Abstract
GD3, a ganglioside expressed on human melanoma, can be recognized by the humoral immune system. In this paper, we demonstrate that immunizing mice with the human melanoma cell line SK-MEL-28 (GD3+ GM2− CD1−) or with syngeneic APCs loaded with GD3 can induce a GD3-reactive natural killer T (NKT) cell response. GD3-reactive NKT cells were detected among splenocytes of immunized mice at frequencies of ∼1:2,000 both by ELISPOT and GD3-loaded mouse CD1d tetramer analysis. GD3-reactive NKT cells did not react with GM2, a closely related ganglioside, and were not detectable in unimmunized mice. GD3-reactive NKT cells initially produced IL-4 and IFN-γ followed by IL-10. They were CD1d restricted in that reactivity was abrogated when APCs were blocked with anti-CD1d monoclonal antibody before being loaded with GD3 or when APCs from CD1d knockout mice were used. Because SK-MEL-28 does not express any isoform of human CD1, GD3 must be cross-presented by murine APCs in vivo. This is the first analysis of a natural ligand for mouse NKT cells and the first definitive paper of cross-presentation to NKT cells. This could be a mechanism for NKT cell recognition of tumor gangliosides in CD1− tumors.
Keywords: NKT cell, α-galactosylceramide, CD1d, tetramer, melanoma
Introduction
CD1 molecules are a family of antigen-presenting molecules with the ability to present lipids and glycolipids to T cells and to natural killer T (NKT)* cells (1). Many of the glycolipids presented by the CD1 family, such as bacterial glycolipids (e.g., glucose monomycolate; reference 2), α-galactosylceramide (αGalCer) from marine sponges (3), and self monosialogangliosides (GM1; reference 4), share a structural motif comprising a hydrophilic head group and two hydrophobic acyl chains (5). CD1d, the only CD1 isoform expressed in the mouse, has two hydrophilic pockets likely to accommodate hydrophobic acyl tails of glycolipid molecules, allowing the hydrophilic carbohydrate portion to be presented to the TCR (6).
In mice, most NKT cells (NK1.1+ TCRαβ+) are reactive to glycolipids in the context of CD1d. They are phenotypically and functionally heterogeneous and may be CD4+, or CD4−8− double negative (DN; reference 7), although they tend to express an invariant TCR α-chain formed by a Vα14-Jα18 rearrangement paired with a limited TCR β chain repertoire (8, 9). αGalCer, a marine sponge glycolipid not present in mammalian cells, has been found to be a universal ligand for NKT cells expressing invariant Vα14-Jα18 TCR (3), and these NKT cells can be identified using αGalCer-CD1d tetramers (10, 11). Although αGalCer is the best-characterized antigen for NKTs, no natural ligands of mouse NKT cells have been identified to date.
GD3 is a relatively tumor-restricted ganglioside highly expressed on human tumors of neuroectodermal origin (e.g., melanoma [12] and small-cell lung carcinoma [13]), but it is expressed on only a few normal tissues in the human or mouse, and at low levels. Although mAb against GD3 can induce antimelanoma responses in animal models and in humans (14–16), there is no information as to whether GD3 can induce a cellular immune response. Indeed, gangliosides have been reported to inhibit T cell responses (17), although some form of T cell help would be anticipated to explain the generation of anti-GD3 IgG in immunized mice.
The structure of GD3 suggested the possibility that GD3 could be presented by members of the CD1 family. Indeed, previous analyses showed that in the human system, T cells reactive against a GM1 ganglioside could be detected (18). We were interested to determine if GD3 can be presented by CD1d to mouse NKT cells.
Materials and Methods
Mice.
Female inbred C57BL/6 mice, 4–6-wk old, purchased from the National Cancer Institute were used throughout this work. CD1d knockout (CD1d−/−) mice were provided by Dr. S.P. Balk (Harvard Medical School, Boston, MA). 2C mice, which are transgenic for the TCR-recognizing peptide SIYRYYGL (19), were provided by Dr. D. SantAngelo (Sloan-Kettering Institute, New York, NY). Mice were maintained in our animal facility under specific pathogen-free conditions.
Immunization Protocol.
Human melanoma cell line, SK-MEL-28, which expresses GD3 but does not express GM2 (20), was used for immunizing mice. For immunization, mice were injected s.c. with 2 × 106 SK-MEL-28 mixed with CFA (Sigma-Aldrich) and boosted 1 wk later with 2 × 106 SK-MEL-28 with IFA (Sigma-Aldrich). In the control group, mice were injected with CFA followed by IFA only without SK-MEL-28. Mice were killed 1 wk after boost, and spleen, liver, and thymus were removed and processed individually.
In some experiments, naive mice were injected s.c. into footpads with 105 syngeneic APCs loaded with 1 μg/ml GD3 or unloaded APCs. On day 7, splenocytes were collected and processed individually, and popliteal and inguinal LN were collected and pooled.
For experiments measuring the kinetics of cytokine production, mice received up to two additional weekly boosts.
Cell Preparation.
Single-cell suspensions of splenocytes, thymocytes or liver cells were prepared by gently pressing the tissue through a sterile 70-μm stainless steel mesh into RPMI 10 (RPMI 1640 supplemented with 10% FCS, 50 μM 2-ME, nonessential amino acids, 100 U/ml penicillin, and 100 U/ml streptomycin). To isolate mononuclear cells from the liver, cell suspensions from liver tissue were further washed once with RPMI 10, resuspended in an isotonic 33% Percoll solution (Sigma-Aldrich) containing 100 U/ml of heparin, and were centrifuged at 500 g for 15 min at room temperature. RBC were lysed with RBC-lysing buffer (containing 150 mM NH4Cl and 10 mM Tris-HCl) and washed twice in RPMI 10.
APCs were splenocytes from naive syngeneic mice injected s.c. with 10 μg Flt3 ligand (Immunex) daily for nine consecutive days. Splenocytes were irradiated (3,000 rad), washed, and resuspended in RPMI 10. APCs were enriched by incubating splenocytes in plastic culture dishes for 2 h at 37°C. The adherent cells were pulsed with ganglioside antigen (1 μg/ml 0.1% DMSO; Matreya) or with control vehicle (0.1% DMSO). After overnight incubation at 37°C, the nonadherent cells were collected, washed, and used as APCs. Approximately 65% of CD11c+ MHC class II+–enriched APCs from Flt3 ligand-treated mice were found to be CD1d+ (unpublished data).
