Abstract
Limited frequencies of T cells express IL-2 in primary antigenic responses, despite activation marker expression and proliferation by most clonal members. To define the basis for restricted IL-2 expression, a videomicroscopic system and IL-2 reporter transgenic model were used to characterize dendritic cell (DC)–T cell interactions. T cells destined to produce IL-2 required prolonged interactions with DCs, whereas most T cells established only transient interactions with DCs and were activated, but did not express IL-2. Extended conjugation of T cells with DCs was not always sufficient to initiate IL-2 expression. Thus, there is intrinsic variability in clonal T cell populations that restricts IL-2 commitment, and prolonged engagement with mature DCs is necessary, but not sufficient, for IL-2 gene transcription.
Keywords: transgenic mice, T lymphocytes, lymphocyte activation, interleukin 2, dendritic cells
Introduction
T lymphocyte activation requires the recognition of specific MHC–peptide complexes expressed on an APC. Within a clonal T cell population, recruitment of individual cells to distinct levels of activation can occur and is contingent on variable signaling through TCR or costimulatory pathways (1–6). In naive CD4 T cells, IL-2 gene transcription is under relatively stringent control compared with other activation-dependent responses, such as surface marker modulation (e.g., increased CD69 and CD25) or cell cycle progression (4, 6–8). Accordingly, IL-2 expression is limited to a subpopulation of antigen-activated naive T cells that modulate activation marker expression and progress through the cell cycle. Using an IL-2 promoter/green fluorescent protein (GFP)* reporter transgenic model to track IL-2–producing T cells, we found that the progeny of antigen-activated T cells that expressed IL-2 in a primary response had enhanced secondary responses in vitro and in vivo compared with descendants of antigen-activated cells that did not express IL-2 (9).
The basis for intraclonal heterogeneity in the T cell response and the restricted expression of IL-2 is not well understood. First, there is undoubtedly a contribution of the strength of activation of individual T cells, which is contingent on the density of peptide–MHC complexes or costimulatory and adhesion molecules expressed on APCs with which the T cell interacts. The most effective APCs to initiate primary immune responses are mature dendritic cells (DCs), which are potent activators of naive T cells due to their high expression of MHC, costimulatory, and adhesion molecules (10). Thus, heterogeneity of the clonal T cell response might reflect heterogeneity in the DC population due to variable antigen loading, maturational state, or both. Alternatively, it is possible that the activation potential of individual T cells in a clonal population is intrinsically diverse, perhaps imparted in a stochastic manner during thymic development, during post-thymic circulation through secondary lymphoid tissues, or during clonal activation (11–13). In this sense, there are no truly “naive” T cells, as there may be “memory” of TCR signaling events during thymic development or during post-thymic circulation that predates recognition of cognate antigen in the periphery.
The initial APC–T cell interaction results in the formation of a defined cell–cell membrane interface, termed the “immunological synapse.” Papers that have examined the kinetics of immunological synapse formation indicate that assembly of a mature synapse requires minutes, and may persist for hours (14, 15). If there is a requirement for mature synapse formation and persistence to fully activate a T cell (an issue of considerable controversy at present), it is conceivable that those T cells that initially engage an APC and form a mature synapse limit the APC's ability to form mature synapses with other T cells, whether by sequestering critical molecules, consuming them, or both (16–19). Competition for APC resources as a mechanism to select the highest affinity clones in a polyclonal T cell response has been reported previously (20, 21), and it is possible that individual T cells within a clonal population might also compete for APC resources on a kinetic basis.
Here, we present studies designed to define the basis for intraclonal heterogeneity of IL-2 gene transcription in naive CD4 T cells. The DO11.IL-2P/GFP transgenic model (9) was used to monitor IL-2 gene expression by individual T cells in the course of a clonal response to antigen presented by mature DCs. Using time-lapse videomicroscopic imaging of individual DC–T cell conjugates, we were able to track the interactions of individual T cells with DCs to define the nature of interactions that led to IL-2 expression. We found that long-term, stable interactions between a T cell and DC were required for IL-2 expression by the T cell, although some long-term DC–T cell conjugates failed to induce IL-2 expression. Furthermore, stable DC–T cell conjugates were formed by a limited fraction of the clonal T cell population, despite frequent transient interactions by most clonal members. Thus, restraints on the activation of individual T cells within a clonal population reflect intrinsic differences between individual T cells with respect to their thresholds of activation and/or capacity to form stable interactions with DCs.
Materials and Methods
Mice.
BALB/c mice were purchased from the Jackson Laboratory or bred in our specific pathogen-free facility and were used at 6–10 wk of age. DO11.10 TCR transgenic mice specific for OVA peptide residues 323–339 (OVAp) and restricted by I-Ad (22) were backcrossed onto the BALB/c background (>16 generations). IL-2P/GFP mice were crossed with DO11.10 TCR transgenic mice for at least nine generations as described previously (9). The IL-2P/GFP and/or DO11.10 transgenics were crossed onto a BALB.RAG-2−/− background (DO11.IL-2P/GFP.RAG-2−/− and DO11.RAG-2−/− mice, respectively) for use in some experiments. All mice were housed and treated according to National Institutes of Health guidelines under the auspices of the University of Alabama at Birmingham Institutional Care and Use Committee.
Antibodies and Reagents.
The KJ1–26 (anti-DO11.10 TCR; reference 23), anti-CD3, and anti-CD28 mAbs were purified from ascites by Dr. R. Lallone (Brookwood Biotech, Birmingham, AL). PE anti-CD4, allophycocyanin-conjugated anti-CD69, and biotin anti-CD25 mAbs were purchased from BD Biosciences. Streptavidin red 670 was purchased from Invitrogen. IL-2 and GM-CSF were purchased from R&D Systems. CellTracker™ Orange CMTMR was purchased from Molecular Probes.
Purification of CD4+ T Cells and DCs.
CD4+ T cells were isolated from spleen and lymph nodes of donor mice by positive sorting using anti-CD4 magnetic beads (Dynal). Greater than 95% of the resulting cells were CD4+.
Splenic DCs were isolated by positive magnetic sorting on CD11c-conjugated beads according to the manufacturer's protocol (Miltenyi Biotec). In brief, four to six BALB/c spleens were treated with 1 mg/ml collagenase D (Sigma-Aldrich) at 37°C and 5% CO2. After 45 min, the spleens were dissociated, the RBCs were lysed using ACK buffer, and the cell suspension was washed and plated in 10 100-mm culture dishes in RPMI 1640, 10% FCS, and 10 mM Hepes (Cellgro, Mediatech) at 37°C and 5% CO2. After 2 h, the supernatants containing nonadherent cells were discarded, the dishes were gently washed, and complete medium was added. After overnight incubation at 37°C and 5% CO2, the DC-enriched supernatant was collected and enriched using mouse CD11c microbeads. The percentage of CD11c+ cells was routinely >90% (unpublished data).
Bone marrow (BM)–derived DCs were prepared by expansion with GM-CSF and LPS (Sigma-Aldrich) maturation after the protocol of Lutz et al. (24). The percentage of CD11c+/I-Ad high cells was >70% after 10 d of culture (unpublished data).
