Abstract
The chemokines are a large family of cytokines that control the recruitment of leukocytes in immune and inflammatory responses. We describe the isolation of a novel murine CC chemokine that, based on its biological and structural features, we have named monocyte chemoattractant protein (MCP)-5. MCP-5 mapped to the CC chemokine cluster on mouse chromosome 11 and was most closely related to human MCP-1 in structure (66% amino acid identity). Purified recombinant MCP-5 protein was a potent chemoattractant for peripheral blood monocytes, was only weakly active on eosinophils at high doses, and was inactive on neutrophils. MCP-5 induced a calcium flux in peripheral blood mononuclear cells, but not in purified murine eosinophils or neutrophils. Consistent with these results, MCP-5 induced a calcium flux in human embryonic kidney (HEK)-293 cells transfected with human and murine CCR2, a CC chemokine receptor expressed on monocytes. MCP-5 did not induce a calcium flux in HEK-293 cells transfected with CCR1, CCR3, or CCR5. Constitutive expression of MCP-5 mRNA was detected predominantly in lymph nodes, and its expression was markedly induced in macrophages activated in vitro and in vivo. Moreover, MCP-5 expression was upregulated in the lungs of mice following aerosolized antigen challenge of sensitized mice, and during the host response to infection with Nippostrongylus brasiliensis. These data indicate that MCP-5 is a novel and potent monocyte active chemokine that is involved in allergic inflammation and the host response to pathogens.
The monocyte chemoattractant proteins (MCP)1 and eotaxin constitute an important subfamily of CC or β-chemokines that share structural and functional features. Four human MCP proteins (-1, -2, -3, and -4) have been identified that share ∼65% amino acid identity (1, 2). Of the four human MCP proteins identified to date, only two have been identified in the mouse: JE (3, 4), the putative orthologue of human MCP-1, and MARC/FIC (5, 6), the putative orthologue of human MCP-3. Human MCP-1, -2, -3, and -4 are all active on monocytes (2, 7, 8), T cells (8– 10), and basophils (2, 11, 12). In addition, human MCP-2, -3, and -4 chemoattract eosinophils (2, 8, 12, 13), and human MCP-3 is chemotactic for dendritic cells (14). Eotaxin, although highly related in sequence to the MCP proteins, is inactive on monocytes, basophils, and lymphocytes and is unique in that it specifically attracts eosinophils (15, 16). MCP-1, MCP-4, and eotaxin are similarly regulated in a variety of cells. For example, in epithelial and endothelial cells, MCP-1, MCP-4, and eotaxin are induced by TNFα, IL-1, and IFNγ (1, 2, 15). IFNγ induces the secretion of MCP-2 from mononuclear cells and fibroblasts, and MARC/FIC is secreted from activated mast cells (1, 5). Other CC chemokines (e.g., RANTES and MIP-1α/β) are more distantly related in sequence to those of the MCPs and eotaxin, although they chemoattract the same spectrum of leukocyte subsets, with variable selectivity.
Chemokines induce leukocyte migration and activation by binding to specific G protein–coupled seven transmembrane spanning cell surface receptors (17). There have been five human CC chemokine receptor (CCR) genes cloned, now being referred to as CCR1 through CCR5. Each of these has an orthologue in the mouse. Human CCR2a and CCR2b are splice variants of the same gene. The chemokine and leukocyte selectivity of CCRs overlap extensively; a given leukocyte often expresses multiple chemokine receptors, and more than one chemokine typically binds to the same receptor.
While chemokines often have overlapping activities in vitro, differences in the timing and location of chemokine production in vivo imply that the redundancy found in vitro may not be biologically relevant. This is supported by chemokine inactivation experiments conducted in animal models of infection and inflammation, such as the targeted deletion of the macrophage inflammatory protein (MIP)- 1α gene (18). Despite the fact that all MIP-1α activities described in vitro are shared by other β-chemokines, including receptor usage, MIP-1α–deficient mice do not mount a normal response to viral infections.
To fully appreciate the role of chemokines in regulating inflammation, the entire spectrum of chemokines needs to be delineated and their functional role analyzed in the context of in vivo immune responses. In this report, we describe the cloning and functional characterization of a new member of the MCP subfamily of β-chemokines, murine MCP-5. The data described below provide evidence that this novel chemokine is a potent monocyte chemotactic factor that signals through CCR2. Further, we demonstrate that MCP-5 is a product of activated macrophages, and its expression is increased in murine models of pulmonary inflammation.
Materials and Methods
Isolation of the Murine MCP-5 Gene.
The human MCP-4 cDNA (2) was 32P labeled and used as a probe to screen a 129SV mouse genomic library (Stratagene Inc., La Jolla, CA). Approximately 106 phages were plated, transferred to GeneScreen Plus (DupontNew England Nuclear, Wilmington, Delaware), hybridized for 18 h at 50°C in a low stringency hybridization buffer (0.6 M NaCl, 80 mM Tris HCl, 4 mM EDTA, 0.1% [wt/vol] sodium pyrophosphate, 0.1% [wt/vol] SDS, 10× Denhardt's, 100 μg/ml denatured herring sperm DNA), and washed at 60°C in 1× SSC/ 0.1% SDS for 40 min. To exclude JE, the putative murine orthologue of human MCP-1, the filters were rehybridized as described above with a full-length murine JE cDNA fragment, and washed at 65°C in 0.1× SSC/0.1% SDS for 40 min. 15 plaques were identified that hybridized more strongly with the human MCP-4 probe than with the murine JE probe. These plaques were analyzed by PCR (30 cycles, 45°C annealing) using a set of degenerate oligomers made from highly conserved regions of murine and human MCP and eotaxin proteins (5′ oligomer in exon 1: CTTCTGKGYCTGCTGYTCA, and 3′ oligomer in exon 2: ACAGCYTYYYDGGGACA). 5 of the 15 plaques amplified an 800-bp PCR product, were subcloned into pCRII vector (Invitrogen, San Diego, CA), and sequenced using Sequenase (United States Biochemical, Cleveland, OH).
5′ and 3′ Rapid Amplification of cDNA Ends of Murine MCP-5 cDNA.
The 5′ and 3′ ends of the cDNA for murine MCP-5 were isolated using a 3′ oligomer in exon 2 (CTGGCTGCTTGTGATTCTCCTGT), a 5′ oligomer at the end of exon 1 (CAGTCCTCAGGTATTGGCTGG), and the Marathon cDNA Amplification Kit (Clontech, Palo Alto, CA). The rapid amplification of cDNA ends (RACE) products were cloned and sequenced, and oligomers were made to amplify the full length cDNA from IFNγ-treated RAW 264.7 cell poly A+ RNA (5′ oligomer: AGCTTTCATTTCGAAGTCTTTG, and 3′ oligomer: TAGATTCGGTTTAATTGGCCC). The PCR products were cloned and sequenced.
Chromosomal Localization.