Antibody Blocking Experiments.
mAb used in this work were purchased from BD Biosciences unless otherwise noted. Before loading with ganglioside, APCs were incubated with 10 μg/ml anti–mouse-CD1d mAb (clone 20H2; American Type Culture Collection), anti-Kb/Db (clone 28–8-6), anti–mouse I-Ab mAb (clone KH74; American Type Culture Collection) mAb, or a negative control Ter-119 mAb (21) against mouse erythroid cells for 30 min at 4°C, and washed with RPMI 10 twice.
ELISPOT Assay.
HA-Multiscreen plates (Millipore) were coated with 10 μg/ml rat anti–mouse IL-4 (clone 11B11) or IFN-γ (clone R4–6A2) mAb and blocked with RPMI 10 for 1 h at 37°C. Splenocytes or T cell subsets from immunized mice were plated in triplicate wells at 1–2 × 105 in 100 μl. Effector cells were stimulated with 1–2 × 104 irradiated APCs in 100 μl pulsed with 1 μg/ml ganglioside (0.1% DMSO) or with control vehicle (0.1% DMSO). Control wells contained effector cells alone, APCs alone, or medium alone. After incubation for 20 h at 37°C, plates were washed with PBS/0.05% Tween 20, followed by incubation with 2 μg/ml biotinylated rat anti–mouse IL-4 (clone BVD6–24G2) or IFN-γ (clone XMG1.2) mAb for 2 h at 37°C. Plates were washed, and reacted with avidin–peroxidase complex (Vectastain Elite Kit; Vector Laboratories) for 1 h at room temperature. After washing, plates were developed with 3-amino-9-ethyl-carbazole substrate in H2O2 at room temperature for 4 min, and the reaction was stopped by washing plates under running water. After the plates were dry, spots were counted by using a stereomicroscope at a 40-fold magnification and an automated ELISPOT reader system (Carl Zeiss MicroImaging, Inc.) with KS ELISPOT 4.0 software.
Flow Immunocytometric Analysis.
mAbs against human CD1a-d (provided by Dr. S. Porcelli, Albert Einstein College of Medicine, Bronx, NY) were biotinylated for use in the flow cytometric analysis. Anti–mouse CD3 (FITC-conjugated, clone 17A2), NK1.1 (PE-conjugated, clone PK136), CD4 (CyChrome–conjugated, clone RM4–5), CD8 (Cy-chrome–conjugated, clone 53–6.7), IL-4 (allophycocyanin-conjugated, clone 11B11), IL-5 (allophycocyanin-conjugated, clone TRFK5), IL-10 (allophycocyanin-conjugated, clone JES5–16E3), IL-12 (allophycocyanin-conjugated, clone C15.6), IFN-γ (allophycocyanin-conjugated, clone XMG1.2), and Tri-Color (TC)–conjugated streptavidin (Caltag Laboratories) were used for staining.
For surface staining, 106 cells were incubated with FITC- or PE-conjugated mAb, or with biotin-conjugated mAb followed by incubating with TC-conjugated streptavidin. Isotype-matched mAb were used as negative controls. All incubations were for 20 min at 4°C, after which the cells were washed with FACS® buffer (containing 0.5% BSA and 0.05% sodium azide in PBS).
For intracellular cytokine staining, splenocytes were incubated with APCs pulsed with 1 μg/ml GD3 or with control vehicle for 1 h at 37°C. 10 μg/ml Brefeldin A (Sigma-Aldrich) was added and incubation was continued overnight at 37°C. 106 cells were surface-stained with fluorescence-conjugated anti-CD3 and NK1.1 mAbs, fixed, and permeabilized by incubating with Cytofix/Cytoperm solution (BD Biosciences), and incubated with allophycocyanin-conjugated mAb against cytokine in 10× Perm/Wash Buffer (BD Biosciences). All incubations were performed for 20 min at 4°C, after which the cells were washed with 1× Perm/Wash Buffer.
Acquisition was performed on a FACSCaliber™ (Becton Dickinson), using forward- and side-scatter characteristics to exclude dead cells. Data were analyzed using CELLQuest™ (Becton Dickinson).
For flow cytometry cell sorting, splenocytes pooled from six to eight immunized mice were stained with fluorescence-conjugated anti–mouse CD3, NK1.1, CD4, and/or CD8 mAbs, and subjected to cell sorting by MoFlow cell sorter. Splenocyte subpopulations were sorted by surface expression of CD3, NK1.1, CD4 and CD8: T cells (CD3+ NK1.1−), NKT cells (CD3+ NK1.1+), CD4 NKT cells (CD4+ CD8− CD3+ NK1.1+), CD8 NKT cells (CD4− CD8+ CD3+ NK1.1+), DN NKT cells (CD4− CD8− CD3+ NK1.1+), and NK cells (CD3− NK1.1+).
Tetrameric Mouse CD1d–GD3 Complexes.
Tetrameric mouse CD1d–β2m complexes were produced using a baculovirus expression system as described previously (22). GD3 (Matreya) was sonicated in 500 μM hexadecyltrimethylammonium bromide (Sigma-Aldrich) and incubated with biotinylated CD1d monomer overnight at a 3:1 molar ratio (GD3/CD1d). To prepare unloaded tetramers, 500 μm hexadecyltrimethylammonium bromide alone was incubated with biotinylated CD1d monomer overnight. To form tetramers, TC-conjugated streptavidin was added at a 4:1 molar ratio (CD1d/streptavidin).
GD3 loading was confirmed by ELISA. Microtiter wells (Maxisorb; Nunc) were coated with tetramers (350 ng CD1d/well). Negative control wells were coated with unloaded tetramers or GD3 alone (GD3 in aqueous solution binds only minimally to the wells). After washing, the wells were blocked with 5% nonfat milk and probed with R24 (mouse IgG3 specific for the carbohydrate portion of GD3) at 5–10 μg/ml. Bound R24 was detected with an alkaline phosphatase–conjugated rabbit anti–mouse IgG3 second antibody. GD3 loading was assumed if the tetramer wells showed an OD405 ≥10-fold higher than the control wells.