The mature DC hybrid line, V-2, was provided by Dr. A. Takashima (University of Texas Southwestern, Dallas, TX; references 25 and 26). This line, produced by fusion of A/J mouse-derived, mature DC line XS106 fused with BALB/c splenic DCs has typical DC morphology and expresses MHC class I and II, CD11c, CD40, CD80, CD86, and CD54 at high levels.
Flow Cytometric Analysis.
CD4+ T cells were stained with the PE-, allophycocyanin-, or biotin-conjugated mAbs indicated in the appropriate figure legends as described previously (8). Cells stained with biotinylated primary antibody were detected with Red 670–labeled streptavidin. GFP expression was detected in the FL1 channel. For analytical flow cytometry, 10,000 events with forward and side scatter properties of lymphocytes were collected on FACScalibur™ and analyzed using CELLQuest™ software (Becton Dickinson).
Primary T Cell Stimulation.
CD4+ T cells isolated from either DO11.IL-2P/GFP or DO11.IL-2P/GFP.RAG-2−/− mice were plated with purified DCs and various concentrations of OVAp and cultured in RPMI 1640 and 10% FCS at 37°C and 5% CO2. 20 h after primary stimulation, cells were recovered, stained, and analyzed by flow cytometry for surface markers and GFP expression.
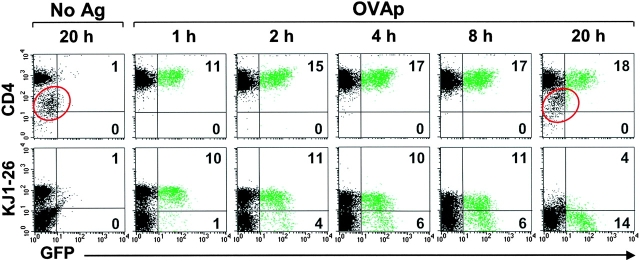
For the T cell–DC contact time determinations, CD4 T cells were separated after the indicated time of culture with DCs by mechanical disruption of the aggregates followed by purification using anti-CD4 magnetic beads (Dynal). The isolated CD4 cells were further incubated for a total of 20 h before measurement of GFP expression. Greater than 96% of the recovered T cells at each time point were CD4hi and negative for expression of CD11c and MHC class II (see Figs. 2 and S1 and unpublished data).
Figure 2.
Kinetics of commitment to IL-2 gene transcription and TCR down-modulation by naive T cells. CD4 T cells from DO11.IL-2P/GFP Tg mice were cultured with purified splenic DCs and 5 μg/ml OVAp after centrifugation onto the bottom of culture wells. At the indicated times, CD4 T cells were recovered by magnetic sorting after mechanical disruption of the T cell–APC clusters, and were returned to culture without DCs for a total of 20 h. Expression of CD4, transgenic TCR (KJ1–26), and IL-2P/GFP transgene was assessed by flow cytometry. Control cultures were as follows: APC and T cells without OVAp (“No Ag”) and an unseparated culture (OVAp/ “20 h”). Cytometric analyses of cells not recovered by magnetic sorting established that >92% of the total CD4+GFP+ and >87% of the CD4+GFP− T cells were recovered for subsequent culture (see also Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1). Red circles denote contaminating DCs.
For the DC exhaustion experiments, purified naive CD4 T cells from DO11.RAG-2−/− mice were labeled with 25 μM CellTracker™ Orange CMTMR dye for 30 min at 37°C, washed, and incubated with splenic DCs and 10 μg/ml OVAp. After 4 h, DO11.IL-2P/GFP.RAG-2−/− CD4 T cells with or without freshly isolated splenic DCs were added to the culture well and further incubated for a total of 20 h before analysis.
For some experiments, the CD4 T cells were activated for 20 h by 1–10 μg/ml immobilized anti-CD3 precoated on culture plates in PBS with 1–10 μg/ml anti-CD28 before analysis.
Time-lapse Cinematography of T Cell–APC Interactions.
Cocultures of 105 DCs and 3 × 105 DO11.IL-2P/GFP CD4 T cells were set up in RPMI 1640, 10% FCS, and 20 mM Hepes without phenol red in a Labtek 8-chamber slide (Nalge Nunc) and stimulated with 10 μg/ml OVAp. Cultures were maintained at 37°C using a custom heating chamber, imaged with an inverted fluorescent microscope (model IX70; Olympus) using 20 or 40× objective, a Photometrics camera (PVCam model 1400 CCD; Roper Scientific), a filter wheel (Ludl Electronic), and a Uniblitz shutter (Vincent Associate) controlled by the IPLab imaging software (Scanalytics). Phase-contrast images were taken every 30 s for a total of 10–12 h. To limit phototoxicity, fluorescent images were collected at 30-min intervals. Phase-contrast images were exposed for 0.2 s and fluorescence images of GFP+ cells were exposed for 10 s. At the end of the experiments, cell viability was assessed by incubation for 10 min with the fluorescent vital dye 7-AAD (BD Biosciences) and was typically >90% (unpublished data); dead cells (7-AAD+) were excluded from statistical and kinetic analysis. Images were merged and combined into a time-lapsed sequence using IPLab. Videos were assembled and annotated using Commotion Pro software (Pinnacle Systems).
Statistical Analysis.
Instant velocity measurements were taken on randomly chosen cells from a representative video using the Warthog Motion Analysis software (M.A. Chappell, University of California Riverside, Riverside, CA); the contact time with DC for each of these cells was measured, and average velocity and contact time were calculated. For statistical analysis, all the cells from the entire field of a representative video (Video 5, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1) were counted every hour. Statistical significance was calculated using unpaired Student's t test in Prism software (GraphPad).
Online Supplemental Material.
Additional studies characterizing the kinetics of IL-2 commitment in primary DO11.IL-2P/GFP.RAG-2−/− T cells and IL-2 frequencies determined by limiting dilution analysis are included in supplemental data online. Time-lapse videos of dendritic cell–T cell interactions are also available at are available at http://www.jem.org/cgi/content/full/jem.20022230/DC1.
Results
Limited IL-2 Expression by Activated T Cells.
Distinct populations of mature DCs were surveyed for their capacity to activate a clonal CD4 T cell response using the DO11.IL-2P/GFP transgenic model. Three populations of mature DCs were examined: (a) purified CD11c+ DCs isolated from spleen; (b) BM-derived mature DCs (24); and (c) the V-2 control DC hybrid line (25, 26). Naive CD4 T cells from DO11.IL-2P/GFP mice were incubated with DCs from each source and the frequency of IL-2/GFP+ cells was assessed 20 h after stimulation with or without OVA peptide (Fig. 1). In all cases, the frequency of GFP+ cells was less than one fourth of the CD4 T cell population (spleen DCs, 23.7%; BM DCs, 20.5%; and V-2 DC line, 20.9%). In contrast, 60–80% of CD4 T cells up-regulated CD25 irrespective of the type of DC used (Fig. 1). Thus, expression of IL-2/GFP by a limited subpopulation of antigen-activated naive T cells was a common feature of the clonal response, and was independent of the type of mature DC population used to present antigen.
