen MNC obtained from poly(I): poly(C)–treated animals (data not shown). IL-2Rβ−/− mice also displayed an absence of Ly-49A+ cells both in peripheral blood and spleen (data not shown), confirming the absence of NK cells in these mice.
Table 2.
Occurence of CD3+ and NK1.1+ Cells in PB and Spleen MNC of IL-2Rβ–deficient Mice
| PB | Spleen MNC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total leukocytes (× 106 ml PB) (n = 3) | CD3+ NK1.1− (Percent, n = 13) | NK1.1+ CD3− | Total MNC (× 106 ml spleen) (n = 3) | CD3+ NK1.1− (Percent, n = 6) | NK1.1+ CD3− | |||||||
| IL-2Rβ+/− | 8.6 ± 0.9 | *33.9 ± 4.4 | *2.3 ± 0.3 | 74.3 ± 5.9 | ‡35.6 ± 4.0 | ‡2.2 ± 0.4 | ||||||
| IL-2Rβ−/− | 2.1 ± 0.5 | 53.2 ± 4.0 | 0.5 ± 0.01 | 24.8 ± 4.6 | 48.5 ± 5.8 | 0.5 ± 0.01 | ||||||
Numbers are mean ± SEM percentage of total leukocytes found in each quadrant as determined by FACS® analysis.
Numbers are mean ± SEM percentage of total spleen MNC found in each quadrant as determined by FACS® analysis.
The IL-2Rβ–deficient mice used in this study were generated using A129/J mice, a strain that is known to be negative for the NK1.1 surface marker of NK cells (28). It is very unlikely that the absence of detectable NK1.1 surface marker on IL-2Rβ−/− cells was due to the presence of A129/J background in these mice, because they had been backcrossed into a C57BL/6 background five times. In all littermates examined during the course of these experiments, IL-2Rβ+/− mice showed consistent numbers of NK1.1+ cells, whereas IL-2Rβ−/− mice always displayed very low levels of NK1.1 staining. Inadvertent deletion of the NK1.1 gene due to colocalization with the the IL-2Rβ gene can also be dismissed, since the NK gene complex (in which the NK1.1, Ly-49, LGL-1, and NKR-P1 genes are clustered) is located on distal mouse chromosome 6 (29), whereas the IL-2Rβ gene is localized on mouse chromosome 15 (30).
Although the β chain subunit is essential for IL-2–mediated signal transduction, a defect in IL-2Rβ expression does not affect the development of classical T cells (10). Several studies have indicated that in both mouse and human, progenitor cells capable of differentiation into either T lymphocytes or NK cells are present in the thymus (31–33). Those progenitors remaining within the thymus differentiate into T cells, while those developing extrathymically become NK cells (31), implying that the immediate microenvironment provides the required signals for a specific differentiation pathway. Within the murine fetal thymus, this bipotential progenitor cell population largely expresses not only IL-2Rβ (34, 35) but also IL-15Rα messenger RNA (34), suggesting that these cells can respond to both IL-2 and IL-15.
It has been demonstrated that IL-2–deficient mice have reduced but inducible NK cell–mediated cytotoxicity (36), suggesting the existence of other compensatory IL-2R binding molecules that may play a role in NK cell development. IL-15 shares many of the properties of IL-2 in its potentiation of NK cell cytokine production and cytotoxic activity (14), and the IL-2Rβ chain is common to the IL-2 and IL-15 receptors (8). The decrease in NK cell number apparent in both IL-2Rβ and γ chain knockout mice, together with the demonstration that IL-2 is dispensable for NK cell development (36), suggests that IL-15 may be the more critical molecule for NK cell development. This is supported by the observation that IL-2Rβ–expressing fetal thymic bipotential T/NK progenitor cells cultured with IL-15 differentiated into functional NK cells capable of lysing YAC-1 target cells (34). High doses of IL-2 also induced differentiation into functional NK cells but did not result in the same level of proliferation (34). It has also recently been reported that IL-15 is a maturation factor for NK cells in vitro, capable of rescuing the development of functional NK cells from mice in which the bone marrow microenvironment (essential for the development of fully functional NK cells) has been disrupted (37).
Investigation of the effect of IL-15 on the differentiation of bipotential T/NK progenitor cells in fetal thymic organ culture has shown that low doses of IL-15, but not IL-2, induce the expansion of TCRγδ CD8αα T cells from this progenitor population (34). This effect is blocked by the addition of anti–IL-2Rβ mAb. Similarly, treatment in utero with anti–IL-2Rβ mAb blocks the development of Vγ3+ dendritic epidermal cells (38), which are the developmental progeny of Vγ3+ fetal thymocytes (39). That this maturation may depend on IL-15–mediated (rather than IL-2– mediated) signaling through the IL-2Rβ chain is suggested by the observation that Vγ3+ dendritic epidermal cells are present in IL-2–deficient mice (40). These results, taken together with the reduction in TCRγδ CD8αα IEL observed in this study, point to the importance of IL-2Rβ in the development of extrathymic TCRγδ lymphocytes, possibly as a component of the IL-15R.