PCR primers in intron 2 and exon 3 of the MCP-5 gene (5′ sense oligomer: TTACAGGTCAGGTCCCCTACT, and 3′ anti-sense oligomer: CTCCTTATCCAGTATGGTCCTG) were used to amplify genomic DNA from 94 interspecific backcross animals (C57BL/6JEi × SPRET/ Ei)F1 × SPRET/Ei (Jackson Laboratory, Bar Harbor, ME) (19). The 32P-radiolabeled PCR products were analyzed on a nondenaturing 5% acrylamide gel as described (20).
RNA Analysis.
RNA was isolated from the organs of a BALB/c mouse by lysing the tissue in guanidinium isothiocyanate and pelleting the RNA through a 5.7 M CsCl2 cushion. The poly(A)+ fraction was isolated from total RNA by oligo dT cellulose chromatography (Pharmacia, Piscataway, NJ). RNA STAT-60 (Tel-Test “B”, Inc., Friendswood, TX) was used to isolate RNA from mouse leukocytes and cell lines. 10 μg of total RNA was fractionated on a 1.2% agarose gel containing 0.7% formaldehyde, transferred to GeneScreen, and hybridized with 32P-dCTP Klenow-labeled random primed cDNA probes encoding MCP-5, JE, and the ribosomal protein (rp) L32 as a control of RNA loading. The membranes were hybridized under conditions of high stringency (50% formamide, 10% dextran sulfate, 5× SSC, 1× Denhardt's solution, 1% SDS, 100 μg/ml denatured herring sperm DNA, and 20 mM Tris at 42°C) and washed at 55°C in 0.2× SSC/0.1% SDS for 40 min. SVEC cells, an SV-40 virus immortalized murine endothelial cell line, and RAW 264.7 (American Type Culture Collection, Rockville, MD) were cultured for 6 and 18 h without additions, or with the addition of 200 U/ml murine IFNγ (Genentech, Inc., San Francisco, CA), 5 ng/ml murine IL-1β (Genzyme Corp., Cambridge, MA), or 10 ng/ml murine IL-4 (Genzyme Corp.). Mouse bone marrow–derived mast cells were activated with IgE anti-TNP and TNP-BSA, or 2.5 mg/ml of Con A for 4 h as described (21).
Mouse Models of Pulmonary Inflammation.
The aerosolized OVA model was performed as described (22). Briefly, BALB/cJ mice between 5 and 10 wks of age were immunized with 10 μg of OVA (Sigma Chemical Co., St. Louis, MO) and 1 mg aluminum hydroxide intraperitoneally on days 0, 7, and 14. Sham-immunized mice received aluminum hydroxide alone. Mice underwent aerosol challenge with OVA (50 mg/ml in sterile saline) 7–10 d after the final immunization. Mice were killed at 3, 6, 24, and 48 h after challenge, and lungs were harvested for RNA extraction. A minimum of three mice were included in each group at each time point. The Nippostronglyus brasiliensis (Nb) model was performed as described (23). Briefly, 12-wk-old female BALB/cJ mice were injected with 750 third stage Nb larvae and the lungs harvested at 7, 10, and 14 days for RNA extraction.
Purification of Monocytes, Macrophages, Eosinophils, and Neutrophils.
PBMC were obtained from 4 normal donors by density gradient centrifugation using 1.077 Histopaque (Sigma Chemical Co.). Murine eosinophils were isolated from the spleens of IL-5 transgenic mice by negative selection through a MACS magnet (Miltenyi Biotech, Auburn, CA) (24). The resulting eosinophil purity was >90% as determined by microscopic examination of Diff Quick (Baxter Scientific, McGaw Park, IL)-stained cytospin preparations; contaminating cells were mononuclear. Neutrophils and macrophages were isolated from the peritoneal cavities of mice, and further purified by centrifugation in self-forming Percoll gradients as described (25). Neutrophil preparations were typically >90% pure with <10% mononuclear cell contamination.
Chemotaxis.
Eosinophils, neutrophils, and mononuclear cells were suspended in HBSS with 0.05% BSA at 2.5, 1, and 5 × 106 cells/ml, respectively, and placed in the top of a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD). A polycarbonate filter with 5-μm pores (eosinophils and mononuclear cells) and a polyvinylpyrrolidine-free filter with 3-μm pores (neutrophils) separated the cells from buffer alone or buffer containing purified recombinant murine eotaxin, human MCP-1, and human IL-8 (PeproTech Inc., Rocky Hill, NJ), or murine MIP-1α, murine MIP-1β, murine JE, and murine KC (R&D Sys. Inc., Minneapolis, MN). Murine MCP-5 was expressed and purified from Escherichia coli by PeproTech as the predicted mature 82– amino acid protein beginning with the NH2-terminal glycine. NH2-terminal sequence analysis of the purified recombinant MCP-5 preparation confirmed its homogeneity and the NH2terminal glycine. Cells were incubated at 37°C for 30 (neutrophils), 60 (eosinophils), or 90 min (mononuclear cells), and the cells that migrated across the filter and adhered to the bottom side of the filter were stained with Diff-Quick.
Calcium Flux in Leukocytes.
Purified cells (107/ml in HBSS with 0.05% BSA) were loaded with 5.0 μM of the acetoxymethyl ester of fura-2 (fura-2 AM) (Molecular Probes Inc., Eugene, OR) for 60 min at 37°C in the dark. Loaded cells were washed twice and resuspended in a buffer containing 145 mM NaCl, 4 mM KCl, 1 mM NaHPO4, 0.8 mM MgCl2, 1.8 mM CaCl2, 25 mM Hepes, and 22 mM glucose. 2 ml of cells (5 × 106 cells/ml) were placed in a continuously stirring cuvette at 37°C in a dual-wavelength excitation source fluorimeter (Photon Technology Inc., South Brunswick, NJ). Changes of cytosolic-free calcium were determined after addition of the chemokines by monitoring the excitation fluorescence intensity emitted at 510 nm in response to sequential excitation at 340 and 380 nm. The data are presented as the relative ratio of fluorescence at 340/380 nm.
Calcium Flux Responses in Chemokine Receptor Transfected Cells.
Human embryonic kidney (HEK)-293 cells stably expressing human CCR2b, CCR1, and CCR3 and murine CCR2 and CCR5 with the “FLAG” epitope at the extreme NH2 terminus, were prepared as described (26–28). For calcium fluorimetry, cells were grown to log phase, loaded with the calcium-specific dye indo-1 AM (Molecular Probes Inc.), and assayed by spectrofluorimetry for changes in the concentration of intracellular calcium in response to addition of chemokines (27).
Results
MCP-5 Genomic Structure and Chromosomal Localization.
