Abstract
Scedosporium apiospermum (Pseudallescheria boydii) is an emerging opportunistic filamentous fungus that causes serious infections in both immunocompetent and immunocompromised patients. To gain insight into the immunopathogenesis of infections due to S. apiospermum, the antifungal activities of human polymorphonuclear leukocytes (PMNs), mononuclear leukocytes (MNCs), and monocyte-derived macrophages (MDMs) against two clinical isolates of S. apiospermum were evaluated. Isolate SA54A was amphotericin B resistant and was the cause of a fatal disseminated infection. Isolate SA1216 (cultured from a successfully treated localized subcutaneous infection) was susceptible to amphotericin B. MDMs exhibited similar phagocytic activities against conidia of both isolates. However, PMNs and MNCs responded differently to the hyphae of these two isolates. Serum opsonization of hyphae resulted in a higher level of superoxide anion (O2−) release by PMNs in response to SA54A (amphotericin B resistant) than that seen in response to SA1216 (amphotericin B susceptible; P < 0.001). Despite this increased O2− production, PMNs and MNCs induced less hyphal damage to SA54A than to SA1216 (P < 0.001). To investigate the potential mechanisms responsible for these differences, hyphal damage was evaluated in the presence of antifungal oxidative metabolites as well as in the presence of a series of inhibitors and scavengers of antifungal PMN function. Mannose, catalase, superoxide dismutase, dimethyl sulfoxide, and heparin had no effect on PMN-induced hyphal damage to either of the two isolates. However, azide, which inhibits PMN myeloperoxidase activity, significantly reduced hyphal damage to SA1216 (P < 0.01) but not to SA54A. Hyphae of SA1216 were slightly more susceptible to oxidative pathway products, particularly HOCl, than those of SA54A. Thus, S. apiospermum is susceptible to antifungal phagocytic function to various degrees. The selective inhibitory pattern of azide with respect to hyphal damage and the parallel susceptibility to HOCl suggests an important difference in susceptibilities to myeloperoxidase products that may be related to the various levels of pathogenicity and amphotericin B resistance of S. apiospermum.
Scedosporium apiospermum, the asexual state of the hyalohyphomycete Pseudallescheria boydii, is an emerging fungal pathogen that causes localized as well as disseminated infections in both immunocompetent and immunocompromised hosts (17, 19, 20, 24, 30, 35, 43). The organism is usually resistant to amphotericin B, whereas it exhibits various degrees of susceptibility to more-recently introduced antifungal compounds such as voriconazole and posaconazole (4, 5, 10, 23, 40). Depending on the degree of immunosuppression and extent of infection, antifungal chemotherapy may have various levels of clinical efficacy in eradication of the organism. Indeed, even immunocompetent patients exhibit various therapeutic responses and immunocompromised patients carry a high risk of intractable infection.
Little is known about phagocytic host defenses against S. apiospermum. Studies of host defenses against filamentous fungi have focused principally upon Aspergillus fumigatus. The main immune component that has been found responsible for host defenses against A. fumigatus consists of phagocytes, including circulating polymorphonuclear leukocytes (PMNs) and monocytes as well as monocyte-derived macrophages (MDMs) (29, 31, 38). These cells are capable of damaging hyphal elements through oxygen-dependent and -independent mechanisms (38). The oxygen-dependent mechanisms consist of a series of reactions starting with the production of superoxide anion (O2−), which is dismutated into hydrogen peroxide (H2O2). Myeloperoxidase (MPO) then catalyzes the conversion of H2O2 and halides to generate hypohalides, such as hypochlorite (HOCl) and chloramines, which exert potent antifungal activities (1, 15). Cationic peptides (represented by two families, the defensins and the cathelicidins) constitute part of the oxygen-independent microbicidal arsenal in phagocytic cells. These peptides also have potent antifungal activity (6, 26, 45).
The extent to which these mechanisms of phagocytic host defenses apply to Scedosporium spp. is unknown. We therefore investigated the human phagocytic cell responses to conidia and hyphae of amphotericin B-resistant and -susceptible isolates of this emerging fungal pathogen.
MATERIALS AND METHODS
Isolation of phagocytes.
PMNs were purified from whole blood of healthy young adult volunteers. The blood was heparinized and immediately allowed to settle with 3% dextran T500 (Pharmacia Biotech AB, Uppsala, Sweden) in a 2:1 (vol/vol) proportion. PMNs were separated by centrifugation over a Ficoll (lymphocyte separation medium; Gibco BRL Life Technologies Ltd., Paisley, Scotland) cushion. Contaminating erythrocytes were hypotonically lysed with distilled water, and the PMN suspension was washed in Hanks' balanced salt solution (Gibco BRL Life Technologies Ltd.) without Ca2+ and Mg2+ (HBSS−). The cells were resuspended in HBSS− and counted on a hemocytometer by trypan blue staining.
For O2− release and hyphal damage assays, mononuclear leukocytes (MNCs) were separated from the blood of healthy adult volunteers by centrifugation over a Ficoll cushion, washed, and resuspended in HBSS−. Cells were counted on a hemocytometer by trypan blue staining, and the percentage of monocytes in the total number of MNCs was defined by May-Grunwald-Giemsa staining.
For phagocytosis assays, monocytes were separated from the blood of healthy adult volunteers by elutriation (28) and resuspended in complete medium (CM) that consisted of RPMI 1640 supplemented with 25% human AB serum (Gibco), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Cells were counted on a hemocytometer and then used in the phagocytosis assays. The percentage of monocytes was consistently >95%.
