Abstract
The granule exocytosis cytotoxicity pathway is the major molecular mechanism for cytotoxic T lymphocyte (CTL) and natural killer (NK) cytotoxicity, but the question of how these cytotoxic lymphocytes avoid self-destruction after secreting perforin has remained unresolved. We show that CTL and NK cells die within a few hours if they are triggered to degranulate in the presence of nontoxic thiol cathepsin protease inhibitors. The potent activity of the impermeant, highly cathepsin B–specific membrane inhibitors CA074 and NS-196 strongly implicates extracellular cathepsin B. CTL suicide in the presence of cathepsin inhibitors requires the granule exocytosis cytotoxicity pathway, as it is normal with CTLs from gld mice, but does not occur in CTLs from perforin knockout mice. Flow cytometry shows that CTLs express low to undetectable levels of cathepsin B on their surface before degranulation, with a substantial rapid increase after T cell receptor triggering. Surface cathepsin B eluted from live CTL after degranulation by calcium chelation is the single chain processed form of active cathepsin B. Degranulated CTLs are surface biotinylated by the cathepsin B–specific affinity reagent NS-196, which exclusively labels immunoreactive cathepsin B. These experiments support a model in which granule-derived surface cathepsin B provides self-protection for degranulating cytotoxic lymphocytes.
Keywords: CTL, cytotoxicity, protease, granule, exocytosis
Introduction
In vitro studies of cytotoxic lymphocytes have demonstrated that they utilize two molecular pathways to kill target cells (1). In vivo studies have shown that both of these pathways play major roles in host defense against infections and tumors, T cell homeostasis, and prevention of autoimmunity (2–5). In one pathway, Fas on target cells is cross-linked by effector cell Fas ligand (FasL),* triggering a relatively well-studied death pathway involving caspase activation. The second, and normally dominant, pathway involves a polarized secretion of preformed perforin and granzymes by effector cell granule exocytosis, leading to rapid target caspase activation as well as a caspase-independent death pathway (6).
Although the basic granule exocytosis mechanism was outlined in the 1980s, an interesting but unresolved issue arose from early findings that CTLs themselves do not normally die while killing target cells, as shown by the demonstration that one CTL can kill multiple targets within a few hours (7). Thus, if perforin and granzymes are secreted in high enough local concentrations to kill target cells, why are the cytotoxic lymphocytes themselves not killed? This issue has been addressed by a number of laboratories without a completely satisfactory explanation (8). There is evidence that cytotoxic lymphocytes may have an inherent resistance to the cytotoxic mediators, particularly perforin. In support of this idea, in vitro–cloned CTLs bearing surface antigens recognized by other CTLs are generally resistant to lytic attack relative to tumor targets (9–11). Cloned CTLs were likewise shown to be relatively resistant to lysis induced by CTL granule extracts as well as purified perforin (11–14). The molecular basis for CTL resistance to perforin remains undefined. One study reported that perforin binding to CTL membranes was defective relative to tumor membranes (15), whereas another showed equivalent perforin binding but provided evidence for a different conformation of membrane-bound perforin on CTLs (14).
Although these studies argue for some kind of protection mechanism for CTLs against their own lethal damage, they also show clear examples of cloned CTLs being readily lysed by other CTLs. Thus, in vivo–derived CTLs and normal lymphocytes were reported to be equally susceptible to CTL attack (16). Cloned CTL specific for a defined peptide restricted by self–MHC class I were shown to kill themselves in a fratricidal process in the presence of the specific peptide (17–19). Fratricide via the granule exocytosis pathway has been shown to limit viral antigen expression in short-term cultured CD8+ T cells from HTLV-1–infected patients (20). Thus, CTLs can clearly sometimes kill other CTLs, and their relative resistance to granule mediators in vitro does not provide a satisfactory explanation for their self-protection during target death.
In our approach to the issue of cytotoxic lymphocyte self-protection, we reasoned that protection is most needed locally at the effector membrane at the time of granule exocytosis and consequently, a granule component was an excellent candidate. Because it is clear that cytotoxic lymphocyte secretory granules contain a full complement of lysosomal enzymes (21), it was interesting to note that the lysosomal thiol cathepsin endoproteases, particularly cathepsin B, can associate with some tumor cell surfaces where they maintain proteolytic activity in the extracellular environment (22, 23). In the process of forming membrane channels, perforin goes through an intermediate membrane-associated stage in which it is highly susceptible to proteolysis before polymerization into a more resistant pore-forming complex (14, 24–26). Because this membrane-associated perforin should be particularly vulnerable to a protease associated with the same membrane, we hypothesized that a membrane-associated granule cathepsin, expressed locally on the effector cell surface after exocytosis, could provide efficient self-protection to effector cells. This study shows that in the presence of nonmembrane permeable cathepsin B inhibitors, cytotoxic lymphocytes undergo a rapid perforin-dependent death when degranulation is triggered, and TCR cross-linking leads to the rapid appearance of active, surface-bound cathepsin B on CTLs. These results provide evidence that proteolysis by surface cathepsin B provides cytotoxic lymphocyte self-protection.
Materials and Methods
Antibodies and Reagents.
Purified hamster anti–mouse CD3 (2C11), hamster IgG, anti–mouse CD45, anti–CD4-FITC, anti–CD8-PE, anti–human CD28, avidin-FITC anti–mouse IgG-FITC, anti–mouse Fas (Jo-2), and recombinant IL-7 were purchased from BD Biosciences. Purified mouse anti–human CD3 (UCHT1) was provided by Julie Titus (NCI, Bethesda, MD). Mouse anti–human cathepsin B antibody was purchased from Oncogene Research Products. Rat anti–mouse perforin was purchased from Kamiya Biomedical Company and goat anti–rat IgG-horseradish peroxidase (HRP) was purchased from Southern Biotechnology Associates, Inc. Mouse anti–human cathepsin L was purchased from Transduction Laboratories. Recombinant human IL-2 was purchased from Roche Diagnostics. The human liver cathepsin B, protease inhibitors ZLLY-DMK, ZFA-FMK, GF-DMK, ZFA(β)-FMK, and Bi-FA-FMK were purchased from Enzyme System Products, dissolved as stock solutions of 50 mM in DMSO and stored at −70°C. CA074 and CA074Me were obtained from the Peptide Institute. CLIK 148 was provided by Giovanni Tonon (NCI, Bethesda, MD). Human cystatin C was purchased from Research Diagnostics. Concanamycin A was obtained from Alexis Biochemicals Corp. EDTA, EGTA, and dextran were purchased from Sigma-Aldrich. Hoechst 33342, propidium iodide, and Streptavidin-HRP were purchased from Molecular Probes. Protein G beads, Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane, and Hyperfilm ECL were obtained from Amersham Biosciences.
Lymphocytes.
C57Bl/6J (B6), BALB/c, and perforin-deficient C57BL/6-Pfptm1Sdz mice were obtained from The Jackson Laboratory. FasL-defective B6Smn.C3H-Faslgld were provided by Paul Chrobak (NCI, Bethesda, MD). The murine lymphomas EL4 and L1210 were maintained in RPMI 1640 supplemented with 10% FCS, 100 IU penicillin, and 10 μg/ml streptomycin. Mouse CTLs were prepared by harvesting spleen cells from B6 or mutant mice, lysing with ACK (Biofluids), and culturing in 24-well plates at 2 × 106 cells/well in RPMI 1640 containing 10% FCS and 25 μM 2-ME with γ-irradiated BALB/c cells (106 cells/well). After 5 d at 37°C in a 5% C02 incubator, viable cells were isolated by Lympholyte (Cedarlane Laboratories) and cultured for an additional 48 h in the same medium containing 0.8 ng/ml rIL-7 and 25 U/ml rIL-2. CD8+ and CD4+ T cells were purified by positive magnetic bead selection using CD8 and CD4 microbeads and the VarioMac cell sorting system (Miltenyi Biotec). For CTL activity assays, BALB/c spleen cells from 2 × 107 EL-4 cells primed intraperitoneally 10–14 d before harvest were cultured for 5 d as described above, with C57Bl/6J spleen cells as stimulators.