Figure 1.
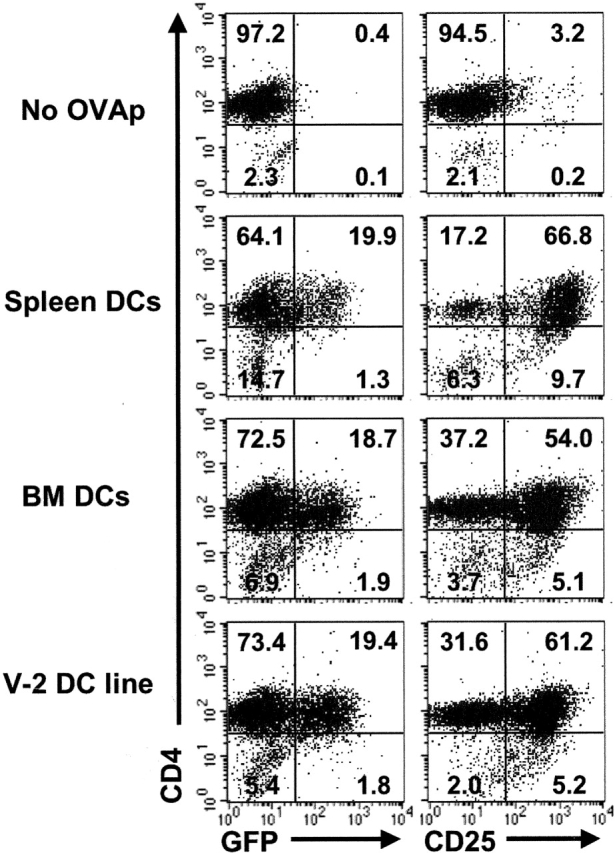
Limited induction of IL-2+ cells is a consistent feature of the primary CD4 T cell response induced by distinct, mature DC populations. DC populations were as follows: splenic DCs purified from BALB/c mice, bone marrow-derived DCs (BM DCs), and a mature hybrid DC line (V-2 DC line). 5 × 105 CD4 T cells from DO11.IL-2P/GFP mice were incubated in culture wells with 1.5 × 105 DCs from each source with or without 10 μg/ml OVAp for 48 h. The frequency of CD4+, GFP+, and CD25+ cells was assessed by flow cytometry on the lymphoid gate.
To determine whether the number of mature DCs might be limiting, we incubated the same number of naive DO11.IL-2P/GFP CD4 T cells with increasing numbers of purified splenic DCs in the presence of nonlimiting concentrations of OVA peptide. As shown in Table I, 20 h of antigenic stimulation resulted in at least partial activation of most of the naive DO11.IL-2P/GFP CD4 T cells (86–91%) as measured by CD25 up-regulation. In contrast, only a limited fraction of T cells (9–16%) was fully activated to express the IL-2/GFP reporter. An increase in APC number did not increase the frequency of clonotypic T cells that expressed IL-2/GFP; in some experiments, it modestly decreased expression of IL-2/GFP at the higher densities of DCs (Table I and unpublished data). Thus, the frequency of fully activated, IL-2–expressing CD4 T cells is restricted to a limited subpopulation of all antigen-stimulated naive CD4 cells, even under conditions in which DCs and antigen are not limiting.
Table I.
Limited IL-2 Frequencies in Primary T Cells Are Not Due to Limiting Numbers of Antigen-presenting Cells
| DC/T cell ratioa | OVAp | CD4+GFP+ b | CD4+CD25+ b |
|---|---|---|---|
| μg/ml | % | % | |
| 1:3 | 10 | 16 | 89 |
| 100 | 17 | 90 | |
| 1:1 | 10 | 12 | 87 |
| 100 | 11 | 89 | |
| 3:1 | – | 0 | 6 |
| 10 | 9 | 91 | |
| 100 | 9 | 86 |
Naive CD4+ T cells were isolated from the lymph nodes and spleens of DO11.IL-2P/GFP Tg mice and cultured at 1.5 × 105 cells/ml with the indicated ratios of purified splenic dendritic cells and antigen doses. Cells were collected after 20 h and stained with anti-CD4 and anti-CD25 for flow cytometric analysis.
Data are the percentage of total CD4+ T cells positive for GFP or CD25.
Kinetics for Commitment to IL-2 Expression.
Although the foregoing experiments precluded a role for limiting mature DC availability as a basis for limited complete T cell activation, they did not directly address the possibility that kinetic factors might be contributory. Assuming that mature synapse formation and persistence are required for IL-2 transcription, T cells that initially engage an APC and form a mature synapse might limit the APC's ability to form mature synapses with other T cells (16–19). To address this, experiments were performed to delineate the kinetics of conjugation time required for maximal induction of IL-2 (Fig. 2 and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1).
DO11.IL-2P/GFP CD4 T cells were cultured with splenic DCs and OVAp, and after the indicated coculture times, DC–T cell conjugates were disrupted and T cells were recovered by magnetic sorting. The recovered T cells were cultured separately for a total of 20 h, and expression of CD4, transgenic TCR, and the IL-2P/GFP transgene was assessed.
As early as 1 h after interaction with DCs, 11% of clonotypic T cells were committed to IL-2/GFP expression (Fig. 2). The maximum frequency of IL-2/GFP+ T cells (17–18%) was achieved between 2 and 4 h of DC–T cell contact time, and was not exceeded by longer conjugate times or when DCs were available for the duration of cultures. Thus, a conjugation time of 1 h or less was sufficient to activate ∼two thirds of the subpopulation of T cells ultimately destined to express IL-2; by 2–4 h, all T cells that would commit to IL-2 gene transcription had done so. These results are consistent with a recent paper that defined minimal conjugation times required to promote T cell progression through the cell cycle (27).
Interestingly, IL-2 commitment was not directly correlated with TCR down-modulation (Fig. 2, bottom, and Fig. S1). By 20 h of continuous culture with DCs, nearly all of the activated clonotypic T cells had significantly down-regulated their TCRs, including a significant fraction of cells that did not express IL-2/GFP. Furthermore, in experiments in which there was nonuniform down-modulation of TCR after antigen-activation, including those performed using T cells from RAG-deficient DO11.IL-2P/GFP mice (Fig. S1), the majority of T cells that down-modulated TCR expression failed to express IL-2/GFP. Thus, in all cases, the fraction of T cells that down-modulated TCR expression exceeded the fraction that expressed IL-2/GFP, suggesting that activation-dependent TCR down-modulation is insufficient to commit T cells to produce IL-2.
DCs Are Not Exhausted by Prior T Cell Conjugation.