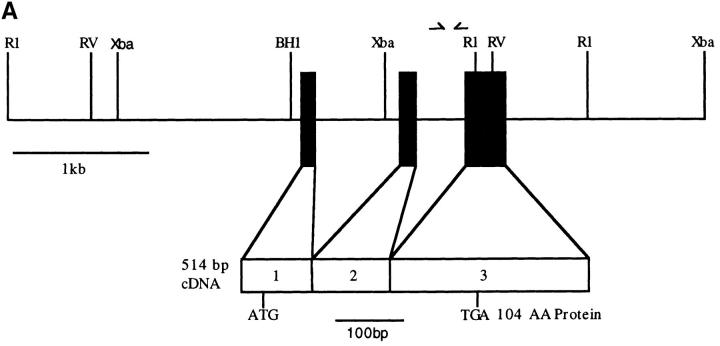
To isolate novel mouse CC chemokine genes, a murine genomic library was sequentially screened with a human MCP-4 cDNA probe under conditions of low stringency and a mouse JE cDNA probe under conditions of high stringency. 15 plaques were identified that hybridized more strongly with the human MCP-4 probe than with the murine JE probe. These plaques were purified and further analyzed by PCR using a set of degenerate primers. A PCR product was generated from five of these clones, and all contained the same novel sequence that had a high degree of homology with the MCP subfamily. Southern blot analysis of mouse genomic DNA established a restriction map (Fig. 1 A) and revealed that MCP-5 was a single copy gene (data not shown). One of the five overlapping MCP-5 genomic clones was partially sequenced to determine the intron/exon structure of the MCP-5 gene (Fig. 1 A), and to confirm the sequence of the PCR products. Like other CC chemokines, the MCP-5 gene contained three exons and two introns.
Figure 1.
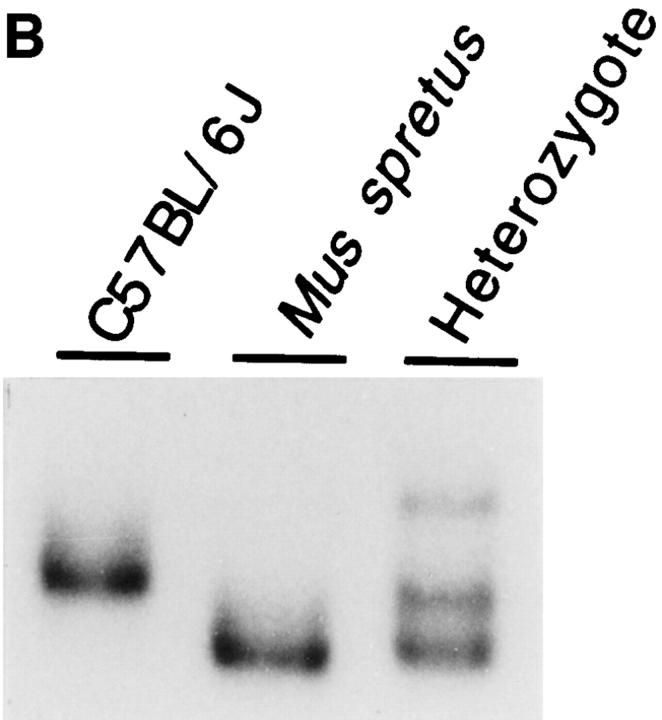
Genomic organization, chromosomal mapping, nucleotide sequence, and predicted amino acid sequence of the murine MCP-5 gene. (A) Partial restriction map and genomic organization of the mouse MCP-5 gene. The mature mRNA is shown schematically below with the positions of the start codon (ATG) and stop codon (TGA). The scales are shown below each drawing. (B) SSCP polymorphism. PCR primers in intron 2 and exon 3, indicated by arrows in A, were used to amplify genomic DNA from C57BL/6J, Mus spretus, or the C57BL/6J × M. spretus heterozygote, and analyzed using SSCP. The complete raw data for this cross with references and notes are available on the World Wide Web at http://www.jax.org/resources/documents/cmdata/BSS11data.html and the Mouse Genome Database accession number is MGD-CREX-697. (C) MCP-5 cDNA sequence. The filled triangles indicate the intron/exon borders. The single underline indicate the ATTTA sequences that have been reported to decrease mRNA stability. The double underlined sequence indicates the predicted polyadenylation signal. The sequence has been deposited in GenBank/EMBL/DDBJ under the accession U66670. The arrow indicates the predicted site for a signal peptidase cleavage.
The chromosomal localization of MCP-5 was determined by single-strand conformation polymorphism (SSCP) analysis using a set of PCR primers in intron 2 and exon 3 that detected a polymorphism when using DNA from C57BL/ 6J and Mus spretus (Fig. 1 B). A panel of genomic DNA from 94 interspecific backcrossed animals was used to map the MCP-5 gene based on this SSCP polymorphism. The MCP-5 gene cosegregated with Scya7 (fic) and Scya11 (eotaxin) in this cross, placing it between D11Mit markers 7 and 36 on chromosome 11 (data not shown). A comparison to the consensus map from the mouse genome database revealed this to be the region of chromosome 11 containing the CC chemokine gene cluster designated Scya1-11. The MCP-5 gene has been assigned the designation Scya12.
Analysis of MCP-5 cDNA.
To determine the complete structure of the MCP-5 cDNA, 5′ and 3′ RACE was performed using RNA isolated from IFNγ-treated RAW 264.7 cells. Once the ends of the cDNA were determined, a full-length cDNA was isolated using reverse transcriptase (RT)-PCR and was sequenced (Fig. 1 C). The cDNA was 514 bp long with an open reading frame that encoded 104 amino acids. The 5′ region of the cDNA encoded a 22– amino acid hydrophobic leader sequence with a predicted cleavage site at a position similar to the other MCPs and eotaxin, resulting in a mature protein of 9.3 kD with a pI of 9.4. The 3′ untranslated region contained a single polyadenylation signal of a rare type also found in human eotaxin (ATTAAA) (15) and four mRNA destabilization signals (ATTTA) (Fig. 1 C) that have been reported to decrease the mRNA stability of other cytokine mRNAs (29).
Sequence analysis revealed that murine MCP-5 was a novel chemokine most homologous to human MCP-1. In fact, MCP-5 is structurally more similar to MCP-1 than JE, the putative murine homologue of MCP-1. This holds true even when JE's unique 49–amino acid serine/threoninerich, highly glycosylated COOH-terminal extension is excluded from the comparison. The mature MCP-5 protein is 66% identical to the mature human MCP-1 protein, while JE is 55% identical to human MCP-1. MCP-5 is unique among the MCP proteins in that its NH2-terminal amino acid is predicted to be glycine, a feature that it shares with human eotaxin (15).
MCP-5 Induced Leukocyte Chemotaxis.
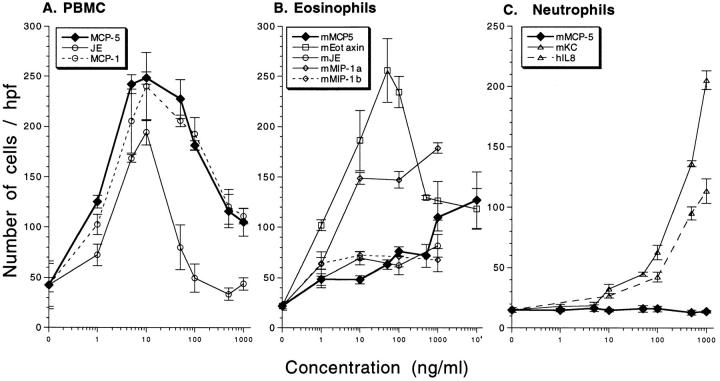
The chemotactic activity of purified recombinant MCP-5 was evaluated on human peripheral blood monocytes, mouse eosinophils, and mouse neutrophils (Fig. 2). MCP-5 was a potent chemoattractant for human peripheral blood monocytes, as was mouse JE and human MCP-1 (Fig. 2 A). MCP-5 had minimal activity on murine eosinophils and only at doses ⩾1,000 ng/ml. These cells were very responsive to the positive controls eotaxin and MIP-1α (peak chemotaxis at 50 ng/ml or 5 nM), and only minimally responsive to the negative controls JE and MIP-1β (Fig. 2 B). Murine neutrophils exhibited no response to MCP-5, but had a strong response to the controls mouse KC and human IL-8 (Fig. 2 C). These results demonstrate that purified MCP-5 was a potent, dose-dependent, chemotactic agent for peripheral blood monocytes.
