Abstract
Several inflammatory cytokines, most notably tumor necrosis factor (TNF) and IL-1, induce anorexia and loss of lean body mass, common manifestations of acute and chronic inflammatory conditions. In C57BL/6 female mice, the administration of TNF, IL-1, and, to a lesser extent, leukemia inhibitory factor (LIF), produced a prompt and dose-dependent increase in serum leptin levels and leptin mRNA expression in fat. IL-10, IL-4, ciliary neurotrophic factor, and IL-2, cytokines not known to induce anorexia or decrease food intake, had no effect on leptin gene expression or serum leptin levels. After administration of Escherichia coli lipopolysaccharide (LPS), leptin gene expression and leptin levels were increased. These findings suggest that leptin levels may be one mechanism by which anorexia is induced during acute inflammatory conditions.
Anorexia and loss of lean body mass are hallmark manifestations of acute or chronic disease including infection or cancer. The role of TNF, IL-1, IL-6, and leukemia inhibitory factor (LIF) as endogenous mediators of the host response to infection or malignancy has been extensively studied (1–8). Chronic administration of TNF or LIF to mice (6, 9, 10), or IL-1 to rats (2, 11) results in significant anorexia and weight loss. Mice engrafted with Chinese hamster ovary (CHO) cells genetically engineered to produce TNF, IL-6, or LIF demonstrate profound wasting comparable to chronic starvation or cancer cachexia (12–14). Alternatively, anti-TNF antibodies and a receptor antagonist against IL-1 ameliorate the manifestations of cancer cachexia in mice (15, 16). Also, cachectic patients with HIV-related lymphoma treated with anti-IL-6 antibodies demonstrate weight gain without change in tumor burden (17).
Though evidence has mounted that inflammatory diseases mediate energy and weight dysregulation through their associated cytokines, the mechanisms are unknown. Many recent studies have suggested that the ob gene product, leptin, may play a central role in energy regulation (18, 19). This protein is expressed specifically in white adipose tissue (18), is strongly correlated with total body fat mass (20, 21), and when given to lean or ob/ob mice reduces food intake and in the ob/ob mice increases basal metabolic rate leading to weight loss (22–24). Therefore, we investigated the hypothesis that inflammatory cytokines might elevate leptin, a potential explanation for their anorectic effects.
Materials and Methods
Animals.
Female C57BL/6 mice (10–12 wk/18–22 g) were housed (5/cage) and raised on open formula rat/mouse ration (Zeigler, Gardners, PA) and water ad libitum, with a 12 h light–dark cycle beginning at 6:30 a.m. All experiments were conducted in compliance with the Animal Care and Use Committee of the National Institute of Health. In the diurnal variation experiment animals had free access to food. In all other experiments, food was withdrawn 2 h before onset of the dark cycle. In the refeeding experiment only, food was reintroduced after 7–7.5 h of fasting. Animals were killed at indicated timepoints for retroperitoneal fat and serum harvest.
Reagents.
The following cytokines were obtained: mTNF-α (Genentech, South San Francisco, CA), hIL-1β (Biologic Response Modifiers Program [BRMP], Frederick, MD), hIL-2 (Cetus, Chiron Corp., Emeryville, CA), mIL-10 (BRMP, Frederick, MD), mIL-4 (BRMP, Frederick, MD), mLIF (Genentech, South San Francisco, CA), mCNTF (R&D Systems, Minneapolis, MN), and mIL-6 (BRMP, Frederick, MD). E. coli lipopolysaccharide (serotype 0127:B8) was purchased from Sigma (St. Louis, MO).
Cytokine Response Studies.
After a 7-h fast, mice were given an i.p. injection of 0.2 ml of a control carrier solution of PBS (Biofluids, Rockville, MD) with 0.5% endotoxin-free fatty acid–poor BSA (Calbiochem-Novabiochem, La Jolla, CA) or LPS, or one of the indicated cytokines.
Leptin/Serum Levels.
Leptin levels were measured by RIA as described (20, 21) using the procedure as directed by Linco (St. Charles, MO), except that all reagents were used at one-half recommended volume.
