Abstract
Streptolysin O (SLO), a major virulence factor of pyogenic streptococci, binds to cholesterol in the membranes of eukaryotic cells and oligomerizes to form large transmembrane pores. While high toxin doses are rapidly cytocidal, low doses are tolerated because a limited number of lesions can be resealed. Here, we report that at sublethal doses, SLO activates primary murine bone marrow-derived mast cells to degranulate and to rapidly induce or enhance the production of several cytokine mRNAs, including tumor necrosis factor alpha (TNF-α). Mast cell-derived TNF-α plays an important protective role in murine models of acute inflammation, and the production of this cytokine was analyzed in more detail. Release of biologically active TNF-α peaked ∼4 h after stimulation with SLO. Production of TNF-α was blunted upon depletion of protein kinase C by pretreatment of the cells with phorbol-12 myristate-13 acetate. Transient permeabilization of mast cells with SLO also led to the activation of the stress-activated protein kinases p38 mitogen-activated protein (MAP) kinase and c-jun N-terminal kinase (JNK), and inhibition of p38 MAP kinase markedly reduced production of TNF-α. In contrast, secretion of preformed granule constituents triggered by membrane permeabilization was not dependent on p38 MAP kinase or on protein kinase C. Thus, transcriptional activation of mast cells following transient permeabilization might contribute to host defense against infections via the beneficial effects of TNF-α. However, hyperstimulation of mast cells might also lead to overproduction of TNF-α, which would then promote the development of toxic streptococcal syndromes.
Streptolysin O (SLO) is a prototypic member of the cholesterol-binding and pore-forming hemolysins. After binding to cholesterol, SLO monomers form homotypic polymers that generate large transmembrane channels (5). SLO is produced by pyogenic streptococci that cause severe diseases ranging from pharyngitis and impetigo to the often fatal states of streptococcal toxic shock syndrome and necrotizing fasciitis. In order to overrun host defenses, streptococci have developed multifaceted virulence mechanisms, including production of pyrogenic exotoxins, which act as superantigens to cause massive production of cytokines that lead to toxic shock (10). Recent studies of mice (26) and chicken embryos (37) have reinforced the old concept that SLO is another virulence factor. Somewhat unexpectedly, SLO was not required for the formation of necrotic lesions or for bacterial dissemination in a mouse model of invasive streptococcal disease (26). Nevertheless, mice infected with an SLO-negative mutant exhibited decreased mortality compared to animals infected with congenic wild-type streptococci (26). This suggested that SLO might provoke deleterious effects via mechanisms other than simple tissue destruction.
Keratinocytes and endothelial cells attacked by staphylococcal alpha-toxin and SLO are able to repair a limited number of transmembrane lesions (39, 40), and this process is accompanied by activation of NF-κB and production of important cytokines (41).
Mast cells are prominent at all potential entry sites for pathogens, especially in skin, intestinal, and respiratory mucosa and around blood vessels (2), and they have recently been recognized to represent sentinels of the immune system (16). Due to their ability to recognize a large spectrum of microbial structures, mast cells are capable of initiating a life-saving inflammatory response in mouse models of acute bacterial inflammation (28, 29).
To complement the observation that mast cells treated with SLO release histamine from their intracellular stores (22), we examined whether these cells are also transcriptionally activated following transient membrane permeabilization. We report transcriptional activation of genes for several cytokines, including tumor necrosis factor alpha (TNF-α), and show that release of biologically active TNF-α occurs as a result of de novo synthesis and not vesicular release of preformed cytokines by bone marrow-derived mast cells (BMMC). TNF-α synthesis, but not toxin-induced granule secretion, was dependent on activation of p38 mitogen-activated protein (MAP) kinase and protein kinase C (PKC).
MATERIALS AND METHODS
Cytokines.
Murine interleukin-3 (mIL-3) was isolated from supernatants of myelomonocytic WEHI-3B cells using DEAE chromatography. Recombinant mIL-4 was a gift of W. Müller, Department of Experimental Immunology, GBF, Braunschweig, Germany.
Generation of BMMC.
BALB/cJ mice were obtained from the Zentralinstitut für Versuchstierforschung, Hannover, Germany; bred in our animal facility (Zentrale Versuchstieranlage, Johannes-Gutenberg-Universität, Mainz, Germany); and used at the age of 5 to 10 weeks.
The mice were sacrificed by cervical dislocation; intact femurs and tibias were removed, and bone marrow cells were harvested by repeated flushing with minimal essential medium.