Figure 2.
Chemotactic response of leukocytes to recombinant murine MCP-5. Human peripheral blood mononuclear cells (A), mouse eosinophils (B), and mouse neutrophils (C) were exposed to increasing concentrations of the indicated chemokines in a modified Boyden chamber, and the number of cells that migrated through the membrane was determined. Data are the number of cells/×400 field. The results shown are representative experiments (n = 9 PBMC, n = 7 eosinophils, and n = 3 neutrophils) and presented as the mean ± standard error of eight fields counted of replicate wells.
Calcium Flux in Leukocytes in Response to MCP-5.
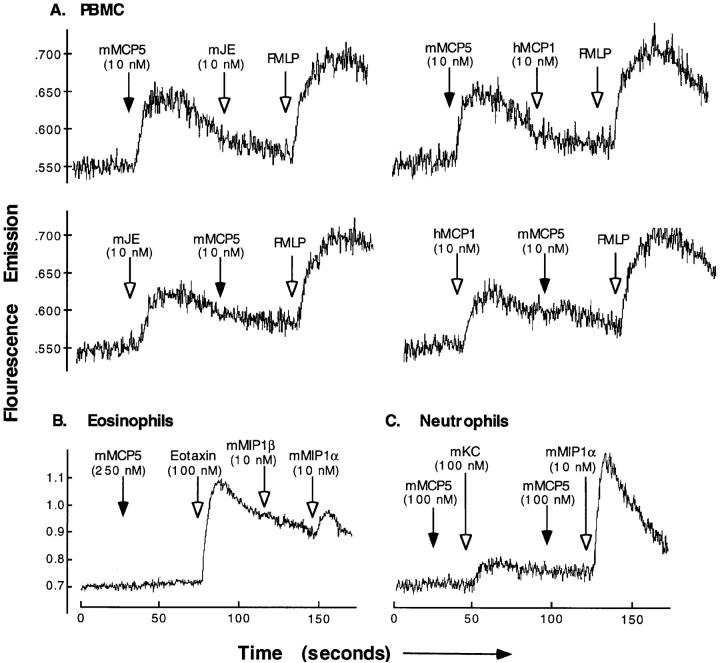
Chemokines induce cell migration and activation by binding to specific G protein–coupled seven transmembrane cell surface receptors on leukocytes. Signaling through these receptors results in a transient rise in intracellular calcium. To determine if MCP-5 induces a calcium flux in responding leukocytes, and thus further examine the leukocyte specificity of MCP-5, purified leukocyte subsets were loaded with the calcium sensitive dye fura-2, and their response to MCP-5 was monitored by fluorimetry. Concordant with the chemotaxis data presented above, MCP-5 induced a dose dependent calcium flux in mononuclear cells (Fig. 3 A), but not eosinophils (even at 50 μg/ml or 5 μM) (Fig. 3 B), or neutrophils (Fig. 3 C). As controls, the purified eosinophils responded appropriately to eotaxin and MIP-1α, but did not respond to MIP-1β (Fig. 3 B) or JE (data not shown), and the purified neutrophils responded appropriately to KC and MIP-1α (Fig. 3 C). The half-maximal effective concentration of the MCP-5 induced mononuclear cell calcium transient was ∼10 ng/ml (1 nM) (data not shown). These results demonstrate that MCP-5 induces a calcium flux in mononuclear cells, but not in eosinophils or neutrophils.
Figure 3.
Calcium flux responses of leukocytes to MCP-5. Fura-2–loaded cells were exposed sequentially to the indicated chemokines with their concentrations in parentheses. Calcium flux is reported as ratio fluorescence of fura-2 in human peripheral blood mononuclear cells (A), murine eosinophils (B), and murine neutrophils (C). The results shown are representative experiments (n ⩾4 for all cell types).
Desensitization Studies.
Rapid successive exposure to the same ligand is known to desensitize the signaling capacity of G protein–linked receptors. This was true for MCP-5 which, at a concentration of 10 nM, completely desensitized PBMC to a subsequent MCP-5 (10 nM) challenge (data not shown). Exposure of cells to different ligands that use the same receptor signaling pathway can also result in desensitization. MCP-5 (10 nM) was able to completely desensitize the cells to a subsequent stimulation with JE (10 nM) or human MCP-1 (10 nM) (Fig. 3 A); however, these cells were still responsive to the unrelated ligand FMLP. This desensitization effect was dose dependent; when the initial dose of MCP-5 was lowered to 0.05 nM, the mononuclear cells were no longer desensitized to subsequent stimulation with either 10 nM JE or MCP-1 (data not shown). Likewise, mononuclear cells initially stimulated with 10 nM JE or MCP-1 were desensitized, although usually not completely, to a subsequent stimulation with 10 nM MCP-5 (Fig. 3 A). As would be expected from the lack of activity of MCP-5 on eosinophils and neutrophils, initial treatment of these cells with MCP-5 (100 nM) had no effect on the ability of eosinophils to respond to eotaxin (10 nM) or MIP-1α (100 nM), or neutrophils to respond to KC (10 nM). These results suggest that MCP-5 shares a receptor with JE and MCP-1 that is distinct from the receptors utilized by MIP-1α, eotaxin, and KC.
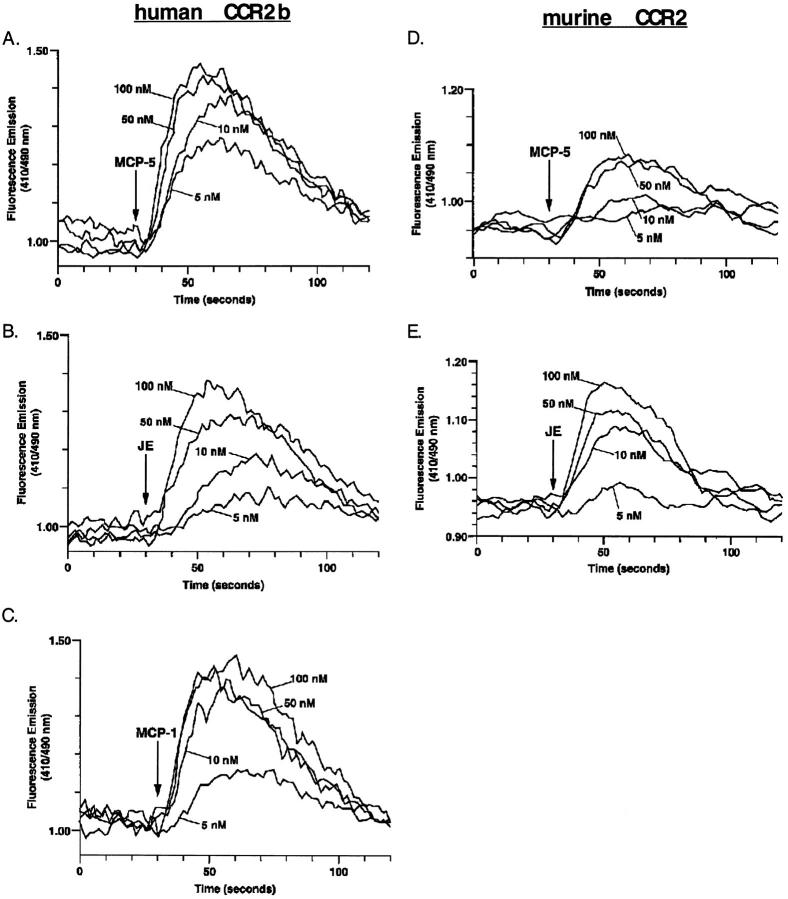
CCR2 is an MCP-5 Receptor.
