Abstract
Our past studies have shown that the mucosal adjuvant cholera toxin (CT) induces T helper type 2 (Th2) responses with systemic IgG1, IgE and mucosal secretory IgA (S-IgA) antibodies (Abs). In this study, recombinant murine IL-12 (rmIL-12) was given either parenterally or orally to mice orally immunized with tetanus toxoid (TT) and CT to determine whether this cytokine could redirect the CT-induced Th2-type responses and what effect this shift would have on S-IgA Ab responses. Intraperitoneal administration of rmIL-12 shifted TT-specific responses toward Th1-type and resulted in CD4+ T cells producing IFN-γ and IL-2 with markedly reduced levels of Th2-type cytokines. This cytokine profile was accompanied by increased delayed-type hypersensitivity (DTH) and shifts in serum IgG1 to IgG2a and IgG3 anti-TT Ab responses. Further, serum IgE and S-IgA Ab responses were markedly reduced by parenteral IL-12. When IL-12 complexed to liposomes was given orally both shifts to IgG2a and IgG3 and low IgE Abs again occurred concomitant with enhanced serum IFN-γ and DTH responses. Interestingly, oral rmIL-12 did not result in significant levels of serum IL-12 nor altered S-IgA Ab responses and resulted in higher levels of some Th2-type cytokines both in vitro and in vivo when compared with parenteral IL-12. Our results show that the shifts in systemic immune responses with intact S-IgA Abs which occur after oral delivery of IL-12-liposomes are due to cytokine effects in the Peyer's patches and suggest new strategies for the targeted manipulation of Th1- and Th2-type responses to mucosal vaccines.
IL-12, a 40 (p40) and 35 (p35) kD dimeric molecule, exhibits remarkable regulatory effects on NK and T cells for enhanced production of IFN-γ (1–4). As a product of macrophages and other APC (5–8), IL-12 appears to set the stage for effective helper type 1 T cell responses which result in IFN-γ production and subsequent cell-mediated immunity (CMI)1 (4, 6, 9–15). A major in vivo effect of IL-12 has been to enhance both NK and CTL lytic activity (1, 16–19). In this regard, IL-12 was shown to restore CMI responses in the peripheral blood of HIV+ individuals (20). One could envision two IL-12 effects, e.g., the initial promotion of Th1-type responses from naive T cell precursors (4, 6), or a reversal of an established Th2-type response by initiation of Th1-type responses (21), and evidence for both pathways has been put forth. The precise mechanisms involved in preferential induction of Th1-type responses and for down regulation of Th2-type responses are still unknown.
It is now well established that selected cytokines from Th1- or Th2-type cells can downregulate the expression of the opposite Th cell phenotype (22–24). For example, IFN-γ production by Th1 cells down regulates IL-4, a major cytokine expressed by Th2 cells, and conversely IL-4 (with IL-10) effectively diminishes IFN-γ production by CD4+ Th1 cells (22–25). Because the in vivo network of Th1- and Th2-type cytokines can determine the profile of antibodies produced during an immune response (26), the biased induction by IL-12 of Th1-type responses would be expected to influence the isotype of antigen-specific antibody responses. In this regard, IL-12 can inhibit production of IgE antibodies, both in vitro (27) and in vivo (15, 28); however, the effects of IL-12 for IgG subclass responses are less clear (15, 28–30). Although IL-12 is considered to be a potent regulatory molecule for systemic immune responses, to our knowledge, no previous study has evaluated the effects of IL-12 on secretory IgA (S-IgA) responses.
Past studies by our group have used the well characterized vaccine protein tetanus toxoid (TT) and the mucosal adjuvant cholera toxin (CT). In this system, co-oral administration of TT and CT to C57BL/6 mice induces CD4+ Th2-type cells in both mucosal (Peyer's patches [PP]) and systemic (spleen) lymphoid tissues (31, 32). This immunization regimen induced brisk antigen-specific mucosal S-IgA Abs together with serum IgG1 (and IgG2b), and IgA Ab responses to TT (32, 33). Furthermore, we (32) and others (34) have shown that CT also induces systemic IgE responses, and the immune responses which are triggered by CT as mucosal adjuvant are dependent upon IL-4 and Th2-type responses (31, 32, 35).
The current study was undertaken to determine if parenterally administered IL-12 could alter mucosal and peripheral immune responses induced by TT and CT as adjuvant, and redirect Th2-type responses toward Th1-type development. We also queried if it would be possible to deliver IL-12 by a mucosal (e.g., oral) route in order to influence the balance of Th1- and Th2-type responses.
Materials and Methods
Mice.
Specific pathogen-free C57BL/6 mice were used throughout this study. The mice were initially obtained from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, MD), and during the past year from the Charles River Laboratories (Wilmington, DE). All mice were received at 5–6 wk of age and were maintained in horizontal laminar flow cabinets in sterile caging conditions of the animal facility provided by the UAB Immunobiology Vaccine Center. The mice were free of bacterial and viral pathogens as determined by routine Ab screening and by histologic analysis of major organs and tissues. The mice were used at 8–12 wk of age in all experiments described here.
Oral Immunization and Treatment with Recombinant Murine IL-12.
Before oral immunization, groups of mice were deprived of food for 2 h, followed by intragastric administration of an isotonic bicarbonate solution (8 parts HBSS and 2 parts 7.5% sodium bicarbonate) to neutralize stomach acidity (31–33). Individual mice were gavaged with a 0.25 ml phosphate-buffered saline solution (PBS, pH 7.2) containing vaccine grade TT (250 μg/mouse), which was kindly provided by Dr. Patricia J. Freda Pietrobon (Connaught Laboratories, Inc., Swiftwater, PA). CT was given (10 μg/mouse) (LIST Biologic Laboratories, Campbell, CA) as mucosal adjuvant. Groups of 5–7 mice were orally immunized with TT plus CT on days 0, 7, and 14 and fecal pellets and blood samples collected as previously described (32, 33).
Recombinant murine IL-12 (rmIL-12) was generously provided by Dr. Maurice K. Gately (Hoffmann-LaRoche, Nutley, NJ) and was given in various doses to mice by the i.p. or oral route (see Fig. 1). For i.p. delivery, rmIL-12 (10, 100, or 1000 ng/dose) was diluted in sterile PBS containing 1% normal mouse serum and given daily (5 times/wk) as indicated in Fig. 1. For oral delivery, rmIL-12 (1,000 ng/dose) was complexed with preformed cationic liposomes (DOTAP; Boehringer Mannheim Corp., Indianapolis, IN). Preliminary experiments established that administration of liposomes alone did not affect immune responses to the vaccine and that rmIL-12 complexed to DOTAP liposomes retained its biologic activity in vitro and in vivo. Moreover, dose-response experiments established that optimal liposome uptake of rmIL-12 was obtained at a 50:1 ratio (wt/wt) of DOTAP liposome mixed with IL-12. Mice receiving oral DOTAP-IL-12 (liposome-IL-12) were deprived of food for 2 h followed by neutralization of stomach acidity as described above. On days where mice were given oral liposome-IL-12 and TT with CT, two separate gavages were made first with oral TT plus CT followed by oral liposome-IL-12 at an ∼30 min interval (Fig. 1).