The cell culture was established at a density of 3 × 106 cells/ml in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum (FCS; inactivated at 56°C), 2 mM l-glutamine, 1 mM pyruvate, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 20 U of mIL-3/ml, and 50 U of mIL-4/ml. Nonadherent cells were transferred to fresh culture plates every 2 to 3 days for a total of at least 21 days to remove adherent macrophages and fibroblasts. Fluorescence-activated cell sorter analyses using an anti-CD13 antibody (R3-242; Pharmingen, San Diego, Calif.) (19) and immunoglobulin E (IgE) plus anti-IgE monoclonal antibody (3, 15, 30), as well as May-Grünwald-Giemsa and toluidine blue staining, revealed that the resulting cell population consisted of >99% BMMC (data not shown).
Preparation of recombinant SLO.
SLO and the SLO mutant N402E were expressed in Escherichia coli and purified as described previously (42). Freshly prepared SLO was stored frozen in aliquots and thawed only once.
In vitro cell stimulation.
The test medium was Iscove's modified Dulbecco's medium supplemented with 5% FCS (previously inactivated at 56°C), 1 mM pyruvate, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml.
Stimulations were conducted in prewarmed test medium using 96-well plates with 105 cells/well for the β-hexosaminidase assay or 2 × 105 cells/well for the TNF-α test in a final volume of 200 μl, including appropriate concentrations of SLO.
PKC was depleted by overnight incubation of mast cells with 80 ng of the phorbol ester phorbol-12 myristate-13 acetate (PMA; Sigma, Deisenhofen, Germany)/ml. The activity of p38 MAP kinase was inhibited by incubating mast cells in the presence of SB203580 (Calbiochem, Schwalbach, Germany).
Determination of cellular ATP.
BMMC were treated with various concentrations of SLO, and intracellular ATP was measured with luciferase (Roche, Mannheim, Germany) as described previously (6).
β-Hexosaminidase release.
Degranulation was quantified by assaying the activity of β-hexosaminidase (33). Briefly, BMMC were activated for 30 min at 37°C. The cells were spun down and lysed in 0.5% Triton X-100. Two 20-μl aliquots from each supernatant and the corresponding lysate were transferred to separate plates. Fifty microliters of substrate solution (1.3 mg of p-nitrophenyl-N-acetyl-β-d-glucosamine/ml in 0.1 M sodium citrate, pH 4.5) was added, and the plates were incubated for 90 min at 37°C. The reaction was stopped by the addition of 150 μl of 0.2 M glycine, pH 10.7. Hydrolysis of the substrate was measured at 405 nm. β-Hexosaminidase activities in the supernatant and lysate were added and defined as the total enzyme content. The results are expressed as the percentage of β-hexosaminidase activity released into the medium.
RNA purification and reverse transcription-PCR.
RNA was isolated using a modification of the protocol of Chomczynski and Sacchi (8) as described previously (14) and was used for reverse transcription with SUPERSCRIPT RNase H− (Life Technologies, Karlsruhe, Germany) following the recommendations of the supplier.
For PCR, the following primers were used: HGPRT forward (GTTGGATACAGGCCAGACTTTGTTG) and reverse (GAGGGTAGGCTGGCCTATAGGCT); GM-CSF forward (GCAGAATTTACTTTTCCTTT) and reverse (AGGGGATATCAGTCAGAAAG); IL-6 forward (GCCAGAGTCCTTCAGAGAGATAC) and reverse (CCCAACGATTCATATTGTCAG); IL-4 forward (GCATGGTGGCTCAGTACTACGAGTA) and reverse (GAATGTACCAGGAGCCATATCCACG); TNF-α forward (GTAGCCCACGTCGTAGCAAACC) and reverse (GGTATATGGGCTCATACCAGGG); MCP-1 forward (ACTGAAGCCAGCTCTCTCTTCCTC) and reverse (TTCCTTCTTGGGGTCAGCACAGAC); and IL-13 forward (GCATGCCATGGCGCTCTGGGTGACTGC) and reverse (CGCGGATTCGAAGGGGCCGTGGCGAAACAG).
Bioassay for TNF-α.