For αGalCer depletion experiments, 107 splenocytes were incubated with PE-conjugated αGalCer-loaded mouse CD1d tetramer (22) or unloaded CD1d tetramer for 30 min at room temperature, washed twice with MACS buffer (0.5% BSA, 2 mM EDTA in PBS), and incubated with anti-PE microbeads (Miltenyi Biotec) for 15 min at 6–12°C. After washing twice with MACS buffer, the cells were suspended in 500 μl MACS buffer and applied to a prewetted MS+/RS+ separation column (Miltenyi Biotec) in the magnetic field. The αGalCer-negative cells were collected in the run-through.
Oligonucleotide Database.
GeneChip® analysis was performed on SK-MEL-28 cell line (for the complete expression database, see www.mskcc.org/genomic/ccsmsp; reference 23). In brief, cryopreserved tumor sections were homogenized under liquid nitrogen by mortar and pestle. Total RNA was extracted in TRIzol reagent and purified using the RNeasy kit (QIAGEN). RNA quality was assessed on ethidium bromide agarose gel electrophoresis. cDNA was synthesized in the presence of oligo(dT)24-T7 from Genset Corp. cRNA was prepared using biotinylated UTP and CTP and hybridized to HG_U95A oligonucleotide arrays (Affymetrix Inc.). Fluorescence was measured by laser confocal scanner (Agilent Technologies) and converted to signal intensity by Affymetrix Microarray Suite v5.0 software. Expression values across each chip were normalized to the 96th percentile of 2,500. CD1a-d signal intensity was compared between SK-MEL-28 and a set of 9 independent melanoma tumors and 19 melanoma cell lines.
Online Supplemental Material.
SK-MEL-28 melanoma cells were analyzed for expression of human CD1a, CD1b, CD1c, or CD1d using FACS® analysis. MOLT-4 cells (CD1+) were used as positive control.
Results
Immunization Against GD3 Induces Lymphocytes That Recognize GD3.
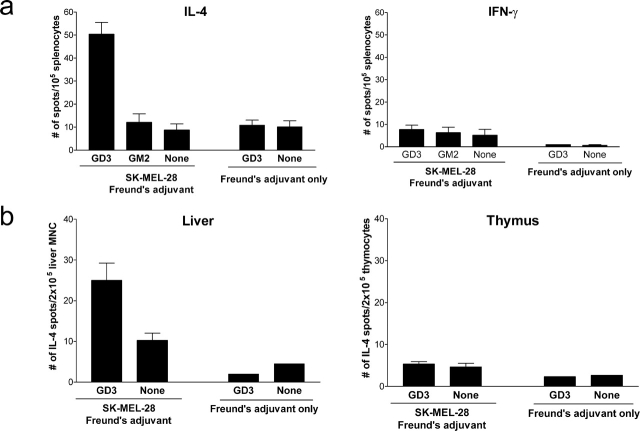
Immunization of mice with human melanoma SK-MEL-28 cells (GD3+, GM2−) expanded a population of splenocytes that produced IL-4 in response to GD3-loaded APCs but did not produce detectable IFN-γ after two immunizations (Fig. 1 a). These GD3-reactive splenocytes were not detectable in the spleens of unimmunized mice, which demonstrated that immunization with the GD3+ melanoma was critical for this response. Reactivity to GD3 was not due to a general, nonspecific reactivity because splenocytes from immunized mice did not recognize unloaded APCs or APCs loaded with GM2, a ganglioside not expressed by SK-MEL-28. This indicates that these splenocytes from immunized mice have some degree of specificity. To exclude the possibility that the splenocyte response against GD3 was induced by lipid components in Freund's adjuvant, we tested splenocytes from mice injected with Freund's adjuvant only. Splenocytes from these mice showed no significant reactivity to unloaded or GD3-loaded APCs, indicating that the splenocyte response to GD3 observed in SK-MEL-28–immunized mice was not due to the Freund's adjuvant. In immunized mice, GD3-reactive lymphocytes were also detected in the liver but not in the thymus (Fig. 1 b).
Figure 1.
Lymphocyte responses induced against GD3 in immunized mice produce IL-4. Mice were immunized with 2 × 106 SK-MEL-28 cells mixed with CFA on day 0, and boosted with 2 × 106 SK-MEL-28 cells mixed with IFA on day 7. Control mice (Freund's adjuvant only) were injected with CFA on day 0 followed by IFA alone on day 7. (a) Splenocytes were collected on day 14 and stimulated with irradiated syngeneic APCs loaded with GD3, GM2, or without ganglioside (None) as indicated. IL-4 and IFN-γ production were detected by ELISPOT assay. (b) In other sets of mice, hepatic mononuclear cells and thymocytes were tested for IL-4 secretion. Data represent mean ± SEM of triplicate wells from four mice and similar results were observed in repeated experiments.
We wished to confirm that it was GD3 and not another molecule expressed by SK-MEL-28 that was responsible for immunization. To do this, mice were immunized by footpad injection with syngeneic APCs loaded with purified GD3; control mice were immunized with unloaded APCs. GD3-reactive lymphocytes were detected in the draining LN, such as popliteal and inguinal LN, (Fig. 2 a) as well as in the spleen (Fig. 2 b) of mice immunized with GD3-loaded APCs. GD3-reactive lymphocytes were not detected in control mice injected with unloaded APCs, or when splenocytes from immunized mice were tested against unloaded APCs in the ELISPOT assay. These studies confirmed that the immunizing molecule was GD3.
Figure 2.