Figure 1.
rmIL-12 treatment schedules for mice which received a combined oral vaccine. Four groups of C57BL/6 mice were given TT plus CT as adjuvant on days 0, 7, and 14 by the oral route. Group A were positive controls receiving oral vaccine but not rmIL-12. Group B received 15 doses of rmIL-12 (day 0 to 5, 7 to 11, and 14 to 18) by the intraperitoneal route. This group was subdivided into mice receiving 10, 100, or 1,000 ng of rmIL-12/dose. Groups C and D received three (days 0, 7, 14) or six (days 0, 3, 7, 10, 14, 17) oral doses of rIL-12 complexed to liposomes.
Analysis of Antibody Isotypes and IgG Subclasses.
An ELISA was used to titrate antibody levels in serum and fecal extracts (32, 33). The extracts from fecal pellets were prepared as described elsewhere (32, 33). In brief, 0.1 g of pellet was mixed with 1 ml of PBS containing 0.1% NaN3 and vortexed for 5–10 min. After centrifugation, supernatants were collected and stored at −70°C until assayed for TT-specific Abs. For assays, plates (Microtest III; Becton Dickinson, Oxnard, CA) were coated with a 100 μl solution of TT (5 μg/ml; 1.25 Lf U/ml) and serial twofold dilutions of serum or fecal extracts added to individual wells. Titers of IgM, IgG or IgA were determined by addition of a 1:2,000 dilution of HRP-conjugated goat anti–mouse μ, γ, or α heavy chain specific antisera (Southern Biotechnology Associates [SBA], Inc., Birmingham, AL). To determine IgG subclass titers, 100 μl of biotin conjugated, rat monoclonal anti–mouse γ1 (G1-7.3; 2 μg/ml), γ2a (R19-15; 1 μg/ml), γ2b (R12-3; 0.5 μg/ml), or γ3 (R40-82; 1 μg/ml) heavy chain–specific antibodies were used (PharMingen, San Diego, CA) as described previously (32). After incubation and washing steps, a 100-μl aliquot of HRP-conjugated streptavidin (GIBCO BRL, Gaithersburg, MD) was added and color developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Moss Inc., Pasadena, MD). The color reaction was terminated after 15 min with 50 μl of 0.5 N HC1 and absorbance at 450 nm was determined with an ELISA plate reader. Data were expressed as reciprocal endpoint titers of the last dilution exhibiting an optical density of ⩾0.1 when compared with negative controls.
Total IgE levels and antigen-specific IgE Ab titers were determined by a sensitive ELISA and a passive cutaneous anaphylaxis (PCA) assay, respectively, as previously described (32). For total IgE measurements, Nunc-Immuno-MaxiSorp plates were coated with 2 μg/ml of rat monoclonal anti-mouse IgE antibody (R35-72; PharMingen). Serial dilutions of immune serum or standard mouse IgE (PharMingen) were then added followed by addition of 100 μl of a biotinylated rat monoclonal anti-mouse IgE antibody (R3592; PharMingen). The HRP-conjugated streptavidin described above was used for detection and plates were then developed with the TMB substrate. The PCA test was performed to determine titers of TT-specific IgE antibodies. Fisher rats (200–250 g) were sensitized by injecting threefold dilutions of mouse sera (starting at 1:10) into their shaved back. After 16 h, the rats received an i.v. injection of 200 μg of TT in 1 ml of 1% Evan's blue dye in PBS. The rats were killed 15 min later and the diameter of blueing resulting from the localized degranulation of mast cells was determined. The end-point titer was selected as the last dilution resulting in a diameter of blueing of ⩾5 mm.
B Cell Enzyme-linked Spot for IgA Antibody-forming Cells.
An enzyme-linked spot (ELISPOT) assay was used to quantitate numbers of IgA antibody-forming cells (AFC) present in the lamina propria (LP) of the small intestine of mice orally immunized with TT and CT either in the presence or absence of IL-12. The LP cells, isolated as previously described (33, 36), were >95% viable as determined by trypan blue dye exclusion, and were resuspended in complete medium (RPMI 1640; Cellgro Mediatech, Washington, DC) containing 10% FCS, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM Hepes, 100 U/ml penicillin and 100 μg/ml streptomycin. Antigen-specific IgA AFC were then determined in LP cell suspensions by TT-specific ELISPOT assay as described previously (33, 36). In brief, 96-well nitrocellulosebased plates were coated with a 100-μl solution of TT (5 μg/ml) diluted in PBS and control wells received PBS without TT. The wells were blocked with 1% BSA in PBS. Serial fivefold dilutions (starting at 1 × 106 cells) were added to the wells in duplicate and incubated for 6 h. Individual AFC were detected with peroxidase-labeled anti-mouse α chain–specific antibodies (1 μg/ml) (SBA) and were visualized by adding the chromogenic substrate, 3-amino9-ethylcarbazole (AEC) (Moss Inc.). Individual AFC were counted with the aid of a dissecting microscope (SZH Zoom Stereo Microscope System; Olympus, Lake Success, NY).
In Vivo Delayed-type Hypersensitivity Responses.
The delayedtype hypersensitivity (DTH) responses were assessed in mice that received oral TT and CT as adjuvant in the presence or in the absence of rmIL-12. 20 μg of TT in 20 μl of sterile PBS (1 mg/ml) was injected into the left ear pinna as described in our previous study (36). The right ear pinna received sterile PBS as a control or an unrelated antigen (OVA). Ear swelling was measured 24 and 48 h later with a spring-loaded dial thickness gauge (Starrett Co., Athol, MA). Positive DTH responses were expressed as an increase in ear swelling (in μm) in TT-injected sites after subtraction of swelling of the control site.
Assessment of Antigen-specific CD4+ T Cell Responses.
Single-cell suspensions of PP and spleen (SP) from immunized and control mice were obtained as previously described (31, 32). In brief, individual PP were excised and individual cells were dissociated by incubation in Joklik's medium containing Dispase® (1.5 mg/ml) (Boehringer-Mannheim Corp.). A total of 4–6 treatments were required for complete cell removal, and dissociated cells were extensively washed in incomplete RPMI 1640 medium to remove excess Dispase®. The isolated PP cells were incubated in complete medium (2–4 h) to allow de novo re-expression of membrane proteins. SP cell suspensions were obtained by teasing small fragments through sterile wire screens, and single cells were obtained from the supernatant after 1× gravity settling. Enriched CD4+ T cells were obtained from PP and SP cell preparations by panning on anti-L3T4 (GK 1.5) mAb coated petri dishes as described elsewhere (31, 32). Two cycles of panning resulted in CD4+ T cell-enriched cultures which were >97% CD3+, CD4+, and CD8− and were >99% viable.
For the stimulation of Ag-specific CD4+ T cells in vitro, TT or OVA was adsorbed to latex microspheres as described previously (31, 32, 36–39) and CD4+ T cells were restimulated in vitro according to previously described methods (31, 32). In brief, CD4+ T cells (2 × 106 cells/ml) were cultured with rIL-2 (10 U/ml, PharMingen), and T cell–depleted, irradiated (3,000 R) feeder SP cells from naive mice in flat-bottom 96-well (200 μl/well) or 24-well (1 ml/well) tissue culture plates (Corning Glass Works, Corning, NY) for proliferation and cytokine synthesis, respectively. To measure antigen-specific T cell proliferation, 0.5 μCi of tritiated [3H]thymidine (Amersham Corp., Arlington Heights, IL) was added after 6 d of culture and 18 h before harvest. Approximately 10 TT-coated particles/cell were found to optimally stimulate CD4+ T cell cultures based on the extent of cellular proliferation as well as levels of cytokines produced in culture supernatants. For the assessment of cytokine production, culture supernatants were harvested after 6 d of incubation. Control wells consisted of cells only or cells incubated with unabsorbed beads or OVA-coated beads. All cell cultures were maintained at 37°C in a 5% CO2 incubator in moist air.