Given that fetal thymic T/NK progenitors require IL2Rβ expression for differentiation into γδ and NK cells (34), it is possible that the block in development of TCRαβ CD8αα and TCRγδ IEL observed in IL-2Rβ–deficient mice lies at an early stage of progenitor differentiation within the thymus. However, the presence of these IEL subsets in athymic mice (41) suggests that it is the gut microenvironment itself that determines the surface phenotype of the cell, and that in the absence of appropriate IL-2Rβ–mediated signaling in this location, extrathymic IEL cannot develop. Likewise, although NK precursors are found in the thymus, NK cell differentiation does not require the presence of a thymus, suggesting that any developmental block seen in IL-2Rβ–deficient mice is probably associated with a block in development or differentiation in the periphery, possibly within the bone marrow microenvironment. A subset of IEL expressing surface markers characteristic of NK cells and capable of natural killing and antibody-dependent cell-mediated cytotoxicity has recently been described (42). Interestingly, this subset is limited exclusively to the thymus-independent TCRαβ CD8αα or TCRγδ population. Therefore, it is fascinating to note that two distinct lineages of lymphocyte capable of natural killing, the thymus-independent IEL (which can now be classed as NK–T IEL), and classical NK cells, are both critically dependent on IL-2Rβ for their normal development.
In conclusion, our observation that TCRαβ CD8αα IEL, TCRγδ IEL, and NK cells fail to develop in IL-2Rβ– deficient mice is supportive of a major role for IL-15 in the development of these extrathymic lymphocyte subsets. The α chain of the IL-15 receptor has recently been identified and cloned (43), manipulation of which may help to clarify the situation. The generation of a mouse with a gene-targeted disruption of the IL-15 gene will no doubt elucidate further the role of IL-15 in NK and IEL cell development.
Acknowledgments
We thank Drs. H.-W. Mittrücker, J. Penninger, and T. Ohteki for critically reviewing the manuscript and Dr. M.E. Saunders for scientific editing.
Footnotes
H. Suzuki was supported by a post doctoral fellowship from the Cancer Research Institute, and T.W. Mak by the Medical Research Council of Canada.
The first two authors contributed equally to this study.
1 Abbreviations used in this paper: CM, complete medium; IEL, intraepithelial cells; MNC, mononulear cells; PB, peripheral blood.
References
- 1.Waldmann TA. The IL-2/IL-2 receptor system: a target for rational immune intervention. Immunol Today. 1993;14:264–270. doi: 10.1016/0167-5699(93)90043-K. [DOI] [PubMed] [Google Scholar]
- 2.Begley CG, Burton JD, Tsudo M, Brownstein BH, Ambrus JL, Waldmann TA. Human β lymphocytes express the p75 component of the interleukin 2 receptor. Leuk Res. 1990;14:263–271. doi: 10.1016/0145-2126(90)90134-u. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa K, Saito S, Mori T, Kato Y, Narita N, Ichijo M, Ohashi Y, Takeshita T, Sugamura K. Differential expression of the interleukin 2 receptor beta (p75) chain on human peripheral blood natural killer subsets. Int Immunol. 1990;2:481–486. doi: 10.1093/intimm/2.6.481. [DOI] [PubMed] [Google Scholar]
- 4.Djeu JY, Liu JH, Wei S, Rui H, Pearson CA, Leonard WJ, Blanchard DK. Function associated with IL-2 receptor-beta on human neutrophils. Mechanism of activation of antifungal activity against Candida albicans by IL-2. J Immunol. 1993;150:960–970. [PubMed] [Google Scholar]
- 5.Espinoza-Delgado I, Ortaldo JR, Winkler-Pickett R, Sugamura K, Varesio L, Longo DL. Expression and role of p75 interleukin 2 receptor on human monocytes. J Exp Med. 1990;171:1821–1826. doi: 10.1084/jem.171.5.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 8.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science (Wash DC) 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 9.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Kündig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJL, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science (Wash DC) 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 11.DiSanto JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 13.Conley ME. Molecular approaches to the analysis of X-linked immunodeficiencies. Annu Rev Immunol. 1992;10:215–238. doi: 10.1146/annurev.iy.10.040192.001243. [DOI] [PubMed] [Google Scholar]
- 14.Carson WE, Giri JG, Lindenmann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caliguri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujihashi K, Taguchi T, McGhee JR, Eldridge JH, Bruce MG, Green DR, Singh B, Kiyono H. Regulatory function for murine intraepithelial lymphocytes. Two subsets of CD3+, T cell receptor-1+intraepithelial lymphocyte T cells abrogate oral tolerance. J Immunol. 1990;145:2010–2019. [PubMed] [Google Scholar]