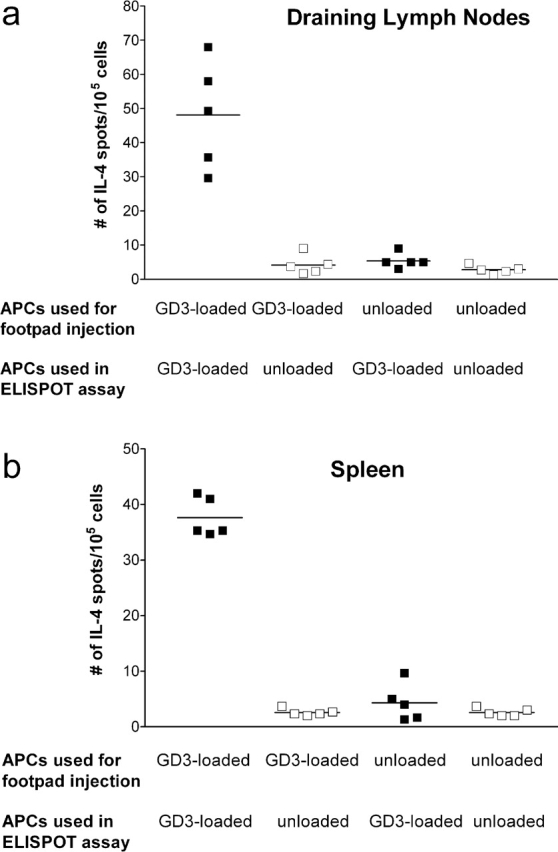
GD3-loaded APCs induced GD3-reactive lymphocytes producing IL-4. Mice were injected with 105 GD3-loaded APCs or unloaded APCs into the footpad, as indicated. Popliteal and inguinal LN (a) or splenocytes (b) were collected on day 7 and tested for reactivity against irradiated syngeneic APCs loaded with GD3 or unloaded APCs (as negative control) as indicated. IL-4 production was detected by ELISPOT assay. Each point represents the mean of triplicate wells from an individual mouse; horizontal lines indicate mean values of five mice.
GD3-reactive Lymphocytes Are CD3+ NK1.1+ NKT Cells.
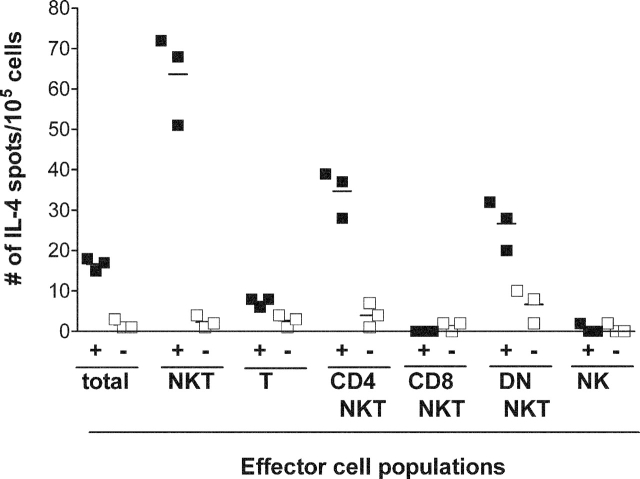
Splenocytes from immunized mice were sorted based on CD3, NK1.1, CD4, and CD8 expression. When each splenocyte subpopulation was tested for reactivity against GD3-loaded APCs or unloaded APCs, GD3 reactivity was observed only among the NK1.1+, CD3+ subpopulations (Fig. 3). Two groups of GD3-reactive NKT cells were detected: CD4+CD8− and CD4−CD8− (DN). We did not detect CD8+ NKT cells reactive to GD3. There was no reactivity against GD3 among T cells (CD3+, NK1.1− lymphocytes) or classical NK cells (CD3−, NK1.1+ lymphocytes).
Figure 3.
GD3-reactive cells are CD4+8− NKT cells and CD4−8− NKTs. Mice were immunized with 2 × 106 SK-MEL-28 cells mixed with CFA on day 0, and boosted with 2 × 106 SK-MEL-28 cells mixed with IFA on day 7. Splenocytes pooled from six to eight immunized mice were separated into subpopulations based on their surface expression of NK1.1, CD3, CD4, and CD8. Total cells (unseparated splenocytes), total NKT cells (CD3+ NK1.1+), total conventional T cells (CD3+ NK1.1−), CD4+ NKT cells (CD4+ CD8− CD3+ NK1.1+), CD8+ NKT cells (CD4− CD8+ CD3+ NK1.1+), DN NKT cells (CD4− CD8− CD3+ NK1.1+), and classic NK cells (CD3− NK1.1+) populations were stimulated with irradiated syngeneic APCs in the presence (+ columns, closed squares) or absence (− columns, open squares) of GD3. IL-4 production by each cell population was detected by ELISPOT assay. Horizontal lines indicate mean values.
Recognition of GD3 by NKT Cells Is CD1d Restricted.
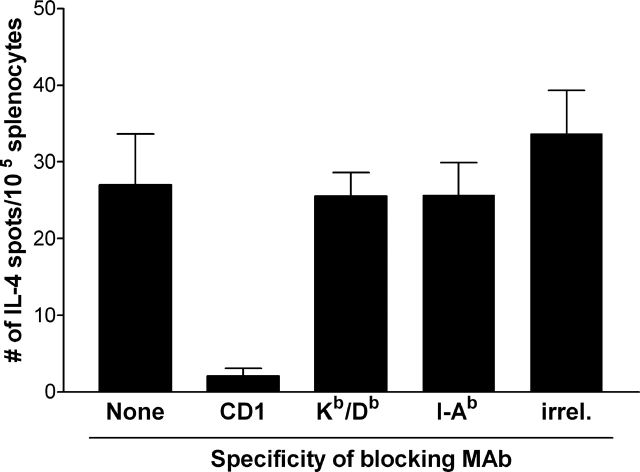
Because NKT cells recognize αGalCer presented by CD1d, we were interested to know if the GD3-reactive NKT cells were restricted by CD1d. Splenocytes from immunized mice were mixed with GD3-loaded APCs that had been preincubated with mAb against CD1d, MHC class I (Kb/Db), MHC class II (I-Ab), or Ter-119, a negative control mAb. The NKT cell response to GD3 was blocked by anti-CD1d mAb, but not by mAb against MHC class I, class II, or control mAb (Fig. 4). Control experiments (unpublished data) demonstrated that the anti-Kb/Db and anti–I-Ab mAb efficiently blocked T cell responses against influenza A/PR/8 virus–derived NP peptide (ASNENMETM) and human TRP2-derived H10 peptide (NESFALPYWNFATGRNECDV), respectively, demonstrating that the inability of these mAbs to block NKT cell responses to GD3 was not due to the lack of blocking capability.
Figure 4.
GD3-reactive NKT cell response is blocked by anti-CD1d mAb. Irradiated APCs were preincubated with PBS alone (none) or with anti-CD1d (CD1d), anti–Kb/Db (Kb/Db), anti–I-Ab (I-Ab), or 10 μg/ml Ter119 (irrel.) mAb before being loaded with GD3. Splenocytes from mice immunized with SK-MEL-28 and Freund's adjuvant were used as effector cells and IL-4 production by splenocytes was detected by ELISPOT assay. Data represent mean ± SEM of triplicate wells from four mice and are indicative of replicate experiments.