Fungal isolates.
Two well-characterized clinical isolates of S. apiospermum were used for these studies. SA54A was isolated from a heart biopsy of a patient with a fatal disseminated infection, and SA1216 was a leg skin biopsy isolate from a patient with a localized subcutaneous infection that was successfully treated. These isolates were cultured and identified to the species level in the Fungus Testing Laboratory of the University of Texas Health Sciences Center at San Antonio and confirmed in two other laboratories (Medical Mycology Research Laboratory, Medical College of Virginia Campus, Virginia Commonwealth University, and the Immunocompromised Host Laboratory, National Cancer Institute). These isolates have also been extensively investigated in previous studies of antifungal drug resistance, pathogenesis, and in vivo host response (11, 12, 40; T. J. Walsh, E. Roilides, C. Gonzalez, D. Shetty, J. Peter, H. Katsifa, and C. A. Lyman, Abstr. 12th Int. Symp. Infect. Immunocompromised Host, Int. J. Infect. Dis. 6:2S49, 2002).
There were distinct differences observed in the colonial morphologies of the two S. apiospermum isolates used in this study when the isolates were grown on potato dextrose agar (PDA; E. Merck AG, Darmstadt, Germany) plates at 37°C for 7 days. Colonies of the SA54A isolate on a PDA plate appeared grey-white in color and lanose, with a slithery mycelium. In contrast, the SA1216 colonies appeared brown-grey in color, with low aerial mycelium. On the reverse side, the SA54A colonies had a dark center with annular rings and dark radial lines surrounded by a white periphery. The SA1216 colonies had a dark brown-grey color, with an annular ring and no radial lines. There were no morphological differences apparent by light microscopy.
The susceptibilities of the clinical isolates to amphotericin B, itraconazole, voriconazole, and posaconazole were determined as the MICs of these drugs according to the National Committee for Clinical Laboratory Standards reference method for broth dilution antifungal susceptibility testing of filamentous fungi (25). Amphotericin B had distinct MICs for the two isolates of S. apiospermum. The MIC of amphotericin B for isolate SA54A was 32-fold higher (MIC = 4.0 μg/ml) than that for SA1216 (MIC = 0.125 μg/ml). The MICs of itraconazole, voriconazole, and posaconazole for SA54A (0.125, 0.125, and 0.125 μg/ml, respectively) and for SA1216 (0.25, 0.06, and 0.25 μg/ml, respectively) differed by ≤2-fold.
To prepare conidia from frozen stocks, fungi were inoculated on PDA plates and grown for 7 days. Conidia were harvested by gently scraping the surfaces of the plates. Conidia were suspended in HBSS, filtered through sterile gauze, and counted on a hemocytometer. They were kept at 4°C for no longer than 3 weeks.
Oxidative pathway products and inhibitors or scavengers of PMN function.
The two fungicidal products of the superoxide pathway (H2O2 and HOCl) were studied at various concentrations. In addition, a number of selective inhibitors of PMN function were examined. These included (i) mannose (MAN) (blocking MAN receptors that mediate attachment) (18), (ii) azide (AZI) (a potent and specific inhibitor of MPO) (8, 9, 36), (iii) catalase (CAT) (an agent that disrupts H2O2) (9, 41), and (iv) heparin (HEP) (a compound that binds to α-defensins and inhibits their function) (2, 16). MAN (100 mM) was dissolved in H2O and stored at −20°C. AZI (0.1 mM) was dissolved in H2O and stored at 4°C. CAT (6,000 U/ml) was stored at 4°C and diluted in HBSS immediately before use. All of the above-named compounds were purchased from the Sigma Chemical Company (St. Louis, Mo.). HEP (Leo Pharmaceutical Products, Ballerup, Denmark) (0.5 U/ml) was diluted in HBSS and stored at 4°C. PMNs were incubated in the presence of 0.1 mM AZI or HBSS at 37°C for 15 min before being added to germinated conidia at an effector-to-target cell (E:T) ratio of 20:1. MAN, CAT, and HEP were added to wells containing hyphae immediately before the addition of PMNs at the same E:T ratio.
In addition, the effects of diphenyleneiodonium (DPI) (21), superoxide dismutase (SOD) (1), and dimethyl sulfoxide (DMSO) (7) on PMN function were examined. DPI and SOD were purchased from Sigma, whereas DMSO was purchased from Riedel-de-Haen, Seelze, Germany. DPI (75 μM) was dissolved in H2O and stored at −20°C. SOD (2,000 U/ml) was dissolved in H2O and stored at 4°C. DMSO (1 μM) was stored at the ambient temperature and diluted in HBSS immediately before use. DPI, SOD, SOD plus CAT, and DMSO each were added to wells containing hyphae immediately before the addition of PMNs at an E:T ratio of 20:1. At the concentrations employed in these studies, none of the above-named compounds (excluding H2O2 and HOCl) had an adverse effect on the viability of PMNs (assessed as the exclusion of trypan blue staining from the PMNs). Control wells contained inhibitors and hyphae without PMNs.
Phagocytosis assay.
