Abstract
Activation of Ras GTPases is a conserved feature of antigen receptor signaling, including FcεR1 activation of mast cells. Antigenic cross-linking of the FcεR1 on mast cells results in secretion of allergic mediators and induction of immediate early and cytokine genes. Here we examine the role of Ras in coupling the FcεR1 to transcriptional regulation. The transcription factors Elk-1, an immediate early gene regulator and the nuclear factor of activated T cells (NFAT), in the context of the IL-4 gene, are identified as Ras targets in mast cells. Ras mediates diverse effects via its diverse effector pathways, which may include other members of the Ras GTPase family such as RhoA and Rac-1. We observe that Elk-1 and NFAT are targeted by distinct Ras effector pathways in mast cells. Activation of the “classical” Ras/Raf-1/MEK/ ERK cascade is necessary and sufficient for FcεR1 induction of Elk-1. Ras function is required, but not sufficient for FcεR1 induction of NFAT. However, activation or inhibition of Ras markedly shifts the antigen dose-response for FcεR1 induction of NFAT. The effector pathway for Ras activation of NFAT is not Raf-1/MEK. We identify that the Rac-1 GTPase is critical in FcεR1 regulation of NFAT, acting either in parallel with or as an effector of Ras. These data place Ras in a crucial position in mast cells, regulating disparate nuclear targets. Moreover, we identify that two GTPases, Ras and Rac-1, are important regulators of NFAT, and therefore of cytokine expression in mast cells.
Antigenic cross-linking of the high affinity receptor for IgE (FcεR1) on mast cells results in expression of inflammatory function (1). In addition to the release of inflammatory mediators from cytoplasmic granules, there is a significant nuclear component to the activation of mast cells after FcεR1 ligation. Expression of various genes is induced, notably leading to de novo synthesis of cytokines such as IL-4, -6, GM-CSF, and TNFα (2).
The FcεR1 complex is tetrameric, comprised of 45-kD α and 30-kD β chains and a homodimer of two disulfidelinked 10-kD γ chains (3). Antigenic cross-linking of receptor-bound IgE results in aggregation of FcεR1 complexes in the plane of the plasma membrane and rapid activation of cytoplasmic protein tyrosine kinases (PTKs)1 (4). Immunoreceptor tyrosine-based activation motifs present in FcεR1 β and γ couple the receptor to the src family PTK p56lyn and to p72syk (3). FcεR1-associated PTKs activate a number of effector pathways which together control mast cell function. These include PLCγ1 activation and subsequent generation of inositol polyphosphate and diacylglycerol second messengers (5, 6). These, in turn, modulate intracellular Ca2+ levels and protein kinase C (PKC) activation, respectively. There are defined roles for Ca2+ and PKC signals in FcεR1 regulation of exocytosis (7, 8), and a Ca2+/ calcineurin dependent pathway is known to be important in the induction of a number of cytokine genes (2, 9).
Our previous work has shown that in addition to Ca2+/ PKC signals, FcεR1-regulated PTKs are coupled to effector pathways via the adaptor molecule Grb2 (10). Grb2 forms protein complexes through interactions of its SH2 and SH3 domains with molecules which may be substrates for receptor-associated PTKs (11). One Grb2 effector molecule in the mast cell is Sos, the mammalian homologue of the Drosophila “Son of Sevenless” protein (10). Sos is a guanine nucleotide exchange factor that promotes GTP-loading, and hence activation of the GTPase Ras and its effector pathways. Studies in various systems have placed Ras in a critical position regulating diverse cellular processes through its regulation of kinase cascades with transcription factor targets.