The signaling and desensitization studies performed on mononuclear cells suggested that MCP-5 activated the same receptor as MCP-1 and JE. Furthermore, the inability of MCP-5 to induce a calcium flux in murine eosinophils suggested that MCP-5 does not signal through the two receptors known to be expressed on eosinophils, CCR3, and CCR1 (21). To directly test these hypotheses, we examined the ability of MCP-5 to activate a number of cloned human CC chemokine receptors stably expressed in HEK-293 cells. MCP-5 (100 nM) reproducibly induced a robust intracellular calcium flux in cells expressing CCR2 (Fig. 4), but not CCR1, CCR3, or CCR5 (data not shown). In control experiments, we confirmed functional expression of the cloned receptors in these MCP-5 unresponsive targets by demonstrating that the CCR1 and CCR5 cell lines responded to MIP-1α, and the CCR3 line responded to eotaxin (data not shown). Doseresponse experiments demonstrated that MCP-5 was an excellent ligand for human CCR2b (EC50 ⩽5 nM), as was MCP-1, and both were more potent ligands than JE (Fig. 4 A–C). Since the MCP-5 we have described is a murine protein, it was of interest to directly compare it to JE for activation of the murine MCP-1 receptor (CCR2). As shown in Fig. 4 B, JE induced a more robust intracellular calcium flux than MCP-5 in HEK-293 cells stably expressing murine CCR2. No response was seen to MCP-5 in cells transfected with murine CCR5, although these cells did respond to the positive control MIP-1α (data not shown). These data raise the possibility that the natural murine receptor for MCP-5 is yet to be cloned. Desensitization studies using cells transfected with human CCR2b revealed that MCP-5 blocked subsequent responses to MCP-5, MCP-1, and JE (Fig. 5). Similar results were obtained when the initial agonist was MCP-1 or JE (Fig. 5). However, MCP-1 did not completely desensitize the CCR2b transfectants to a subsequent MCP-5 stimulation (Fig. 5). These data are consistent with the desensitization responses observed on human PBMC (Fig. 3), and with the hypothesis that MCP-5 is a full agonist for the human MCP-1 receptor (CCR2b).
Figure 4.
Identification of CCR2 as a functional MCP-5 receptor. HEK-293 cells stably expressing human CCR2b (A–C) or murine CCR2 (D and E) were loaded with indo-1 AM, and intracellular calcium concentrations were monitored by ratio fluorescence in response to the indicated concentrations of MCP-5 (A and D), JE (B and E), and MCP-1 (C). Shown is one of three similar experiments.
Figure 5.
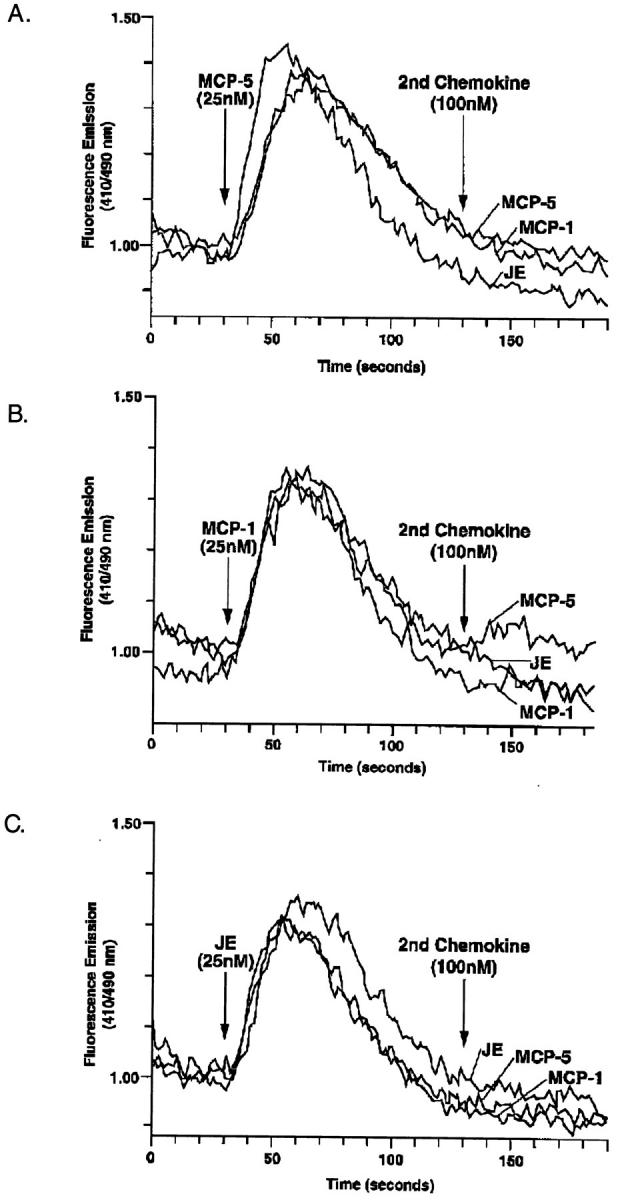
MCP-5 induces desensitization of CCR2b. HEK-293 cells stably expressing human CCR2b were exposed to MCP-5 (25 nM) (A), and subsequently challenged with MCP-5 (100 nM), MCP-1 (100 nM), or JE (100 nM). Intracellular calcium concentrations were monitored by ratio fluorescence in response to the indicated ligand. Identical experiments were performed in which the CCR2b expressing cells were initially exposed to MCP-1 (25 nM) (B), or JE (25 nM) (C). Shown is one of three experiments.
MCP-5 mRNA Expression in Mouse Tissues and Cells.