Following incubation with SLO, BMMC were centrifuged at 500 × g for 5 min, and the cells were lysed in fresh medium (equal to the starting amount) by freezing and thawing. The biological activity of TNF-α was assayed using WEHI-164 target cells cultured in RPMI- 10% FCS; 40,000 cells/50 μl in medium containing 2 μg of actinomycin D/ml were added to equal volumes of serially diluted TNF-α containing supernatants or cell lysates and incubated at 5% CO2 and 37°C for 24 h. Serial dilutions of human recombinant TNF-α (a gift of G. R. Adolf, Firma Bender, Vienna, Austria) were used as a reference. Fifty microliters of 0.25% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (Sigma) in phosphate-buffered saline was added, and the cells were further incubated for 2 h. One hundred microliters of sodium dodecyl sulfate-dimethylformamide (SDS-DMF) was then added, and the optical density was measured at 570 nm (reference, 660 nm) after a 3-h incubation. SDS-DMF was prepared by mixing 2 volumes of 30% SDS and 1 volume of DMF, and the pH was adjusted to 4.7. The specificity of the assay was verified using the ammonium-precipitated rat monoclonal antibody V1q raised against mTNF-α at a concentration of 50 μg/ml (13). To exclude any influence of SLO, SB203580, or PMA on the cytotoxicity assay, these substances were added in comparable concentrations to the WEHI-164 cells alone. The measured effects were negligible.
Assay of MAP kinase activation.
Cells were lysed in 1% SDS- 15% glycerol- 4 M urea- 50 mM Tris, pH 6.8 (150 μl/106 cells), boiled for 3 min, and then passed through a syringe several times in order to reduce the viscosity. After determination of the protein concentration using the DC protein assay (Bio-Rad, Munich, Germany), bromophenol blue and β-mercaptoethanol were added to final concentrations of 100 ng/μl and 1%, respectively, and samples containing 10 to 15 μg of protein were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting (25, 38). Activated MAP kinases were detected using phosphospecific antibodies for p38 MAP kinase and c-jun N-terminal kinase (JNK). Detection of total p38 with a p38-specific antibody served as an equal-loading control (Cell Signaling Technology; New England Biolabs, Frankfurt am Main, Germany).
RESULTS
Treatment of mast cells with SLO induces transcriptional activation leading to the production of biologically active TNF-α.
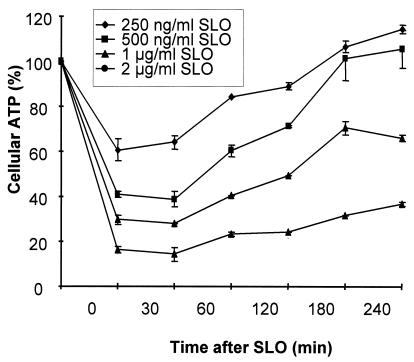
Transient permeabilization of cells with SLO can be followed by monitoring cellular ATP content, and pilot experiments were performed in serum-containing medium (5% FCS) to determine the SLO concentrations that allow cellular recovery. Following exposure to SLO, the ATP levels dropped within 30 min and, at SLO concentrations which permit cellular survival, gradually recovered and reached nearly 100% of the original value after 4 h (Fig. 1). The transient permeabilization of cells can also be followed by monitoring trypan blue uptake. At SLO concentrations which do not permit survival of the cells, dead cells are irreversibly stained with the dye (not shown).
FIG. 1.
Recovery of cellular ATP levels following treatment of mast cells with SLO. Mast cells were treated with SLO at the indicated concentrations, and ATP was quantified at the given times. Cellular ATP is given as the percentage of luminescence relative to untreated control cells. Shown are the means of three experiments. The error bars represent standard deviations.
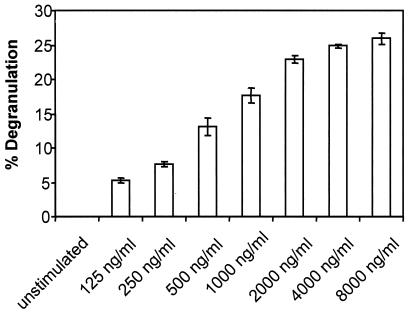
It has been reported that treatment of mast cells with cytocidal doses of SLO results in granule exocytosis (22). We quantified the release of β-hexosaminidase, and the dose-response curve is shown in Fig. 2. Release of the granular enzyme occurred at low SLO concentrations and remained constant thereafter, irrespective of cellular recovery.
FIG. 2.
Dose-response titration for the SLO-induced degranulation of mast cells. Mast cells were stimulated for 30 min in the presence of various concentrations of SLO as indicated. Degranulation was measured as the release of β-hexosaminidase as described in Materials and Methods. Shown are the means of three experiments. The error bars represent standard deviations.