It is recognized that Ras is able to activate multiple effector signaling pathways. An effector pathway mediated by the Raf-1 serine/threonine kinase has been extensively characterized in numerous systems (12). Raf-1 is recruited to the plasma membrane by active Ras.GTP. This recruitment results in Raf-1 activation and subsequent activation of the mitogen-activated protein (MAP) kinases Erk1 and Erk2 by the Erk-activating kinases (MEKs). Like the B and T cell antigen receptors, the FcεR1 activates the Ras/Raf-1/ Erk cascade, but its importance in antigen receptor signaling is not clear. Moreover, studies of the role of Ras in regulating fibroblast transformation have concluded that the Raf-1/MEK pathway does not mediate all Ras effector functions (13). Similarly, in T lymphocytes, the Raf-1/MEK pathway has been shown to mediate Ras effects on positive selection of thymocytes but apparently is not required for Ras control of T cell proliferative responses (14). Alternative effectors for Ras are less defined than the “classical” Raf-1/MEK/Erk cascade, but include the Ras GTPase activating proteins (Ras-GAPs) (15), PtdIns-3′ hydroxyl kinase (16), and GDS proteins for the GTPase Ral (17). There is also a consensus that Ras responses are coupled to signaling networks mediated by Rho family GTPases such as Rac-1, CDC42, and RhoA (18, 19). The role of these Rho-family GTPases in antigen receptor responses is not well characterized, but there is an increasing awareness from both biochemical and genetic analyses that these molecules will have important functions in the immune system. Vav, which is one of the immediate substrates for PTKs associated with antigen receptor complexes (20), has a domain homologous to Dbl, a guanine nucleotide exchange factor for Rho family proteins (21). Recent studies have shown that Vav can induce Rac-1–mediated activation of the MAP kinase JNK-1 (22). In a clinical context, the WiskottAldrich Syndrome immunodeficiency has been mapped to a defect in expression of an effector protein for the GTPase CDC42 (23).
Previous studies have shown that FcεR1 induction of a number of cytokine genes is sensitive to the imunosuppressive drug cyclosporin A (CsA) (24). The CsA sensitivity of cytokine gene induction reflects the importance of the CsA target molecule, the calcium dependent phosphatase calcineurin (CN), in this process. CN is critical for the regulation of the nuclear factor of activated T cells (NFAT), a transcription factor involved in the regulation of various cytokine genes (25, 26). In addition to the role of calcium/ CN, a previous report has shown that Ras signals link the FcεR1 to regulation of the IL-5 cytokine gene (27). However, there is no description of Ras effector pathways involved in coupling the FcεR1 to nuclear events, or in determining the repertoire of such targets for Ras signals. The present report identifies the transcription factors Elk-1 and NFAT as targets for Ras signals in mast cells. Moreover, we explore the Ras effector pathways used to link the FcεR1 to these targets. The data show that the FcεR1 regulates Elk-1 transactivation via a Ras/MEK-dependent pathway. The FcεR1 activation of Elk-1, described herein for FcεR1 signaling, provides the paradigm for involvement of the “classical” Ras/Raf-1/MEK cascade in coupling antigen receptors to gene transcription. We also show that NFAT in the context of the IL-4 promoter is another nuclear target for Ras signals downstream of the FcεR1. However, in contrast to Elk-1, FcεR1 regulation of NFAT is accomplished by an effector pathway for Ras distinct from Raf-1/ MEK. The present study also provides the first evidence that a member of the Rho family of GTPases, Rac-1, can regulate FcεR1 induction of NFAT. These data reveal the complexity and efficacy of Ras and Rho family GTPases in coupling antigen receptors to diverse nuclear events.
Materials and Methods
Reagents.
KLH-DNP conjugate, ionomycin calcium salt, CsA and phorbol-12,13 dibutyrate were from Calbiochem (La Jolla, CA). Monoclonal IgE anti-DNP was from Sigma Chemical Co. (St. Louis, MO), as were all reagents for chloramphenicol acetyl transferase (CAT) assays except 14C acetyl coenzyme A from Amersham International (Buckinghamshire, England). MEK inhibitor PD095089 was from New England Biolabs (Beverly, MA). The PKC inhibitor Ro-318425 was a gift from Dr. David Williams (Roche Pharmaceuticals, Welwyn, UK). Anti-pan–Erk antibody was from Affiniti (UK).
Cell Culture and Stimulation.
The rat basophilic leukemia cell line RBL2H3 as maintained in DMEM supplemented with 10% (vol/vol) heat-inactivated (56°C for 30 min) fetal bovine serum. Only the adherent fraction of the cell cultures was passaged or used in experiments. Cells were detached from culture substrate using cell scrapers, washed once, and primed with 1 μg/ml IgE anti-DNP in DMEM, 10% fetal bovine serum for 2 h at 37°C. Receptor cross-linking was effected using 500 ng/ml KLH-DNP conjugate (Calbiochem) at 37°C. Other stimuli were applied as follows: 50 ng/ml PdBu, 500 ng/ml ionomycin.
Western Blotting.