For early differentiation to MDMs, 1 million monocytes purified as mentioned above and resuspended in CM were placed on 18-mm-diameter sterile round glass coverslips in 12-well plates and incubated at 37°C with 5% CO2 for 2 to 3 days. After this incubation, the wells with the coverslips in them were washed with warm HBSS and 1 ml of a suspension containing 106 conidia/ml of CM was added. After incubation at 37°C with 5% CO2 for 1 h, the wells were again washed with warm HBSS three times. For measurement of the percentage of phagocytosis and the phagocytic index, the coverslips were removed, dried, and subjected to modified May-Grunwald-Giemsa staining. Cells having or not having phagocytosed conidia were counted microscopically as previously described (14). Conidia that remained attached after three washes were counted only if they had been ingested at a rate of more than 50%. The results were calculated according to the following formulas:
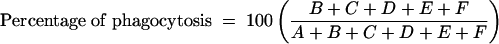 |
(1) |
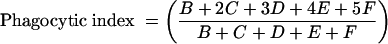 |
(2) |
where the phagocytic index represents the average number of phagocytosed conidia per phagocytosing cell, A represents the number of cells that ingested no conidia, B represents the number of cells that ingested one conidium, C represents the number that ingested two conidia, D represents the number that ingested three conidia, E represents the number that ingested four conidia, and F represents the number that ingested more than four conidia.
Superoxide anion release assay.
The oxidative burst evidenced by the production of O2− was measured as the SOD-inhibitable reduction of cytochrome c. A total of 200 μl of a suspension containing 1.5 × 106 conidia/ml in yeast nitrogen base medium (YNB; Difco Laboratories, Detroit, Mich.) was plated in each of 96 flat-bottom wells in cell culture clusters (Corning Inc., New York, N.Y.) and incubated at 32°C for 18 h. In the case of O2− assays with serum-opsonized hyphae, YNB was replaced by 50% pooled human serum in HBSS and incubated for 30 min at 37°C for opsonization. Pooled human serum was obtained from healthy young adult volunteers and kept at −35°C for no longer than 1 month. The plates were washed three times, and phagocytes were added at an E:T ratio of 1:1 to a final volume of 300 μl of HBSS containing 50 μM cytochrome c (Sigma) (from horse heart) with serum-opsonized or unopsonized hyphae. Basal O2− production was assessed in the absence of stimuli. After incubation at 37°C with 5% CO2 for 1 h, 200 μl was read in a spectrophotometer at a wavelength of 550 nm. The extinction coefficient of ferricytochrome c at 550 nm was taken as 29.5 × 104 M−1 cm−1.
Hyphal damage assay.
Phagocyte-induced hyphal damage was assessed with XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]2H-tetrazolium-5-carboxanilide sodium salt; Sigma) plus coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone; Sigma). A total of 200 μl of a suspension containing 7.5 × 104 conidia/ml in YNB was plated in each of 96 flat-bottom wells in cell culture clusters (Corning) and incubated at 32°C for 18 h. YNB was then replaced by HBSS, and phagocytes (either PMNs or MNCs) were added at different E:T ratios. Following incubation at 37°C with 5% CO2 for 2 h, cells were lysed by washing with H2O and shaking for 5 min three times. A total of 150 μl of phosphate-buffered saline (Biochrom KG, Berlin, Germany) containing 0.25 mg of XTT/ml with 40 μg of coenzyme Q0/ml was added, and plates were again incubated at 37°C with 5% CO2 for 1 h. A total of 100 μl of aliquots from each well was transferred to a new plate and read at 450 nm with a reference wavelength of 690 nm. Antifungal activity was calculated according to the following formula: percentage of hyphal damage = [(1 − X)/C] × 100, where X represents the optical density of test wells and C represents the optical density of control wells with hyphae only. Each set of conditions was tested in duplicate, control wells were set up in quadruplicate, and the results were averaged.
In studies examining the antifungal activity of individual oxidative compounds (H2O2 and HOCl), conidia were germinated as described above. YNB was then replaced by HBSS, and H2O2 or HOCl was added at different concentrations. Following incubation at 37°C with 5% CO2 for 2 h, 150 μl of phosphate-buffered saline containing 0.25 mg of XTT/ml with 40 μg of coenzyme Q0/ml was added and plates were again incubated at 37°C with 5% CO2 for 1 h. Supernatants were read, and antifungal activity was calculated as described above.
Statistical analysis.
Each experiment was performed with the cells of one donor and studied in duplicate or quadruplicate wells for each condition. The mean value of these replicate wells was taken as the value for the particular donor and experiment. The means of the replicate wells of each experiment were then used in the data analysis to calculate the means ± standard errors of the means (SE) of all the experiments at the same conditions. The statistical program GraphPad Instat (GraphPad Inc., San Diego, Calif.) was used for analysis. Unless otherwise stated, paired Student t tests with Bonferroni's correction for repeated measures were used for comparisons between the two strains, as these data followed a normal distribution. Repeated measurement of analysis of variance (ANOVA) with Dunnett's correction for multiple comparisons was used for comparisons of each strain with the control. A two-sided P value of <0.05 indicated statistical significance.
RESULTS
Phagocytosis of conidia by MDMs.
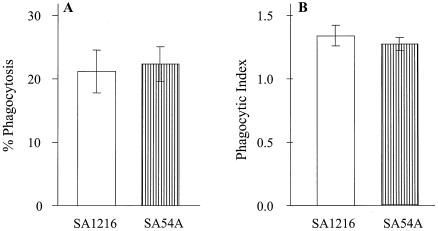
To evaluate the ability of MDMs to phagocytose conidia of S. apiospermum, MDMs were incubated with conidia at an E:T ratio of 1:1 for 1 h (Fig. 1). No differences were detected in the percentages of MDMs that phagocytosed conidia of the two strains (21.2% ± 3.4% for strain SA1216 and 22.3% ± 2.7% for strain SA54A). Similarly, the phagocytic indices were similar for the two strains (1.3 ± 0.08 and 1.3 ± 0.05 for SA1216 and SA54A, respectively).