Human lymphocytes were used for most of the experiments in this study. PBMC were purified by Ficoll-Paque (Amersham Biosciences) from buffy coat or lymphocyte apheresis preparations obtained from normal healthy donors. For most experiments, PBMC were stimulated with 2.5 μg/ml PHA (Sigma-Aldrich) or irradiated PBMC from HLA-mismatched donors at a 2:1 responder/stimulator ratio, followed by culture for 7–10 d in culture medium containing RPMI 1640 supplemented with 10% FCS, 20 U/ml rIL-2, antibiotics, and nonessential amino acids. For purification of CD4+ and CD8+ subsets, blasts were positively selected magnetically with CD4 or CD8 microbeads (Miltenyi Biotec), resulting in >90% purity by flow cytometry. After purification, the blasts were maintained in culture medium with 20 U/ml rIL-2 for at least 3 d before the experiments were performed. Resting peripheral CD4+ and CD8+ T cells were purified from PBMC by negative selection using the antibody cocktails in CD4+ and CD8+ isolation kits (Miltenyi Biotec), again with >90% purity by flow cytometry.
CD8+ CTL clone RS-56, recognizing HTLV-1 tax peptide in the context of HLA-A2, was provided by William Biddison (National Institute of Neurological Disorders and Stroke, Bethesda, MD). Human NK cells purified from human blood were activated for 6 d by culture with irradiated RPMI 8866 cells (27), and purified using the Vario-MACS NK isolation kit (Miltenyi Biotec) provided by Alessandra Mazzoni and David Segal, NCI, Bethesda, MD).
In Vitro Suicide Experiments.
Flat-bottom 96-well plates were coated with 10 μg/ml anti-CD3 or isotype-matched IgG in bicarbonate buffer, pH 8.5, overnight at 4°C, and then washed with medium. Standard conditions for suicide experiments used 200 μl activated CD8+ T blasts of 106 cells/ml complete medium with and without cathepsin inhibitors incubated for 4 h in a CO2 incubator. The plates were then spun and the supernatant was removed, followed by staining with 5 μg/ml propidium iodide or Hoechst 33342 in HBSS for 10 min at 37°C before fluorescence microscopy.
Flow Cytometry.
CD4/CD8 phenotyping was performed by incubating 1 μg appropriate antibody with 106 cells in 100 μl, followed by washing and flow cytometry with a FACScan™ (BD Biosciences). For cathepsin B and cathepsin L staining, CTLs were harvested and incubated with 10 μg/ml anticathepsin mAbs or control IgG for 1 h on ice. After washing, cells were incubated with 5 μg/ml of FITC-anti–mouse IgG for 30 min and samples were analyzed by flow cytometry.
Cytotoxicity Assays.
51Cr-labeled B6 anti-BALB/c MLR cells were mixed with varying numbers of L1210 cells in the presence or absence of 10 μM CA074 for 4 h at 37°C in 5% CO2 in 96-well plates (see Fig. 3, A and B) . After 4 h, 100 μl supernatant were harvested for counting. EL-4 target cells were labeled with Na2 51Cr2O7 (200 μCi in 0.5 ml HBSS plus 10% FCS) for 45 min at 37°C (see Fig. 3, C–E). After washing twice with complete medium, 104 target cells were incubated with varying numbers of BALB/c anti-B6 MLR cells in the presence or absence of 10 μM CA074 for 4 h at 37°C in 5% CO2 in 96-well plates. After 4 h, 125 μl supernatant were harvested for counting and replaced with 125 μl medium for ∼12 h of additional incubation. As a toxicity control for CA074, CTLs were mixed with 10 μM CA074 for 16 h before carrying out cytotoxicity assays with 51Cr-labeled EL-4 cells.
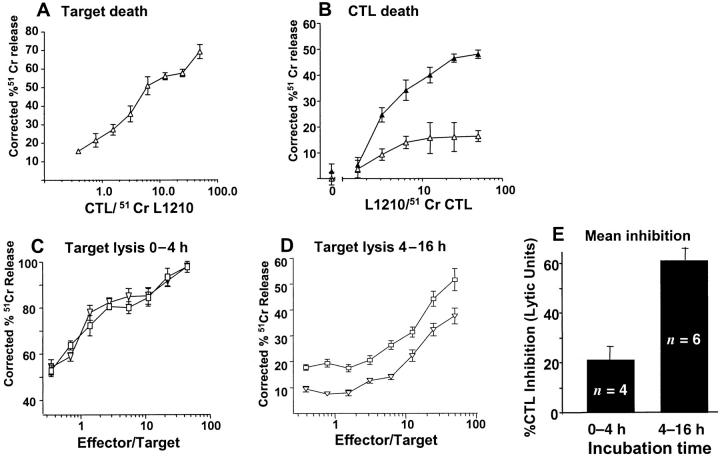
Figure 3.
CTL suicide during target cell lysis. (A) Lysis of H-2d–bearing L1210 tumor cells by H-2d–reactive CTL in a 4-h assay. (B) Lysis of 51Cr-labeled CTL (104/well) used in A when incubated for 4 h with L1210 target cells in the presence of 10 μM CA074. ▴, CA074; ▵, no inhibitor. (C–D) H-2b–reactive CTL were mixed with 51Cr-labeled EL4 cells in the presence or absence of 10 μM CA074. After 4 h, 125 μl supernatant were harvested for counting (C) and replaced with 125 μl of medium for ∼12 h of additional incubation. D shows the second harvest. As a toxicity control for CA074 in D, the CTLs in C were incubated with 10 μM CA074 for 16 h before the cytotoxicity assay with 51Cr-labeled EL-4 cells. □, no inhibitor; ▿, 10 μM CA074. (E) Mean inhibition of CTL lytic activity from multiple experiments like those shown in C and D, calculated by lytic units (horizontal shift between cytotoxicity dose response curves).
Surface Cathepsin B Characterization.
Human RS-56 CTLs were incubated on wells coated with anti-CD3 or control IgG for 2 h at 37°C. After washing with cold HBSS, they were incubated in HBSS with or without 2 mM Mg-EGTA or Ca-EDTA for 10 min at 37°C. Supernatants were harvested, concentrated with a Savant SpeedVac (Savant Instruments), dissolved, dialyzed, run on a 12% reduced SDS gel, and blotted onto nitrocellulose membranes. These were then incubated with 10 μg/ml anticathepsin B mAb, probed with HRP-anti–mouse IgG, and developed using ECL (Amersham Biosciences). For detection of surface cathepsin B, CTLs were cultured on plate-bound anti-CD3 for 2 h at 37°C. Cells were washed with PBS and incubated with 0.5 mM EDTA in PBS for 10 min at 37°C. Cells were spun down, washed with PBS, and stained with anti-cathepsin B mAb followed by FITC-anti–mouse IgG.
To detect the active form of cathepsin B, CTL clone RS-56 was cultured on plate-bound anti-CD3 or isotype Ig for 2 h at 37°C and stained with 1 μM membrane-impermeant biotinylated cathepsin B–specific epoxysuccinyl peptide affinity label NS196 for 45 min at 37°C. Cells were washed twice with HBSS followed by staining with 10 μg/ml streptavidin-FITC for 45 min on ice. Samples were analyzed by flow cytometry.
To show that cathepsin B–specific affinity label NS-196 reacts with one 32-kD protein corresponding to cathepsin B single chain form, human CTL clone RS56 was stimulated with plate-bound anti-CD3 or isotype Ig for 2 h at 37°C. Cells were harvested, washed twice with HBSS, and counted for cell number. Samples with equal cell numbers were pulsed with or without 0.1 μM NS196 for 1 h at 37°C and then washed twice with HBSS. Cells were lysed with 200 μl lysis buffer (0.5% Triton X-100 in HBSS) for 30 min at room temperature. Supernatants were harvested by centrifugation and biotinylated cathepsin B was immunodepleted with 10 μg/ml rabbit polyclonal antihuman cathepsin B or rabbit Ig plus protein G beads. Supernatants were harvested, concentrated with a Savant SpeedVac, and dissolved in nonreducing SDS sample buffer. Samples were run on 12% SDS gel and blotted onto a nitrocellulose membrane. For labeling whole CTLs, cells were lysed with lysis buffer and incubated with 0.1 μM NS196 for 1 h at 37°C. The lysate proteins were precipitated with 3.3% TCA, washed twice with acetone, and dissolved in HBSS. Samples were mixed with nonreducing SDS sample buffer and run on 12% SDS gel and then blotted onto a nitrocellulose membrane. The membrane was blocked with 3% nonfat dry milk in TBST (Tris-buffered saline with 0.1% Tween 20) overnight at 4°C. The membrane was incubated with Streptavidin-HRP (0.16 μg/ml in TBST) for 30 min at room temperature and then washed three times with TBST and developed using ECL (Amersham Biosciences).