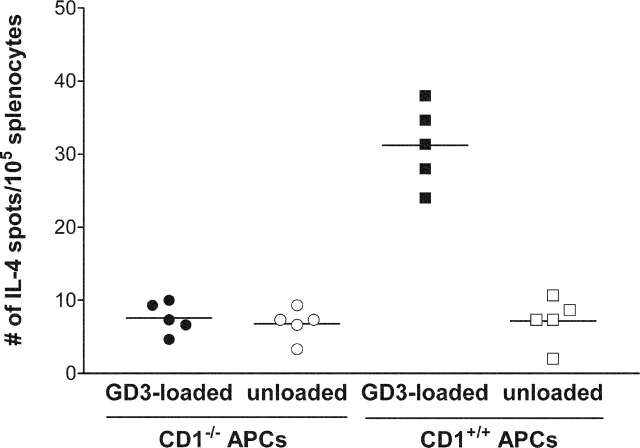
To confirm that GD3 recognition by NKT cells was CD1d dependent, splenocytes from immunized mice were stimulated with GD3-loaded APCs from CD1d knockout (CD1d−/−) or wild-type (CD1d+/+) mice. As shown in Fig. 5, GD3-loaded APCs derived from CD1−/− mice were not able to present GD3 to GD3-reactive NKTs. These results confirm that CD1d is required for presentation of GD3 to the NKTs.
Figure 5.
CD1d expression by APCs is required for presentation of GD3 to NKT cells. Splenocytes from mice immunized with SK-MEL-28 and Freund's adjuvant were used as effector cells and IL-4 production by splenocytes was detected by ELISPOT assay. APCs were isolated from the spleen of mCD1d knockout mice (CD1−/−) or from wild-type mice (CD1+/+) as indicated and loaded with GD3. Control wells received unloaded APCs. Each point represents the mean of triplicate wells from an individual mouse; horizontal lines indicate mean values.
Quantification of the NKT Cell Response to GD3 Using GD3-loaded Mouse CD1d Tetramers.
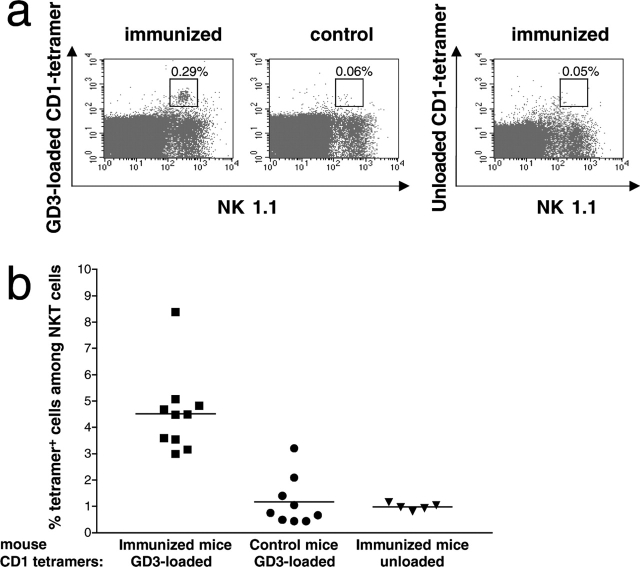
We wished to confirm the NKT cell response against GD3 and quantitate it using a technique other than ELISPOT. To do this, we analyzed the NKT cell response against GD3 using GD3-loaded mouse CD1d tetramers. CD3+ NK1.1+ tetramer+ cells were detected among the splenocytes of immunized mice, but not among splenocytes of control mice injected only with Freund's adjuvant (Fig. 6 a, left and middle). As an important control, splenocytes from immunized mice stained with unloaded CD1d tetramers showed only background levels of tetramer staining, indicating the specificity of the GD3-loaded CD1d tetramers (Fig. 6 a, right). Overall, among the CD3+ NK1.1+ splenocytes in immunized mice, a mean of 4.5% tetramer+ NKT cells was compared with a mean of 1.2% in unimmunized mice or in immunized mice stained with unloaded CD1d tetramers, (P < 0.001; Fig. 6 b). These experiments confirmed that immunization expanded CD1d-restricted NKT cells recognizing GD3.
Figure 6.
Detection of GD3-reactive NKT cells using CD1d-GD3 tetramers. Splenocytes from mice immunized with SK-MEL-28 cells and Freund's adjuvant (immunized) or mice injected with Freund's adjuvant only (control) were analyzed by flow cytometry for binding to FITC-conjugated anti-CD3 mAb, PE-conjugated anti-NK1.1 mAb and TC-conjugated GD3-loaded mCD1d tetramers or unloaded mCD1d tetramers. Gating was performed on CD3+ cells. (a) Double immunofluorescence staining of cell surface expression of NK1.1 (PE, abscissa) and tetramer (TC, ordinate) from representative immunized and control mice. Splenocytes from an immunized mouse were stained with unloaded tetramer (right). Double-positive cells are indicated by the framed window. (b) Percent tetramer+ cells among total CD3+ NK1.1+ splenocytes from immunized and control mice stained with GD3-loaded CD1d tetramers, and immunized mice stained with unloaded tetramer. Each point represents a single mouse. The difference in the mean values (horizontal lines) was highly significant (P < 0.001, Kruskal-Wallis test).
GD3-reactive NKT Cells Are a Subset of αGalCer-reactive NKT Cells.