Conditions for RBL2H3 lysis and Western blotting were as described previously (10). Briefly, postnuclear lysates were prepared, and cellular proteins were acetone precipitated and then resolved by 15% SDS-PAGE. Proteins were transferred to polyvinyl difluoride membrane which was blocked in 5% nonfat milk for 1 h at room temperature before probing with 1 μg/ml anti-Erk (Affiniti). The membrane was washed three times in PBS/0.02% Tween-20, and incubated with 0.5 μg/ml goat anti–mouse horseradish peroxidase conjugate. After washing as above, Erk bands were visualized by enhanced chemiluminescence (Amersham International).
Plasmids.
LexA OP.tk CAT comprises two copies of the LexA reporter. In conjunction with the pEF-NLexA Elk-1C fusion protein, its use has already been described (28). This system involves the transfection of a reporter gene construct of a LexA binding site upstream of the CAT reporter gene under a thymidine kinase minimal promoter (LexA OP.tk CAT). A plasmid encoding a fusion protein of the LexA DNA binding domain and Elk-1 COOH-terminal region under a constitutive promoter is cotransfected (pEF-NLexA Elk-1C). The LexA region of the fusion protein binds constitutively to its site on the reporter gene construct. Activation signals phosphorylate the Elk-1C region of the fusion protein, leading to recruitment of transcriptional machinery and CAT gene induction. The IL-4 NFAT CAT reporter was a gift of E. Serfling (Wurzburg University, Wurzburg, Germany) and comprised a trimerized NFAT/AP-1 site derived from purine box B of the murine IL-4 promoter (GATCCTGAGTTTACATTGGAAAATTTTATAGAGCGAGTTG). Plasmids encoding signaling proteins were as follows: pEF-BOSv-HaRas (constitutively active V12Ras), RSVN17Ras (dominant inhibitory Ras), and pEFdnRaf-1 (dominant inhibitory Raf-1, Raf-1 residues 1-257). The active pEF-V12Rac, dominant inhibitory pEF-N17Rac, and active pEF-V14Rho mutants have all been previously described (29). The constitutively active membrane targeted pEF-Raf-1CAAX was a gift of J. Downward (Imperial Cancer Research Fund). Plasmid DNA was prepared by CsCl density gradient centrifugation.
Transient Transfection Assays Using CAT Reporter Gene Constructs.
Transient transfection was carried out using a Beckman Gene-Pulser electroporation apparatus. RBL2H3 monolayer cells were detached from the culture flask using a cell scraper, and resuspended at 2 × 107 cells/0.6 ml DMEM + 10% FCS at 37°C. Cell were pulsed in 0.4 cm cuvettes (Beckman) at 310 V, 960 μF before pooling, dilution in complete medium, and division at 1 ml/ well between wells of a 24-well tissue culture plate. Cells were allowed to recover for 6 h at 37°C before priming and stimulation as indicated.
For CAT reporter gene assays 5 × 106 cells were lysed in 150 μl buffer containing 0.65% (vol/vol) NP-40, 10 mM Tris, pH 8.0, 1 mM EDTA, and 150 mM NaCl for 15 min on ice. Lysates were then transferred to a 68°C water bath for 10 min. Cell debris was pelleted, and aliquots of lysate were removed to a fresh tube in an assay volume of 100 μl to which 40 μl of a start solution containing 0.5 mM acetyl CoA, 5 mM chloramphenicol, 0.5 M Tris, pH 8.0, and 1 μl/point of 50 μCi/ml 14C acetyl coenzyme A was added. The assay was incubated for 16 h at 37°C before chloramphenicol was extracted using ethyl acetate. The amount of radioactivity in the acetylated product and nonacetylated substrate for each reaction was determined by liquid scintillation counting of organic and aqueous phases, respectively. Results are expressed as percentage conversion of chloramphenicol to the acetylated form.
Results
Elk-1 Transactivation Is Induced by the FcεR1 and Phorbol Esters, But Not by the Action of Calcium Ionophore.