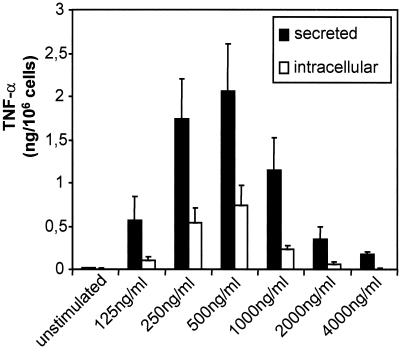
A different response pattern was observed when TNF-α was assayed using a bioassay based on the cytotoxic activity of TNF-α on WEHI-164 target cells. As shown in Fig. 3, the amounts of both secreted and cell-associated TNF-α were highest when SLO was applied at concentrations that led to transient permeabilization. The potency of mast cells to initiate life-saving inflammatory responses in murine models of acute bacterial inflammation has been ascribed mainly to their unique property of storing preformed TNF-α within their secretory granules, which can be released upon demand (17). However, in our experiments, unstimulated cells contained virtually no TNF-α, which was in accord with the observations of other laboratories that TNF-α is not present in granules of unstimulated BMMC and most mast cell lines (17, 18). Slight batch-to-batch variations of different SLO preparations used to generate the data reported here were found in these experiments, with the SLO concentrations used to achieve maximal production of TNF-α varying between 500 and 1,000 ng/ml.
FIG. 3.
Dose-response titration for the SLO-induced production of TNF-α. Mast cells were stimulated for 1 h in the presence of various concentrations of SLO as indicated. Thereafter, biologically active TNF-α was determined in the supernatants (secreted) and in the cell lysates (intracellular) obtained by freezing and thawing. Shown are the means of three experiments. The error bars represent standard deviations.
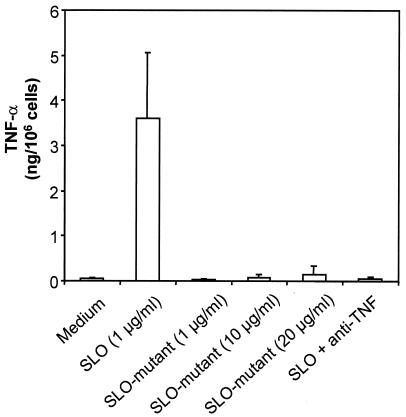
To exclude the possibility that bacterial contaminations in the recombinant SLO preparations might lead to the activation of mast cells, we tested the recombinant SLO mutant N402E in parallel. This mutant had been generated by introducing a point mutation in SLO which still allowed membrane binding but abolished the oligomerization of protein monomers and hence the pore-forming capability of SLO (1). Treatment of mast cells with N402E, even at concentrations 20-fold higher than those of wild-type SLO, did not induce any production of TNF-α (Fig. 4). In this experiment, as a further control, the cytotoxic activities of supernatants derived from mast cells which had been treated with SLO could be abolished by the addition of a neutralizing anti-TNF-α monoclonal antibody (Fig. 4). Since this antibody does not cross-react with human TNF-α, the neutralization of mTNF-α in the mast cell supernatants could be compensated for by the addition of the human cytokine (data not shown).
FIG. 4.
The SLO mutant N402E does not induce the production of biologically active TNF-α. Mast cells were activated with the indicated concentrations of SLO or the SLO mutant N402E for 2 h, and a bioassay for biologically active TNF-α in the supernatants was performed. As an additional internal control, the biological activity of TNF-α was blocked by the addition of a neutralizing anti-TNF-α antibody (anti-TNF). Shown are the means of three experiments. The error bars represent standard deviations.
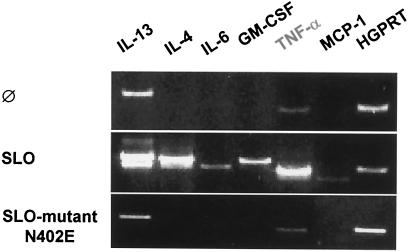
Since our finding that TNF-α release was abrogated due to cell death at high concentrations of SLO indicated that toxin-induced TNF-α secretion was due to de novo protein synthesis, we addressed the question of whether mast cells are transcriptionally activated to produce mRNA for various cytokines. As shown in Fig. 5, treatment of BMMC with SLO rapidly enhanced or induced the production of mRNAs for the cytokines IL-4, IL-13, IL-6, granulocyte-macrophage colony-stimulating factor, and TNF-α and for monocyte chemoattractant protein 1 (MCP-1), whereas the SLO mutant N402 had no effect on cytokine mRNA expression.
FIG. 5.
Treatment of mast cells with SLO induces the transcription of various cytokines. Cells were left untreated (Ø) or incubated with SLO (0.5 μg/ml) or the SLO mutant N402E (0.5 μg/ml) for 1 h, and reverse transcription-PCR was performed as described in Materials and Methods. The experiment shown is representative of three independent experiments with equivalent results. HGPRT, hypoxanthine-quanine phosphoribosyltransferase.