Perforin Cleavage by Purified Cathepsin B.
B6 anti-BALB/c MLR cells (25 × 106/ml in PBS plus 0.5 M NaCl) were freeze-thawed six times and the supernatant was harvested by centrifugation. Aliquots of 1.2 × 105 cell equivalents were treated with 258 ng/ml purified human liver cathepsin B in 50 mM phosphate buffer, pH 6.0, for various time periods, and mixed with SDS sample buffer. Reduced 12% SDS gels were run and blotted onto a nitrocellulose membrane. After blocking with 3% nonfat dry milk in TBST overnight at 4°C, the membranes were incubated with 0.5 μg/ml antiperforin mAb followed by HRP-anti–rat IgG. The blots were developed using ECL (Amersham Biosciences).
Results
CD8+ Blasts Undergo Rapid Suicide via Granule Exocytosis When Cultured on Anti-CD3 in the Presence of Cathepsin Inhibitors.
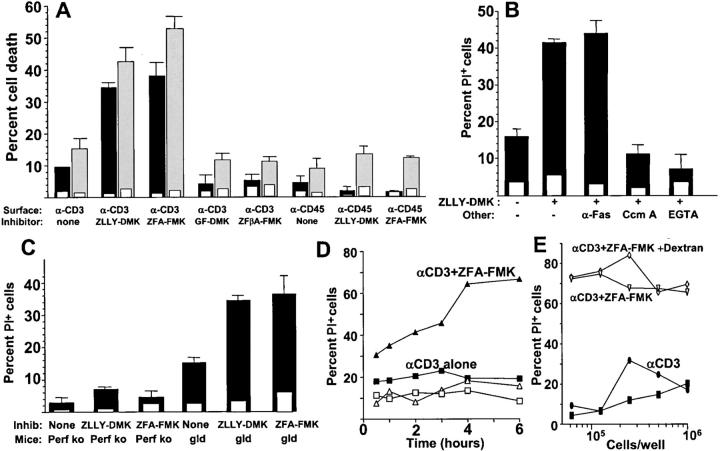
If thiol cathepsin endoproteases participate in cytotoxic lymphocyte self-protection, inhibiting them during degranulation would be expected to induce effector cell suicide. The experiment in Fig. 1 A shows that surface-bound anti-CD3 induces death within 4 h of ∼10–15% of murine CD8+ T cells from a primary MLR culture. However, in the presence of two irreversible inhibitors of thiol cathepsin endoproteases, ZFA-FMK (28) and ZLLY-DMK (29), 35–55% of these cells died, with similar numbers obtained by measuring apoptotic nuclear morphology or lytic membrane damage. These peptide-based cathepsin inhibitors displayed no toxicity in the absence of CD3 cross-linking (inset bars). As specificity controls for the reactivity of these inhibitors, we used the β amino acid–containing homologue ZF(β)A-FMK and the cathepsin C inhibitor GF-DMK, which contain identical reactive groups but do not inactivate cathepsin endoproteases. These control compounds failed to promote CTL death when CD3 was cross-linked (Fig. 1 A). As additional controls, CTLs were incubated on wells coated with anti-CD45, which induces attachment without degranulation. Cathepsin inhibitors did not promote death under these conditions (Fig. 1 A).
Figure 1.
Alloactivated mouse CD8+ T cell death induced by anti-CD3 in the presence of cathepsin inhibitors. (A) CD8+ T cells from B6 mice activated by a primary MLR culture were incubated for 4 h with and without the indicated cathepsin inhibitors on wells coated with anti-CD3 or anti-CD45 (filled bars) or control hamster or mouse IgG (inset open bars). Cell death was measured microscopically by nuclear propidium iodide staining (measuring membrane integrity, solid bars) or apoptotic nuclear morphology using Hoechst 33342 (gray bars). The cathepsin inhibitors ZLLY-DMK, ZFA-FMK, and controls GF-DMK and ZFβA-FMK, were used at final concentrations of 50 μM. Error bars show SEM of triplicate samples. (B) Murine-alloactivated CD8+ MLR blasts were incubated with 50 μM cathepsin inhibitor ZLLY-DMK in the presence of 10 μg/ml IgG anti-Fas antibody, 1μM concanamycin A (Ccm A), or 2.5 mM EGTA. Cell death was assessed after 4 h by propidium iodide staining in anti-CD3– (solid bars) or control hamster IgG–coated wells (inset open bars). (C) Alloactivated CD8+ MLR blasts from perforin knockout or gld mice were incubated 4 h with or without 50 μM cathepsin inhibitor ZLLY-DMK. Cell death by propidium iodide was measured in wells coated with anti-CD3 (solid bars) or control IgG (open inset bars). (D) Kinetics of death of human CTL clone RS-56 induced by plate-bound anti-CD3 in the presence of 50 μM cathepsin inhibitor ZFA-FMK. □, no anti-CD3 or ZFA-FMK. (E) Density dependence of CTL death as in D, measured at 4 h. ▿, CTL incubated with 50 μM ZFA-FMK on plate-bound anti-CD3; ⋄, same as ▿ with 2 × 106 kD dextran added to a final concentration of 5% to increase medium viscosity to block fratricidal killing; ▪, anti-CD3 with no ZFA-FMK; •, ZFA-FMK with no anti-CD3.
This TCR-induced CTL death in the presence of cathepsin inhibitors could be interpreted as a form of activated cell death, which in T cells has been strongly associated with the FasL/Fas death pathway. Although such activation-induced cell death is typically observed only 12–16 h after TCR ligation, several approaches were taken to address the relative roles of the FasL–Fas and granule exocytosis pathways in this T cell death. An IgG anti-Fas mAb that blocks the FasL–Fas death pathway (6) had no effect on CTL death induced by anti-CD3 in the presence of the cathepsin inhibitor ZLLY-DMK (Fig. 1 B). In contrast, clear inhibition was observed in the presence of the granule proton pump inhibitor concanamycin A, which blocks the granule exocytosis cytotoxicity death pathway (30), and in the presence of EGTA, which blocks CTL degranulation (31) and perforin function.
The role of the granule exocytosis pathway in this death was additionally confirmed using T cells from perforin knockout and gld (FasL-mutant) mice. As shown in Fig. 1 C, activated CD8+ T cells from the former did not show significant death when incubated on anti-CD3–coated wells in the presence of ZLLY-DMK or ZFA-FMK, whereas the latter showed death induction similar to control mice.
Fig. 1 D illustrates the kinetics of death in the cloned human CTL line RS-56 induced by anti-CD3 in the presence of cathepsin inhibitor ZFA-FMK, which increases between 1 and 4 h, paralleling the secretion of granule enzymes under these conditions (32). Similar results were obtained with mouse CTL (unpublished data). To probe whether this death is cell autonomous (suicidal) or involves an interaction between two cells (fratricidal), we used a previous approach for activation-induced cell death via the FasL–Fas pathway (33). Unlike the latter case of fratricide, the activation-induced death of CTL in the presence of cathepsin inhibitor was not dependent on cell concentration, nor was it inhibited by viscous dextran solutions that inhibit standard CTL killing assays (Fig. 1 E). Thus, in the presence of cathepsin inhibitors, anti-CD3 induces a cell-autonomous suicidal death, as expected for a failure in CTL self-protection.
Rapid Activation-induced Death of Cytotoxic Effector Cells Occurs in the Presence of Membrane-impermeant, Cathepsin B–specific Inhibitors.