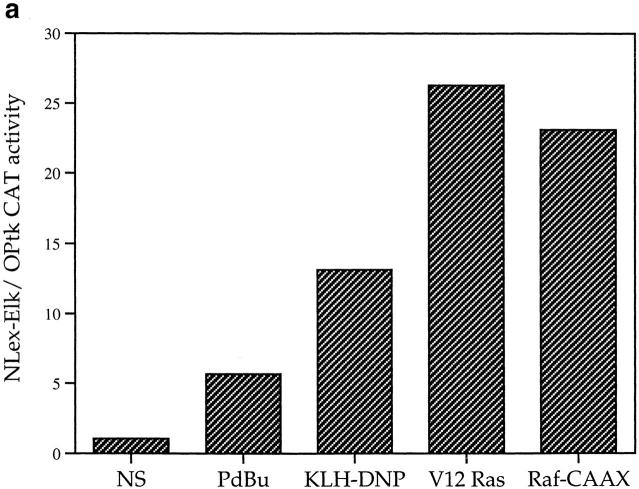
The effects of various stimuli on Elk-1 activity in mast cells were assessed using a CAT reporter gene assay for Elk-1 transactivation. The data in Fig. 1 a shows that antigenic cross-linking of the FcεR1 using KLH-DNP strongly induces Elk-1 transactivation in a dose-dependent manner. In addition, the phorbol ester PdBu that directly activates PKC, induces ninefold induction of Elk-1 transactivation over basal levels. Fig. 1 a also shows that elevation of intracellular calcium levels using the ionophore Ionomycin is not sufficient for Elk-1 activation. Stimulation by FcεR1 or the phorbol ester PdBu does not induce LexA OPtk.CAT activity in the absence of a coexpressed LexA Elk-1C fusion protein (Fig. 1 b).
Figure 1.
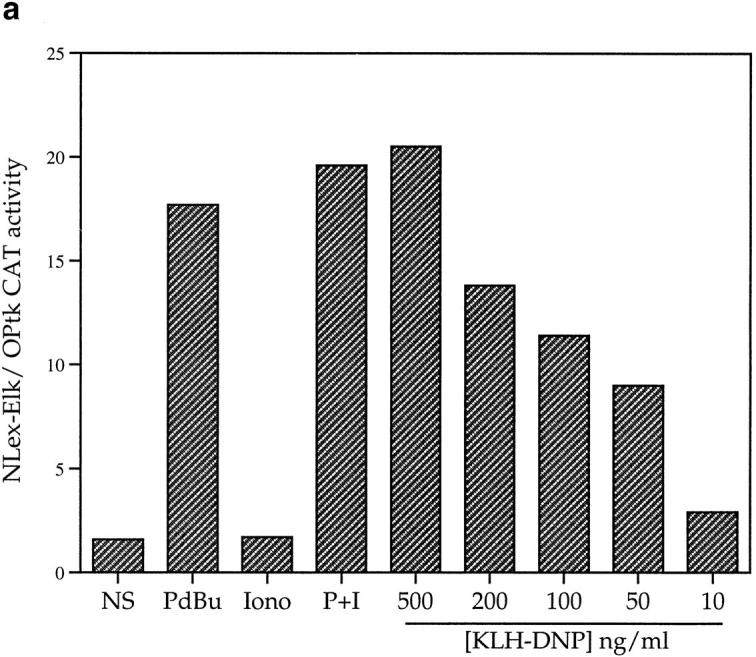
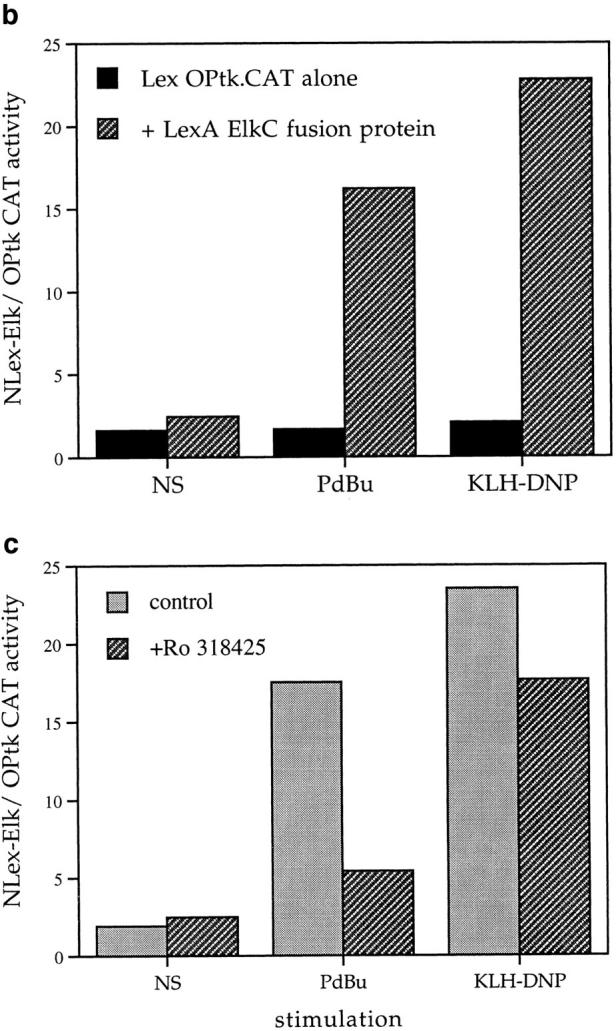
(a) FcεR1 activation stimulates Elk-1 transactivation in mast cells. 12 μg LexA OPtk.CAT and 6 μg LexA Elk-1C plasmids were used per 1 × 107 cells in electroporation at 310 V/960 μF. Cells were recovered as described in Materials and Methods before stimulation for 16 h with PdBu (50 ng/ml), Ionomycin (500 ng/ml) alone or in combination (P+I), or IgE priming followed by antigenic cross-linking of the FcεR1 with the indicated dose of KLH-DNP. Control cells (NS) were left unstimulated. (b) Stimulation of LexA OP.tk CAT activity is dependent on the presence of the LexA Elk-1C fusion protein. 1 × 107 cells per stimulus were electroporated as described with 12 μg LexA OPtk.CAT alone or in combination with 6 μg LexA Elk-1C. Cells were recovered, and then stimulated for 16 h as described above. (c) FcεR1 activation of Elk-1 is not PKC-dependent. Cells were transfected with the Elk-1 reporter system as described, and recovered for 6 h. Preincubation with either 1 μM Ro-318425 or DMSO carrier was 30 min at 37°C before stimulation with either PdBu (50 ng/ml) or KLH-DNP (250 ng/ml).