- 16.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521–553. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- 17.Poussier P, Julius M. Intestinal intraepithelial lymphocytes: the plot thickens. J Exp Med. 1994;180:1185–1189. doi: 10.1084/jem.180.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha B, Guy-Grand D, Vassalli P. Extrathymic T cell differentiation. Curr Opin Immunol. 1995;7:235–242. doi: 10.1016/0952-7915(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto Y, Sasaki K, Kato Y, Kojima K, Tsuji T, Miyama A. Rapid killing of murine lymph node T blasts by intestinal intraepithelial lymphocytes in vitro. Eur J Immunol. 1996;26:653–658. doi: 10.1002/eji.1830260322. [DOI] [PubMed] [Google Scholar]
- 20.Ohtsuka K, Iiai T, Watanabe H, Tanaka T, Miyasaka M, Sato K, Asakura H, Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994;153:52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- 21.He Y-W, Malek TR. Interleukin-7 receptor α is essential for the development of γδ+T cells, but not natural killer cells. J Exp Med. 1996;184:289–293. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki J-I, Ikuta K. Interleukin 7 receptor–deficient mice lack γδ T cells. Proc Natl Acad Sci USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy-Grand D, Rocha B, Mintz P, Malassis-Seris M, Selz F, Malissen B, Vasalli P. Different use of T cell receptor transducing modules in two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J Exp Med. 1994;180:673–679. doi: 10.1084/jem.180.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinchierei G. Biology of natural killer cells. Adv Immunol. 1989;47:187–386. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips JH, Takeshita T, Sugamura K, Lanier LL. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989;170:291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka T, Kitamura F, Nagasaka Y, Kuida K, Suwa H, Miyasaka M. Selective long-term elimination of natural killer cells in vivo by an anti–interleukin 2 receptor β chain monoclonal antibody in mice. J Exp Med. 1993;178:1103–1107. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giorda R, Weisberg EP, Ip TK, Trucco M. Genomic structure and strain-specific expression of the natural killer receptor NKR-P1. J Immunol. 1992;149:1631–1635. [PubMed] [Google Scholar]
- 29.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 30.Malek TR, Vincek V, Gatalica B, Bucan M. The IL-2 receptor beta chain gene (IL-2Rβ) is closely linked to the PDGFβ locus on mouse chromosome 15. Immunogenetics. 1993;38:154–156. doi: 10.1007/BF00190904. [DOI] [PubMed] [Google Scholar]
- 31.Rodewald HR, Moingeon P, Lucich JL, Dosion C, Lopez P, Reinherz EL. A population of early fetal thymocytes expressing FcγRII/III contain precursors of T lymphocytes and natural killer cells. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez MJ, Muench MO, Roncarolo MG, Lanier LL, Phillips JH. Identification of a common T/natural killer cell progenitor in human fetal thymus. J Exp Med. 1994;180:569–576. doi: 10.1084/jem.180.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez MJ, Spits H, Lanier LL, Phillips JH. Human natural killer cell committed thymocytes and their relation to the T cell lineage. J Exp Med. 1993;178:1857–1866. doi: 10.1084/jem.178.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclerque G, Debacker V, DeSmedt M, Plum J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bi-potential T/natural killer progenitor cells. J Exp Med. 1996;184:325–226. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk I, Levelt CN, Eichmann K. Lineage relationships of the fetal thymocyte subset that expresses the β chain of the interleukin-2 receptor. Eur J Immunol. 1993;23:3373–3376. doi: 10.1002/eji.1830231248. [DOI] [PubMed] [Google Scholar]
- 36.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2–deficient mice. Science (Wash DC) 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 37.Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J Immunol. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 38.Tanaka T, Takeuchi Y, Shiohara T, Kitamuro F, Nagaska Y, Hamamura K, Yagita H, Miyasaka M. In utero treatment with monoclonal antibody to IL-2 receptor β chain completely abrogates development of Thy-1+dendritic epidermal cells. Int Immunol. 1992;4:487–491. doi: 10.1093/intimm/4.4.487. [DOI] [PubMed] [Google Scholar]
- 39.Havran WL, Allison JP. Origin of Thy-1+dendritic epidermal cells of adult mice from fetal thymic precursors. Nature (Lond) 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 40.Schimpl, A., T. Hünig, A. Elbe, I. Berberich, S. Krämer, H. Merz, A.C. Feller, B. Sadlack, H. Schorle, and I. Horak. 1994. Development and function of the immune system in mice with targeted disruption of the IL-2 gene. In Transgenics and Targeted Mutagenesis in Immunology. H. Bluethman and P. Ohasi, editors. Academic Press, London, UK. 191–201.
- 41.Rocha B, Vassalli P, Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992;13:449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- 42.Guy-Grand D, Cuenod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- 43.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO (Eur Mol Biol Organ) J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]