FIG. 1.
Percentages of phagocytosis (A) and phagocytic indices (B) of S. apiospermum conidia by MDMs. Strains SA1216 (open columns) and SA54A (dashed columns) were used as targets of phagocytosis. Vertical bars represent means ± SE of the results of six experiments.
Oxidative burst in response to hyphae.
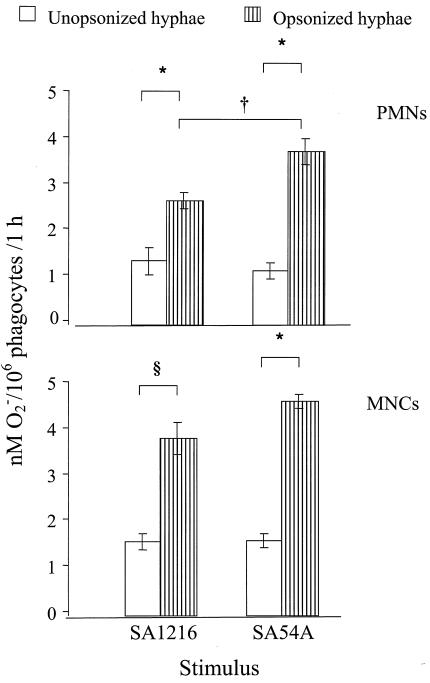
The O2− released by PMNs and MNCs in response to the presence of both serum-opsonized and nonopsonized hyphae of both isolates was measured (Fig. 2). Opsonization of hyphae of both isolates with human serum induced a significant increase in PMN O2− production over that seen with nonopsonized hyphae (P < 0.001). Moreover, serum-opsonized hyphae of the SA54A isolate elicited significantly more O2− from PMNs than those of SA1216 (P < 0.001).
FIG. 2.
Superoxide anion expressed as nanomolar concentrations of O2− produced by 106 PMNs (upper panel) or MNCs (lower panel) in 1 h in response to isolates SA1216 and SA54A of S. apiospermum. Open columns, O2− produced by phagocytes in response to nonopsonized hyphae; dashed columns, O2− produced by phagocytes stimulated with opsonized hyphae. The results are presented as means ± SE of the results of six experiments with PMNs and four experiments with MNCs. The symbol * indicates a level of O2− produced by phagocytes in response to nonopsonized hyphae significantly different from that produced in response to serum-opsonized hyphae (P < 0.001). The symbol § indicates a level of O2− produced by monocytes in response to nonopsonized SA1216 hyphae significantly different from that produced in response to opsonized SA1216 hyphae (P = 0.011). The symbol † indicates a level of O2− produced by PMNs in response to opsonized hyphae of SA54A significantly different from that produced in response to opsonized hyphae of SA1216 (P < 0.001).
When the oxidative burst of MNCs was evaluated (Fig. 2, lower panel), opsonization of hyphae with pooled human serum significantly increased superoxide release over that seen with nonopsonized hyphae of isolates SA1216 and SA54A (P < 0.011). There also was a tendency for the opsonized SA54A isolate to elicit more O2− from MNCs than the SA1216 isolate (P = 0.07).
Damage of hyphae.
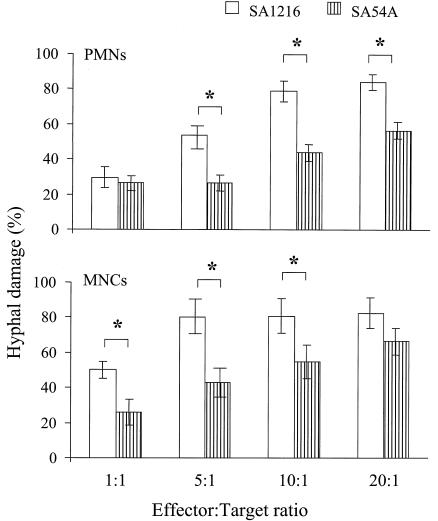
Hyphal damage induced by PMNs (Fig. 3, upper panel) or by MNCs (Fig. 3, lower panel) was assessed at E:T ratios of 1:1, 5:1, 10:1, and 20:1. When challenged with either type of phagocytic cell, the isolates under investigation were damaged in a concentration-dependent manner, with a statistically significant linear trend (P < 0.001 for both strains and both types of phagocytes, as determined by repeated measurement of ANOVA and posttest for linear trend).
FIG. 3.
Percentages of hyphal damage to strain SA1216 (open bars) or SA54A (dashed bars) of S. apiospermum induced by PMNs (upper panel) or MNCs (lower panel). Vertical columns represent means ± SE of the results of eight experiments with PMNs and six experiments with MNCs. The symbol * indicates a significant difference between SA54A and SA1216 in the levels of hyphal damage to these two strains induced by PMNs or MNCs at corresponding E:T ratios (P < 0.001).
However, the SA54A isolate was more resistant to PMN- and MNC-mediated hyphal damage than the SA1216 isolate. For instance, while PMNs caused 53.7% ± 6.4% hyphal damage to SA1216 at an E:T ratio of 5:1 they caused only 26.7% ± 4.5% hyphal damage to SA54A hyphae (P < 0.001, as determined by repeated measurement using ANOVA with Dunnett's correction for multiple comparisons). With MNCs at an E:T ratio of 5:1, the hyphal damage was 80.2% ± 7.9% and 44.2% ± 5.9% for SA1216 and SA54A, respectively (P < 0.001).