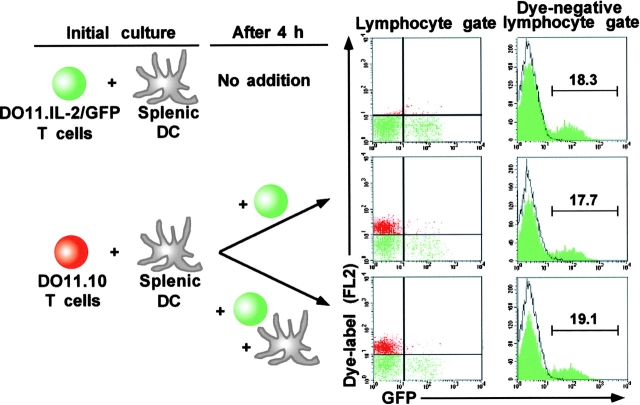
Having established the minimal DC–T cell conjugation time required for maximal IL-2 recruitment, we sought to determine whether T cells that engaged antigen-bearing DCs early in the antigenic response had a competitive advantage over those that engaged DCs late. A two-step culture system was devised, wherein splenic DCs were preincubated with DO11.RAG-2−/− CD4 T cells and OVAp for 4 h before the addition of fresh DO11.IL-2P/GFP.RAG-2−/− T cells or fresh DO11.IL-2P/GFP.RAG-2−/− T cells, plus fresh DCs (Fig. 3). The addition of the second T cell population was delayed 4 h after initiation of the preculture to allow formation of DC–T cell conjugates sufficient to fully activate the first wave of T cells, and the cultures were continued an additional 20 h before analysis. Because only the T cells added late to the cultures bore the GFP reporter transgene, IL-2 expression could be uniquely detected in this population. To further distinguish the “early” and “late” T cell populations to permit calculation of IL-2P/GFP transgene expression frequencies, DO11.RAG-2−/− T cells were prelabeled with a red fluorescent dye that identified them.
Figure 3.
Limited complete activation of naive T cell population is not due to APC exhaustion. Naive CD4 T cells isolated from DO11.RAG-2−/− mice were labeled with an FL2-detectable vital dye (“DO11.10 T cells” in red) before culture with purified splenic DCs and OVAp as described in Fig. 2. 4 h after initiation of culture, a second aliquot of DO11.RAG-2−/− T cells bearing the IL-2P/GFP transgene (“DO11.IL-2P/GFP” in green), which was not dye-labeled, was added to the initial culture, with (middle) or without (bottom) fresh splenic DCs. Incubation was continued for an additional 20 h, and cells were recovered and analyzed for GFP expression in lymphocyte or dye-negative lymphocyte gates. A culture that contained only DO11.IL-2P/GFP and DCs for the duration of the total incubation period was included as a positive control for reporter induction. The percentage of GFP+ cells in the unlabeled, DO11.IL-2P/GFP fraction is indicated in the histograms (right).
After 24 h of total culture, recovered cells were stained for expression of CD4, and gated into CD4+dye+ and CD4+dye− populations for analysis of GFP expression. No difference in frequency or intensity of IL-2/GFP+ cells was evident in either group with delayed T cell addition compared with a control culture that did not receive a competing T cell population. Thus, “exhaustion” of APCs by prior T cell interactions is probably not a mechanism limiting the frequency of naive T cells that express IL-2, and mature DCs may be competent to fully activate multiple T cells sequentially.
Prolonged DC–T Cell Conjugation Is Necessary, but Not Sufficient, for IL-2 Induction.
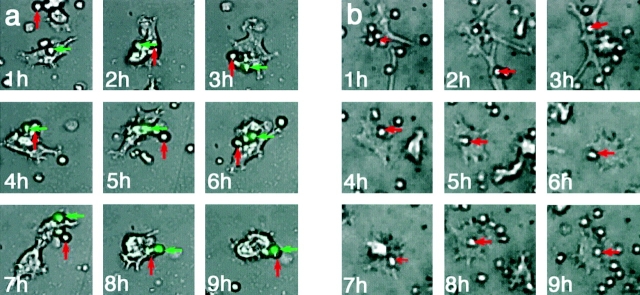
To further characterize the kinetic interactions between T cells and DCs, prolonged videomicroscopic imaging of DC–T cell interactions was performed. For these studies, the V-2 DC hybrid line was used because it provided easier morphological discrimination from T cells, although comparable results were obtained with either splenic or BM-derived DCs (unpublished data). As shown in Fig. 4 a, and in time-lapse videos (Videos 1–3 and 5, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1), individual T cells and DCs could be continuously tracked throughout a 9–12-h culture period, and the expression of IL-2/GFP by individual T cells could be visualized. Under conditions of antigenic stimulation, two general subpopulations of T cells could be defined: a highly motile population that generally engaged in only intermittent, transient (<15 min) contact with DCs before detaching, moving away, and often sequentially contacting other DCs transiently; and a population of T cells that were stably conjugated to a DC for prolonged periods (>4 h). Within the population of T cells that formed long-term conjugates (LTCs), a fraction expressed detectable GFP as early as 3–4 h after contact, whereas another fraction of LTC never expressed GFP during the period of observation (10–12 h). In the absence of antigen, far fewer DC–T cell conjugates formed and GFP expression was not induced (Fig. 4 B and Video 4, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1).
Figure 4.
Time-lapse videomicroscopy of DO11.IL-2P/GFP Tg T cells and DCs interaction with or without OVAp. (a) Naive CD4 T cells were isolated from DO11.IL-2P/GFP mice and cultured with DC hybrid cells and OVAp as described in Materials and Methods, and were imaged sequentially at 0.5-min intervals by phase-contrast microscopy for a total of for 10 h. The GFP fluorescent signal was collected at 30-min intervals and has been digitally superimposed over the transmitted light images. A green arrow identifies a T cell that ultimately expressed GFP; the red arrow identifies a cell that remained GFP− (localized under the GFP+ cell at 8 and 9 h), despite intimate contact with a DC shared with a GFP+ cell. (b) Representative images of a T cell–DC conjugate in the absence of OVAp. Same experimental condition as in a. Red arrow identifies the only T cell that established a prolonged interaction with a DC. No GPF signal was detected during the 10-h incubation.
To facilitate the tracking and analysis of different T cell subpopulations in some experiments, individual T cells were pseudo-colored based on their patterns of movement and GFP expression (Fig. 5 A and Video 5). Instantaneous velocities were calculated and differences between the GFP+ cells and the motile GFP− cells were obvious. For individual motile GFP− cells, the measurements were limited to the duration of their transit across the fixed field of observation (30–180 min). Instantaneous velocities of GFP+ and LTC GFP− T cells were comparable and could be tracked over a prolonged period, reflecting their intimate association with DCs adherent to the culture dish (Fig. 5 B).
Figure 5.