Reverse Transcriptase–PCR.
Total RNA was extracted from frozen fat tissue samples by the guanidinum–thiocyanate/CsCl method. An ob gene cDNA probe for Northern blotting was generated as follows. First strand cDNA was synthesized from 3 μg of total RNA derived from an untreated control group, using Moloney murine leukemia virus reverse transcriptase (GIBCO BRL, Gaithersburg, MD) and oligo(dT) priming according to the directions of the manufacturer. The reaction was carried out at 40°C for 1 h in a final volume of 60 μl PCR mix. Amplification primers were synthesized using the mouse ob gene sequence: sense, 5′-AATGTGCTGCAGACCCCTGTG-3′, and antisense, 5′-CATTCAGGGCTAACATCCAAC-3′. Hot-start PCR was performed using the protocol for Ampliwax in the following reaction: 2.5 μl PCR buffer (10×), 10.5 μl sterile water, 5 μl of each primer (4 μM), 2 μl dNTP mix (10 mM each dNTP) were mixed in a PCR microfuge tube, followed by a single Ampliwax PCR Gem, heated to 80°C, and allowed to cool at room temperature. On the solidified wax layer, 7.5 μl PCR buffer (10×), 56.5 μl sterile water, 10 μl cDNA template, and 1 μl Taq polymerase (5 U/μl) were mixed and heated at 95°C for 5 min, followed by 37 cycles using a 1-min denaturation step at 94°C, a 1-min annealing step at 60°C, and a 2-min extension step at 72°C. An additional 8-min extension step at 72°C was added after 37 cycles. PCR reactions were performed in a thermocycler (Perkin-Elmer Cetus model 480, Norwalk, CT). A single predicted 500-bp PCR product was obtained as resolved on a 2% agarose gel. The product was cloned with the Invitrogen T/A cloning kit (San Diego, CA), produced in quantity, purified, random prime labeled with P32, and used as a cDNA probe for our Northern blots. A quantity of the product was subjected to restriction enzyme analysis for confirmation of specificity.
Northern Blot Analysis.
Total RNA was extracted from frozen fat tissue pooled from each experimental group as described above. Equal amounts of total RNA (25 μg/lane) were subjected to gel electrophoresis using 1% agarose gels containing 0.6 M formaldehyde. RNA was blotted onto Duralon-UV (Strategene, Inc., La Jolla, CA) nylon membranes and ultraviolet cross-linked. Hybridization and autoradiography were performed using standard techniques. Equal loading of RNA was confirmed by ethidium bromide staining of the agarose gel or hybridization of nylon membranes with a chicken β-actin cDNA probe (Oncor, Gaithersburg, MD). Each lane represents RNA from tissue pooled from five or six mice. Each experiment was done between three to six times with consistent results.
Statistical Methods.
Individual comparisons were evaluated with Student's t test using the Statview™ program.
Results
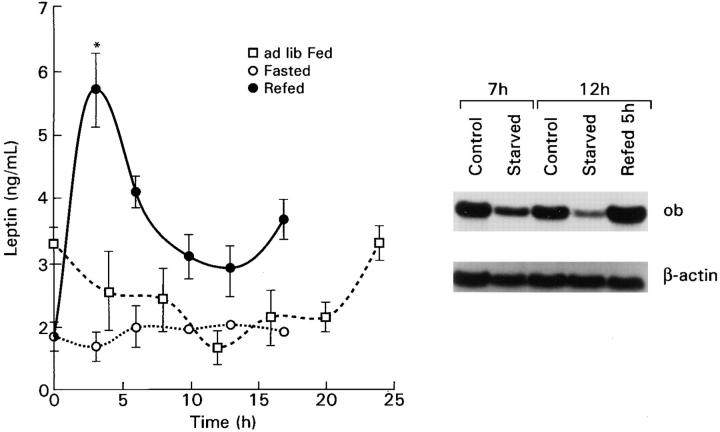
We first examined the characteristics of leptin gene expression and serum levels under simple physiological manipulations, including circadian variation in freely feeding mice and the response to acute starvation and refeeding. As previously demonstrated at the mRNA level (25), leptin levels under conditions of ad libitum feeding were lowest in the middle of light cycle and highest in the middle of the dark cycle (Fig. 1), consistent with the well-established diurnal but primarily nocturnal food intake behavior of rodents (26). When animals were fasted beginning 2 h before the night cycle, the nocturnal rise was abolished. When 7-h fasted animals were refed, there was an exuberant increase in leptin gene expression and serum levels higher than freely fed controls and were manifest as early as 3 h after feeding (Fig. 1).