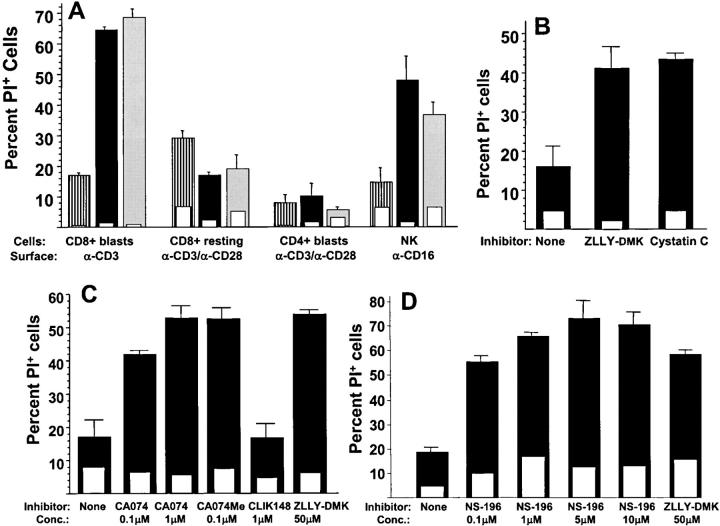
The experiments described above indicate that activated mouse and human CD8+ T cells die when induced to degranulate in the presence of cathepsin inhibitors. To define the cell types that are capable of undergoing this death, purified subpopulations of human blood lymphocytes were cultured under activating conditions to induce degranulation. As shown in Fig. 2 A, human CD8+ T cell blasts, highly active as cytotoxic effector cells, died within 4 h when incubated on anti-CD3–coated wells in the presence of cathepsin inhibitors. On the other hand, resting human CD8+ T cells and CD4+ blasts did not die when incubated on wells coated with both anti-CD3 and anti-CD28. Highly cytolytic CD56+ cultured human NK cells showed a pronounced death when triggered to degranulate with immobilized anti-CD16 (34) in the presence of cathepsin inhibitors. As with CTLs, these inhibitors showed no evidence of toxicity in the absence of the degranulating stimulus. Thus, the lymphocyte death response after a degranulation stimulus in the presence of cathepsin inhibitors reflects their cytotoxic potential via the granule exocytosis pathway.
Figure 2.
Activation-induced suicide of cytotoxic effectors in the presence of membrane-impermeant cathepsin B inhibitors. (A) Human CD8+ T cell blasts, resting blood CD8+ cells, CD4+ T cell blasts, and NK cells were incubated for 4 h with and without 50 μM cathepsin inhibitors ZLLY-DMK or ZFA-FMK. Cell death was assessed by propidium iodide from wells coated with control IgG (open inset bars) or the indicated degranulating stimuli (open bars). Striped bars, no inhibitor; solid bars, ZLLY-DMK; gray bars, ZFA-FMK.(B) Alloactivated mouse CD8+ T cell blasts were incubated for 4 h on anti-CD3–coated wells (solid bars) or control IgG (open inset bars) with or without 10 μM cystatin C or 50 μM ZLLY-DMK, and cell death was assessed by propidium iodide. (C) Alloactivated CD8+ BALB/c T cells were incubated on wells coated with anti-CD3 (solid bars) or control IgG (open inset bars) for 4 h in presence or absence of the indicated cathepsin inhibitors and stained with propidium iodide. (D) Same conditions as in C, comparing NS-196 and ZLLY-DMK.
These cathepsin inhibitors are small hydrophobic peptides that can readily permeate cells and inactivate intracellular thiol proteases including cathepsins B, L, and H, as well as calpain. However, cathepsin protection of degranulating cytotoxic lymphocytes against perforin attack is expected to occur in an extracellular location. Fig. 2 B shows that the membrane-impermeant 13-kD protein cathepsin inhibitor cystatin C facilitates activation-induced CD8+ T cell suicide as well as ZLLY-DMK, arguing that cathepsin inhibition at an extracellular location is sufficient for this death.
Although cathepsins B, L, and H generally have a similar proteolytic cleavage specificity, detailed structural studies of cathepsins B and L have recently allowed the design of a new generation of highly selective inhibitors (35). These compounds are based on the general thiol protease inhibitor E64 with the covalently reactive epoxysuccinyl moiety linked to peptides that bind selectively to the active sites of these proteases. The cathepsin B–specific inhibitor CA074 is available as a cell-permeant methyl ester (CA074Me) as well as the membrane-impermeant anionic form (CA074; reference 36). As shown Fig. 2 C, both CA074 and CA074Me facilitated CD8+ T cell death after TCR cross-linking. Concentrations of <1 μM were sufficient for these cathepsin B inhibitors, whereas concentrations of >25 μM were required in the case of ZLLY-DMK and ZFA-FMK (unpublished data). In contrast to the potent effect of the cathepsin B–specific inhibitor, the cathepsin L–specific homologous epoxysuccinyl-based inhibitor CLIK 148 failed to induce suicide in degranulating T cells at micromolar concentrations (Fig. 2 C). Thus, these functional experiments indicate that surface cathepsin B is required for activation-induced suicide of cytotoxic effector cells.
NS-196 is another epoxysuccinyl-based cathepsin B–specific inhibitor, which was synthesized as a biotinylated peptide affinity reagent and shown to be impermeable to cell membranes (37). Similar to CA074, this reagent sensitized CTLs to activation-induced suicide at submicromolar concentrations (Fig. 2 D). Thus, two distinct, highly cathepsin B–specific membrane-impermeable inhibitors potently showed this ability, implicating extracellular cathepsin B in self-protection.
CTLs Die upon Target Cell Recognition in the Presence of Surface Cathepsin B Inhibitor CA074.
To test whether surface cathepsin B inhibition causes CTLs to die upon target cell recognition, we performed an experiment in which either CTLs or target cells were labeled with 51Cr. Allo-specific mouse CTLs that potently killed L1210 tumor targets in a 4-h assay (Fig. 3 A) were killed by incubation with these target cells for 4 h in the presence of CA074 (Fig. 3 B). Thus, triggering the CTL TCR with antibodies or antigen gives equivalent suicide when surface cathepsin B is inhibited.
Previous experiments using the cathepsin inhibitor ZFA-FMK as a control for caspase inhibitors showed that it failed to inhibit CTL-mediated target lysis or apoptotic injury (6, 38). When CA074 was tested for its ability to block CTL-mediated target death in a standard 4-h assay, little or no inhibition was found at concentrations up to 50 μM (Fig. 3 C). If most of the target death in this assay resulted from initial encounters of CTL with their targets, this antigen-triggered suicide would not be expected to influence levels of target lysis. However, when we later examined target lysis in the same wells by replacing the supernatants harvested at 4 h with fresh medium and reharvesting after an additional overnight incubation, CA074 was seen to have a clear inhibitory effect on this delayed target lysis (Fig. 3 D). When calculated by lytic units (horizontal shift in the curves), 10 μM CA074 inhibits ∼75% of this late cytotoxicity. To control the possibility that CA074 exerts a delayed inhibition of CTL function, the inhibitor was preincubated with CTL for 16 h before adding target cells. Averaging several experiments of this type shows that CA074 inhibits delayed target death at ∼60%, compared with ∼20% inhibition during a 4-h assay. Thus, surface cathepsin B inhibition blocks CTL function, as expected for an agent that defeats self-protection.
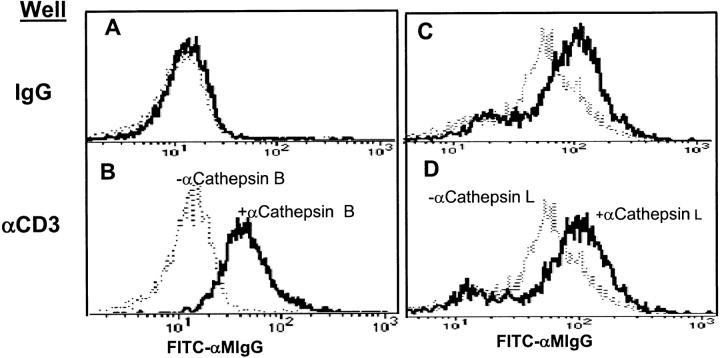
Cathepsin B Is Rapidly Expressed on the CTL Surface upon TCR Stimulation.
Using flow cytometry, cells from a human CD8+ CTL clone were examined for surface expression of cathepsin B after a degranulation stimulus. Fig. 4 shows that nonstimulated CTL did not express significant levels of surface cathepsin B, although it was minimally detectable in some experiments. However, within 2 h after exposure to plate-bound anti-CD3, cathepsin B was clearly increased with a homogenous peak of positive cells. In contrast, cathepsin L showed weak surface expression on unstimulated CTL, which was not increased after CD3 cross-linking.