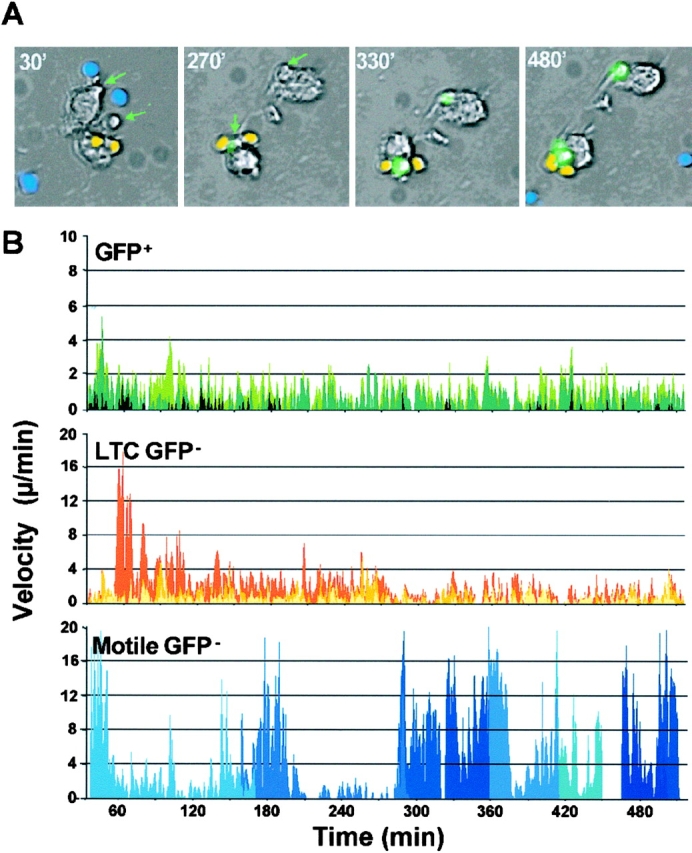
Motion analysis of individual cells imaged by time-lapse videomicroscopy. (A) Identification of the T cell subpopulations based on their IL2/GFP expression and motion characteristics. Representative frames from Video 5, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1. The GFP− cells were pseudo-colored in yellow (LTC GFP−) or blue (Motile GFP−). The GFP+ cells indicated by the green arrows express detectable amount of GFP after 4 h (green fluorescent signal). (B) Instant velocity plots of DO11.IL-2/GFP.RAG-2−/− CD4 T cells committed to IL-2 expression (GFP+), cells establishing long-term conjugates (LTCs) with DC hybrid cells without expressing IL-2 (LTC GFP−) and cells with no or very brief interaction with DC hybrid cells (Motile GFP−). The instant velocity of three to six representative cells in each group was measured at 1 min intervals and plotted for the entire duration of the time-lapse videomicroscopic analysis.
Analysis of the average velocity and total DC–T cell conjugation times of GFP+ and GFP− subpopulations confirmed the visual impressions from time-lapse videos (Fig. 6 A). The GFP− population stayed in contact with a DC for 102 ± 23 min on average with a majority of the GFP− cells that either did not interact with a DC at all, or did so very briefly (<15 min). In contrast, the GFP+ population remained firmly attached to DCs 554 ± 19 min on average, with 77% of the cells staying in intimate contact with one or, rarely, two DCs during the entire experiment (∼10 h). The striking difference in conjugation time was correlated with a significant difference (P < 0.01) in average velocity between the GFP− and GFP+ population (3.2 ± 0.2 and 0.6 ± 0.07 μm/min, respectively) (Fig. 6, B and C), even though the velocity measurement does not distinguish between the intrinsic motility of isolated T cells and the motility of DCs, to which T cells were attached.
Figure 6.
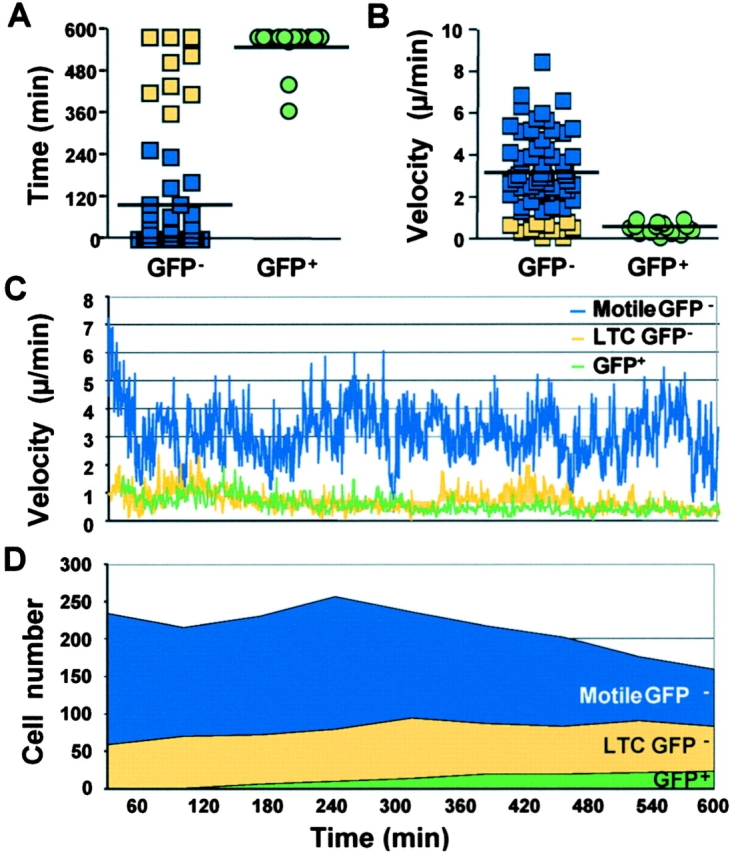
Quantitative analysis of the movements of the different T cell subpopulations imaged by time-lapse videomicroscopy. (A) The total conjugation time with DCs over the 10-h incubation period of 50 Motile GFP−, 9 LTC GFP−, and 15 GFP+ cells is plotted as follows: Motile GFP−, blue squares; LTC GFP−, yellow squares; and GFP+, green circles. The horizontal bars indicate the average conjugation time of the GFP− and GFP+ populations. (B) Average velocity. The average velocity of individual T cells analyzed in A over the 10-h incubation period is represented as follows: Motile GFP+, blue squares; LTC GFP−, yellow squares; and GFP+, green circles. The horizontal bars indicate the mean velocity of the GFP− and GFP+ populations. (C) Variation over time in average velocity of the CD4 T cells in each subpopulation. The instantaneous velocity of individual T cells analyzed in A was measured every minute for 10 h. Average velocity for Motile GFP− (blue line), LTC GFP− (yellow line), and GFP+ (green line) T cells was calculated and plotted over time. (D) Cell number distribution over time. The total cell numbers from the entire microscopic field (20×) of a representative experiment were counted every hour for 10 h. The cells were classified in three subpopulations: Motile GFP− (blue), LTC GFP− (yellow), and GFP+ (green).
Of the total fraction of T cells that were conjugated with a DC continuously for a minimum of 4 h, less than one third expressed detectable GFP, whereas a number of cells remained conjugated with a single DC for >8 h without expressing GFP (Fig. 6 D). There were multiple examples in which the same DC supported GFP induction in more than one conjugated T cell, whereas other T cells stably conjugated with the same DC remained GFP− (Fig. 4 A and Videos 1B, 2, and 5). In each experiment, nearly all GFP+ cells remained conjugated to a DC after 10–12 h and only rarely migrated away from their DC partner (Video 3). In six separate experiments, none of the highly motile cells that formed only transient interactions with DCs were found to express GFP.