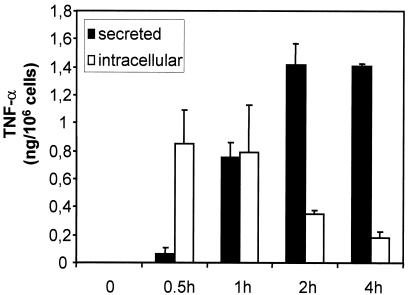
Since mast cell-derived TNF-α is critical for the early and life-saving recruitment of neutrophils in mouse models of bacterial infections, we analyzed the SLO-induced production of this cytokine in more detail. With respect to the kinetics of TNF-α production, it could readily be detected as early as 30 min poststimulation, and maximum release was reached between 2 and 4 h (Fig. 6). It can further be concluded from the data shown in Fig. 6 that the de novo production of TNF-α obviously ceased ∼1 h after stimulation, and accumulation of TNF-α in the supernatant was paralleled by a decrease in intracellular TNF-α.
FIG. 6.
Kinetics of TNF-α production and secretion induced by SLO. Mast cells were treated with SLO (500 ng/ml), and biologically active TNF-α was determined in the supernatants (secreted) and in the cell lysates (intracellular), obtained by freezing and thawing, at the indicated times. Shown are the means of three experiments. The error bars represent standard deviations.
SLO-induced production of TNF-α strongly depends on activation of p38 MAP kinase and PKC.
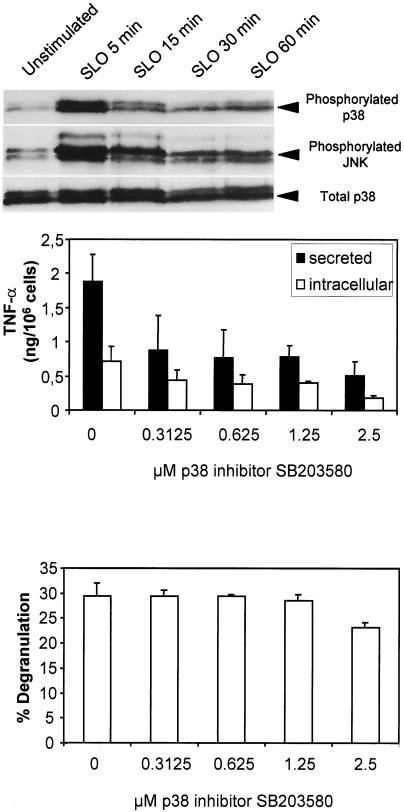
We next addressed the question of which intracellular signaling pathways might contribute to the SLO-induced production and secretion of TNF-α. Likely candidates were the stress-activated protein kinases p38 MAP kinase and JNK, whose activation can be detected by using antibodies specific for the phosphorylated proteins (12, 32). As shown in Fig. 7, top, both p38 MAP kinase and JNK were indeed activated upon treatment of mast cells with SLO. Activation occurred very rapidly and was transient, with maximum phosphorylation of both kinases observed 5 min after toxin application. To assess whether the activation of p38 and JNK was critical for SLO-induced degranulation and production of TNF-α, we stimulated mast cells with SLO in the presence of MAP kinase inhibitors. Inhibition of JNK activation using the PI3K inhibitor wortmannin (23) had no effect on either SLO-induced degranulation or production of TNF-α (data not shown). In contrast, inhibition of p38 MAP kinase with SB203580 (11) profoundly impaired the production of TNF-α without affecting degranulation (Fig. 7, middle and bottom). Semiquantitative mRNA analyses also revealed that in the presence of 2.5 μM SB203580, SLO-induced TNF-α mRNA expression is reduced to 25% (data not shown). Therefore, we concluded that SLO-induced production of TNF-α is at least partially driven by activation of p38 MAP kinase.
FIG. 7.
SLO-induced activation of p38 MAP kinase is critical for the production of TNF-α but not for the degranulation of mast cells. (Top) Mast cells were activated with SLO (1 μg/ml), and cell lysates were prepared at the indicated times. Activation of the stress-activated MAP kinases p38 and JNK was assessed by Western blotting using antibodies specific for the phosphorylated kinases. After stripping of the blot, reprobing with an antibody against total p38 MAP kinase served as an equal-loading control. The experiment shown is representative of two experiments with comparable results. (Middle and bottom) Mast cells were stimulated with SLO (1 μg/ml) for 1 h in the presence of the indicated concentrations of the p38 MAP kinase inhibitor SB203580. Thereafter, biologically active TNF-α was determined in the supernatants (secreted) and in the cell lysates (intracellular) obtained by freezing and thawing. Degranulation was measured as the release of β-hexosaminidase after 30 min. Shown are the means of three experiments. The error bars represent standard deviations.