Figure 1.
Leptin levels in freely feeding mice at intervals throughout a 24-h period and after short-term fasting and refeeding. The initial point represents midnight, 5.5 h after beginning the dark cycle in the ad libitum fed diurnal experiment, 7 h after the commencement of the fast in the fasting and refeeding experiments, and the beginning of refeeding in the latter experiment. Each point represents the mean ± SEM of 6–8 individually measured mice. Northern blot shows ob gene expression in adipose tissue from freely fed mice (control), decreased expression after a 7- or 12-h fast (starved), and increased 5 h after refeeding groups of mice starved for 7 h (refed 5 h).
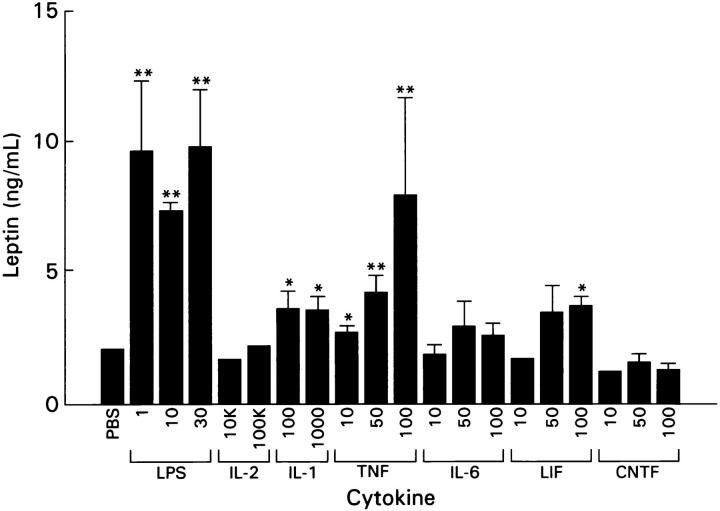
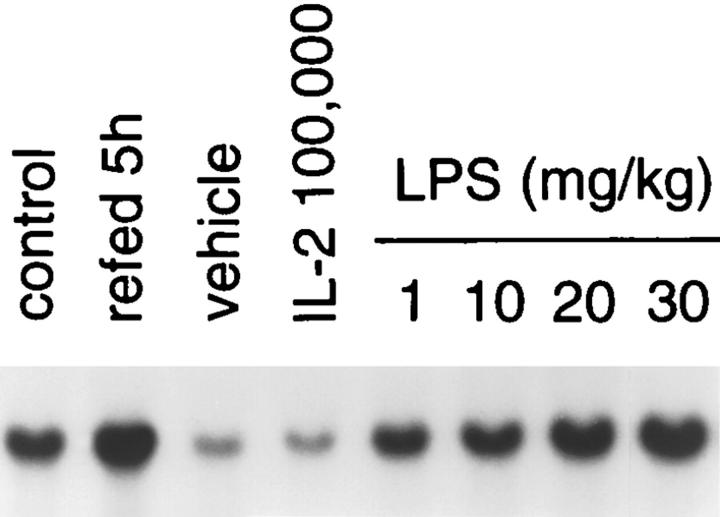
After a 7-h evening fast, mice were treated with a single intraperitoneal injection of LPS, or multiple cytokines at doses that have an anorectic effect (Ma, G. and H.R. Alexander, manuscript in preparation). Fig. 2 shows that leptin levels are significantly increased by LPS, TNF, IL-1, and LIF in a doserelated manner. LPS and TNF increased leptin levels nearly five fold to levels greater than that seen in animals acutely fasted and refed. IL-1 and LIF increased fasting leptin by approximately twofold, a level similar to that observed in fed animals. IL-6 had a trend to increase leptin, which did not reach significance. LPS administration at sublethal (1 and 10 mg/kg) and lethal doses (20 and 30 mg/kg) produced a dose-dependent increase in ob gene expression in fasted mice (Fig. 3). In this model, 20–30 mg/kg of LPS resulted in a 30–40% lethality by 72 h after adminstration (data not shown). These effects were specific, because IL-10 and IL-4, which generally exhibit anti-inflammatory characteristics, as well as IL-2 and ciliary neurotrophic factor (CNTF), had no effect on leptin mRNA expression in retroperitoneal fat (data not shown) or serum leptin levels (see Fig. 2).