Figure 4.
TCR cross-linking rapidly increases CTL surface expression of cathepsin B, but not cathepsin L. (B and D) CD8+ cloned human CTL were incubated on surface-bound anti-CD3, or (A and C) isotype IgG for 2 h at 37°C. (A and B) Cells were then stained with anti-cathepsin B or (C and D) with anti-cathepsin L antibody followed by FITC-anti–mouse IgG (heavy lines). Dashed lines show staining by normal IgG controls.
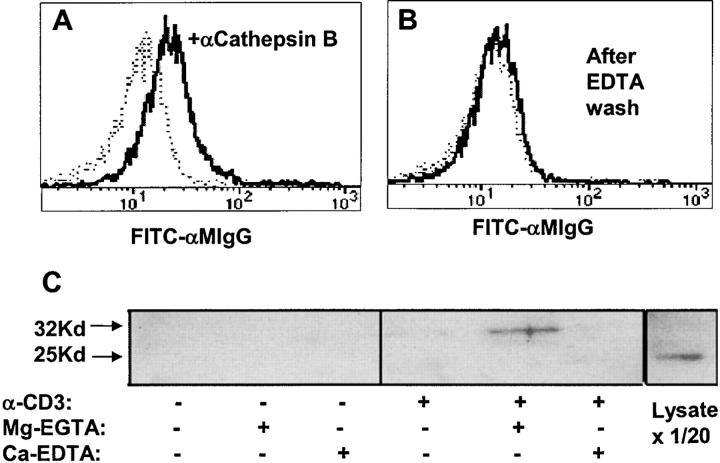
Because the association of active cathepsin B with tumor cell surfaces has been shown to be dependent on divalent cations (22), we tested whether this was also the case with CTL. Fig. 5, A and B , show that the EDTA treatment that removes surface cathepsin B from tumor cells also removes it from recently degranulated CTL. Fig. 5 C shows an experiment in which the released cathepsin B was immunoprecipitated from supernatants after treating such CTL under selective conditions for Ca+2 and Mg+2 chelation. Western blots of such supernatants (Fig. 5 C) showed that the 32-kD “one-chain” form of cathepsin B was only detectable when CTL had been precultured on anti-CD3–coated wells to trigger degranulation, and when they were subsequently incubated in Mg-EGTA to chelate free calcium.
Figure 5.
Surface cathepsin B on TCR-activated CTL is released by EGTA and is the single chain form. CD8+ cloned human CTL were stimulated with plate-bound anti-CD3 for 2 h at 37°C, washed with PBS, and (A) incubated with PBS or (B) 0.53 mM EDTA in PBS for 10 min at 37°C. After washing, cells were incubated with anti-cathepsin B (heavy line) followed by anti–mouse Ig-FITC (dashed line shows control with no anticathepsin B). (C) Anti-CD3–treated CTL as in A and B were incubated with 2 mM Mg-EGTA or Ca-EDTA in HBSS for 10 min at 37°C. Supernatants were analyzed by blotting with anti-cathepsin B. The last lane shows whole CTL dissolved in SDS sample buffer and run directly (1/20 of the cell-equivalent input of other lanes).
Western blots of human CTL lysates show that cathepsin B expression in these cells occurs as either the 32-kD one-chain form or the additionally processed 25-kD “two-chain” form. In some CTL preparations these two forms are expressed in roughly equal proportions, whereas in other preparations one or the other isoforms is dominant. In the experiment shown in Fig. 5, the whole CTL lysate expresses ∼10 times more 25-kD cathepsin B, although the 32-kD form is visible in longer exposures.
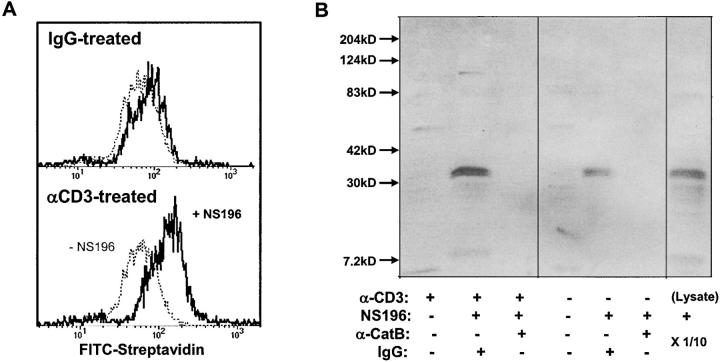
CTL Surface Cathepsin B Is Enzymatically Active and the Sole Target of NS-196.
Because lysosomal cathepsin B might be unstable and inactive at neutral pH, the hypothesis that it provides self-protection for cytotoxic lymphocytes by cleaving perforin is open to question. On the other hand, it has been shown that cathepsin B on the surface of tumor cells is enzymatically active (23). We probed for the activity of CTL surface cathepsin B by testing whether it is reactive with the biotinylated cathepsin B–specific affinity reagent NS-196, which sensitizes CTL to activation-induced suicide (Fig. 2 D). This reagent undergoes covalent reaction with the active site cysteine in a step that mimics proteolysis. As shown by flow cytometry in Fig. 6 A, the NS-196 reagent biotinylates resting CTL surfaces to a small extent (variable in different experiments), but after degranulation a greatly increased biotinylation is observed, as predicted if the immunoreactive cathepsin B (Fig. 4) is enzymatically active. Fig. 6 B shows streptavidin blots of NS-196–treated CTL with and without degranulating stimuli. NS-196 biotinylates a single 32-kD protein in untreated CTL (compatible with the slight biotinylation seen in Fig. 6 A), and this band is greatly enhanced by the degranulation stimulus. Immunodepletion of the lysate with anticathepsin B specifically removes the biotinylated 32-kD band, demonstrating that the only protein significantly labeled by NS-196 during its inactivation of self-protection is cathepsin B.
Figure 6.
Surface cathepsin B on TCR-activated T cells is active and the target of NS-196. (A) Detection of biotinylated NS-196 on the CTL surface after degranulation. Flow cytometry of CD8+ cloned human CTL RS-56 after culture on wells coated with anti-CD3 or isotope control for 2 h, followed by treatment with or without 1 μM NS-196, which was detected by FITC-streptavidin. (B) Identification of cathepsin B as the molecular target of NS-196. CTL clone RS-56 was incubated for 2 h on anti-CD3– or IgG-coated wells, followed by incubation with or without 0.1 μM NS-196. Cells were lysed with Triton X-100 and immunodepleted with beads containing anticathepsin B antibody or control rabbit IgG. The remaining lysate was run on a 12% nonreduced SDS gel, blotted onto nitrocellulose, probed with Streptavidin-HRP, and developed using ECL. The right lane shows the biotinylation pattern when the whole CTL lysate was labeled with 0.1 μM NS-196 and run directly (1/10 of the cell-equivalent input of other lanes).
Cathepsin B Cleaves Perforin.
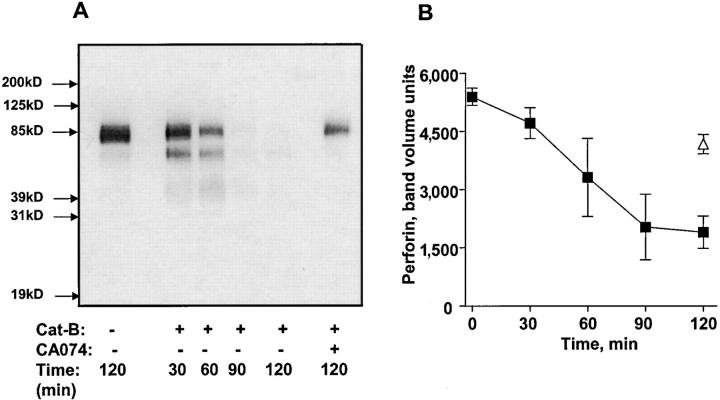
The above data clearly implicate surface cathepsin B in cytotoxic lymphocyte self-protection, but do not directly address the molecular target of cathepsin B. Given previous data showing that membrane-associated perforin is highly susceptible to proteolysis (26), and the proximity of degranulated surface cathepsin B to perforin associated with effector cell membranes, perforin is an obvious candidate. To see if this protease is capable of degrading perforin, we incubated purified cathepsin B with CTL extracts containing perforin and monitored digestion with Western blots. The results show that perforin is efficiently digested by cathepsin B, a process that is inhibited by CA074 (Fig. 7) .