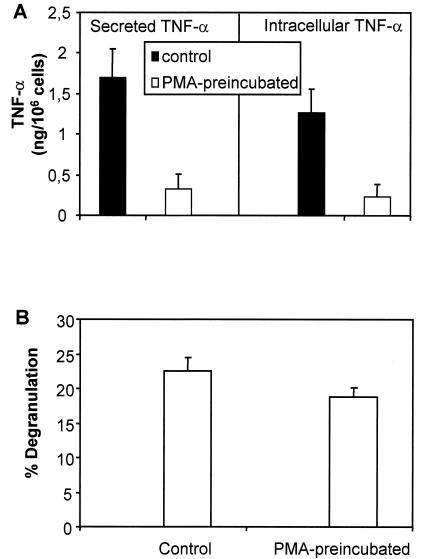
It has been reported that PKC is critical for both production of TNF-α and degranulation following cross-linking of IgE (4, 31, 34), so we next examined whether depletion of PKC by long-term (24-h) incubation with PMA (35) had any effect on the SLO-induced activation of mast cells. As depicted in Fig. 8A, depletion of PKC prior to the activation of mast cells with SLO strongly decreased production and secretion of TNF-α. However, preincubation with PMA did not influence the production of TNF-α mRNA (not shown). This observation is compatible with a previous report that PKC activity is required for TNF-α secretion by RBL-2H3 mast cells stimulated through the high-affinity IgE receptor but is dispensable for TNF-α mRNA expression (34). This finding implied that activation of PKC was also a critical event for the production of TNF-α induced by SLO, at least at the posttranscriptional level. In contrast, depletion of PKC did not significantly influence the SLO-induced degranulation of mast cells (Fig. 8B).
FIG. 8.
Depletion of PKC reduces the SLO-induced production of TNF-αbut does not affect the degranulation of mast cells.(A)After overnight preincubation with PMA, mast cells were activated with SLO (1 μg/ml) for 1 h,and biologically active TNF-αwas determined in the supernatants(Secreted TNF-α)and in the cell lysates(Intracellular TNF-α).(B)After overnight preincubation with PMA, mast cells were activated with SLO (1 μg/ml) for 30 min,and degranulation was measured as the release of β-hexosaminidase. Shown are the meansof threeexperiments. The error bars represent standard deviations.
DISCUSSION
There is growing evidence that many bacterial toxins possess cytokine-inducing activities and that the host proinflammatory cytokine response may contribute to pathogenesis (20).
With respect to pore-forming toxins, this was shown for the aerolysin-related enterotoxin (Act), an important virulence factor of Aeromonas hydrophila. In macrophages, Act caused increased levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and activated arachidonic acid metabolism and transcription of inducible nitric oxide synthase (9). Pneumolysin, a pore-forming hemolysin produced by Streptococcus pneumoniae, also was capable of increasing TNF-α and IL-6 levels in macrophages and initiating the production of NO (7).
A role for SLO in infection was suggested based on the finding that keratinocytes infected with SLO-deficient Streptococcus pyogenes displayed reduced expression of IL-1β, IL-6, IL-8, and prostaglandin E2 compared with the wild-type strain (36). Indeed, it was shown that reversible permeabilization of keratinocytes and endothelial cells with SLO is followed by the release of IL-6 and IL-8 and that production of these cytokines is preceded by activation of NF-κB (41). With respect to the cytocidal action of SLO, it has recently been reported that SLO directs the translocation of S. pyogenes NAD-glycohydrolase into the cytosol of keratinocytes (27). Translocation is contact dependent and equivalent to type III secretion, which has previously been described only in gram-negative bacterial species. The effector molecule NAD-glycohydrolase triggers a cytocidal response in infected cells and probably also interacts with a host signaling pathway required to repair damage caused by SLO. However, at present it is unclear whether this process also modulates cytokine production by infected cells.
It has been reported that rat mast cells permeabilized with SLO release histamine, β-hexosaminidase, and lactate dehydrogenase in the presence of micromolar concentrations of Ca2+. Since the release of both histamine and β-hexosaminidase had an additional requirement for nucleoside triphosphates, it was concluded that these substances are secreted via an exocytotic pathway, while lactate dehydrogenase simply leaks from the cells through the toxin-generated transmembrane pores (22).