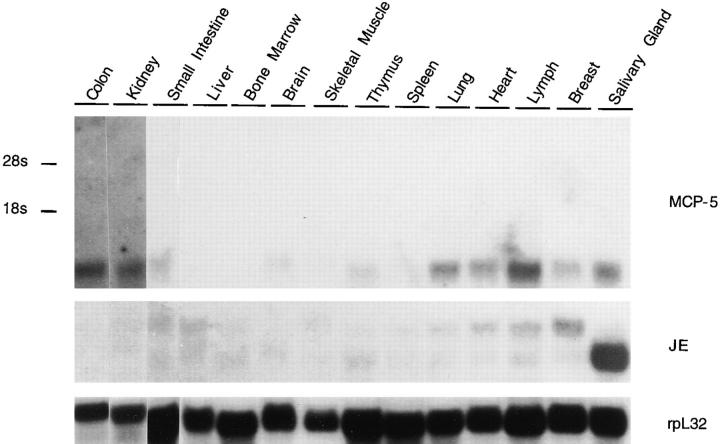
To investigate the expression of murine MCP-5 in normal murine tissues, a Northern analysis of mRNA obtained from mouse organs was performed (Fig. 6). This analysis revealed an ∼550-bp transcript that corresponds to the mature mouse MCP-5 mRNA. The highest levels of constitutive MCP-5 expression were observed in normal lymph nodes. MCP-5 mRNA levels were also seen in breast and salivary glands containing lymph nodes, heart, lung, thymus, brain, small intestines, kidney, and colon. However, no expression was detected in spleen, skeletal muscle, bone marrow, or liver. The expression of MCP-5 and JE were compared in these mouse tissues since MCP-5 had a similar biological profile to JE and signaled through the same receptor. Highest constitutive expression of JE was seen in the salivary gland with low levels detected in other organs (e.g., lymph nodes) (Fig. 6). The ∼550- and 800-bp JE transcripts correspond to known 3′ splice variants (4).
Figure 6.
Expression of MCP-5 in normal mouse organs. Northern analysis of 10 μg total RNA isolated from various tissues of a normal BALB/c mouse. Salivary gland and breast tissue contained lymph nodes. The blot was hybridized sequentially with MCP-5, JE, and rpL32 cDNA probes and exposed for 29 d, 21 d, and 18 h, respectively. The positions of 18s and 28s RNA are indicated on the left.
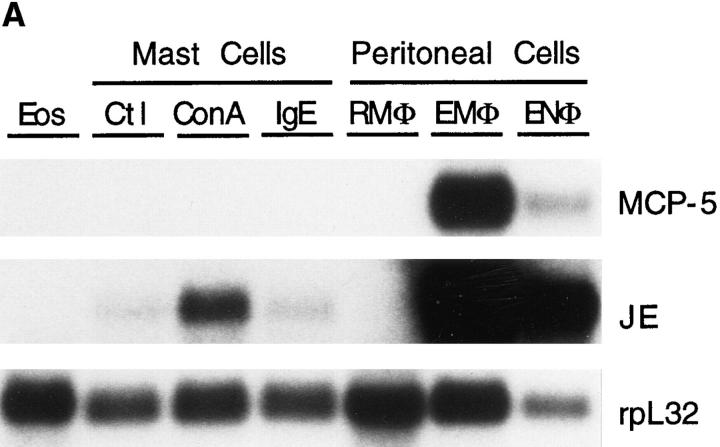
The expression of MCP-5 was examined in purified leukocyte subsets isolated from normal mice (Fig. 7 A). MCP-5 expression was not seen in eosinophils, bone marrow–derived mast cells, or resident peritoneal macrophages. Stimulation of these mast cells with either IgE or Con A did not induce MCP-5 expression. Con A upregulated the expression of JE (Fig. 7 A), and both treatments induced the expression of murine CCR1 (21). Activated macrophages elicited into the peritoneal cavity by thioglycollate expressed significant levels of MCP-5 mRNA. Neutrophils elicited into the peritoneum by treatment with sodium casein also expressed MCP-5 mRNA; however, expression from contaminating elicited macrophages cannot be excluded.
Figure 7.
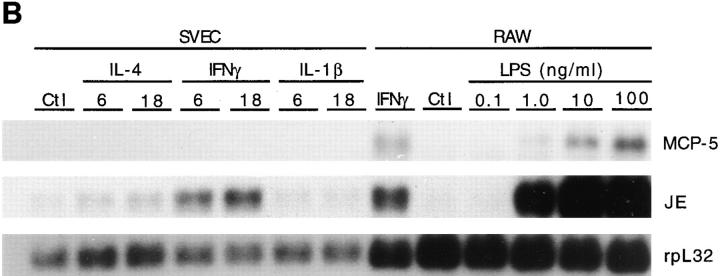
MCP-5 mRNA expression in murine leukocytes and endothelial cells. (A) Northern analysis of total RNA isolated from eosinophils purified from the spleens of CD5-IL5 transgenic mice (Eos), bone marrow–derived mast cells either untreated (Ctl) or stimulated with IgE antiTNP and TNP-BSA (IgE), or Con A, resident peritoneal macrophages (RMφ) (93% mononuclear, 7% granulocytes), elicited peritoneal macrophages (EMφ) (76% mononuclear, 24% neutrophils), and elicited peritoneal neutrophils (95% neutrophils, 5% mononuclear). Blots were hybridized with MCP-5, JE, and rpL32 cDNA probes and exposed for 14 d, 3 d, and 18 h, respectively. (B) Northern analysis of total RNA isolated from SVEC cells treated for 6 and 18 h with IFNγ (200 U/ml), IL-4 (10 ng/ml), and IL-1β (5 ng/ml) and 10 μg of total RNA isolated from RAW 264.7 macrophage cell line, either untreated or treated for 18 h with IFNγ (200 U/ml) or the indicated concentrations of LPS. Blots were hybridized sequentially with MCP-5, JE, and rpL32 cDNA probes and exposed for 7 d, 3 d, and 18 h, respectively.
Since it appeared that activated macrophages were a primary source of MCP-5 mRNA, we evaluated the ability of various stimuli to induce MCP-5 mRNA in the RAW 264.7 macrophage cell line. IFNγ and LPS induced MCP-5 expression (Fig. 7 B), while IL-4 and TNFα had no apparent effect (data not shown). JE was similarly expressed in activated macrophages and induced in RAW cells by LPS and IFNγ (Fig. 7 B).
The expression of MCP-5 was also examined in cytokine stimulated murine endothelial cells (SVEC) to further explore the regulation and cellular sources of this chemokine. MCP-5 was not induced in SVEC endothelial cells by IFNγ, IL-4, or IL-1β, but JE was induced by IFNγ and IL-4 (Fig. 7 B), and eotaxin was induced by IFNγ (24).
MCP-5 mRNA Expression in Murine Models of Pulmomary Inflammation.