Cytokine ELISA.
Cytokine levels in culture supernatants and sera were determined by ELISA using the appropriate combination of mAbs described in our previous study (36). In brief, Falcon Microtest III plates (Becton Dickinson) were coated with 100 μl of anti-cytokine Ab diluted in 0.1 M bicarbonate buffer (pH 8.2) and incubated overnight at 4°C. The wells were blocked with PBS containing 1% BSA at room temperature for 1 h. Serial twofold dilutions of supernatants or sera were added to duplicate wells and incubated overnight at 4°C. The wells were then washed with PBS-Tween (PBS-T) and incubated with the appropriate biotinylated anti-cytokine mAb diluted in PBS-T with 1% BSA for 1 to 2 h. After three rinses, wells were incubated with peroxidase-labeled anti-biotin Ab (0.5 μg/ml; Vector Laboratories, Inc., Burlingame, CA) for 1 h and developed with TMB reagent (Moss, Inc.). Standard curves were generated using murine rIFN-γ, rIL-5, rIL-6, and rIL-10 (Genzyme, Cambridge, MA); rIL-2 (PharMingen); and rIL-4 (Endogen, Boston, MA). The ELISA assays were capable of detecting 15 pg/ml for IFN-γ, 5 pg/ml for IL-2, IL-4, and IL-5, 100 pg/ml for IL-6 and 200 pg/ml for IL-10. Serum IL-12 levels were measured by using a combination of antibodies (for coating and detection) kindly provided by Dr. David H. Presky (Hoffman-LaRoche).
Statistics.
The results are expressed as the mean ± one standard deviation. Statistical significance (P <0.05) was analyzed by Student's t test and by ANOVA followed by Fisher least significant difference test. For statistical analysis of cytokine levels, those below the detection limit were recorded as one-half the detection limit (e.g., IFN-γ = 7.5 pg/ml).
Results
Parenteral rmIL-12 Downregulates Serum IgE and Shifts IgG Subclasses in Response to an Oral Vaccine.
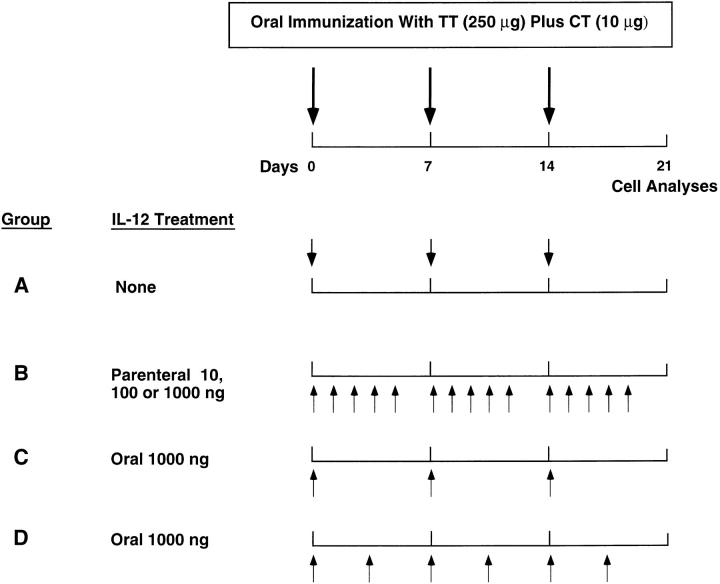
Since IL-12 plays an important role in the induction of IFN-γ production and subsequent Th1-type responses, we queried whether IL-12 treatment would alter Th2-type responses and the resultant serum IgE and IgG subclass responses in mice receiving oral TT plus CT as adjuvant (31, 32, 40, 41). Frequent i.p. injections of rmIL-12 downregulated both polyclonal and TT-specific IgE Abs (see Fig. 2 A). The effect of rmIL-12 was dose dependent because IgE responses were not significantly affected by administration of 10 ng of rmIL-12, while a significant decrease in both polyclonal and TT-specific IgE Abs was observed in mice receiving 100 ng/dose (see Fig. 2 A). The distribution of anti-TT IgG subclass Ab responses was also affected by parenteral rmIL-12 treatment and small increases in IgG2a anti-TT Abs were observed in mice given a 10 ng/dose of rmIL-12 (see Fig. 2 B). When the dose of rmIL-12 was increased to 100 ng, a significant change in the profile of IgG subclasses was observed with concomitant down regulation of IgG1 and increases in IgG2a and IgG3 Ab titers. Despite this shift in IgG subclass distribution, serum TT-specific IgG, IgA, and IgM titers were similar in mice orally immunized with TT plus CT in the presence or in the absence of IL-12, suggesting that rmIL-12 did not affect the potency of CT as adjuvant for serum Ab responses (see Fig. 2 C). Antibody responses of all isotypes were abrogated in mice receiving 1,000 ng/dose of rmIL-12, indicating that high doses of rmIL-12 impaired the ability of CT to act as a mucosal adjuvant (results not shown). However, ∼40% of mice which received 10–14 doses of 1,000 ng of rmIL-12 did not survive, perhaps due to combined toxic effects of CT and IL-12.
Figure 2.
The effect of parenteral administration of rmIL-12 on polyclonal and TT-specific serum Ab responses in mice orally immunized with TT and CT as mucosal adjuvant. Mice received the oral vaccine alone (unshaded) or together with rmIL-12 by the i.p. route (shaded, 10 ng/dose or striped, 100 ng/dose). (A) Polyclonal and TT-specific serum IgE Abs were measured on day 14 by ELISA and PCA assays, respectively. (B) The distribution of TT-specific serum IgG subclasses were analyzed on day 21 by ELISA. (C) Serum TT-specific IgM, IgA, and IgG Abs were measured by ELISA on day 21. Results are expressed as the mean ± one SD from three different experiments (five mice per group).
Oral IL-12-Liposome Effects on Systemic Ab Responses to an Oral Vaccine.