In this study, degranulation of mast cells, measured by the release of the granular enzyme β-hexosaminidase, was initiated at low doses of SLO when virtually no cell death was observed and remained constant at high concentrations when the cells were killed. Of note, our observation that PKC is not involved in SLO-induced degranulation suggests that this process profoundly differs from degranulation triggered by cross-linking of IgE.
A novel aspect reported here is the transcriptional activation of mast cells occurring at low doses of SLO that allow cellular recovery. The production and secretion of mast cell-derived TNF-α harbor potentially important implications. Upon local infection with pyogenic streptococci, production and release of TNF-α might contribute to host defense, as demonstrated in previous studies (28, 29). However, upon severe and systemic infection, overproduction of TNF-α would be expected to exert a detrimental influence and might then contribute to the development of toxic streptococcal syndromes. Thus, dual and counteractive effects—activation of a curative immune response versus the induction of septic shock—may be provoked by SLO, as has been observed for other cytokine-inducing microbial products, e.g., lipopolysaccharide. In general, recognition of bacterial constituents primarily leads to cell activation and production of proinflammatory mediators (e.g., TNF-α and IL-6) necessary for an effective innate inflammatory response, but when this beneficial response gets out of control, as in septic shock, these mediators can be extremely harmful (21, 24). In both circumstances, SLO likely produces sophisticated effects involving mechanisms other than simple destruction of cells and tissues.
Acknowledgments
We thank Erwin Rüde for critical and fruitful discussions and Sandra Gerecht, Alexandra Hobel, and Marion Krail for expert technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft grants SCHM 1014-4-1 and SFB 490 (projects C1 and C2).
Editor: V. J. DiRita
REFERENCES
- 1.Abdel, G. E., S. Weis, I. Walev, M. Kehoe, S. Bhakdi, and M. Palmer. 1999. Streptolysin O: inhibition of the conformational change during membrane binding of the monomer prevents oligomerization and pore formation. Biochemistry 38:15204-15211. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, S. N., and R. Malaviya. 1997. Mast cells in infection and immunity. Infect. Immun. 65:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniyash, M., and Z. Eshhar. 1984. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur. J. Immunol. 14:799-807. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner, R. A., K. Yamada, V. A. Deramo, and M. A. Beaven. 1994. Secretion of TNF from a rat mast cell line is a brefeldin A-sensitive and a calcium/protein kinase C-regulated process. J. Immunol. 153:2609-2617. [PubMed] [Google Scholar]
- 5.Bhakdi, S., H. Bayley, A. Valeva, I. Walev, B. Walker, M. Kehoe, and M. Palmer. 1996. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch. Microbiol. 165:73-79. [DOI] [PubMed] [Google Scholar]
- 6.Bhakdi, S., M. Muhly, S. Korom, and G. Schmidt. 1990. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J. Clin. Investig. 85:1746-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Chopra, A. K., X. Xu, D. Ribardo, M. Gonzalez, K. Kuhl, J. W. Peterson, and C. W. Houston. 2000. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 68:2808-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, C., R. J. Davis, and R. A. Flavell. 2001. Signaling by the JNK group of MAP kinases. c-jun N-terminal kinase. J. Clin. Immunol. 21:253-257. [DOI] [PubMed] [Google Scholar]
- 13.Echtenacher, B., W. Falk, D. N. Mannel, and P. H. Krammer. 1990. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145:3762-3766. [PubMed] [Google Scholar]
- 14.Erlich, H. A. 1989. PCR technology: principles and applications. Stockton Press, New York, N.Y.