Figure 7.
Perforin cleavage by cathepsin B. (A) Perforin from CTL extracts was treated with 258 ng/ml purified cathepsin B for various times and analyzed by blotting with anti-perforin antibody. (B) Mean densitometric analysis of perforin degradation by cathepsin B. Three experiments are shown.
Discussion
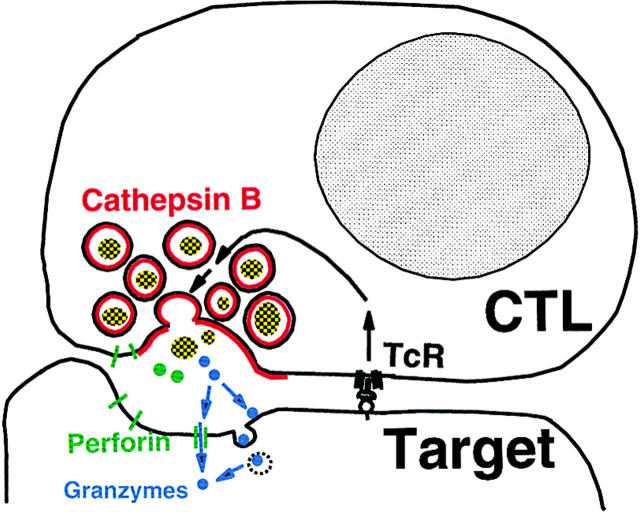
The experiments described above provide strong support for the model of cytotoxic lymphocyte self-protection shown in Fig. 8 . The essential feature of this model is that self-protection is provided by surface membrane cathepsin B expressed as a result of degranulation. The data we have presented support this model with functional evidence that cathepsin B inhibitors sensitize cytotoxic lymphocytes to activation-induced suicide, as well as evidence for the cell surface expression of active cathepsin B triggered by degranulation. This model provides a rational explanation for the considerable literature bearing on cytotoxic lymphocyte self-protection (8). Because it postulates that expression of the critical cathepsin B self-protective molecule is local and transient, cytotoxic lymphocytes would be vulnerable to fratricidal attack. The model postulates that self-protection occurs before perforin-mediated membrane damage, which is the most upstream of the series of damaging events leading to target cell death.
Figure 8.
Surface cathepsin B model for cytotoxic lymphocyte self-protection.
Our functional data show that cytotoxic lymphocytes induced to degranulate in the presence of membrane-impermeant cathepsin B–specific protease inhibitors die rapidly via a perforin-dependent suicide process. T his death process occurs in cytotoxic T and NK cells, but not in T cells lacking the granule exocytosis cytotoxicity pathway. This suicide does not occur in perforin-deficient CTL and is inhibited by the granule proton pump poison concanamycin A and calcium chelation, all of which implicate the granule exocytosis cytotoxicity pathway.
Extracellular cathepsin B is implicated by the data in Fig. 2, which shows that two different membrane-impermeant cathepsin B–specific protease inhibitors sensitize CTL to this activation-induced suicide. Cathepsin B is the only protease inactivated by all the inhibitors sensitizing CTL activation suicide, which include general thiol cathepsin inhibitors such as fluoromethyl ketones (28), diazomethyl ketones (39), epoxysuccinyl peptide compounds (35), and cystatins (40). Other cysteine proteases expressed in mammalian cells, such as caspases and calpains, do not react with most of these inhibitors. The thiol cathepsin family is associated with lysosomes and shares sequence homology, but different family members display different enzymatic properties and tissue expression patterns (41, 42). Lymphocytes express the general lysosomal endoprotease cathepsins B, H, and L, as well as the exopeptidase cathepsin C (DPPI). Cytotoxic lymphocytes also express cathepsin W (43–46), whose enzymatic properties remain undescribed. However, CA074 and NS-196 are cathepsin B–specific reagents and virtually inactive against cathepsins H or L (36, 37, 47, 48). As these compounds are the most potent inhibitors in sensitizing CTL activation suicide, the functional data implicate cathepsin B as the major component of CTL self-protection. Although cathepsin W, with its uncharacterized enzyme activity, could be the target of CA074 and NS-196, the experiments discussed below appear to rule out this possibility. However, our data do not rigorously exclude the possible role of other minor NS-196–reactive cathepsins in cytotoxic lymphocyte self-protection.
The second line of evidence implicating cathepsin B comes from our studies of its surface expression on CTL, which indicate that this protease is minimally expressed on the surface of resting CTL, but rapidly detectable there after degranulation (Fig. 4). This is compatible with cathepsin B secretion from the secretory granule, where it normally resides as one of the lysosomal granule components. The issue of how cathepsin B associates with the surface membrane is not fully resolved. This protein has no hydrophobic membrane domain and is normally found as a soluble protein in lysosomes, but active cathepsin B is expressed on the surface of some tumor cells (23). Because in the latter case the protease was released by EDTA, we performed the experiment shown in Fig. 5, which shows that cathepsin B bound to degranulated CTL is released by calcium chelation. However, calcium is not known to bind to cathepsin B and it is not clear what CTL surface component might be involved. A surface complex of procathepsin B and annexin II as found on tumor cell surfaces (22) would not be relevant to cytotoxic lymphocyte self-protection, as the proenzyme lacks the critical proteolytic activity. In our survey of various human CTL lines, Western blots detected both the 32-kD one-chain and the 25-kD two-chain cathepsin B in varying proportions in lysates of different lines (unpublished data). However, the cathepsin B eluted off these degranulated CTL with EGTA always consisted of only the 32-kD form (e.g., Fig. 5), which was also the only NS-196–reactive molecule in CTL lysates (e.g., Fig. 6). These experiments show that the surface-expressed 32-kD cathepsin B in CTL is enzymatically active and seems to be specifically selected for surface expression, even in cells expressing a large amount of 25-kD cathepsin B (Fig. 5). One possible explanation is that a subpopulation of cathepsin B molecules in cytotoxic lymphocyte granules is bound to the granule membrane and that the molecular interactions providing membrane attachment also provide protection against the additional processing cleavages, giving rise to the two-chain form of cathepsin B in those granules. In any case, the role of cathepsin B in self-protection is definitively established by the experiment shown in Fig. 6, in which the NS-196 reaction conditions that defeat self-protection (Fig. 2 D) were shown to result in a single biotinylated 32-kD protein that was reactive with anticathepsin B.
The model in Fig. 8 proposes that cathepsin B cleaves membrane-associated perforin before it forms a pore. This is based on previous studies indicating that perforin initially binds to membranes via a reversible intermediate (14, 24, 25) that is highly susceptible to proteolysis by trypsin, chymotrypsin, and pronase (14, 26), proteases with distinctly different cleavage specificities. Because the surface-associated forms of cathepsin B and perforin appear to have different properties from the soluble proteins, it is difficult to experimentally mimic the local environment illustrated in Fig. 8. However, the experiment in Fig. 7 shows that cathepsin B readily cleaves perforin and that having both the protease and perforin associated with the same membrane would greatly enhance the efficiency of perforin degradation.
One other molecular model for cytotoxic lymphocyte self-protection that has been proposed involves the serpin granzyme B inhibitor, PI-9 (49, 50). This protein is expressed in the cytoplasm of cytotoxic lymphocytes and other cells, such as dendritic cells (51), and has been shown to be capable of blocking lymphocyte cytotoxicity when overexpressed in MCF-7 cells (50). Although it is possible that PI-9 plays a role in cytotoxic lymphocyte self-protection, its role remains speculative because (a) there is no direct evidence for a PI-9 role in self-protection, and (b) PI-9 specifically blocks granzyme B, and cytotoxic lymphocytes lacking granzyme B retain potent cytotoxic activity even if their ability to induce target apoptosis is diminished (52). Unless PI-9 protein is polarized within the effector cytoplasm after target recognition, it is hard to envision how this granzyme B inhibitor could provide self-protection while allowing fratricide. Nevertheless, PI-9 or other internal protease inhibitors could contribute to effector self-protection by mechanisms that are compatible with and complementary to surface cathepsin B protection.