Figure 2.
Leptin levels after i.p. administration of various cytokines and LPS. Mice were injected with LPS (mg/kg) or recombinant cytokine in the doses shown (IL-2, IL-1: U/mouse; TNF, IL-6, LIF, CNTF: μg/kg) or PBS after a 7-h fast and sera and adipose tissue harvested 5 h later. Further details are in Materials and Methods. Each bar represents the mean ± SEM serum leptin level of 6–7 mice. Significance compared with the PBS-treated animals is indicated as *P <0.05, **P <0.01.
Figure 3.
Effect of LPS on ob gene expression. After a 7-h fast mice were injected i.p. with LPS at the doses indicated and adipose tissue harvested 5 h later as described. ob gene expression is low in fasted animals treated with PBS and increased in a dose-dependent manner after LPS.
We next examined the time course of effects of TNF and IL-1 on leptin levels (Fig. 4). After a 7-h fast, mice were injected with 100 μg/kg of TNF, or 1,000 U of IL-1. Expression of the leptin gene in retroperitoneal fat increased within 2 h after injection and was maximal 6–8 h after TNF administration (data not shown), while leptin serum levels reached a maximal three-fold elevation by 7 h, and subsequently returned toward baseline at 18 h. IL-1 induced a slower increase in leptin to a maximal twofold increase by 10 h, which persisted for at least another 8 h.
Figure 4.
Kinetics of leptin in sera from mice injected with 100 μg/kg of TNF or 1,000 U of IL-1, after a 7-h fast. All animals were food-deprived during the experimental period. Each point represents mean ± SEM leptin level of 5–6 mice, except for the 13-h points, which represent 10–14 mice. Compared with fasted controls, significant differences are indicated as *P <0.05.
Discussion
The time course and dose-dependent effects of the inflammatory cytokines TNF and IL-1 on leptin gene expression suggest that their anorectic effects may be mediated in part by regulation of leptin gene expression. The finding that IL-6 and LIF are not as potent as TNF or LPS in inducing leptin levels or gene expression is not surprising. Although IL-6 and LIF have pleiotropic inflammatory properties, have been detected in various acute and chronic diseases (2, 10), and have been shown to induce tissue wasting in hosts bearing implantable tumors secreting either cytokine (13, 14), the evidence that IL-6 or LIF induce anorexia or mediate cachexia in various disease states is not consistent (27–29). CNTF, IL-2, IL-4 and IL-10, the latter two being primarily counterinflammatory cytokines, have not been shown to have anorectic effects in mice (30– 33) and had no effect on leptin levels or gene expression.
The full syndrome of leptin insufficiency is seen in ob/ob mice that lack functional leptin. These mice have increased food-seeking behavior, insulin resistance, hypothermia, decreased sympathetic drive, elevated corticosterone, and infertility, all of which tend to normalize with exogenous leptin (21–23, 34, 35). Acute food restriction in mice, associated with low leptin levels, is characterized by the same abnormalities. In an acutely infected mouse, these abnormalities (except infertility) might be anticipated to impair survival in the face of bacterial infection. In particular, leptin support of brown fat, basal metabolism, and prevention of hypothermia may be important in preserving the adaptive febrile response. We conjecture that cytokine stimulation of leptin to levels at or above seen in the fed state may have developed to prevent the abnormalities of leptin deficiency during infection, regardless of the food intake.