In absence of specific antigen (OVAp), only few T cells made prolonged (>1 h) interaction with the DC hybrids (Fig. 4 B and Video 4). Most of the cells stayed motile and the few cells that interacted behaved like the LTC GFP− cells described previously. Analysis of the cell distribution shows that the fraction of T cells interacting with DCs is similar to that in the presence of antigen for the first couple of hours (28–40% of the T cells), but it decreased rapidly to 5–10% of the total T cell population by 5 h (unpublished data). The GFP− cells that interacted long-term with the DCs established contact for 190 ± 165 min on average as compared with 308 ± 210 min in the presence of OVAp. In three separate experiments done in the absence of OVAp, none of the T cells crawled on the surface of the DC or established intimate membrane contact as seen in the presence of a specific antigen (Videos 1 and 2). Thus, as reported previously (12, 27, 28), the presence of a specific antigen is not necessary for establishing T cell–DC interaction, but seems to reinforce and change the characteristics of the DC–T cell interaction.
Discussion
In this paper, we present a novel experimental system for tracking the dynamic interactions of individual T cells and DCs, using a reporter transgene to monitor a high-stringency parameter of naive CD4 T cell activation–IL-2 gene transcription. Our results support two general conclusions. First, the limited expression of IL-2 by antigen-activated naive T cells reflects a T cell–intrinsic component that stratifies the clonal response even under nonlimiting conditions of antigen and APC availability. Second, although transient, sequential DC–T cell interactions are sufficient to prime naive T cells for IL-2 responsiveness, prolonged, stable DC–T cell conjugation consistent with mature synapse formation is associated with IL-2 gene transcription in a minority of antigen-activated T cells.
At the outset of these studies, we favored the hypothesis that limited recruitment of IL-2 expression in a naive T cell population primarily reflected limiting APC function of DCs. This might be due to a limited number of fully mature, fully activated DCs (e.g., limited numbers of cells that coexpress high levels of appropriate MHC molecules, costimulatory activity, and adhesion molecules). Alternatively, there might be kinetically regulated effects on T cell activation that reflected heterogeneity with respect to the timing of an individual T cell's interaction with a competent DC. Recent papers have suggested that MHC–peptide complexes and costimulatory molecules can be actively removed from the APC by interacting T cells, perhaps depleting the APC's functional capacity as a function of productive interactions with the T cell (18, 19). Finally, it was possible that the T cell might sequester critical APC surface molecules, such as MHC–peptide complexes during the formation of an immunological synapse, thereby limiting availability of these molecules to other T cells (16, 17). In essence, this amounts to a “first come, first served” model for T cell activation, in which T cells that are latecomers to physical conjugation with DCs engaged previously by another T cell are incompletely activated due to diminished availability of MHC–peptide complexes, costimulatory factors, or both. This model predicts that there is exhaustion of DC function due to prior productive interactions with T cells, either via sequestration or down-modulation of critical DC molecules, or outright apoptotic death of the DC (29).
The data in the present work do not support this model. Irrespective of the population of mature DCs used to elicit the T cell response, the frequency of IL-2 producers was restricted and remarkably similar under nonlimiting conditions of antigen or APC availability. Moreover, preincubation of the DCs with antigen-specific naive T cells did not reduce their ability to further induce IL-2/GFP expression in a second cohort of T cells, thus arguing against an exhaustion of their APC function. This is consistent with our time-lapse studies showing that mature DCs are competent to fully activate multiple T cells sequentially. Furthermore, in a set of experiments designed to determine whether T cells activated early in the response might actively inhibit their clonal partners through an APC-independent mechanism, we found no evidence of T cell–mediated suppression. Thus, activation of naive T cells by optimal anti-CD3/anti-CD28 stimulation under conditions of limiting dilution elicited no increase in the frequency of IL-2/GFP+ recruited, and closely paralleled that induced by mature DCs (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20022230/DC1). Finally, in preliminary experiments, we have found that the addition of CD4+CD25+ regulatory T cells does not affect the frequency of IL-2/GFP+ cells in our system (unpublished data). Undoubtedly, under conditions of limited antigen or APC availability, or weak innate immune signals to induce the maturation and activation of DCs, there will be a limited clonal T cell response. However, collectively these results strongly implicate a T cell–intrinsic mechanism that limits the extent of T cell activation within a clonal population, independent of possible APC limitations or regulatory T cell effects.
To further characterize the basis for limited IL-2 recruitment, a system was developed to perform time-lapse videomicroscopy of individual DC–T cell interactions during a relatively prolonged period of the early antigenic response. Although a number of papers have examined supramolecular events that transpire at the interface, or immunological synapse, that forms between the T cell and APC during the onset of TCR signaling (14, 15, 30, 31), details of the spatiotemporal reorganization of signaling molecules in the synapse are controversial and it has been difficult to correlate details of synapse formation with functional outcomes of T cell activation. Although most data indicate that there is rapid conversion between an immature and mature synapse within minutes of TCR engagement, detailed analyses of the stability and longevity of the mature synapse are limited. A prevailing model posits that stable, prolonged maintenance of the mature synapse for many hours is required for complete T cell activation, and may be necessary for reciprocal delivery of activating signals to the APC from the T cell (14, 32–34). In contrast, Gunzer et al. reported videomicroscopic studies of DC–T cell interactions in collagen gel matrices (35), and found multiple, transient contacts between T cells with DCs that resulted in intracellular calcium fluxes, blast transformation, activation marker up-regulation, and proliferation of the responding T cells. This led to a serial encounter model, in which the T cell integrates transient activation events that it receives in serial encounters with multiple DCs (36).
More recently, two groups have used time-lapse, laser confocal imaging to visualize DC–T cell interactions in explanted lymph nodes under antigen-dependent and -independent conditions (37, 38). Each of these papers emphasized the presence of a subpopulation of antigen-specific T cells that was relatively stationary (37) or established prolonged contact with antigen-loaded DCs (38). Miller et al. identified long-lived clusters and “swarms” of T cells that were presumed to be interacting with DCs (37). Stoll et al. found that stable (>15 h), one-on-one interactions were maintained between an antigen-loaded DCs and a single T cell, and identified exclusion of CD43 from the contact zone between T cells and DCs, consistent with synapse formation (38). These papers lend support to a prolonged synapse model and raise interesting points of difference with the collagen gel studies (39).