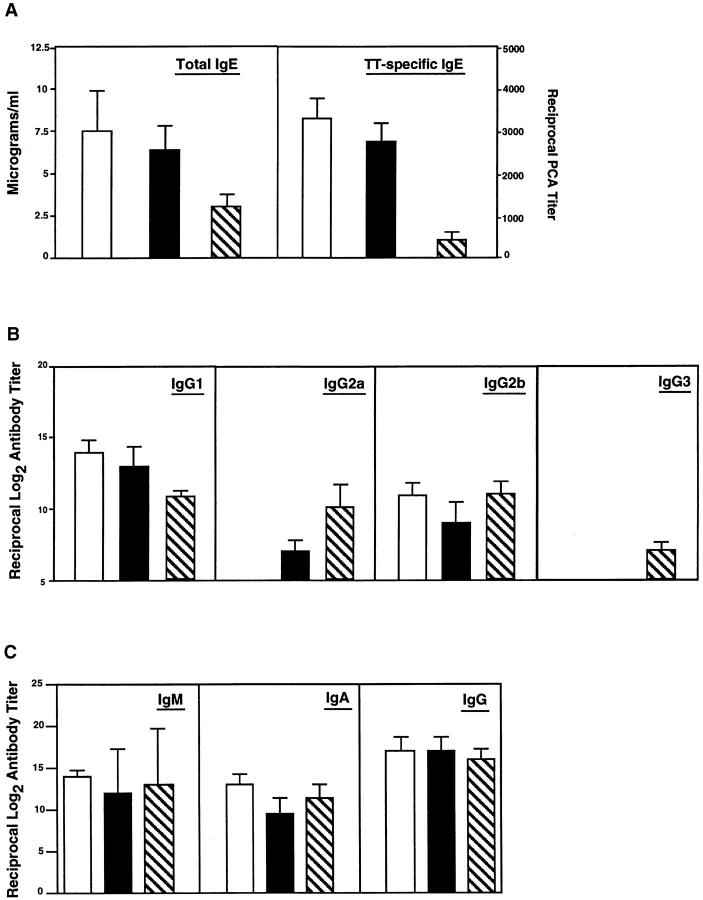
We next determined if rmIL-12 could be given by the oral route. To obviate potential degradation in the gastrointestinal (GI) tract, mice were given 1,000 ng of rmIL-12/dose complexed to liposomes. The efficacy of oral rmIL-12-liposomes was assessed in two schedules, both of which included the time of oral immunization (Fig. 1), since it has been shown that shifts toward Th1-type responses require IL-12 delivery at the time of antigen administration (15, 42). Analysis of serum IgE responses in mice orally immunized with TT plus CT and receiving oral rmIL-12–liposomes showed that three doses (days 0, 7, and 14) of rmIL-12 significantly downregulated both polyclonal and TT-specific IgE responses, an effect which was further increased when six oral doses (days 0, 3, 7, 10, 14, and 17) of rmIL-12–liposomes were given (Fig. 3 A). Administration of three doses of rmIL-12 also resulted in moderate increases in serum IgG2a and IgG3 anti-TT Ab responses (see Fig. 3 B); however, six oral doses of rmIL-12– liposomes elicited high IgG2a and IgG3 anti-TT Ab titers and downregulated TT-specific IgG1 Ab responses (see Fig. 3 B). Interestingly, the inhibition of serum IgE responses and the changes in IgG subclass responses after administration of six oral doses of rmIL-12–liposomes (1,000 ng/dose) were comparable to or greater than those observed after parenteral rmIL-12 (100 ng/dose) (Fig. 2). Despite the effects of rmIL-12 on IgG subclass responses, the total levels of serum IgG, IgM and IgA were not significantly affected by oral administration of this cytokine (Fig. 3 C).
Figure 3.
The effect of oral administration of rmIL-12 on polyclonal and TT-specific serum Ab responses. Mice received the oral combined vaccine alone (unshaded) or vaccine together with rmIL-12 complexed to liposomes by the oral route (striped, 1,000 ng/dose three times and shaded, 1,000 ng/dose six times). (A) Polyclonal and TT-specific serum IgE Abs were measured on day 14 by ELISA and PCA assays, respectively. (B) The distribution of TT-specific serum IgG subclasses were determined on day 21 by ELISA. (C) Serum TT-specific IgM, IgA, and IgG Abs were measured by ELISA on day 21. Results are expressed as the mean ± one SD from four different experiments (five mice per group).
Parenteral Versus Oral rmIL-12 Delivery Have Different Effects on Mucosal IgA Responses.
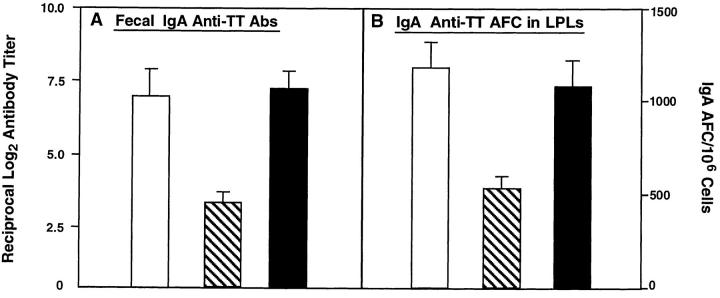
We next compared the effect of rmIL-12 given i.p. or orally on TT-specific mucosal IgA Ab responses to oral TT plus CT as adjuvant. Three oral doses of TT with CT at weekly intervals led to brisk fecal IgA Abs and elevated numbers of IgA anti-TT AFC in isolated lamina propria lymphocytes (LPLs) (Fig. 4, A and B). Intraperitoneal delivery of rmIL-12 resulted in an eightfold decrease in IgA Ab titers, which correlated with an ∼50% reduction in IgA AFC in isolated LPLs (Fig. 4, A and B). In sharp contrast to the significant inhibition of mucosal IgA anti-TT Ab responses induced by parenteral rmIL-12, neither IgA anti-TT Abs nor numbers of IgA AFC in isolated LPLs were affected by oral delivery of rmIL-12 (Fig. 4, A and B). Thus, both oral and parenteral rmIL-12 reduced IgE responses and induced shifts from IgG1 to IgG2a and IgG3 anti-TT Ab responses in serum, while only the oral route of delivery allowed mucosal IgA anti-TT Ab responses.
Figure 4.
The effect of oral and parenteral administration of rmIL-12 on mucosal IgA responses to an oral vaccine consisting of TT and CT as mucosal adjuvant. TT-specific S-IgA Ab titers in fecal extracts were measured on day 21 by ELISA. The frequency of TT-specific IgA AFCs in LPLs was determined on day 21 by ELISPOT. Mice received the oral vaccine alone (unshaded) or together with rmIL-12 by i.p. injection (striped, 100 ng/dose) or six oral administrations (shaded, 1,000 ng/dose). The results are expressed as the mean ± one SD from four different experiments (five mice per group).
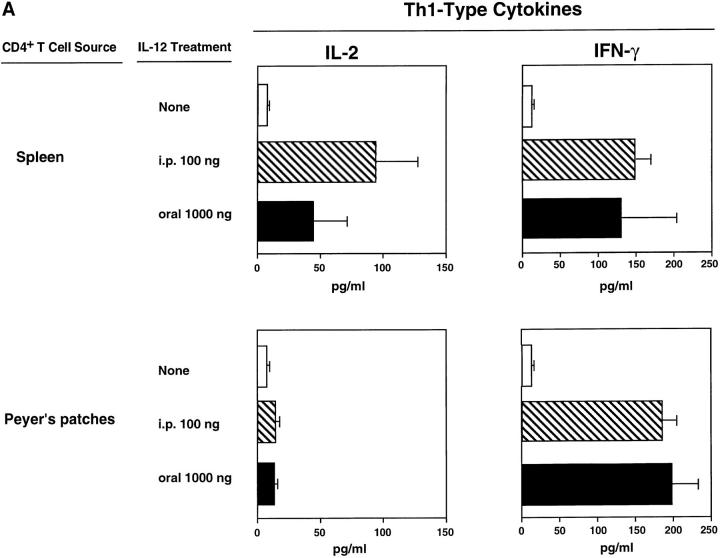
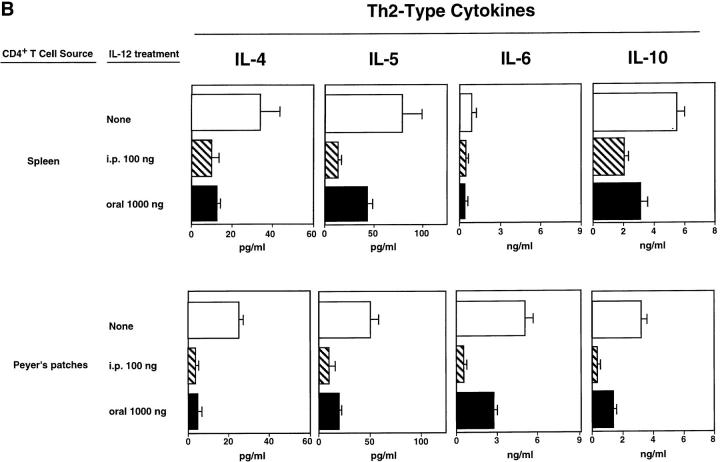
Cytokine Profiles of TT-specific CD4+ T Cells of Mice Given Combined Oral Vaccine and rmIL-12.