The leptin receptor, closely related to gp-130 (heterodimer of IL-6 and LIF), is a member of the class I cytokine group (36). Thus, it seems quite likely that the leptin system is ancestrally related to the cytokines. In this context, it is perhaps not surprising that multiple cytokines influence leptin levels, as we have demonstrated. The possibility of the reverse, leptin influence on other cytokines, is plausible based on the wide distribution of some forms of the leptin receptor (36), including primitive hematopoetic stem cells and lymphohematopoetic cell lines (37), and deserves investigation.
In summary, we have demonstrated that multiple cytokines documented to induce anorexia increase leptin, a protein demonstrated to produce anorexia and confirm similar findings with LPS (38). These results strongly advance the hypothesis that cytokine induction of leptin may play a significant role in the anorexia and cachexia of inflammatory diseases such as infections, collagen vascular disease, and cancer. If the cytokine–leptin hypothesis is supported by further studies, it opens a novel approach to combating this significant comorbidity of many common diseases.
Acknowledgments
This work was supported in part by National Institutes of Health grants P30 DK46200 and K08 HL02564. D.J. Rivet III is a Howard Hughes Medical Institute–National Institutes of Health Research Scholar.
Footnotes
The authors wish to thank Michael Nishimura, Rudy Pozzatti, and James Gnarra for their technical advice and support. The assistance of Aida Ordoubadi and Aaron Rosenfeld is gratefully acknowledged. We also wish to thank Dr. Carl Grunfeld for valuable discussions and sharing of data before publication. Finally, we thank Barbara Owen for her help in preparation of the manuscript.
References
- 1.Moldawer LL, Georgieff M, Lundholm K. Interleukin 1, tumour necrosis factor-alpha(cachectin) and the pathogenesis of cancer cachexia. Clin Physiol. 1987;7:263–274. doi: 10.1111/j.1475-097x.1987.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 2.Bendtzen K. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett. 1988;19:183–192. doi: 10.1016/0165-2478(88)90141-1. [DOI] [PubMed] [Google Scholar]
- 3.Evans RD, Argiles JM, Williamson DH. Metabolic effects of tumour necrosis factor-α (cachectin) and inteleukin-1. Clin Sci. 1991;77:357–364. doi: 10.1042/cs0770357. [DOI] [PubMed] [Google Scholar]
- 4.Mori M, Yamaguchi K, Honda S, Nagasaki K, Ueda M, Abe O, Abe K. Cancer cachexia syndrome developed in nude mice bearing melanoma cells producing leukemia-inhibitory factor. Cancer Res. 1991;51:6656–6659. [PubMed] [Google Scholar]
- 5.Jensen JC, Buresh CM, Fraker DL, Langstein HN, Doherty GD, Alexander HR, Norton JA. Enhanced hepatic cytokine gene expression in cachectic tumor bearing rats. Surg Forum. 1990;41:469–472. [Google Scholar]
- 6.Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, Kuo GC, Beuhler B, Cotran RS, Cerami A, Lowry SF. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strassman G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Socher SM, Friedman A, Martinez D. Recombinant human tumor necrosis factor induces acute reductions in food intake and body weight in mice. J Exp Med. 1988;167:1957–1962. doi: 10.1084/jem.167.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraker DL, Stovroff MC, Merino MJ, Norton JA. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med. 1988;168:95–105. doi: 10.1084/jem.168.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalf D. The leukemia inhibitory factor (LIF) Int J Cell Cloning. 1991;9:95–108. doi: 10.1002/stem.5530090201. [DOI] [PubMed] [Google Scholar]
- 11.Butler LD, Layman NK, Cain RL, Riedl RE, Mohler KM, Bobbitt JL, Belagajie R, Sharp J, Bendele AM. Interleukin l-induced pathophysiology: induction of cytokines, development of histopathologic changes, and immunopharmacologic intervention. Clin Immunol Immunopathol. 1989;53:400–421. doi: 10.1016/0090-1229(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 12.Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987;50:555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- 13.Black K, Garrett IR, Mundy GR. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene cause hypercalcemia as well as cachexia, leukocytosis and thrombocytosis in tumor-bearing nude mice. Endocrinology. 1991;128:2657–2659. doi: 10.1210/endo-128-5-2657. [DOI] [PubMed] [Google Scholar]