Effects of superoxide pathway products.
There was a direct concentration-response relationship between the oxidative metabolites (H2O2 and HOCl) and percent hyphal damage to both isolates of S. apiospermum. Although the SA54A isolate exhibited slightly more susceptibility to H2O2-mediated hyphal damage at 1.0 mM, SA1216 was more susceptible to HOCl-mediated hyphal damage at 0.1 mM (Table 1). At high concentrations, however, both strains were highly susceptible (hyphal damage > 94%) to both H2O2 and HOCl.
TABLE 1.
Percentage of hyphal damage induced by antifungal oxidative metabolites
| Hyphae or antifungal compound concentration (mM) | % of hyphal damage toa:
|
|
|---|---|---|
| SA1216 | SA54A | |
| Control hyphae | 0 | 0 |
| H2O2 | ||
| 0.01 | 38.8 ± 0.01 | 34.0 ± 0.01 |
| 0.1 | 86.0 ± 0.001 | 89.6 ± 0.001 |
| 1b | 94.4 ± 0.001 | 98.7 ± 0.001 |
| 10 | 98.9 ± 0.000 | 98.7 ± 0.001 |
| HOCl | ||
| 0.01 | 29.2 ± 0.01 | 31.0 ± 0.01 |
| 0.1c | 87.7 ± 0.001 | 61.8 ± 0.01 |
| 1 | 98.9 ± 0.001 | 98.5 ± 0.001 |
| 10 | 99.1 ± 0.001 | 98.7 ± 0.001 |
The results are indicated as means + SE derived from six experiments. All the differences from the results for control hyphae are highly significant (P < 0.01).
P = 0.009 for the differences in the results for isolates SA1216 and SA54A.
P < 0.001 for the differences in the results for isolates SA1216 and SA54A.
Effects of inhibitors and scavengers of PMN antifungal functions on hyphal damage.
To further investigate the differences of isolates in host response, we assessed the damage to both isolates induced by PMNs at an E:T ratio of 20:1 in the presence of various inhibitors and scavengers of PMN functions, i.e., DPI, MAN, CAT, SOD, DMSO, AZI, and HEP. In these experiments, MAN, CAT, SOD (or CAT plus SOD), and HEP had no effect on PMN-induced hyphal damage to the two isolates (Table 2). In addition, DMSO (2 and 20 mM) had no significant effect on PMN-induced hyphal damage to the two isolates. None of the above-named compounds had any adverse effect on damage to hyphae of either strain. By comparison, at 5 μM DPI alone had a direct adverse effect on hyphae of both strains, causing 62.2% damage to the SA54A isolate and 41.8% to SA1216. When DPI was added to PMNs and hyphae, the level of hyphal damage was similar to that seen with DPI alone.
TABLE 2.
Percentage of hyphal damage to S. apiospermum strain SA1216 or SA54A induced by PMNs in the presence of inhibitors and scavengers
| Inhibitor or scavenger | % Hyphal damage toa:
|
|
|---|---|---|
| SA1216 | SA54A | |
| None | 79.8 ± 7.1b | 34.9 ± 8.0 |
| MAN | 80.5 ± 7.2 | 35.5 ± 7.1 |
| CAT | 73.0 ± 5.2 | 44.0 ± 8.0 |
| SOD | 53.0 ± 6.2 | 23.1 ± 7.4 |
| SOD + CAT | 65.0 ± 6.0 | 26.1 ± 7.0 |
| AZI | 27.4 ± 8.0b | 27.0 ± 3.5 |
| HEP | 75.0 ± 6.0 | 42.2 ± 6.4 |
PMNs were added to germinated conidia at E:T ratio 20:1. PMNs were preincubated for 15 min in the presence of 0.1 mM AZI or HBSS at 37°C before being added to germinated conidia. The inhibitors MAN (100 mM), CAT (6,000 U/ml), SOD (2,000 U/ml) and HEP (0.5 U/ml) were added immediately before incubating the PMNs with the hyphae at the same E:T ratio. The results are indicated as means ± SE derived from six experiments. None of the inhibitors and scavengers listed caused direct hyphal damage. However, DPI caused 62.2 and 41.8% direct hyphal damage to SA54A and SA1216, respectively. The level of hyphal damage caused when DPI was added to PMNs and hyphae was similar to that caused by DPI alone.
Significant (P < 0.01) difference in the damage to strain SA1216 induced by PMNs in the presence of AZI from that seen with control PMNs without inhibitors.
In contrast, AZI exerted a significant effect on PMN-induced damage to SA1216 hyphae. At 0.1 mM, a concentration previously reported to inhibit MPO activity (8, 9, 36), the presence of AZI resulted in a significant inhibition (from 79.8% ± 7.1% to 26.6% ± 3.5%) of the damage to isolate SA1216 hyphae (P < 0.01). The presence of AZI resulted in only an insignificant reduction (from 34.9% ± 8.0% to 26.6% ± 3.5%) of the damage to SA54A hyphae caused by PMNs (P > 0.05). In the presence of AZI, the levels of damage to both isolates induced by PMNs became equivalent and SA1216 then displayed resistance to hyphal injury similar to that demonstrated by SA54A.
DISCUSSION
In this study, we found that phagocytes respond actively to challenge by S. apiospermum. Specifically, (i) macrophages are capable of phagocytosing S. apiospermum conidia, (ii) both PMNs and MNCs release O2− in response to the presence of serum-opsonized hyphae of this fungal pathogen, and (iii) these cells damage hyphae of S. apiospermum in a concentration-dependent manner. Additionally, we have observed that the hyphae of two strains that caused infections with different clinical outcomes in patients had different levels of susceptibility to MPO products.