It was important to determine cytokine profiles exhibited by TT-specific CD4+ T cells from both peripheral (SP) and mucosal (PP) immune compartments since mice treated parenterally or orally with rmIL-12 exhibited comparable effects on serum Ab responses but different effects on mucosal IgA Ab responses. Culture supernatants from PP and SP CD4+ T cells restimulated with Ag in vitro revealed that T cells from mice treated either orally or parenterally with rmIL-12 secreted more IFN-γ than did T cells from mice orally immunized with TT and CT only (Fig. 5 A). The two different treatment routes both led to induction of comparable IFN-γ synthesis at the mucosal level (PP) and in SP. IL-12 treatment also increased IL-2 secretion by splenic T cells and to a lesser extent by PP T cells. Levels of IL-4, IL-5, IL-6, and IL-10 assessed in culture supernatants of TT-specific CD4+ T cells from SP and PP were significantly downregulated after parenteral administration of rmIL-12 (Fig. 5 B). Oral rmIL-12–liposomes downregulated IL-4 secretion but reduced IL-5, IL-6, and IL-10 secretion by SP and PP CD4+ T cells less markedly (Fig. 5 B).
Figure 5.
The induction of Th1-type (IL-2 and IFN-γ) and Th2-type (IL-4, IL-5, IL-6, and IL-10) cytokine secretion by CD4+ T cells isolated from mucosally immunized mice which had been given oral (shaded) or parenteral (striped) rmIL-12. SP and PP CD4+ T cells, were purified from mice receiving the combined oral vaccine only (unshaded) or combined vaccine together with rmIL-12 orally (six times, 1,000 ng/dose) or parenterally (15 times, 100 ng/dose) and were restimulated in vitro for 6 d in the presence of feeder cells and TT-coated beads. Th1type (A) and Th2-type (B) cytokine production in culture supernatants were analyzed by ELISA. Cytokine levels are representative of three separate experiments.
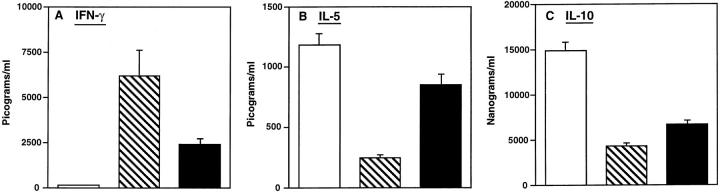
Effects of Oral and Parenteral IL-12 on Serum Cytokine Levels.
To directly compare the in vivo effects of oral or i.p. delivery of rmIL-12, we measured serum cytokine levels (IL-2, IFN-γ, IL-4, IL-5, IL-6, and IL-10) in mice that received the oral vaccine and oral or parenteral rmIL-12. While serum IL-2 and IFN-γ were undetectable in mice receiving the oral vaccine alone, i.p. delivery of 100 ng/ dose of rmIL-12 induced high levels of serum IFN-γ (Fig. 6 A). Interestingly, oral administration of rmIL-12–liposomes (6 doses; 1,000 ng/dose) also resulted in significant serum IFN-γ levels in mice orally immunized with TT plus CT as adjuvant; however, systemic rmIL-12 induced the highest levels of serum IFN-γ (Fig. 6 A). When serum IL-4, IL-5, IL-6 and IL-10 levels were assessed in the three different mouse groups, oral TT and CT as adjuvant induced high levels of IL-5 and IL-10 (Fig. 6, B and C), while both IL-4 and IL-6 were undetectable in all groups tested (data not shown). Serum IL-5 and IL-10 levels were significantly decreased in mice receiving parenteral IL-12 (Fig. 6 B), while mice fed oral IL-12–liposomes exhibited higher levels of both IL-5 and IL-10. These findings again show that the route of rmIL-12 delivery affects the pattern of cytokines secreted in vitro and in vivo.
Figure 6.
The induction of Th1-type (IFN-γ) and Th2-type (IL-5 and IL-10) cytokines in sera of mice receiving rmIL-12 and orally immunized with TT and CT as adjuvant. Mice received the combined oral vaccine (unshaded) together with rmIL-12 by the i.p. route (striped, 15 times, 100 ng/dose) or by the oral route (shaded six times, 1,000 ng/dose). Levels of IFN-γ, IL-5, and IL-10 in the serum were determined on day 21 by ELISA. The results are expressed as the mean ± one SD and are representative of three different experiments.
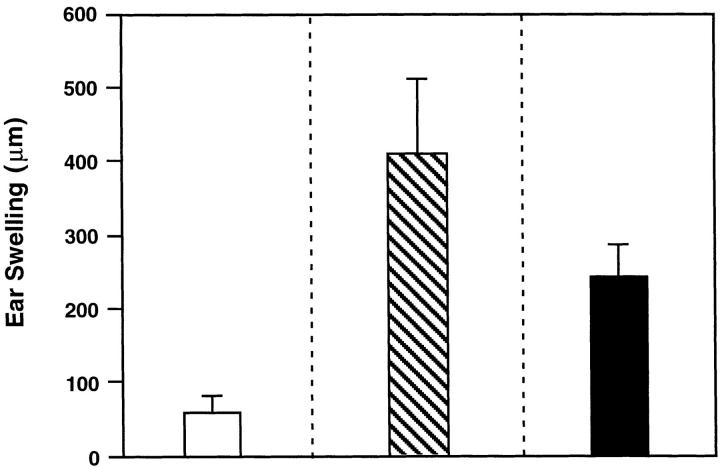
Both Oral and Parenteral IL-12 Induce DTH Responses.
To assess changes in CMI responses resulting from the shift in cytokine production, we measured DTH responses to TT in mice orally or parenterally treated with rmIL-12. Mice given the combined oral vaccine alone did not develop significant DTH responses, supporting the presence of a predominant Th2-type immune response (Fig. 7). In contrast, mice treated either orally or parenterally with rmIL-12 during the immunization protocol exhibited significantly higher DTH responses 24 h after TT challenge when compared with mice receiving the oral vaccine and adjuvant only.
Figure 7.
Induction of delayed-type hypersensitivity responses in rmIL-12–treated mice given the combined oral vaccine. Three groups of mice were assessed and included combined oral vaccine only (unshaded), those receiving combined oral vaccine and rmIL-12 by the i.p. (striped; 15 times 100 ng/dose) route, or mice given rmIL-12 by the oral route (shaded; six times 1,000 ng/dose). The results are expressed as the mean ± one SD of the difference in the ear swelling between the TT-injected and OVA-injected ear pinna and are representative of three separate experiments.
Kinetics of Serum IL-12 After Parenteral or Oral Administration of rmIL-12.