A limitation in each of these previous works has been the lack of a functional read-out for activation that could be correlated with a significant population of specific individuals within the T cell response. Although transient calcium flux and surface receptor redistribution are clearly associated with T cell activation, they are very early events that can precede mature synapse formation (27). They also occur in abortive, or nonantigen-dependent interactions between T cells and APCs, and, therefore, are not necessarily predictive of productive T cell activation (12, 28, 40, 41). The present work is the first to quantitate by sequential analysis the expression of a relatively late, stringent parameter of T cell activation (e.g., IL-2) by individual cells engaged in antigen recognition on DCs, and complements in vivo papers that have demonstrated IL-2 expression by T cells in contact with DCs (42). It offers a unifying explanation for the apparently disparate observations in previous works and provides support for both the serial encounter and synapse models. Our data indicate that a majority of antigen-specific T cells undergo transient, sequential interactions with multiple DCs, consistent with the serial encounter model. These T cells fail to establish prolonged conjugates despite the availability of fully competent DCs, and whereas this population does not express IL-2, it is sufficiently activated to up-regulate CD25. In contrast, a minority of T cells engages individual DCs for prolonged periods, well exceeding the minimal period required for commitment to IL-2 gene expression. Among this subpopulation of T cells, a significant fraction receives, or is competent to receive, signals sufficient to activate IL-2 gene transcription, thereby providing a growth factor that can be used by IL-2 producers and non–IL-2 producers alike. Indeed, a small fraction of T cells were reported to undergo long-lived interaction with DCs (1–4 h) by Gunze et al. (35), and we speculate that these might represent the IL-2 producers.
The basis for limited IL-2 recruitment by the population of T cells that were stably conjugated to DCs for prolonged periods (>4 h) is unclear, but it reinforces the notion that there is intrinsic heterogeneity in the naive T cell population that restricts IL-2 competency. Importantly, the limited frequency of GFP+ cells does not reflect a lack of antigen-presenting capacity of the DC population, because in numerous examples a single DC was able to induce IL-2 production from one or more conjugated T cells without inducing IL-2 expression by other T cells in the same DC–T cell cluster. Notably, there was no simple correlation between the duration of the DC–T cell contact and the probability that an individual T cell would initiate IL-2 production, although without exception, prolonged conjugation was required for IL-2 gene expression. Accordingly, prolonged, stable contact with the DC appears to be necessary, but not sufficient, to initiate a program of T cell activation that results in IL-2 gene recruitment.
Because this work did not correlate synapse formation with IL-2 production, we have not established that mature synapse formation is a requirement for cells destined to express IL-2. Conversely, we do not know whether those cells destined to remain GFP− despite long-term conjugation with a DC failed to assemble a synapse, or whether mature synapses were formed but were uncoupled from IL-2 gene transcription. However, the fractions of T cells in our studies that form stable conjugates with DCs are in good agreement with recent studies by Bromley et al., who found that ∼35% of T cells formed stable conjugates with antigen-pulsed DCs (43). Assuming that the fraction of T cells that form mature synapses on ICAM-1 and MHC–peptide containing planar lipid bilayers approximates that formed in association with mature DCs (∼50%), the observed frequencies of IL-2/GFP+ cells in this work would be consistent with a requirement for mature synapse formation to induce IL-2 gene transcription, although this will need to be directly tested.
An interesting observation in this paper concerns the kinetics of IL-2 commitment and its relationship to TCR down-modulation. TCR down-modulation has been correlated with various T cell activation markers (44–48), and it was recently reported that TCR down-regulation occurred rapidly after APC–T cell conjugation, preceding mature synapse formation (27). Our work showed a dissociation between TCR down-modulation and commitment to IL-2 expression in that the fraction of the cells that lost TCR expression far exceeded that which expressed IL-2/GFP (Fig. 2; references 49, 50). Although the current work cannot exclude the possibility that rapid TCR down-modulation and replenishment occur in cells destined to express IL-2, our data are consistent with models in which TCR down-modulation serves to terminate, rather than initiate, a program of T cell activation leading to IL-2 expression.
This paper supports a model of naive T cell activation in which intrinsic intraclonal heterogeneity limits stable interactions with DCs, thereby restricting the recruitment of clonal precursors to complete activation and IL-2 expression. Given the reported previously link between IL-2 gene expression and enhanced effector T cell differentiation (9), this may represent an important mechanism for limiting commitment of naive T cells to an effector response. Papers exploring effector T cell survival indicate that these cells may be terminally differentiated and relatively short-lived (51, 52). Thus, it is likely that the T cell response has evolved to place restraints on the fraction of clonal precursors that can establish prolonged interactions with DCs perhaps as a means to conserve clonal progeny for recall, or memory, responses, and thereby ensure immunological reserve.
Acknowledgments
The authors thank Drs. M. Anderson, R.P. Bucy, and D. Chaplin, and all members of the Weaver lab for helpful comments and/or manuscript review. We thank N. Le Lievre for manuscript preparation and editorial critique, J.S. Thomas for excellent technical assistance, and Drs. K. Keyser and D. Kucik for expert advice concerning microscopic studies.
This work was supported by grants from the National Institutes of Health (RO1 AI35783) and Sankyo Co. Ltd.
The online version of this article includes supplemental material.
Footnotes
Abbreviations used in this paper: BM, bone marrow; DC, dendritic cell; GFP, green fluorescent protein; LTC, long-term conjugate.
References
- 1.Evavold, B.D., and P.M. Allen. 1991. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 252:1308–1310. [DOI] [PubMed] [Google Scholar]
- 2.Evavold, B.D., J. Sloan-Lancaster, and P.M. Allen. 1993. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol. Today. 14:602–609. [DOI] [PubMed] [Google Scholar]
- 3.Viola, A., and A. Lanzavecchia. 1996. T cell activation determined by T cell receptor number and tunable thresholds. Science. 273:104–106. [DOI] [PubMed] [Google Scholar]
- 4.Itoh, Y., and R.N. Germain. 1997. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cyto-kine responses of CD4+ T cells. J. Exp. Med. 186:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldrop, S.L., K.A. Davis, V.C. Maino, and L.J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284–5295. [PubMed] [Google Scholar]
- 6.Weaver, C.T., A. Saparov, L.A. Kraus, W.O. Rogers, R.D. Hockett, and R.P. Bucy. 1998. Heterogeneity in the clonal T cell response. Implications for models of T cell activation and cytokine phenotype development. Immunol. Res. 17:279–302. [DOI] [PubMed] [Google Scholar]