It is possible that surface proteases, including cathepsin B, play a role in controlling the in vivo susceptibility of various cells to cytotoxic lymphocyte death. In many cases, virally infected cells decrease their recognizability by cytotoxic lymphocytes by either suppressing surface MHC or surface peptide expression in the case of CTL, or by interfering with NK cell activation (53). An alternative means of resisting cytotoxic lymphocyte attack is to induce resistance to the lethal damage itself. One such example may be the human leukemia cell line ML-2, which is recognized by NK cells but resistant to killing by either NK cells or perforin, apparently because of defective perforin binding (54). Expression of a surface protease such as cathepsin B could explain such tumor cell resistance to cytotoxic lymphocytes. Although the expression of surface cathepsins on tumor cells has been thought of as a means to free cells from tissue adhesions and allow metastasis (55), our results suggest that this protease may also allow resistance to attack by cytotoxic lymphocytes, which recent experiments clearly show are important in tumor surveillance (56).
Our finding that CTL and NK cells utilize surface cathepsin B for self-protection opens the possibility that its activity could be subject to physiological regulation. Its inhibition in vivo may lead to activation-induced suicide accompanying degranulation similar to what we have demonstrated experimentally. For example, Figs. 1 and 2 show a 10–18% background activation-induced death detectable at 4 h with both CTL and NK cells in the absence of added cathepsin inhibitors (Figs. 1 and 2). This death seems compatible with the slow perforin-dependent activation-induced death previously reported with activated CD8+ T cells in vitro and in vivo (57, 58), and may reflect a physiological pathway for deleting CD8+ T cells. The failure of normal CD8+ T cell homeostasis after chronic viral infection in perforin knockout mice (59) suggests that CTL may kill themselves or each other when antigen persists. The fatal hyperproliferative syndrome in humans with perforin defects is characterized by infiltrating activated T cells and macrophages (4), suggesting that CTL cytotoxicity normally restrains these populations, possibly by third-party, fratricidal, and suicidal mechanisms. Thus, the role of surface cathepsin B in self-protection raises the question of whether naturally occurring protease inhibitors may compromise the efficiency of self-protection in vivo and therefore modulate T cell homeostasis.
Acknowledgments
We thank Charles Mainhart for suggestions and help with multiple experiments.
Footnotes
Abbreviations used in this paper: ECL, enhanced chemiluminescence; FasL, Fas ligand; HRP, horseradish peroxidase.
References
- 1.Henkart, P.A. 1994. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1:343–346. [DOI] [PubMed] [Google Scholar]
- 2.Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. [DOI] [PubMed] [Google Scholar]
- 3.Smyth, M.J., K.Y. Thia, S.E. Street, D. MacGregor, D.I. Godfrey, and J.A. Trapani. 2000. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stepp, S.E., R. Dufourcq-Lagelouse, F. Le Deist, S. Bhawan, S. Certain, P.A. Mathew, J.I. Henter, M. Bennett, A. Fischer, G. de Saint Basile, et al. 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 286:1957–1959. [DOI] [PubMed] [Google Scholar]
- 5.Straus, S.E., M. Sneller, M.J. Lenardo, J.M. Puck, and W. Strober. 1999. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann. Intern. Med. 130:591–601. [DOI] [PubMed] [Google Scholar]
- 6.Sarin, A., M.S. Williams, M.A. Alexander-Miller, J.A. Berzofsky, C.M. Zacharchuk, and P.A. Henkart. 1997. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 6:209–215. [DOI] [PubMed] [Google Scholar]
- 7.Rothstein, T.L., M. Mage, G. Jones, and L.L. McHugh. 1978. Cytotoxic T lymphocyte sequential killing of immobilized allogeneic tumor target cells measured by time-lapse microcinematography. J. Immunol. 121:1652–1656. [PubMed] [Google Scholar]
- 8.Martz, E. 1993. Overview of CTL-target adhesion and other critical events in the cytotoxic mechanism. In Cytotoxic Cells. Recognition, Effector Function, Generation, and Methods. M.V. Sitkovsky and P.A. Henkert, editors. Birkhauser, Boston. 9–45.
- 9.Luciani, M.F., J.F. Brunet, M. Suzan, F. Denizot, and P. Golstein. 1986. Self-sparing of long-term in vitro–cloned or uncloned cytotoxic T lymphocytes. J. Exp. Med. 164:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kranz, D.M., and H.N. Eisen. 1987. Resistance of cytotoxic T lymphocytes to lysis by a clone of cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA. 84:3375–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakely, A., K. Gorman, H. Ostergaard, K. Svoboda, C.C. Liu, J.D. Young, and W.R. Clark. 1987. Resistance of cloned cytotoxic T lymphocytes to cell-mediated cytotoxicity. J. Exp. Med. 166:1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verret, C.R., A.A. Firmenich, D.M. Kranz, and H.N. Eisen. 1987. Resistance of cytotoxic T lymphocytes to the lytic effects of their toxic granules. J. Exp. Med. 166:1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, C.C., S. Jiang, P.M. Persechini, A. Zychlinsky, Y. Kaufmann, and J.D. Young. 1989. Resistance of cytolytic lymphocytes to perforin-mediated killing. Induction of resistance correlates with increase in cytotoxicity. J. Exp. Med. 169:2211–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller, C., and J. Tschopp. 1994. Resistance of CTL to perforin-mediated lysis. Evidence for a lymphocyte membrane protein interacting with perforin. J. Immunol. 153:2470–2478. [PubMed] [Google Scholar]
- 15.Jiang, S., D.M. Ojcius, P.M. Persechini, and J.D. Young. 1990. Resistance of cytolytic lymphocytes to perforin-mediated killing. J. Immunol. 144:998–1003. [PubMed] [Google Scholar]
- 16.Schick, B., and G. Berke. 1990. The lysis of cytotoxic T lymphocytes and their blasts by cytotoxic T lymphocytes. Immunology. 71:428–433. [PMC free article] [PubMed] [Google Scholar]
- 17.Walden, P.R., and H.N. Eisen. 1990. Cognate peptides induce self-destruction of CD8+ cytolytic T lymphocytes. Proc. Natl. Acad. Sci. USA. 87:9015–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su, M.W., P.R. Walden, D.B. Golan, and H.N. Eisen. 1993. Cognate peptide-induced destruction of CD8+ cytotoxic T lymphocytes is due to fratricide. J. Immunol. 151:658–667. [PubMed] [Google Scholar]
- 19.Dutz, J.P., P.R. Walden, and H.N. Eisen. 1992. Effects of cognate peptides on cytolytic and proliferative activities of cloned cytotoxic T lymphocytes. Int. Immunol. 4:571–580. [DOI] [PubMed] [Google Scholar]
- 20.Hanon, E., J.C. Stinchcombe, M. Saito, B.E. Asquith, G.P. Taylor, Y. Tanaka, J.N. Weber, G.M. Griffiths, and C.R. Bangham. 2000. Fratricide among CD8(+) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity. 13:657–664. [DOI] [PubMed] [Google Scholar]
- 21.Page, L.J., A.J. Darmon, R. Uellner, and G.M. Griffiths. 1998. L is for lytic granules: lysosomes that kill. Biochim. Biophys. Acta. 1401:146–156. [DOI] [PubMed] [Google Scholar]
- 22.Mai, J., R.L. Finley, Jr., D.M. Waisman, and B.F. Sloane. 2000. Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J. Biol. Chem. 275:12806–12812. [DOI] [PubMed] [Google Scholar]
- 23.Hulkower, K.I., C.C. Butler, B.E. Linebaugh, J.L. Klaus, D. Keppler, V.L. Giranda, and B.F. Sloane. 2000. Fluorescent microplate assay for cancer cell-associated cathepsin B. Eur. J. Biochem. 267:4165–4170. [DOI] [PubMed] [Google Scholar]