We also determined if the effect of oral IL-12 was due to the passage of the cytokine into the periphery or was due to a localized action in the GI tract. To this end, we measured serum IL-12 levels at different time points after oral (1,000 ng) or intraperitoneal (10, 100, and 1,000 ng) administration of rmIL-12. No detectable levels of IL-12 were found in sera before administration of this cytokine (Table 1); however, mice which received rmIL-12 by the i.p. route exhibited serum IL-12 by 30 min, and levels were maintained over a 2-h interval, and then gradually decreased over the next 48 h (Table 1). The serum concentration of IL-12 after intraperitoneal administration was dose dependent and increasing doses prolonged the survival of cytokine in the circulation. On the other hand, mice receiving rmIL-12 by the oral route exhibited negligible levels of serum IL-12 which peaked 2 h after administration and had markedly decreased by 24 h. These results suggested that the effects of oral IL-12 on the immune response to the vaccine did not result primarily from the passage of the cytokine into the circulation. Furthermore, oral administration of CT did not alter either the kinetics or the amount of serum IL-12 detected in mice receiving either oral or parenteral IL-12.
Table 1.
Kinetics of Serum IL-12 after Intraperitoneal or Oral Administration*
| Route of IL-12 administration | Dose of IL-12 (ng) | Serum IL-12 levels (ng/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 2 h | 24h | 48 h | ||||||||
| Intraperitoneal | 10 | <0.05 | 0.496 | 0.384 | <0.05 | <0.05 | ||||||
| Intraperitoneal | 100 | <0.05 | 10.4 | 8.8 | 3.9 | 0.42 | ||||||
| Intraperitoneal | 1000 | <0.05 | 93.7 | 86.5 | 56.3 | 6.1 | ||||||
| Oral | 1000 | <0.05 | <0.05 | 0.175 | 0.079 | <0.05 | ||||||
Groups of C57BL/6 mice received 10, 100, or 1,000 ng of rmIL-12 by the intraperitoneal or 1,000 ng of rmIL-12 by the oral route. Sera were collected at different intervals and IL-12 levels were measured by an IL-12-specific ELISA. Results are representative of three separate experiments (six mice per group).
Discussion
CT has been the most widely used mucosal adjuvant to induce mucosal S-IgA and parenteral immune responses to coadministered proteins in experimental animals (43) and our recent studies have shown that Th2-type responses to coadministered protein antigens are responsible for the isotype and subclass of Ab response when CT is used as adjuvant (31, 32). The major findings of the present study are that oral as well as parenteral administration of rmIL-12 redirects the CT-induced Th2 cell–mediated responses to IFN-γ producing Th1 cells with enhanced DTH and serum IgG2a and reduced IgE Ab responses. However, oral delivery of rmIL-12 complexed to liposomes, unlike parenteral rmIL-12 did not impair mucosal S-IgA Ab responses. The effects of IL-12 on induction of Th1-type and suppression of Th2-type responses are well documented (21, 42, 44, 45); however, no previous studies have addressed the effect of this cytokine on mucosal S-IgA responses or the possibility of its mucosal (e.g., oral) delivery. Therefore, we investigated the role of IL-12 in a model which purposely employed the delivery of this regulatory cytokine either by a parenteral (i.p.) or by the same (oral) route as antigen and CT adjuvant.
Increasing doses of rmIL-12 given i.p. progressively suppressed systemic IgE and IgG1 responses, further, this was accompanied by enhanced IgG2a Abs to TT given orally with the adjuvant CT. This finding added support to previous observations that the administration schedule (dose and frequency) of IL-12 during the period of antigen-induced immune stimulation is critical for induction of maximum effects in vivo (15, 42) and that IL-12 can inhibit both in vitro (27) and in vivo (28) production of IgE Abs. However, past work on the role of IL-12 on serum IgG subclasses yielded conflicting results (15, 28–30). In this regard, mice injected with anti-IgD Abs exhibited inhibition of IgG1, IgG2a and IgG3 subclasses after IL-12 treatment (15), while coadministration of IL-12 to mice immunized with haptenated-protein markedly inhibited IgG1, but was without effect on other IgG subclasses (29). In contrast, others have observed enhanced IgG2a Ab responses to hen egg white lysozyme (30) as well as IgG2a, IgG2b, and IgG3 Ab responses to several protein antigens (28). The finding that parenteral administration of rmIL-12 (100 ng/dose) during the initiation of immune responses to an oral vaccine significantly enhanced IgG2a and IgG3 and downregulated IgG1 Abs are consistent with the fact that IL-12 induces IFN-γ production which would support Th1-type cell differentiation with specific help for IgG2a and IgG3 Ab responses (23, 24, 26). Differences in antigen and polyclonal stimulation systems and in IL-12 administration schedules used in previous studies could explain the differences observed for IgG subclass responses.
The finding that oral delivery of rmIL-12 induced similar regulatory effects on serum IgE and IgG subclass Ab responses suggested that this cytokine could be efficiently delivered to mucosal inductive sites, i.e., the PP. To our knowledge this is the first evidence that IL-12 can resist enzymatic degradation in the GI tract and exert a regulatory effect on systemic immune responses to an oral vaccine. In support of this observation, previous reports have shown that hormones (46–49) and interferons (α and β) (50–52) retain biological activity after oral administration even in the absence of detectable levels of these molecules in serum. In addition, in a separate study we have observed that PP are the major site of rmIL-12–liposome uptake in the GI tract after oral delivery and that increased expression of Ly6A/E occurred on PP lymphocytes after oral administration of rIL-12–liposomes (Marinaro M., P.N. Boyaka, R.J. Jackson, F.D. Finkelman, H. Kiyono, E. Jirillo, and J.R. McGhee, manuscript in preparation). This was consistent with the findings of others which showed that oral delivery of liposomes to rats led to a preferential uptake by PP (53). Further, M cells in the epithelium overlying PP promoted the passage of liposomes to underlying lymphoid cells (54).
Of particular interest was the finding that the numbers of IgA antibody-forming cells (AFC) in the lamina propria of the small intestine as well as IgA Ab titers in fecal extracts were not affected by oral administration of IL-12 in contrast to parenterally administered cytokine which did reduce the levels of specific mucosal IgA responses. This finding suggested that differences occur in the ability of orally versus parenterally administered rmIL-12 to regulate mucosal IgA responses. It has been shown that mucosal IgA responses are optimally induced by Th2-type cell-derived cytokines, e.g., IL-4, IL-5, IL-6, and IL-10 (36, 37, 55–57). Furthermore, IL-4-deficient mice and IL-6-deficient mice exhibited impaired mucosal immunity (32, 35, 41, 57). Although oral delivery of rmIL-12–liposomes resulted in the induction of TT-specific CD4+ T cells producing IFN-γ and IL-2, we also found that significant production of select Th2-type cytokines still occurred. Thus, stimulated CD4+ T cells secreted IL-5, IL-6, and IL-10, while insignificant levels of IL-4 were present. On the other hand, parenteral administration of IL-12 profoundly suppressed Th2-type cytokines. Thus, we propose that maintenance of S-IgA Ab responses when IL-12 was delivered orally was due to the presence of TT-specific CD4+ T cells which produce select Th2-type cytokines. In support of this, serum IL-5 and IL-10 levels were also higher in mice receiving oral versus parenteral IL-12, confirming that these cytokines were maintained at higher levels in vivo. Thus, the S-IgA Ab response following oral treatment with IL-12– liposomes was most likely due to the presence of CD4+ T cells producing IL-5, IL-6, and IL-10, which provided effective help for this isotype. In this regard, IL-6 has been shown to be the most efficient terminal differentiation factor for murine IgA committed B cells to become IgA secreting plasma cells (56, 57) and both IL-5 and IL-10 have also been associated with IgA responses in experimental animals (55, 58) and in humans (59).