S. apiospermum is a filamentous fungus characterized by oval, 6- to 12-μm-long conidia. The in vitro susceptibility of this fungus to conventional and novel antifungal drugs has been studied extensively (4, 5, 10, 23, 40). Despite interlaboratory discrepancies, in general these studies suggest that S. apiospermum is frequently resistant to amphotericin B. Determination of antifungal drug MICs for the two clinical isolates used in this study showed that strain SA54A was 32-fold more resistant to amphotericin B than strain SA1216 (amphotericin B MICs = 4 and 0.125 μg/ml, respectively).
As macrophages are the first phagocytic cells to encounter conidia of A. fumigatus, their primary role in antifungal defense appears to be prevention of conidial germination (38). When macrophages fail to prevent germination, hyphae transfix these cells and grow through them. In that case, PMNs and circulating MNCs attempt to damage hyphae extracellularly by potent oxidative and nonoxidative fungicidal mechanisms (31, 38).
The fact that host defenses against S. apiospermum are poorly understood prompted us to evaluate the antifungal activities of phagocytes against conidia and hyphae of this organism. To gain insight into the immunopathogenesis of infection due to S. apiospermum, two clinical strains (isolated from human infections with very different outcomes and characterized by distinct colonial morphologies and antifungal drug susceptibility features) were used to compare the innate immune responses to each of them.
Phagocytosis of S. apiospermum conidia by MDMs does not differ from phagocytosis of A. fumigatus conidia by MDMs, as assessed in similar experiments using the same methodology in our laboratory (27). While macrophages phagocytosed conidia of the two S. apiospermum isolates in equivalent manners, hyphae of the two isolates studied showed distinct differences in their interactions with and susceptibilities to PMNs and MNCs. For example, when the oxidative burst in response to opsonized hyphae of both isolates was evaluated the SA54A isolate elicited a significantly higher amount of O2− from PMNs than SA1216. While MNCs displayed a significant oxidative burst in response to opsonized hyphae of both isolates, the level of O2− generated in response to SA54A tended to be higher than that seen with SA1216. Although SA54A elicited production of more O2− than did SA1216, this did not translate into more hyphal damage, suggesting a relatively high level of resistance of this strain to oxidative injury.
When hyphal damage induced by both PMNs and MNCs was assessed, the level of damage inflicted on isolate SA54A was found to be significantly lower than that seen with SA1216. Under some experimental conditions this difference in susceptibility to hyphal damage approached 40%. As the patient infected with SA54A had a fatal outcome whereas the patient infected with SA1216 was cured, we hypothesized that the difference in the hyphal damage was related to distinct differences in the susceptibilities of these isolates to certain components of the antifungal host defense mechanism. To further investigate this, we used oxidative pathway antifungal products and inhibitors or scavengers of various steps of PMN antifungal functions to determine whether this difference resides in one or more of these pathways.
Hyphae of the SA1216 strain appeared to be slightly more susceptible to HOCl at ≥0.1 mM than those of SA54A. This finding in a cell-free system correlates with the finding of a higher level of susceptibility of SA1216 to MPO products (as shown by AZI inhibition) in our PMN-hyphal system. By (presumably) acting on the hyphal surface, both antifungal oxidative compounds of phagocytes damaged hyphae of the susceptible strain more efficiently, a finding similar to that of the higher degree of susceptibility to amphotericin B, an agent also acting on the surface of the fungi (ergosterol of the fungal membrane).
The differences in susceptibility to amphotericin B between these isolates of S. apiospermum parallel the differences in vulnerability to oxidative metabolites and to hyphal damage. As susceptibility to amphotericin B-induced membrane injury is dependent upon lipoperoxidation (3), it is possible that the greater susceptibility to oxidative injury by some isolates of S. apiospermum is related. Resistance to lipoperoxidative injury by amphotericin B may be enhanced by increased expression of CAT (32). However, this appears not to be the mechanism of resistance of SA54A to amphotericin B or to H2O2 since there was a concentration-dependent response to H2O2 and even a trend toward heightened H2O2 activity at 1 mM. Assessment of potential differences in membrane sterol composition may further elucidate these mechanisms.
MAN receptors have been shown to mediate attachment and subsequent phagocytosis of various fungi (13, 33, 34, 39) and can be inhibited by the use of MAN (18). It appears that MAN receptors are not involved in the attachment of PMNs to S. apiospermum hyphae.
Although expected to create a cellular environment similar to that of chronic granulomatous disease PMNs by inhibiting the NADPH oxidase activity (21), DPI exerted a direct adverse effect on survival of Scedosporium hyphae, thus precluding an assessment of its effects on PMN hyphal damage. This may have been due to an effect of DPI on NADPH-like enzymes of Scedosporium hyphae (21).
Further, in previous studies CAT was successfully used to disrupt H2O2 at concentrations similar to those used in the present study (9, 41). H2O2 has been found to play a role in inducing destruction of fungi, including damage to A. fumigatus conidia and hyphae (8, 9, 22, 44). Using H2O2 as the substrate, MPO generates hypohalides, such as HOCl and chloramines, which are highly toxic compounds (1, 15). It is unclear whether the antifungal role of H2O2 is due to its own fungicidal activity or to the activity of its downstream metabolic products. Wagner et al. reported similar results when assessing the damage to C. albicans pseudohyphae caused by PMNs (37). They proposed that CAT might be excluded from the area of attack at the fungal surface, since it has been previously demonstrated that proteins with a molecular mass over 50 kDa are excluded from sites of attachment of macrophages (42) and CAT is a high-molecular-mass protein.