- 14.Metcalf D, Gearing DP. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc Natl Acad Sci USA. 1989;86:5948–5952. doi: 10.1073/pnas.86.15.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelin J, Moldawer LL, Lonnroth C, Sherry B, Chizzonite R, Lundholm K. Role of endogenous tumor necrosis factor α and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991;51:415–421. [PubMed] [Google Scholar]
- 16.Sherry BA, Gelin J, Fong Y, Marano M, Wei H, Cerami A, Lowry SF, Lundholm KG, Moldawer LL. Anticachectin/tumor necrosis factor-α antibodies attenuate development of cachexia in tumor models. FASEB J. 1989;3:1956–1962. doi: 10.1096/fasebj.3.8.2721856. [DOI] [PubMed] [Google Scholar]
- 17.Emilie D, Wijdenes J, Gisselbrecht C, Jarrousse B, Billaud E, Blay J-Y, Gabarre J, Gaillard J-P, Brochier J, Raphael M, Boue F, Galanaud P. Administration of an anti-interleukin-6 monoclonal antibody to patients with acquired immunodeficiency syndrome and lymphoma: Effect on lymphoma growth and on B clinical symptoms. Blood. 1994;84:2472–2479. [PubMed] [Google Scholar]
- 18.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obesegene and its human homologue. Nature (Lond) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 19.Flier JS. The Adipocyte: Storage depot or node on the energy information superhighway? . Cell. 1995;80:15–18. doi: 10.1016/0092-8674(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 20.Frederich RC, Löllmann B, Hamann A, NapolitanoRosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 22.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/obmice. Science (Wash DC) 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 23.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science (Wash DC) 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 24.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science (Wash DC) 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 25.Saladin R, De Vos P, Guerve-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese-gene expression after food intake or insulin administration. Nature (Lond) 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 26.Nelson W, Scheving L, Halberg F. Circadian rhythms in mice fed a single daily meal at different stages of lighting regimen. J Nutr. 1995;105:171–184. doi: 10.1093/jn/105.2.171. [DOI] [PubMed] [Google Scholar]
- 27.McNamara MJ, Alexander HR, Norton JA. Cytokines and their role in the pathophysiology of cancer cachexia. J Parenter Enteral Nutr. 1992;16:505–535. doi: 10.1177/014860719201600603. [DOI] [PubMed] [Google Scholar]
- 28.Jablons DM, McIntosh JK, Mulé JJ. Induction of interferon-2/interleukin-6 by cytokine administration and detection of circulating interleukin-6 in the tumor bearing state. Ann NY Acad Sci. 1989;557:157–160. doi: 10.1111/j.1749-6632.1989.tb24008.x. [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science (Wash DC) 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 30.Truitt, R.L., C.A. Keever, and E.C. Borden. 1995. Role of IL-4, IL-6, and IL-12 in cancer therapy. In Biologic Therapy of Cancer. V.T. DeVita, S. Hellman, and S.A. Rosenberg, editors. J.B. Lippincott, Philadelphia. 279–293.
- 31.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann NY Acad Sci. 1993;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 32.deWaal Malefyt, R., H. Yssel, M.-G. Roncarolo, H. Spits, and J.E. deVries. Interleukin-10. Curr Opin Immunol. 1992;4:314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 33.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature (Lond) 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 35.Chehab PF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 36.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 37.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/ OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nature Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 38.Grunfeld C, Zhao C, Fuller J, Pollock A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the obgene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]