The lack of suppression of Th2-type cytokines both in vitro and in vivo induced by oral IL-12–liposomes concomitant with a normal mucosal IgA response are in contrast with the finding that the oral route of rmIL-12 delivery induced major effects on systemic Ab responses. To further investigate possible mechanisms whereby oral rmIL-12–liposomes induced systemic regulatory effects, we measured serum IL-12 levels after oral or parenteral administration. Oral delivery of IL-12 increased serum IL-12 to a much lesser extent than did parenteral delivery. This implies that the activity of oral IL-12 was more likely due to initial effects on lymphoid cells in the mucosal immune compartment rather than to passage of cytokine into the blood circulation. Since it has been shown that PP dendritic cells can stimulate CD4+ T cells with an increased propensity to support IgA synthesis (60, 61), it was possible that IFN-γ synthesis after oral rmIL-12–liposome administration may result in the activation of mucosal dendritic cells with subsequent induction of selected cytokines (e.g., IL-5 and IL-6) for support of IgA production.
Further support for direct effects of rmIL-12 in the PP or gut-associated lymphoreticular tissue (GALT) was the finding that IgE responses were essentially abrogated by oral administration of six doses of rmIL-12 liposomes. Furthermore, IgE responses were markedly down regulated after administration of only three oral doses of rmIL-12–liposomes. Indeed, it has been suggested that IgE-specific B cells arise in GALT, irregardless of the route of immunization (62). In that study, surface IgE-positive (sIgE+) B cells and hapten-specific IgE AFC were analyzed during an immune response to benzylpenicilloyl keyhole limpet hemocyanin administered by several routes (i.p., gavage, intravenous, subcutaneous, and intramuscular) with aluminum hydroxide adjuvant. It was found that commitment of murine B cells to express IgE (sIgE+) first occurred in PP with subsequent differentiation of sIgE+ B cells into IgE AFC (62). Our results suggest that uptake of IL-12 by PP results in the induction of CD4+ T cells producing IFN-γ which suppressed IgE secretion by downregulating IL-4 production (22–24, 26). The targeted delivery of IL-12 with induction of IFN-γ to the site where IgE responses arise (i.e., the PP) may explain the substantial downregulation of IgE Abs which occurs following oral administration of IL-12.
The finding that serum IFN-γ levels measured in mice receiving the oral vaccine and either oral or parenteral IL-12 differed in magnitude also merits discussion. We found that both splenic and PP CD4+ T cells from mice receiving either oral or parenteral rmIL-12 secreted comparable amounts of IFN-γ after antigenic stimulation in vitro. Thus, although the potential of antigen-specific T cells to produce IFN-γ was similar in both groups of mice, differences in serum IFN-γ levels were related to the different administration schedules. In this regard, we have shown that increasing doses of IL-12 delivered parenterally not only increased the total amount of this cytokine in the serum but also prolonged survival in the circulation. Thus, a longer persistence of IL-12 in the sera together with the different timing of administration could be responsible for the higher levels of IFN-γ detected in the sera of mice receiving parenteral IL-12 during the immunization protocol.
In summary, our study has provided the first evidence that IL-12 can be administered by the oral route for regulation of systemic immune responses to an oral vaccine and suggests that oral IL-12 can also exert immunomodulatory effects at the mucosal level. This latter finding has important implications for the targeted induction of immune responses to mucosal vaccines. Furthermore, the differential effect of oral versus parenteral IL-12 on S-IgA Ab responses also emphasizes the unique features of the mucosal and systemic immune compartments, respectively. Whether mucosal as well as parenteral immune responses to mucosal vaccines can be manipulated by delivering IL-12 or other cytokines through additional mucosal routes is currently under investigation.
Acknowledgments
We thank Dr. Maurice K. Gately for the generous supply of rmIL-12 and for critical review of this work, Dr. David H. Presky for monoclonal anti-IL-12 Abs, and Dr. Patricia J. Freda Pietrobon for the vaccine grade tetanus toxoid. We also thank Ms. Sheila D. Turner for preparation of the manuscript.
Footnotes
This work was supported by National Institutes of Health grants AI 18958, DK 44240, DE 04217, AI 35344, DE 09837, and NIAID-DMID contract N01 AI 65299.
1 Abbreviations used in this paper: AFC, antibody-forming cells; CMI, cellmediated immunity; CT, cholera toxin; ELISPOT, enzyme-linked spot; GALT, gut-associated lymphoreticular tissue; GI, gastrointestinal; LPL, lamina propria lymphocytes; PCA, passive cutaneous anaphylaxis; PP, Peyer's patches; S-IgA, secretory IgA; SP, spleen; TMB, 3,3′,5,5′-tetramethylbenzidine; TT, tetanus toxoid.
References
- 1.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, Familletti PC, Gately MK, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 4.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+T cells to enhance priming for interferon γ production and diminished interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste-Amezaga M, Chan SH, Kobayashi M, Young D, Nickberg E, et al. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+T cells through IL-12 produced by Listeria-induced macrophages. Science (Wash DC) 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 7.Mengel J, DarDé L, DarDé GM, Delgacto M, Nomizo A, Silva JS, Campos-Neto A. An activated murine B cell lymphoma line (A-20) produces a factor-like activity which is functionally related to human natural killer cell stimulatory factor. Eur J Immunol. 1992;22:3173–3178. doi: 10.1002/eji.1830221222. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida A, Koide Y, Uchijima M, Yoshida TO. IFN-γ induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–861. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]
- 9.Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 10.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of IFN-γ production by NK cell stimulatory factor (NKSF): characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manetti R, Parronchi P, Giudizi MG, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (NKSF/IL-12) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni M, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. Interleukin-12 induces stable priming for interferon-γ (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu CY, Demeure D, Kiniwa M, Gately M, Delespesse G. IL-12 induces the production of IFN-γ by neonatal human CD4 T cells. J Immunol. 1993;151:1938–1949. [PubMed] [Google Scholar]
- 14.Finkelman FD, Madden KB, Cheveer AW, Katona IM, Morris SC, Gately MK, Hubbard BR, Gause WC, Urban JF. Effects of Interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris SC, Madden KB, Adamovicz JJ, Gause WC, Hubbard BR, Gately MK, Finkelman FD. Effects of IL-12 on in vivocytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–1056. [PubMed] [Google Scholar]
- 16.Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, Herrmann SH, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnema JD, Rivlin KA, Ting AT, Schoon RA, Abraham RT, Leibson PJ. Cytokine-enhanced NK cell-mediated cytotoxicity. Positive modulatory effects of IL-2 and IL-12 on stimulus-dependent granule exocytosis. J Immunol. 1994;152:2098–2104. [PubMed] [Google Scholar]
- 18.Gately MK, Wolitzky AG, Quinn PM, Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 1992;143:127–142. doi: 10.1016/0008-8749(92)90011-d. [DOI] [PubMed] [Google Scholar]
- 19.Bloom ET, Horvath JA. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T cell activity. Implications for the age-related decline in CTL. J Immunol. 1994;152:4242–4254. [PubMed] [Google Scholar]