- 24.Young, J.D., A. Damiano, M.A. DiNome, L.G. Leong, and Z.A. Cohn. 1987. Dissociation of membrane binding and lytic activities of the lymphocyte pore-forming protein (perforin). J. Exp. Med. 165:1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuta, A.E., C.R. Reynolds, and P.A. Henkart. 1989. Mechanism of lysis by large granular lymphocyte granule cytolysin: generation of a stable cytolysin-RBC intermediate. J. Immunol. 142:4378–4384. [PubMed] [Google Scholar]
- 26.Kuta, A.E., C.L. Bashford, C.A. Pasternak, C.W. Reynolds, and P.A. Henkart. 1991. Characterization of non-lytic cytolysin-membrane intermediates. Mol. Immunol. 28:1263–1270. [DOI] [PubMed] [Google Scholar]
- 27.Perussia, B., C. Ramoni, I. Anegon, M.C. Cuturi, J. Faust, and G. Trinchieri. 1987. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat. Immun. Cell Growth Regul. 6:171–188. [PubMed] [Google Scholar]
- 28.Rasnick, D. 1985. Synthesis of peptide fluoromethyl ketones and the inhibition of human cathepsin B. Anal. Biochem. 149:461–465. [DOI] [PubMed] [Google Scholar]
- 29.Crawford, C., R.W. Mason, P. Wikstrom, and E. Shaw. 1988. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem. J. 253:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678–3686. [PubMed] [Google Scholar]
- 31.Fortier, A.H., C.A. Nacy, and M.V. Sitkovsky. 1989. Similar molecular requirements for antigen receptor-triggered secretion of interferon and granule enzymes by cytolytic T lymphocytes. Cell. Immunol. 124:64–76. [DOI] [PubMed] [Google Scholar]
- 32.Haddad, E.K., X. Wu, J.A. Hammer, and P.A. Henkart. 2001. Defective granule exocytosis in Rab27a-deficient lymphocytes from ashen mice. J. Cell Biol. 152:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfoco, E., P.M. Stuart, T. Brunner, T. Lin, T.S. Griffith, Y. Gao, H. Nakajima, P.A. Henkart, T.A. Ferguson, and D.R. Green. 1998. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 9:711–720. [DOI] [PubMed] [Google Scholar]
- 34.Milella, M., A. Gismondi, P. Roncaioli, L. Bisogno, G. Palmieri, L. Frati, M.G. Cifone, and A. Santoni. 1997. CD16 cross-linking induces both secretory and extracellular signal-regulated kinase (ERK)-dependent cytosolic phospholipase A2 (PLA2) activity in human natural killer cells: involvement of ERK, but not PLA2, in CD16-triggered granule exocytosis. J. Immunol. 158:3148–3154. [PubMed] [Google Scholar]
- 35.Matsumoto, K., K. Mizoue, K. Kitamura, W.C. Tse, C.P. Huber, and T. Ishida. 1999. Structural basis of inhibition of cysteine proteases by E-64 and its derivatives. Biopolymers. 51:99–107. [DOI] [PubMed] [Google Scholar]
- 36.Buttle, D.J., M. Murata, C.G. Knight, and A.J. Barrett. 1992. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 299:377–380. [DOI] [PubMed] [Google Scholar]
- 37.Schaschke, N., I. Assfalg-Machleidt, T. Lassleben, C.P. Sommerhoff, L. Moroder, and W. Machleidt. 2000. Epoxysuccinyl peptide-derived affinity labels for cathepsin B. FEBS Lett. 482:91–96. [DOI] [PubMed] [Google Scholar]
- 38.Sarin, A., E.K. Haddad, and P.A. Henkart. 1998. Caspase dependence of target cell damage induced by cytotoxic lymphocytes. J. Immunol. 161:2810–2816. [PubMed] [Google Scholar]
- 39.Shaw, E. 1990. Cysteinyl proteinases and their selective inactivation. Adv. Enzymol. Relat. Areas Mol. Biol. 63:271–347. [DOI] [PubMed] [Google Scholar]
- 40.Barrett, A.J., N.D. Rawlings, M.E. Davies, W. Machleidt, G. Salvesen, and V. Turk. 1986. Cysteine proteinase inhibitors of the cystatin superfamily. In Proteinase Inhibitors. A.J. Barrett and G.S. Salvesen, editors. Elsevier, New York. 515–569.
- 41.McGrath, M.E. 1999. The lysosomal cysteine proteases. Annu. Rev. Biophys. Biomol. Struct. 28:181–204. [DOI] [PubMed] [Google Scholar]
- 42.Turk, V., B. Turk, and D. Turk. 2001. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 20:4629–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linnevers, C., S.P. Smeekens, and D. Bromme. 1997. Human cathepsin W, a putative cysteine protease predominantly expressed in CD8+ T lymphocytes. FEBS Lett. 405:253–259. [DOI] [PubMed] [Google Scholar]
- 44.Brown, J., E. Matutes, A. Singleton, C. Price, H. Molgaard, D. Buttle, and T. Enver. 1998. Lymphopain, a cytotoxic T and natural killer cell-associated cysteine protease. Leukemia. 12:1771–1781. [DOI] [PubMed] [Google Scholar]
- 45.Wex, T., B. Levy, H. Wex, and D. Bromme. 1999. Human cathepsins F and W: a new subgroup of cathepsins. Biochem. Biophys. Res. Commun. 259:401–407. [DOI] [PubMed] [Google Scholar]
- 46.Wex, T., F. Buhling, H. Wex, D. Gunther, P. Malfertheiner, E. Weber, and D. Bromme. 2001. Human cathepsin W, a cysteine protease predominantly expressed in NK cells, is mainly localized in the endoplasmic reticulum. J. Immunol. 167:2172–2178. [DOI] [PubMed] [Google Scholar]
- 47.Otto, H.H., and T. Schirmeister. 1997. Cysteine proteases and their inhibitors. Chem. Rev. 97:133–171. [DOI] [PubMed] [Google Scholar]
- 48.Schaschke, N., I. Assfalg-Machleidt, W. Machleidt, and L. Moroder. 1998. Substrate/propeptide-derived endo-epoxysuccinyl peptides as highly potent and selective cathepsin B inhibitors. FEBS Lett. 421:80–82. [DOI] [PubMed] [Google Scholar]
- 49.Sun, J., C.H. Bird, V. Sutton, L. McDonald, P.B. Coughlin, T.A. DeJong, J.A. Trapani, and P.I. Bird. 1996. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J. Biol. Chem. 271:27802–27809. [DOI] [PubMed] [Google Scholar]
- 50.Bird, C.H., V.R. Sutton, J. Sun, C.E. Hirst, A. Novak, S. Kumar, J.A. Trapani, and P.I. Bird. 1998. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the fas cell death pathway. Mol. Cell. Biol. 18:6387–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bladergroen, B.A., M.C. Strik, N. Bovenschen, O. van Berkum, G.L. Scheffer, C.J. Meijer, C.E. Hack, and J.A. Kummer. 2001. The granzyme B inhibitor, protease inhibitor 9, is mainly expressed by dendritic cells and at immune-privileged sites. J. Immunol. 166:3218–3225. [DOI] [PubMed] [Google Scholar]
- 52.Shresta, S., D.M. MacIvor, J.W. Heusel, J.H. Russell, and T.J. Ley. 1995. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc. Natl. Acad. Sci. USA. 92:5679–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorenzo, M.E., H.L. Ploegh, and R.S. Tirabassi. 2001. Viral immune evasion strategies and the underlying cell biology. Semin. Immunol. 13:1–9. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann, C., M. Zeis, N. Schmitz, and L. Uharek. 2000. Impaired binding of perforin on the surface of tumor cells is a cause of target cell resistance against cytotoxic effector cells. Blood. 96:594–600. [PubMed] [Google Scholar]
- 55.Mai, J., D.M. Waisman, and B.F. Sloane. 2000. Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim. Biophys. Acta. 1477:215–230. [DOI] [PubMed] [Google Scholar]
- 56.Smyth, M.J., D.I. Godfrey, and J.A. Trapani. 2001. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2:293–299. [DOI] [PubMed] [Google Scholar]
- 57.Spaner, D., K. Raju, L. Radvanyi, Y. Lin, and R.G. Miller. 1998. A role for perforin in activation-induced cell death. J. Immunol. 160:2655–2664. [PubMed] [Google Scholar]
- 58.Spaner, D., K. Raju, B. Rabinovich, and R.G. Miller. 1999. A role for perforin in activation-induced T cell death in vivo: increased expansion of allogeneic perforin-deficient T cells in SCID mice. J. Immunol. 162:1192–1199. [PubMed] [Google Scholar]
- 59.Matloubian, M., M. Suresh, A. Glass, M. Galvan, K. Chow, J.K. Whitmire, C.M. Walsh, W.R. Clark, and R. Ahmed. 1999. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]