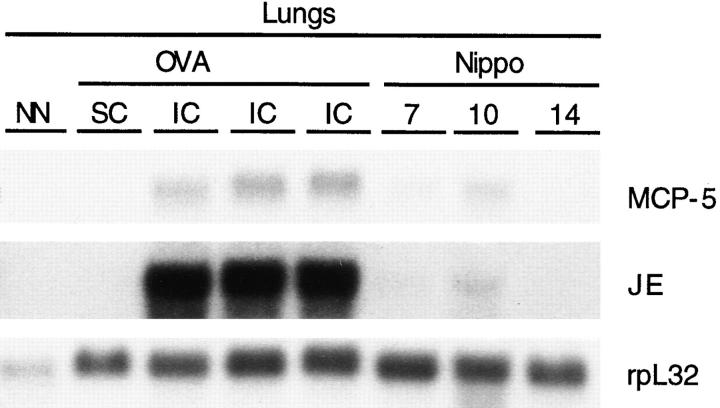
To examine the regulation of MCP-5 during in vivo immune responses, we examined its expression in the lungs of mice following infection with Nb and in a murine model of OVA-induced pulmonary inflammation (Fig. 8). Following infection with Nb, MCP-5 lung mRNA levels were markedly increased by day 7, peaked by day 10, and returned to baseline by day 14. The kinetics of MCP-5 mRNA accumulation preceded the peak recruitment of pulmonary macrophages, neutrophils, and eosinophils that characterize the granulomatous immune response to this nematode pathogen (23). MCP-5 mRNA was also detected in OVA-immunized mice 3 h after aerosol challenge (Fig. 8), and remained elevated at 48 h (data not shown). MCP-5 mRNA remained at prechallenge levels in challenged sham-immunized mice (Fig. 8). Again, the kinetics of MCP-5 mRNA accumulation preceded the peak accumulation of pulmonary macrophages, lymphocytes, and eosinophils that characterize the immune response in this model (22). Like MCP-5, JE was induced in both murine models of pulmonary inflammation; however, in the OVA model, levels of JE mRNA returned to baseline by 48 h, while MCP-5 levels remained elevated (data not shown).
Figure 8.
MCP-5 mRNA expression in murine models of pulmonary inflammation. Northern analysis of total RNA isolated from the lungs of nonimmunized nonchallenged mice (NN), sham-immunized mice challenged with aerosolized OVA (SC), OVA-immunized mice challenged with aerosolized OVA (IC), or from the lungs of mice at 7, 10, and 14 d after infection with Nb. All OVA challenged tissue was harvested at 3 h (n = 3). Each lane represents an individual mouse. Blots were hybridized sequentially with MCP-5, JE, and rpL32 cDNA probes and exposed for 14 d, 3 d, and 18 h, respectively.
Discussion
As the complexity of the chemokine system continues to increase, it is apparent that to fully appreciate the role of chemokines in development, homeostasis, and host response to infection and inflammation, the entire spectrum of chemokines needs to be known, and their functions deciphered in animal models. In this report, we describe the molecular isolation and functional characterization of a novel mouse β-chemokine, most closely related to the MCP and eotaxin subfamily of β-chemokines, which we have named MCP-5. We have demonstrated that MCP-5 is expressed constitutively in lymph nodes of normal mice, and its expression is increased in activated macrophages and in the lungs of mice following allergen challenge and Nb infection. Purified recombinant MCP-5 protein was potently chemotactic for monocytes and induced a calcium flux in these cells. In addition, we identified CCR2 (the MCP-1 receptor), which is expressed preferentially on monocytes and activated lymphocytes (30, 31) as an MCP-5 receptor.
Although JE is widely regarded as the murine orthologue of human MCP-1, MCP-5 is more homologous to human MCP-1 than is JE. It is noteworthy that both murine MCP-5 and human MCP-1 were more potent and more efficacious agonists of human CCR2b than JE. In contrast, JE was a better agonist of murine CCR2 than murine MCP-5. This suggests that there may be as yet an unidentified receptor for MCP-5 in the mouse and perhaps additional MCP-like ligands (e.g., MCP-5) and receptors in humans.
Like other β-chemokines, MCP-5 was inactive on neutrophils. However, we were also interested in testing the activity of MCP-5 on another granulocyte, the eosinophil. Aside from MCP-1, which lacks activity on eosinophils, the other MCP proteins characterized to date, MCP-2, -3, and -4, are active on eosinophils. We were surprised that MCP-5 had essentially no activity on eosinophils since it shares an NH2-terminal glycine with human eotaxin. The small amount of chemotaxis seen at high doses of MCP-5 (1 and 10 μg/ml) was not associated with a detectable calcium flux in these cells. Furthermore, MCP-5 was not a functional ligand for human CCR1 and CCR3, the only two CC chemokine receptors that have been demonstrated to be expressed on eosinophils. Therefore, the weak chemotaxis of eosinophils induced by high doses of MCP-5 may be secondary to a low affinity interaction with a different receptor that does not induce a calcium flux or induces one below the limit of detection.
The mature NH2-terminal amino acid is thought to be important for the biological activity and leukocyte selectivity of the MCP and eotaxin proteins. For example, addition of a single amino acid before MCP-1's NH2-terminal glutamine, or the deletion of this glutamine residue, reduces MCP-1's biological activity on monocytes 100–1,000-fold (32). Furthermore, deletion of this NH2-terminal glutamine residue converts MCP-1 from an activator of basophils to an eosinophil chemoattractant (33). However, if the NH2-terminal glutamine of MCP-1 is replaced by other small amino acids, like asparagine and alanine, it still retains activity on monocytes (32). We have also found that an NH2-terminal extension of eotaxin or MCP-4 completely inactivated these molecules (2, 16). Our findings that MCP-5 shares a receptor with MCP-1, -3, and -4, namely CCR2, even though it does not share the NH2-terminal glutamine, is consistent with the hypothesis that the NH2terminal residue of this family of molecules is not the sole determinant of leukocyte specificity.
Understanding the regulation of chemokine expression in vitro and in vivo is essential in order to begin to appreciate the mechanisms whereby this family of molecules controls the recruitment of immune cells in the host response. In this regard, the expression of MCP-5 was examined in normal tissues, cytokine activated cells in vitro, and inflammatory responses in vivo. The pattern of MCP-5 expression differs considerably from other related chemokines. For example, MCP-5 was expressed at the highest constitutive levels in the lymph nodes of normal mice, while JE was expressed at highest levels in the salivary gland. MCP-4 and eotaxin are constitutively expressed at the highest levels in organs with large epithelial surfaces, such as the small and large intestines and the lungs (2, 15). MCP-5 was induced in thioglycollate elicited macrophages and in a macrophage cell line induced by IFNγ and LPS. However, MCP-5 was not expressed in activated mast cells, though these cells expressed MARC/FIC and JE. Furthermore, while we did not detect MCP-5 expression in activated endothelial cells, these cells express MCP-1, JE, MCP-4, and eotaxin after stimulation with inflammatory cytokines like IL-1, IL-4, TNF, and IFNγ (2, 15, 24). These results suggest that MCP-5 has a unique pattern of expression that reflects its unique role in regulating the immune and inflammatory responses.
The expression of MCP-5 was also examined in two murine models of inflammation. A kinetic pattern of expression was observed consistent with the role of MCP-5 in recruiting the early mononuclear phase of the inflammatory response. In the Nb model, MCP-5 mRNA expression peaked at day 10 and was back down to baseline by day 14. Nb larvae pass through and molt in the lung during the first few days of infection. The time of peak histologic change in the lungs is at day 14, and is characterized by focal aggregates of monocytes, neutrophils, and eosinophils (23). In the aerosolized ovalbumin model, MCP-5 mRNA expression was detected 3 h after antigen challenge, a time when the inflammatory infiltrate is just beginning to be seen (22). We have determined that several chemokines such as eotaxin, JE, MIP-1α, and MARC/FIC are also upregulated following antigen challenge, whereas the level of RANTES is unchanged (22). While MCP-5 is not directly chemotactic for eosinophils, its association with pulmonary eosinophilia in these two models may be due to its role in recruiting the initial waves of mononuclear cells which control the subsequent recruitment of eosinophils.