- 20.Clerici M, Lucey DR, Berzofsky JA, Pinto LA, Wynn TA, Blatt SP, Dolan MJ, Hendrix CW, Wolf SF, Shearer GM. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. . Science (Wash DC) 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 21.Nabors GS, Afonso LCC, Farrel JP, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania majorinfection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 24.Coffman RL, Varkila K, Scott P, Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. . Immunol Rev. 1991;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 25.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte IFN-γ production by suppressing natural killer cell stimulatory factor/interleukin-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckman MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivoimmunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 27.Kiniwa M, Gately M, Gubler U, Chizzonite R, Fargeas C, Delespesse G. Recombinant interleukin-12 suppresses the synthesis of immunoglobulin E by interleukin 4 stimulated human lymphocytes. J Clin Invest. 1992;90:262–266. doi: 10.1172/JCI115846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski FJ, Gately MK, Rude E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. . Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 29.McKnight AJ, Zimmer GJ, Fogelman I, Wolf SF, Abbas AK. Effects of IL-12 on helper T cell-dependent immune responses in vivo. . J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 30.Buchanan JM, Vogel LA, Van Cleave VH, Metzger DW. Interleukin 12 alters the isotype-restricted antibody response of mice to hen eggwhite lysozyme. Intern Immunol. 1995;7:1519–1528. doi: 10.1093/intimm/7.9.1519. [DOI] [PubMed] [Google Scholar]
- 31.Xu-Amano J, Jackson RJ, Staats HF, Fujihashi K, Kiyono H, Burrows PD, Elson CO, Pillai S, McGhee JR. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa-associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 33.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, McGhee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immunol. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153:647–657. [PubMed] [Google Scholar]
- 35.Vajdy M, Kosco-Vilbois MH, Kopf M, Köhler G, Lycke N. Impaired mucosal immune response in interleukin 4-targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 36.Van Cott, J.L., H.F. Staats, D.W. Pascual, M. Roberts, S.N. Chatfield, M. Yamamoto, M. Coste, P.B. Carter, H. Kiyono, and J.R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. <JNL>J. Immunol. 156:1504–1514. [PubMed]
- 37.Okahashi N, Yamamoto M, VanCott JL, Chatfield SN, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee JR. Oral immunization of IL-4 knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+Th2 cells producing IL-6 and IL-10 are associated with mucosal IgA responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu-Amano J, Jackson RJ, Fujihashi K, Kiyono H, Staats HF, McGhee JR. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994;12:903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 39.Wu J-Y, Riggin CH, Seals JR, Murphy MI, Newman MJ. In vitromeasurement of antigen-specific cellmediated immune responses using recombinant HIV-1 proteins adsorbed to latex microspheres. J Immunol Methods. 1991;143:1–9. doi: 10.1016/0022-1759(91)90266-i. [DOI] [PubMed] [Google Scholar]
- 40.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Holmes J, O'Hara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivoIgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 41.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (Lond) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 42.Sypek JP, Chung CL, Nayor SEH, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous Leishmaniasis: Interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staats HF, Jackson RJ, Marinaro M, Takahashi I, Kiyono H, McGhee JR. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 44.Yanagida T, Kato T, Igarashi O, Inoue T, Nariuchi H. Second signal activity of IL-12 on the proliferation and IL-2R expression of a T helper cell-1 clone. J Immunol. 1994;152:4919–4928. [PubMed] [Google Scholar]
- 45.Wynn TA, Cheveer AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by Schistosome infection. Nature (Lond) 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 46.Patel HM, Ryman BE. Oral administration of insulin by encapsulation within liposomes. FEBS Lett. 1976;62:60–63. doi: 10.1016/0014-5793(76)80016-6. [DOI] [PubMed] [Google Scholar]
- 47.Patel HM, Ryman BE. The gastrointestinal absorption of liposomally entrapped insulin in normal rats. Biochem Soc Trans. 1977;5:1054–1055. doi: 10.1042/bst0051054. [DOI] [PubMed] [Google Scholar]
- 48.Dapergolas G, Gregoriadis G. Hypoglycemic effect of liposome-entrapped insulin administered intragastrically into rats. Lancet. 1976;2:824–827. doi: 10.1016/s0140-6736(76)91209-5. [DOI] [PubMed] [Google Scholar]
- 49.Ryman BE, Jewkes RF, Jeyasingh K, Osbourne MP, Patel HM, Richardson VJ, Tattersall MHN, Tyrell DA. Potential applications of liposomes to therapy. Ann NY Acad Sci. 1978;308:281–307. doi: 10.1111/j.1749-6632.1978.tb22031.x. [DOI] [PubMed] [Google Scholar]
- 50.Nelson PA Y, Akselband, Dearborn SM, Al-Sabbagh A, Tian ZJ, Gonnella PA, Zamvil SS, Chen Y, Weiner HL. Effect of oral beta interferon on subsequent immune responsiveness. Ann NY Acad Sci. 1996;778:145–155. doi: 10.1111/j.1749-6632.1996.tb21123.x. [DOI] [PubMed] [Google Scholar]
- 51.Brod SA, Burns DK. Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology. 1994;4:1144–1148. doi: 10.1212/wnl.44.6.1144. [DOI] [PubMed] [Google Scholar]
- 52.Cummins JM, Tompkins MB, Olsen RG, Tompkins WA, Lewis MG. Oral use of human alpha interferon in cats. J Biol Response Modif. 1988;7:513–523. [PubMed] [Google Scholar]
- 53.Aramaki Y, Tomizawa H, Hara T, Yachi K, Kikuchi H, Tsuchiya S. Stability of liposomes in vitroand their uptake by rat Peyer's patches following oral administration. Pharm Res. 1993;10:1228–1231. doi: 10.1023/a:1018936806278. [DOI] [PubMed] [Google Scholar]
- 54.Childers NK, Denys FR, McGee NF, Michalek SM. Ultrastructural study of liposome uptake by M cells of rat Peyer's patch: an oral vaccine system for delivery of purified antigen. Reg Immunol. 1990;3:8–16. [PubMed] [Google Scholar]
- 55.Beagley KW, Eldridge JH, Kiyono H, Everson MP, Koopman WJ, Honjo T, McGhee JR. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J Immunol. 1988;141:2035–2042. [PubMed] [Google Scholar]
- 56.Beagley KW, Eldridge JH, Kiyono H, Everson MP, Koopman WJ, Hirano T, Kishimoto T, McGhee JR. Interleukins and IgA synthesis: Human and murine IL-6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramsay AJ, Husband AJ, Ramshaw IA, Boa S, Matthaei KI, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. . Science (Wash DC) 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 58.Murray PD, McKenzie DT, Swain SL, Kagnoff MF. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987;139:2669–2674. [PubMed] [Google Scholar]
- 59.Briére F, Bridon J-M, Chevet D, Souillet G, Bienvenu F, Guret C, Martinez-Valdez H, Banchereau J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J Clin Invest. 1994;94:97–104. doi: 10.1172/JCI117354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spalding DM, Williamson SI, Koopman WJ, McGhee JR. Preferential induction of polyclonal IgA secretion by murine Peyer's patch dendritic cell-T cell mixtures. J Exp Med. 1984;160:941–946. doi: 10.1084/jem.160.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrader CE, George A, Kerlin RL, Cebra JJ. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Intern Immunol. 1990;2:563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- 62.Auci DL, Chice SM, Heusser C, Athanassiades TJ, Durkin HG. Origin and fate of IgE-bearing lymphocytes. II. Gut-associated lymphoid tissue as sites of first appearance of IgE-bearing B lymphocytes and hapten-specific IgE antibody-forming cells in mice immunized with benzylpenicilloyl-keyhole limpet hemocyanin by various routes: relation to asialo GM1 ganglioside+cells and IgE/CD23 immune complexes. J Immunol. 1992;149:2241–2248. [PubMed] [Google Scholar]