SOD (alone or in combination with CAT) as well as DMSO did not induce significant differences in hyphal damage between the two strains. It is possible that SOD with or without CAT is unable to eliminate all superoxide and H2O2 in the presence of PMNs. As noted in Table 1, a concentration of H2O2 of <0.1 mM was able to inflict hyphal injury on Scedosporium spp. It is possible that at low concentrations, the oxidative metabolites O2−, H2O2, HOCl, and MPO function additively or synergistically to mediate hyphal damage in the presence of these inhibitors.
Furthermore, HEP has been shown to bind to α-defensins (members of the cationic proteins) and inhibit their function (2, 16). We did not find an inhibitory effect of HEP on PMN-induced hyphal damage to either the SA54A or SA1216 isolate. Supporting our results, Diamond et al. suggested that although cationic proteins may be involved in damaging Rhizopus oryzae hyphae, they do not appear to have an effect on PMN-induced damage to A. fumigatus hyphae (9). Since S. apiospermum is more closely related to A. fumigatus than to R. oryzae, it is probable that α-defensins are not a key component in cell damage of this organism.
Among all seven inhibitors and scavengers used, only AZI exerted an inhibitory effect on PMN-induced hyphal damage and this inhibition was significant only with SA1216 isolate. Since the aim of these experiments was to maximize differences in hyphal damage between the two isolates, we used a relatively high E:T ratio. Smaller differences would be expected at lower E:T ratios. The concentration of AZI used in this study has been shown previously to inhibit MPO enzyme without altering other PMN functions (8, 9, 36). This finding suggests that the isolate SA54A is resistant to MPO products. This resistance may occupy an important role in the differences in susceptibility to innate host defense and, thus, may be partially responsible for the differences in clinical outcome for the patients infected with these isolates.
MPO deficiency is a common phagocytic disorder in the general population and is asymptomatic in the vast majority of persons. These individuals are not at significant risk for invasive infection due to filamentous fungi. However, when exposed to an uncommon pathogen such as S. apiospermum, an MPO-deficient host may be at greater risk for refractory infection.
In conclusion, this study has demonstrated a selective activity of phagocytic host defense against S. apiospermum, enhancing our understanding of the immunopathogenesis of infection due to this emerging fungal pathogen. The study findings indicate the need for further studies on the effects of immunomodulatory agents to augment host response and thus improve therapy to combat the devastating infections caused by S. apiospermum.
Acknowledgments
C.G.-L. has been a research fellow supported by European Commission Training and Mobility of Researchers grant FMRX-CT970145 Eurofung.
We thank Joanne Peter for performing antifungal drug susceptibility studies.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. [DOI] [PubMed] [Google Scholar]
- 2.Bdeir, K., W. Cane, G. Canziani, I. Chaiken, J. Weisel, M. L. Koschinsky, R. M. Lawn, P. G. Bannerman, B. S. Sachais, A. Kuo, M. A. Hancock, J. Tomaszewski, P. N. Raghunath, T. Ganz, A. A. Higazi, and D. B. Cines. 1999. Defensin promotes the binding of lipoprotein(a) to vascular matrix. Blood 94:2007-2019. [PubMed] [Google Scholar]
- 3.Brajtburg, J., W. G. Powderly, G. S. Kobayashi, and G. Medoff. 1990. Amphotericin B: current understanding of mechanisms of action. Antimicrob. Agents Chemother. 34:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109, 496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 6.De Lucca, A. J., and T. J. Walsh. 1999. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 43:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, R. D., and R. A. Clark. 1982. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect. Immun. 38:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond, R. D., E. Huber, and C. C. Haudenschild. 1983. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J. Infect. Dis. 147:474-483. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, R. D., R. Krzesicki, B. Epstein, and W. Jao. 1978. Damage to hyphal forms of fungi by human leukocytes in vitro: a possible host defense mechanism in aspergillosis and mucormycosis. Am. J. Pathol. 91:313-328. [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff, A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., K. Dawson, M. Pfaller, E. Anaissie, B. Breslin, D. Dixon, A. Fothergill, V. Paetznick, J. Peter, and M. Rinaldi. 1995. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob. Agents Chemother. 39:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezekowitz, R. A., D. J. Williams, H. Koziel, M. Y. Armstrong, A. Warner, F. F. Richards, and R. M. Rose. 1991. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155-158. [DOI] [PubMed] [Google Scholar]
- 14.Gil-Lamaignere, C., A. Maloukou, J. L. Rodriguez-Tudela, and E. Roilides. 2001. Human phagocytic cell responses against Scedosporium prolificans. Med. Mycol. 39:169-175. [DOI] [PubMed] [Google Scholar]
- 15.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 16.Higazi, A. A., T. Nassar, T. Ganz, D. J. Rader, R. Udassin, K. Bdeir, E. Hiss, B. S. Sachais, K. J. Williams, E. Leitersdorf, and D. B. Cines. 2000. The alpha-defensins stimulate proteoglycan-dependent catabolism of low-density lipoprotein by vascular cells: a new class of inflammatory apolipoprotein and a possible contributor to atherogenesis. Blood 96:1393-1398. [PubMed] [Google Scholar]