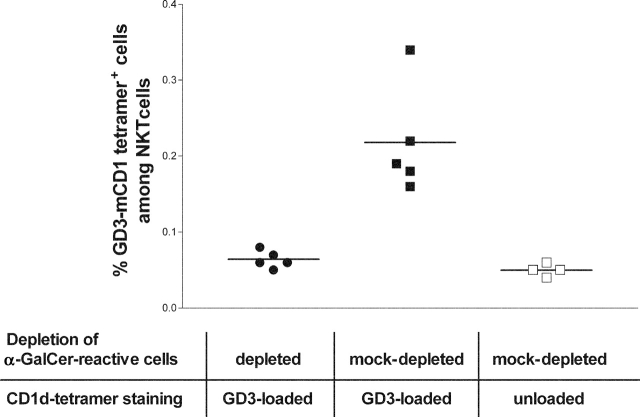
Most CD1d-restricted mouse NKT cells express an invariant TCR encoded by Vα14 and Jα18 segments and react with αGalCer. We wished to determine if GD3-reactive NKT cells were a subset of αGalCer-reactive NKTs. Splenocytes from SK-MEL-28–immunized mice were stained with PE-conjugated αGalCer-loaded CD1d tetramer and depleted using anti-PE magnetic beads. Depletion was highly efficient reducing the αGalCer-CD1d tetramer+ CD3+ population from 2.58% ± 0.171 (±SEM) after mock depletion (splenocytes stained with PE-conjugated unloaded CD1d tetramer before depletion) to 0.04% ± 0.002 (±SEM). αGalCer-depleted and mock-depleted cells were stained with TC-conjugated GD3-loaded mouse CD1d tetramers. Fig. 7 illustrates that, after mock depletion, a mean of 0.21% of the CD3+ cells were positive for GD3-loaded CD1d tetramer staining compared with a mean of 0.06% after depletion of αGalCer-reactive cells (P = 0.005, Kruskal-Wallis test). These experiments show that GD3-loaded mouse CD1d tetramers bind to a subpopulation of αGalCer-reactive NKT cells.
Figure 7.
Effects of αGalCer-reactive NKT cell depletion on GD3 reactivity. Splenocytes from immunized mice were either depleted of αGalCer-loaded CD1d tetramer-reactive cells and stained with TC-conjugated GD3-loaded CD1d tetramer (•), or mock-depleted of αGalCer-loaded CD1d tetramer-reactive cells and stained with TC-conjugated GD3-loaded CD1d tetramer (▪) or with TC-conjugated unloaded mouse CD1d tetramer (□). Gating was performed on CD3+ cells and each point represents a single mouse. P = 0.005, Kruskal-Wallis test. Horizontal lines indicate mean values.
Cytokine Profile of NKT Cells Against GD3.
The kinetics of cytokine production by GD3-reactive NKT cells was determined by intracellular cytokine FACS® analysis after immunizing mice weekly for up to three immunizations. After a single immunization on day 0, we detected production of IL-4, IL-10, and IFN-γ, which peaked on day 7 and diminished to baseline by day 10 (IL-4 and IFN-γ) or by day 17 (IL-10; Fig. 8 a). We did not detect IL-5 or IL-12 production by GD3-reactive NKT cells. These results indicate that, after a single immunization, the NKT cell response against GD3 is limited. In mice receiving weekly immunizations (Fig. 8 b), IFN-γ production by NKT cells was not sustained beyond day 10, despite booster immunizations on day 7 and day 14. This is consistent with our previous observations (Fig. 1 a), in which little IFN-γ secretion was detected after two immunizations and indicates that the IFN-γ response in these cells is transient despite further immunizations. IL-4 production by GD3-reactive NKT cells was detectable after the first immunization and peaked after the second immunization. However, despite a third immunization on day 14, IL-4 production decreased, indicating that the NKT cell response against GD3 could not be sustained. No IL-4 production was detected if splenocytes were incubated with unloaded APCs or if splenocytes from mice immunized with adjuvant alone were used (unpublished data), confirming the specificity of the response.
Figure 8.
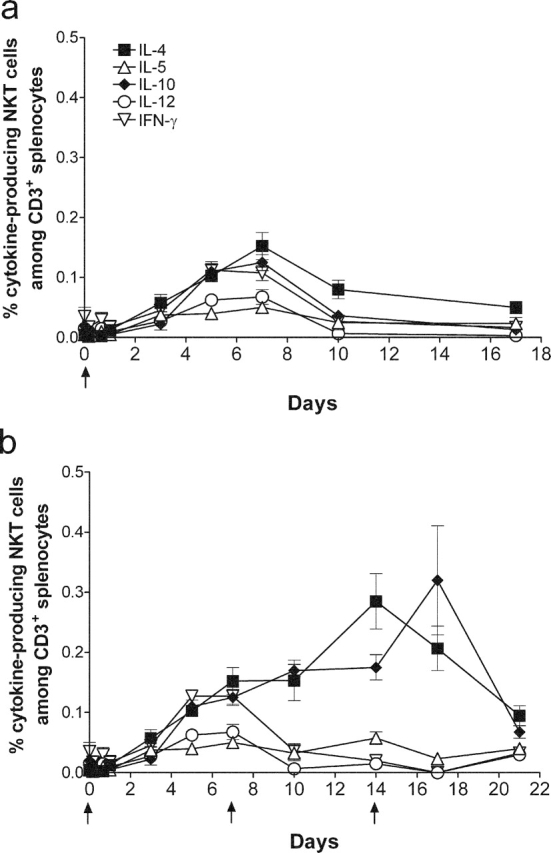
Cytokine production by GD3-reactive NKTs. Mice were immunized with 2 × 106 SK-MEL-28 cells mixed with CFA on day 0 without further boost (a), or were immunized with 2 × 106 SK-MEL-28 cells mixed with CFA on day 0 and boosted with 2 × 106 SK-MEL-28 cells mixed with IFA on day 7 and day 14 (b). Splenocytes were collected at the time points indicated. Intracellular production of IL-4 (▪), IL-5 (▵), IL-10 (♦), IL-12 (○) and IFN-γ (▿) by NK1.1+ CD3+ cells was measured gating on CD3+ cells. Arrows indicate time of immunization. Data represent mean ± SEM from four mice, and similar results were observed in repeated experiments.
GD3-reactive NKT cells also produced IL-10, which was detected after a single immunization (Fig. 8 a). With weekly immunizations, the number of IL-10–secreting NKT cells expanded, reaching a peak on day 17, which was 2 d after the third immunization. Without additional immunizations, the number of IL-10–secreting NKT cells reacting to GD3 diminished afterwards.
GD3 Is Cross-presented by Host CD1+ APCs.