- 15.Eshhar, Z., M. Ofarim, and T. Waks. 1980. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J. Immunol. 124:775-780. [PubMed] [Google Scholar]
- 16.Galli, S. J., M. Maurer, and C. S. Lantz. 1999. Mast cells as sentinels of innate immunity. Curr. Opin. Immunol. 11:53-59. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, J. R., and S. J. Galli. 1990. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 346:274-276. [DOI] [PubMed] [Google Scholar]
- 18.Gordon, J. R., and S. J. Galli. 1991. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 174:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen, A. S., O. Noren, H. Sjostrom, and O. Werdelin. 1993. A mouse aminopeptidase N is a marker for antigen-presenting cells and appears to be co-expressed with major histocompatibility complex class II molecules. Eur. J. Immunol. 23:2358-2364. [DOI] [PubMed] [Google Scholar]
- 20.Henderson, B., M. Wilson, and B. Wren. 1997. Are bacterial exotoxins cytokine network regulators? Trends Microbiol. 5:454-458. [DOI] [PubMed] [Google Scholar]
- 21.Heumann, D., M. P. Glauser, and T. Calandra. 1998. Molecular basis of host-pathogen interaction in septic shock. Curr. Opin. Microbiol. 1:49-55. [DOI] [PubMed] [Google Scholar]
- 22.Howell, T. W., and B. D. Gomperts. 1987. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim. Biophys. Acta 927:177-183. [DOI] [PubMed] [Google Scholar]
- 23.Ishizuka, T., K. Chayama, K. Takeda, E. Hamelmann, N. Terada, G. M. Keller, G. L. Johnson, and E. W. Gelfand. 1999. Mitogen-activated protein kinase activation through Fc epsilon receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J. Immunol. 162:2087-2094. [PubMed] [Google Scholar]
- 24.Karima, R., S. Matsumoto, H. Higashi, and K. Matsushima. 1999. The molecular pathogenesis of endotoxic shock and organ failure. Mol. Med. Today 5:123-132. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Limbago, B., V. Penumalli, B. Weinrick, and J. R. Scott. 2000. Role of streptolysin O in a mouse model of invasive group A streptococcal disease. Infect. Immun. 68:6384-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 28.Malaviya, R., T. Ikeda, E. Ross, and S. N. Abraham. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77-80. [DOI] [PubMed] [Google Scholar]
- 29.Mannel, D. N., L. Hultner, and B. Echtenacher. 1996. Critical protective role of mast cell-derived tumour necrosis factor in bacterial infection. Res. Immunol. 147:491-493. [DOI] [PubMed] [Google Scholar]
- 30.Mazurek, N., H. Schindler, T. Schurholz, and I. Pecht. 1984. The cromolyn binding protein constitutes the Ca2+ channel of basophils opening upon immunological stimulus. Proc. Natl. Acad. Sci. USA 81:6841-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nechushtan, H., and E. Razin. 2001. Studies of different aspects of the role of protein kinase C in mast cells. Int. Arch. Allergy Immunol. 124:130-132. [DOI] [PubMed] [Google Scholar]
- 32.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 33.Ortega, E., B. Hazan, U. Zor, and I. Pecht. 1989. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur. J. Immunol. 19:2251-2256. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier, C., C. Guerin-Marchand, B. Iannascoli, F. Marchand, B. David, A. Weyer, and U. Blank. 1998. Specific signaling pathways in the regulation of TNF-alpha mRNA synthesis and TNF-alpha secretion in RBL-2H3 mast cells stimulated through the high affinity IgE receptor. Inflamm. Res. 47:493-500. [DOI] [PubMed] [Google Scholar]
- 35.Razin, E., Z. Szallasi, M. G. Kazanietz, P. M. Blumberg, and J. Rivera. 1994. Protein kinases C-beta and C-epsilon link the mast cell high-affinity receptor for IgE to the expression of c-fos and c-jun. Proc. Natl. Acad. Sci. USA 91:7722-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz, N., B. Wang, A. Pentland, and M. Caparon. 1998. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol. Microbiol. 27:337-346. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, K. H., D. Gerlach, K. Gubbe, A. Geyer, E. Birch-Hirschfeld, E. Straube, and A. Podbielski. 2001. Virulence of group A streptococci in fertile hens eggs is mainly effected by M protein and streptolysin O. Int. J. Med. Microbiol. 291:45-56. [DOI] [PubMed] [Google Scholar]
- 38.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valeva, A., I. Walev, A. Gerber, J. Klein, M. Palmer, and S. Bhakdi. 2000. Staphylococcal alpha-toxin: repair of a calcium-impermeable pore in the target cell membrane. Mol. Microbiol. 36:467-476. [DOI] [PubMed] [Google Scholar]
- 40.Walev, I., S. C. Bhakdi, F. Hofmann, N. Djonder, A. Valeva, K. Aktories, and S. Bhakdi. 2001. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. USA 98:3185-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walev, I., M. Hombach, W. Bobkiewicz, D. Fenske, S. Bhakdi, and M. Husmann. 2002. Resealing of large transmembrane pores produced by streptolysin O in nucleated cells is accompanied by NF-κB activation and downstream events. FASEB J. 16:237-239. [DOI] [PubMed] [Google Scholar]
- 42.Weller, U., L. Muller, M. Messner, M. Palmer, A. Valeva, J. Tranum-Jensen, P. Agrawal, C. Biermann, A. Dobereiner, M. A. Kehoe, and S. Bhakdi. 1996. Expression of active streptolysin O in Escherichia coli as a maltose-binding-protein- streptolysin-O fusion protein. The N-terminal 70 amino acids are not required for hemolytic activity. Eur. J. Biochem. 236:34-39. [DOI] [PubMed] [Google Scholar]