- 7.Bucy, R.P., L. Karr, G.-Q. Huang, J. Li, D. Carter, K. Honjo, J.A. Lemons, K.M. Murphy, and C.T. Weaver. 1995. Single cell analysis of cytokine gene co-expression during CD4+ T-cell phenotype development. Proc. Natl. Acad. Sci. USA. 92:7565–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers, W.O., C.T. Weaver, L.A. Kraus, J. Li, L. Li, and R.P. Bucy. 1997. Visualization of antigen-specific T cell activation and cytokine expression in vivo. J. Immunol. 158:649–657. [PubMed] [Google Scholar]
- 9.Saparov, A., F.H. Wagner, R. Zheng, J.R. Oliver, H. Maeda, R.D. Hockett, and C.T. Weaver. 1999. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 11:271–280. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 11.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 290:92–97. [DOI] [PubMed] [Google Scholar]
- 12.Reay, P.A., K. Matsui, K. Haase, C. Wulfing, Y.H. Chien, and M.M. Davis. 2000. Determination of the relationship between T cell responsiveness and the number of MHC-peptide complexes using specific monoclonal antibodies. J. Immunol. 164:5626–5634. [DOI] [PubMed] [Google Scholar]
- 13.Lanzavecchia, A., and F. Sallusto. 2001. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2:487–492. [DOI] [PubMed] [Google Scholar]
- 14.Bromley, S.K., W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. [DOI] [PubMed] [Google Scholar]
- 15.Richie, L.I., P.J. Ebert, L.C. Wu, M.F. Krummel, J.J. Owen, and M.M. Davis. 2002. Imaging synapse formation during thymocyte selection: inability of CD3zeta to form a stable central accumulation during negative selection. Immunity. 16:595–606. [DOI] [PubMed] [Google Scholar]
- 16.Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- 17.Dustin, M.L., and J.A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23–29. [DOI] [PubMed] [Google Scholar]
- 18.Hwang, I., J.F. Huang, H. Kishimoto, A. Brunmark, P.A. Peterson, M.R. Jackson, C.D. Surh, Z. Cai, and J. Sprent. 2000. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 191:1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang, I., and J. Sprent. 2001. Role of the actin cytoskeleton in T cell absorption and internalization of ligands from APC. J. Immunol. 166:5099–5107. [DOI] [PubMed] [Google Scholar]
- 20.Kedl, R.M., B.C. Schaefer, J.W. Kappler, and P. Marrack. 2002. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat. Immunol. 3:27–32. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia, A. 2002. Lack of fair play in the T cell response. Nat. Immunol. 3:9–10. [DOI] [PubMed] [Google Scholar]
- 22.Murphy, K.M., A.B. Heimberger, and D.Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+ CD8+TCRlo thymocytes in vivo. Science. 250:1720–1723. [DOI] [PubMed] [Google Scholar]
- 23.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex–restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz, M.B., A.L. Kukutsch, S. Ogilvie, F. Rossner, N. Koch, K. Okumura, L. Yagita, and A. Takashima. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 223:77–92. [DOI] [PubMed] [Google Scholar]
- 25.Matsue, H., K. Matsue, M. Kusuhara, T. Kumamoto, K. Okumura, H. Yagita, and A. Takashima. 2001. Immunosuppressive properties of CD95L-transduced “killer” hybrids created by fusing donor- and recipient-derived dendritic cells. Blood. 98:3465–3472. [DOI] [PubMed] [Google Scholar]
- 26.Kusuhara, M., K. Matsue, D. Edelbaum, J. Loftus, A. Takashima, and H. Matsue. 2002. Killing of naive T cells by CD95L-transfected dendritic cells (DC): in vivo study using killer DC-DC hybrids and CD4(+) T cells from DO11.10 mice. Eur. J. Immunol. 32:1035–1043. [DOI] [PubMed] [Google Scholar]
- 27.Lee, K.H., A.D. Holdorf, M.L. Dustin, A.C. Chan, P.M. Allen, and A.S. Shaw. 2002. T cell receptor signaling precedes immunological synapse formation. Science. 295:1539–1542. [DOI] [PubMed] [Google Scholar]
- 28.Kondo, T., I. Cortese, S. Markovic-Plese, K.P. Wnadninger, C. Carter, M. Brown, S. Leitman, and R. Martin. 2001. Dendritic cells signal T cells in the absence of exogenous antigen. Nat. Immunol. 2:932–938. [DOI] [PubMed] [Google Scholar]
- 29.Ingulli, E., A. Mondino, A. Khoruts, and M.K. Jenkins. 1997. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 185:2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- 31.Shaw, A.S., and M.L. Dustin. 1997. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 6:361–369. [DOI] [PubMed] [Google Scholar]
- 32.Delon, J., and R.N. Germain. 2000. Information transfer at the immunological synapse. Curr. Biol. 10:R923–R933. [DOI] [PubMed] [Google Scholar]
- 33.Krummel, M.F., and M.M. Davis. 2002. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr. Opin. Immunol. 14:66–74. [DOI] [PubMed] [Google Scholar]
- 34.van der Merwe, P.A. 2002. Formation and function of the immunological synapse. Curr. Opin. Immunol. 14:293–298. [DOI] [PubMed] [Google Scholar]
- 35.Gunzer, M., A. Schafer, S. Borgmann, S. Grabbe, K.S. Zanker, E.B. Brocker, E. Kampgen, and P. Friedl. 2000. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 13:323–332. [DOI] [PubMed] [Google Scholar]
- 36.Friedl, P., and M. Gunzer. 2001. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 22:187–191. [DOI] [PubMed] [Google Scholar]
- 37.Miller, M.J., S.H. Wei, I. Parker, and M.D. Cahalan. 2002. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 296:1869–1873. [DOI] [PubMed] [Google Scholar]
- 38.Stoll, S., J. Delon, T.M. Brotz, and R.N. Germain. 2002. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 296:1873–1876. [DOI] [PubMed] [Google Scholar]
- 39.von Andrian, U.H. 2002. Immunology. T cell activation in six dimensions. Science. 296:1815–1817. [DOI] [PubMed] [Google Scholar]
- 40.Inaba, K., and R.M. Steinman. 1986. Accessory cell–T lymphocyte interactions. Antigen-dependent and -independent clustering. J. Exp. Med. 163:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delon, J., N. Bercovici, G. Raposo, R. Liblau, and A. Trautmann. 1998. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J. Exp. Med. 188:1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichert, P., R.L. Reinhardt, E. Ingulli, and M.K. Jenkins. 2001. Cutting edge: in vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J. Immunol. 166:4278–4281. [DOI] [PubMed] [Google Scholar]
- 43.Bromley, S.K., and M.L. Dustin. 2002. Stimulation of naive T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms. Immunology. 106:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valitutti, S., S. Muller, M. Dessing, and A. Lanzavecchia. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachmann, M.F., A. Oxenius, D.E. Speiser, S. Mariathasan, H. Hengartner, R.M. Zinkernagel, and P.S. Ohashi. 1997. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur. J. Immunol. 27:2195–2203. [DOI] [PubMed] [Google Scholar]
- 46.Hemmer, B., I. Stefanova, M. Vergelli, R.N. Germain, and R. Martin. 1998. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J. Immunol. 160:5807–5814. [PubMed] [Google Scholar]
- 47.Itoh, Y., B. Hemmer, R. Martin, and R.N. Germain. 1999. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J. Immunol. 162:2073–2080. [PubMed] [Google Scholar]
- 48.Germain, R.N., and I. Stefanova. 1999. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu. Rev. Immunol. 17:467–522. [DOI] [PubMed] [Google Scholar]
- 49.Cai, Z., H. Kishimoto, A. Brunmark, M.R. Jackson, P.A. Peterson, and J. Sprent. 1997. Requirements for peptide-induced T cell receptor down-regulation on naive CD8+ T cells. J. Exp. Med. 185:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salio, M., S. Valitutti, and A. Lanzavecchia. 1997. Agonist-induced T cell receptor down-regulation: molecular requirements and dissociation from T cell activation. Eur. J. Immunol. 27:1769–1773. [DOI] [PubMed] [Google Scholar]
- 51.Sprent, J., and C.D. Surh. 2002. T cell memory. Annu. Rev. Immunol. 20:551–579. [DOI] [PubMed] [Google Scholar]
- 52.Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. [DOI] [PubMed] [Google Scholar]