Because GD3 is presented to NKT cells by CD1d, and the immunizing cell was a human GD3+ cell line, we considered it likely that the GD3 was cross-presented by CD1d+ mouse APCs. However, it was formally possible that the GD3 could be presented by SK-MEL-28 if it expressed human CD1 because there is evidence that human CD1 can present glycolipid antigens to mouse NKT cells (24). To test this, we probed SK-MEL-28 for human CD1a, CD1b, CD1c, and CD1d expression using FACS® analysis. We found that SK-MEL-28 cells did not express any isoforms of human CD1 (Fig. S1 available at http://www.jem.org/cgi/content/full/jem.20030446/DC1). Our previous experiments indicated that some inflammatory cytokines (i.e., IFN-α, IFN-γ, and TNF-α) could up-regulate CD1d expression on some of human melanoma cell lines, but treatment of SK-MEL-28 with those cytokines did not result in CD1 expression. Consistent with these observations, cDNA array experiments with SK-MEL-28 demonstrated that there was no expression of mRNA encoding any of the human CD1 isoforms (unpublished data). Therefore, on both the mRNA and protein levels, we are satisfied that SK-MEL-28 does not express human CD1 and could not have presented GD3 to mouse NKTs. As a result, we conclude that GD3 was cross-presented by CD1d+ mouse APCs.
Discussion
GD3 is a ganglioside abundantly expressed on human tumors of neuroectodermal origin such as melanoma, sarcoma, neuroblastoma, and small cell lung cancer cells. Because GD3 is not expressed, or expressed at low levels, on normal human tissues, it has been an attractive target for immunotherapy. Attempts to immunize animals (25) and patients (26–28) against GD3 have focused exclusively on antibody responses because gangliosides are T cell–independent antigens and not recognized by classical MHC-restricted T cells. There is evidence in humans that group 1 CD1 molecules (CD1a, CD1b, and CD1c) can present glycolipids, but aside from αGalCer, glycolipids presented by group 2 CD1 molecules (i.e., CD1d) have not been identified. Although T cells have been described that can recognize GM1 ganglioside presented by group 1 CD1 molecules (18), natural ligands recognized by NKT cells have not been described. In this paper, we demonstrate that mice immunized with GD3+ human melanoma developed a CD1d-restricted NKT cell response against GD3 and that the GD3 was cross-presented by mouse APCs. We also show that a similar response could be generated by immunizing mice with syngeneic APCs loaded with GD3, confirming that the response was to GD3 and not to other molecules on human melanoma. Crowe et al. showed that NKT cells from wild-type mice could prevent outgrowth of the MCA sarcoma induced in NKT–deficient mice that had been transplanted into NKT–deficient mice, but could not prevent outgrowth when the sarcoma was transplanted into CD1−/− mice (29). Although cross-presentation was not demonstrated formally and a specific antigen recognized by the NKT cells was not identified, these data are consistent with our observations and support the notion that antigens can be cross-presented to NKTs.
GD3-reactive NKT cells were found to be a subset of αGalCer-reactive NKTs. Although we were not able to carry out an exhaustive specificity analysis, the NKT cells failed to recognize GM2, a structurally related ganglioside, which suggests some degree of specificity. This is the first example of NKT cells recognizing a natural ligand.
We were able to quantify the CD1d-restricted GD3-reactive NKT cell response using both ELISPOT and mouse CD1d tetramers loaded with GD3. Previous tetramer analyses of NKT cells have largely focused on tetramers loaded with αGalCer, which mark virtually all NKTs (10, 11, 22). Our tetramer studies indicated that 0.29% of CD3+ cells in splenocytes of immunized mice (∼1:345 CD3+ cells) were tetramer-positive NKT cels. When considering total splenocytes, this corresponded to a mean of 54.4 ± 7.3 tetramer-positive NKT cells per 105 splenocytes (1:1,828). By ELISPOT analysis, the frequency of NKT cells that produced IL-4 in response to GD3 was ∼1:2,000 splenocytes, which matches almost exactly the frequency measured by tetramer. This validates these two assays and indicates that there were no anergic NKT cells detected by tetramer analysis. It is interesting to note that this is the same frequency of glycolipid-reactive T cells observed in patients with multiple sclerosis (18) and suggests that this frequency of NKT cells could be a clinically relevant.
GD3-reactive NKT cells secreted IL-4, IL-10, and IFN-γ, but not IL-5, or IL-12. With further immunization, the NKT cell response to GD3 became polarized toward a TH2-like response with subsequent extinguishing of the IFN-γ response. The IL-4 response peaked after two immunizations and began to wane despite additional booster immunizations. GD3-reactive NKT cells also produced IL-10, which peaked after three immunizations. From the kinetics of this response, we speculate that the IL-10 response may turn off the NKT cell response against GD3 despite further immunizations.
The NKT cell response we observed against GD3 is typical of TH2-like cells, although the cells also produced some IFN-γ transiently. A similar cytokine profile of elevated IL-4 and/or IL-10 production and low level IFN-γ production has been observed in several models of NKT–mediated immune protection from autoimmune diseases. In the model of anterior chamber–associated immune deviation, IL-10–producing NKT cells are required to generate regulatory T cells that suppress delayed-type hypersensitivity, although the antigen recognized by the NKT cells is unknown (30). In another model (experimental autoimmune encephalomyelitis [EAE]), preimmunization of mice with an analogue of αGalCer activated NKT cells secreting IL-4 that blocked development of EAE (31). Although we do not have any additional information about the precise immunological effects of the GD3-reactive NKT cells observed in this work, we have noted that IgG antibodies against GD3 are easily induced in the mouse (32) and one speculation is that NKT cells could provide T cell help for IgG class switching during the humoral response to GD3. There is evidence that NKT cells can provide T cell help to B cells resulting in enhanced immunoglobulin secretion (33).
In summary, we have shown that mouse TH2-like NKT cells can recognize GD3 in a CD1d-restricted manner and that the GD3 is cross-presented by mouse APCs. The response seems to be self-limited, possibly due to IL-10 production that correlated with extinguishing of the IL-4 response. Extrapolating to the human system, we speculate that in patients with GD3+ tumors, the GD3 could be cross-presented and may induce an NKT cell response. We are currently studying whether NKT cells against GD3 can be detected in melanoma patients immunized against GD3.
Acknowledgments
We thank Dr. G. Ritter for helpful suggestions, Dr. S. Porcelli for mAbs against human CD1 and for valuable discussions, and S. Lu for technical assistance.
This paper was supported with funds from the National Cancer Institute under grants K24 CA81293 (to P.B. Chapman) and RO1 CA2511 (to M. Kronenberg), and the Swim Across America Foundation.
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: αGalCer, α-galactosylceramide; DN, double negative; NKT, natural killer T cell; TC, Tri-Color.