These findings add to the growing understanding of the chemokine system by describing the isolation of MCP-5, a potent monocyte chemoattractant, and CCR2 agonist that is expressed in activated macrophages and models of pulmonary inflammation. As such, the functional role of MCP-5 will need to be considered when trying to understand the in vivo immune response to allergens and pathogens.
Acknowledgments
We would like to thank L. Rowe from the Jackson Laboratory for her help with the chromosome mapping, M. Rothenberg for the generous gift of mast cell RNA, hybridomas, and for his helpful discussions, R. Coffman for the generous gift of lungs infected with Nb, C. Sanderson for the IL-5 transgenic mice, C. Franci for calcium fluorimetry on transfected cell lines, B. Rollins for the JE cDNA clone, and K. O'Connell for the SVEC cells.
Footnotes
This work was supported by National Institutes of Health grants to A.D. Luster (CA69212), I.F. Charo (HL52773), and J. MacLean (AI01245), a Cancer Research Institute/Benjamin Jacobson Family Investigator Award to A.D. Luster and a Fogarty International Fellowship to E.A. Garcia-Zepeda.
1 Abbreviations used in this paper: CCR, CC chemokine receptor; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; Nb, Nippostronglyus brasiliensis; RACE, rapid amplification of cDNA ends; rp, ribosomal protein; SSCP, single-strand conformation polymorphism; SVEC, SV-40 immortalized murine endothelial cell line.
References
- 1.Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J Leukocyte Biol. 1996;59:67–74. doi: 10.1002/jlb.59.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Zepeda EA, Combardiere C, Rothenberg ME, Sarafi M, Hamid Q, Murphy PM, Luster AD. Human monocyte chemoattractant protein (MCP)-4: a novel CC chemokine with activities on monocytes, eosinophils, and basophils, induced in allergic and non-allergic inflammation, that signals through the CC chemokine receptors CKR-2 and CCR-3. J Immunol. 1996;157:5613–5626. [PubMed] [Google Scholar]
- 3.Rollins BJ, Morrison ED, Stiles CD. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci USA. 1988;85:3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawahara RS, Deuel TF. Platelet-derived growth factor-inducible gene JE is a member of a gene family of small inducible genes related to platelet factor 4. J Biol Chem. 1989;264:679–682. [PubMed] [Google Scholar]
- 5.Kulmburg PA, Huber NE, Scheer BJ, Wrann M, Baumruker T. Immunoglobulin E plus antigen challenge induces a novel intercrine/chemokine in mouse mast cells. J Exp Med. 1992;176:1773–1778. doi: 10.1084/jem.176.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinrich JN, Ryseck R-P, MacDonald-Bravo H, Bravo R. The product of a novel growth factor-activated gene, fic, is a biologically active “C–C”-type cytokine. Mol Cell Biol. 1993;13:2020–2030. doi: 10.1128/mcb.13.4.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uguccioni M, D'Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α and MIP-1β on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 8.Uguccioni M, Loetscher P, Forssmann U, Dewald B, Li H, Lima SH, Li Y, Kreider B, Garotta G, Thelen M, Baggiolini M. Monocyte chemotactic protein 4 (MCP-4), a novel structural and functional analogue of MCP-3 and eotaxin. J Exp Med. 1996;183:2379–2384. doi: 10.1084/jem.183.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, Van Damme J. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95:1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994;8:1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff SC, Krieger M, Brunner T, Dahinden CA. Monocyte chemotactic protein 1 is a potent activator of human basophils. J Exp Med. 1992;175:1271–1275. doi: 10.1084/jem.175.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber M, Uguccioni M, Ochennsberger B, Baggiolini M, Clark-Lewis I, Dahinden CA. Monocyte chemotactic protein MCP-2 activates human basophil and eosinophil leukocytes similar to MCP-3. J Immunol. 1995;154:4166–4172. [PubMed] [Google Scholar]
- 13.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 15.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;4:449–456. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 16.Rothenberg ME, Ownbey R, Mehlhop P, Loiselle PM, van de Rijn JM, Bonventre JV, Oettgen HC, Leder P, Luster AD. Eotaxin triggers eosinophil selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin-5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 18.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science (Wash DC) 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 19.Rowe LB, Nadeau JH, Turner R, Frankel WN, Letts VA, Eppig JT, Ko MS, Thurston SJ, Birkenmeier EH. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource (erratum published 5:463) Mamm Genome. 1994;5:253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- 20.Beier DR, Dushkin H, Sussman DJ. Mapping genes in the mouse using single-strand conformation polymorphism analysis of recombinant inbred strains and interspecific crosses. Proc Natl Acad Sci USA. 1992;89:9102–9106. doi: 10.1073/pnas.89.19.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J-L, Sen AI, Tiffany L, Yoshi O, Rothenberg ME, Murphy PM, Luster AD. Identification of a mouse eosinophil-selective receptor for the CC chemokine eotaxin. Biochem Biophys Res Commun. 1996;223:679–684. doi: 10.1006/bbrc.1996.0955. [DOI] [PubMed] [Google Scholar]
- 22.MacLean JA, Ownbey R, Luster AD. T cell– dependent regulation of eotaxin in antigen-induced pulmomary eosinophilia. J Exp Med. 1996;184:1461–1469. doi: 10.1084/jem.184.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminthinduced eosinophilia in mice. Science (Wash DC) 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 24.Rothenberg ME, Luster AD, Leder P. Murine eotaxin: an eosinophil chemoattractant inducible in endothelial cells and in IL-4-induced tumor suppression. Proc Natl Acad Sci USA. 1995;92:8960–8964. doi: 10.1073/pnas.92.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, Laning J, Devi S, Mak J, Schall TJ, Dorf ME. Biologic activities of the murine β-chemokine TCA3. J Immunol. 1994;153:4616–4624. [PubMed] [Google Scholar]
- 26.Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1α receptor confers chemokine selectivity. J Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- 27.Boring L, Gosling J, Monteclaro FS, Lusis AJ, Tsou CL, Charo IF. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein-1 alpha receptors: evidence for two closely linked C–C chemokine receptors on chromosome 9. J Biol Chem. 1996;271:7551–7558. doi: 10.1074/jbc.271.13.7551. [DOI] [PubMed] [Google Scholar]
- 28.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 29.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 30.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong J-H, Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J Exp Med. 1995;181:631–640. doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber M, Uguccioni M, Baggiolini M, Clark-Lewis I, Dahinden CA. Deletion of the NH2-terminal residue converts monocyte chemotactic protein 1 from an activator of basophil mediator release to an eosinophil chemoattractant. J Exp Med. 1996;183:681–685. doi: 10.1084/jem.183.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]