- 17.Jabado, N., J. L. Casanova, E. Haddad, F. Dulieu, J. C. Fournet, B. Dupont, A. Fischer, C. Hennequin, and S. Blanche. 1998. Invasive pulmonary infection due to Scedosporium apiospermum in two children with chronic granulomatous disease. Clin. Infect. Dis. 27:1437-1441. [DOI] [PubMed] [Google Scholar]
- 18.Karbassi, A., J. M. Becker, J. S. Foster, and R. N. Moore. 1987. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J. Immunol. 139:417-421. [PubMed] [Google Scholar]
- 19.Kiraz, N., Z. Gulbas, Y. Akgun, and O. Uzun. 2001. Lymphadenitis caused by Scedosporium apiospermum in an immunocompetent patient. Clin. Infect. Dis. 32:E59-E61. [DOI] [PubMed] [Google Scholar]
- 20.Kusne, S., S. Ariyanayagam-Baksh, D. C. Strollo, and J. Abernethy. 2000. Invasive Scedosporium apiospermum infection in a heart transplant recipient presenting with multiple skin nodules and a pulmonary consolidation. Transpl. Infect. Dis. 2:194-196. [DOI] [PubMed] [Google Scholar]
- 21.Lesuisse, E., M. Casteras-Simon, and P. Labbe. 1996. Evidence for the Saccharomyces cerevisiae ferrireductase system being a multicomponent electron transport chain. J. Biol. Chem. 271:13578-13583. [DOI] [PubMed] [Google Scholar]
- 22.Lyman, C. A., E. R. Simons, D. A. Melnick, and R. D. Diamond. 1988. Induction of signal transduction in human neutrophils by Candida albicans hyphae: the role of pertussis toxin-sensitive guanosine triphosphate-binding proteins. J. Infect. Dis. 158:1056-1064. [DOI] [PubMed] [Google Scholar]
- 23.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montero, A., J. E. Cohen, M. A. Fernandez, G. Mazzolini, C. R. Gomez, and J. Perugini. 1998. Cerebral pseudallescheriasis due to Pseudallescheria boydii as the first manifestation of AIDS. Clin. Infect. Dis. 26:1476-1477. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Ramanathan, B., E. G. Davis, C. R. Ross, and F. Blecha. 2002. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 4:361-372. [DOI] [PubMed] [Google Scholar]
- 27.Roilides, E., A. Dimitriadou, I. Kadiltsoglou, T. Sein, J. Karpouzas, P. A. Pizzo, and T. J. Walsh. 1997. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J. Immunol. 158:322-329. [PubMed] [Google Scholar]
- 28.Roilides, E., A. Holmes, C. Blake, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1994. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-γ. J. Infect. Dis. 170:894-899. [DOI] [PubMed] [Google Scholar]
- 29.Roilides, E., H. Katsifa, and T. J. Walsh. 1998. Pulmonary host defences against Aspergillus fumigatus. Res. Immunol. 149:454-465. [DOI] [PubMed] [Google Scholar]
- 30.Rollot, F., P. Blanche, B. Richaud-Thiriez, F. Le Pimpec-Barthes, M. Riquet, D. Dusser, D. Salmon, and D. Sicard. 2000. Pneumonia due to Scedosporium apiospermum in a patient with HIV infection. Scand. J. Infect. Dis. 32:439. [DOI] [PubMed] [Google Scholar]
- 31.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus: observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol-Anderson, M. L., J. Brajtburg, and G. Medoff. 1986. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 154:76-83. [DOI] [PubMed] [Google Scholar]
- 33.Stehle, S. E., R. A. Rogers, A. G. Harmsen, and R. A. Ezekowitz. 2000. A soluble mannose receptor immunoadhesin enhances phagocytosis of Pneumocystis carinii by human polymorphonuclear leukocytes in vitro. Scand. J. Immunol. 52:131-137. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, T., N. Ohno, Y. Ohshima, and T. Yadomae. 1998. Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol. Med. Microbiol. 21:223-230. [DOI] [PubMed] [Google Scholar]
- 35.Tadros, T. S., K. A. Workowski, R. J. Siegel, S. Hunter, and D. A. Schwartz. 1998. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum. Pathol. 29:1266-1272. [DOI] [PubMed] [Google Scholar]
- 36.Tan, A. S., and M. V. Berridge. 2000. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods 238:59-68. [DOI] [PubMed] [Google Scholar]
- 37.Wagner, D. K., C. Collins-Lech, and P. G. Sohnle. 1986. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect. Immun. 51:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldorf, A. 1989. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol. Ser. 47:243-271. [PubMed] [Google Scholar]
- 39.Walenkamp, A. M., J. Scharringa, F. M. Schramel, F. E. Coenjaerts, and I. M. Hoepelman. 2000. Quantitative analysis of phagocytosis of Cryptococcus neoformans by adherent phagocytic cells by fluorescence multi-well plate reader. J. Microbiol. Methods 40:39-45. [DOI] [PubMed] [Google Scholar]
- 40.Walsh, T. J., J. Peter, D. A. McGough, A. W. Fothergill, M. G. Rinaldi, and P. A. Pizzo. 1995. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob. Agents Chemother. 39:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Washburn, R. G., J. I. Gallin, and J. E. Bennett. 1987. Oxidative killing of Aspergillus fumigatus proceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect. Immun. 55:2088-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, S. D., and S. C. Silverstein. 1984. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature 309:359-361. [DOI] [PubMed] [Google Scholar]
- 43.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum. Report of two cases and review of treatment. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]
- 44.Wysong, D. R., L. Christin, A. M. Sugar, P. W. Robbins, and R. D. Diamond. 1998. Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect. Immun. 66:1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]