References
- 1.Porcelli, S.A., and R.L. Modlin. 1999. The CD1 System: Antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297–329. [DOI] [PubMed] [Google Scholar]
- 2.Moody, D.B., B.B. Reinhold, M.R. Guy, E.M. Beckman, D.E. Frederique, S.T. Furlong, S. Ye, V.N. Reinhold, P.A. Sieling, R.L. Modlin, G.S. Besra, and S.A. Porcelli. 1997. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 278:283–286. [DOI] [PubMed] [Google Scholar]
- 3.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 4.Shamshiev, A., A. Donda, T.I. Prigozy, L. Mori, V. Chigorno, C.A. Benedict, L. Kappos, S. Sonnino, M. Kronenberg, and G. De Libero. 2000. The αβ T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 13:255–264. [DOI] [PubMed] [Google Scholar]
- 5.Porcelli, S.A., B.W. Segelke, M. Sugita, I.A. Wilson, and M.B. Brenner. 1998. The CD1 family of lipid antigen-presenting molecules. Immunol. Today. 19:362–368. [DOI] [PubMed] [Google Scholar]
- 6.Zeng, Z., A.R. Castano, B.W. Segelke, E.A. Stura, P.A. Peterson, and I.A. Wilson. 1997. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 277:339–345. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac, A., M.N. Rivera, S.H. Park, and J.H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535–562. [DOI] [PubMed] [Google Scholar]
- 8.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4-8–T cells in mice and humans. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac, A., O. Lantz, M.E. Quimby, J.W. Yewdell, J.R. Bennink, and R.R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865. [DOI] [PubMed] [Google Scholar]
- 10.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdin, N., L. Brossay, Y. Koezuka, S.T. Smiley, M.J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161:3271–3281. [PubMed] [Google Scholar]
- 12.Pukel, C.S., K.O. Lloyd, L.R. Travassos, W.G. Dippold, H.F. Oettgen, and L.J. Old. 1982. GD3, a prominent ganglioside of human melanoma. Detection and characterization by mouse monoclonal antibody. J. Exp. Med. 155:1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusano, A., S. Ohta, K. Shitara, and N. Hanai. 1993. Immunocytochemical study on internalization of anti-carbohydrate monoclonal antibodies. Anticancer Research. 13:2207–2212. [PubMed] [Google Scholar]
- 14.Chapman, P.B., M. Lonberg, and A.N. Houghton. 1990. Light chain variants of an IgG3 anti-GD3 monoclonal antibody and the relationship between avidity, effector functions, tumor targeting, and antitumor activity. Cancer Res. 50:1503–1509. [PubMed] [Google Scholar]
- 15.Vadhan-Raj, S., C. Cordon-Cardo, E.A. Carswell, D. Mintzer, L. Dantis, C. Duteau, M.A. Templeton, H.F. Oettgen, L.J. Old, and A.N. Houghton. 1988. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: induction of inflammatory responses at tumor sites. J. Clin. Oncol. 6:1636–1648. [DOI] [PubMed] [Google Scholar]
- 16.Nasi, M., M. Meyers, P. Livingston, A. Houghton, and P. Chapman. 1997. Anti-melanoma effects of R24, a monoclonal antibody against GD3. Vaccine Research. 7:S155–S162. [PubMed] [Google Scholar]
- 17.Ladisch, S., H. Becker, and L. Ulsh. 1992. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim. Biophys. Acta. 1125:180–188. [DOI] [PubMed] [Google Scholar]
- 18.Shamshiev, A., A. Donda, I. Carena, L. Mori, L. Kappos, and G. De Libero. 1999. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29:1667–1675. [DOI] [PubMed] [Google Scholar]
- 19.Tallquist, M.D., T.J. Yun, and L.R. Pease. 1996. A single T cell receptor recognizes structurally distinct MHC–peptide complexes with high specificity. J. Exp. Med. 184:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan, S., and K.O. Lloyd. 1992. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 52:5725–5731. [PubMed] [Google Scholar]
- 21.Kina, T., K. Ikuta, E. Takayama, K. Wada, A.S. Majumdar, I.L. Weissman, and Y. Katsura. 2000. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109:280–287. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda, J.L., O.V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C.R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal, N.H., P. Pavlidis, W.S. Noble, C.R. Antonescu, A. Viale, U.V. Wesley, K. Busam, H. Gallardo, D. DeSantis, M.F. Brennan, et al. 2003. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J. Clin. Oncol. 21:1775–1781. [DOI] [PubMed] [Google Scholar]
- 24.Brossay, L., M. Chioda, N. Burdin, Y. Koezuka, G. Casorati, P. Dellabona, and M. Kronenberg. 1998. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman, P.B., and A.N. Houghton. 1991. Induction of IgG antibodies against GD3 in rabbits by an anti-idiotypic monoclonal antibody. J. Clin. Invest. 88:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritter, G., E. Boosfeld, R. Adluri, M. Calves, H.F. Oettgen, L.J. Old, and P.O. Livingston. 1991. Antibody response to immunization with ganglioside GD3 and GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int. J. Cancer. 48:379–385. [DOI] [PubMed] [Google Scholar]
- 27.McCaffery, M., T.-J. Yao, L. Williams, P.O. Livingston, A.N. Houghton, and P.B. Chapman. 1996. Enhanced immunogenicity of BEC2 anti-idiotypic monoclonal antibody that mimics GD3 ganglioside when combined with adjuvant. Clin. Cancer Res. 2:679–686. [PubMed] [Google Scholar]
- 28.Ragupathi, G., M. Meyers, S. Adluri, L. Howard, L. Musselli, and P.O. Livingston. 2000. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int. J. Cancer. 85:659–666. [DOI] [PubMed] [Google Scholar]
- 29.Crowe, N.Y., M.J. Smyth, and D.I. Godfrey. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 196:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonoda, K.H., D.E. Faunce, M. Taniguchi, M. Exley, S. Balk, and J. Stein-Streilein. 2001. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J. Immunol. 166:42–50. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto, K., S. Miyake, and T. Yamamura. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 413:531–534. [DOI] [PubMed] [Google Scholar]
- 32.Yan, X., S.V. Evans, M.J. Kaminski, S.D. Gillies, R.A. Reisfeld, A.N. Houghton, and P.B. Chapman. 1996. Characterization of an Ig VH idiotope that results in specific homophilic binding and increased avidity for antigen. J. Immunol. 157:1582–1588. [PubMed] [Google Scholar]
- 33.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]