FcεR1 ligation is known to activate PKC, and previous studies have established that PKC mediated signals are important for FcεR1 induction of mast cell degranulation. PKC activation is therefore a candidate mechanism for FcεR1 induction of Elk-1. We used a broad-spectrum inhibitor of PKC isozymes, Ro-318425 (30), to assay the contribution of PKC to Elk-1 induction by the FcεR1. Ro-318425 inhibits the activity of the major PKC isozymes shown to translocate to the plasma membrane following FcεR1 ligation. The data in Fig. 1 c show that a 500 nM dose of Ro-318425 inhibits PdBu activation of Elk-1 by 85%. In contrast, FcεR1 stimulation can potently induce Elk-1 transactivation, despite the presence of Ro318425.
The data in Fig. 1 a demonstrate that signals derived from the FcεR1 regulate the transcriptional activity of Elk-1. The insensitivity of FcεR1 stimulation of Elk-1 to the PKC inhibitor Ro-318425 suggests that PKC-mediated signals are not essential for Elk-1 regulation by the FcεR1. Raising of intracellular calcium levels by ionomycin treatment or the mimicking of this effect by the cotransfection of an activated mutant of the calcium-dependent phosphatase calcineurin (data not shown) did not induce Elk-1 activity. Hence, we investigated candidate noncalcium/ PKC pathways for Elk-1 activation.
Elk-1 Activation in Mast Cells Is Induced by Constitutively Active Mutants of Ras and Raf-1.
Elk-1 could be a target for a kinase cascade initiated by Ras. The prototypical kinase cascade transducing Ras signals is the Raf-1/MEK/ Erk pathway, and there is a precedent for Elk-1 activation via this mechanism in the fibroblast (31). To explore the role of Ras and Raf-1 signals in Elk-1 activation in mast cells, we examined the effects of activated Ras and Raf-1 mutants on Elk-1 activity in mast cells. The Raf-1 construct used here is a fusion protein of Raf-1 with a CAAX box motif that targets Raf-1 to the plasma membrane, rendering the exogenously expressed Raf-1 constitutively active (32). In these experiments, plasmids bearing the indicated mutants were cotransfected with the LexA Elk-1C fusion protein expression plasmid and the LexA OP.tkCAT reporter gene. The data in Fig. 2 a show that activated forms of both Ras and a Ras effector, Raf-1, are capable of inducing Elk-1 transactivation in RBL2H3 cells in the absence of other stimuli.
Figure 2.
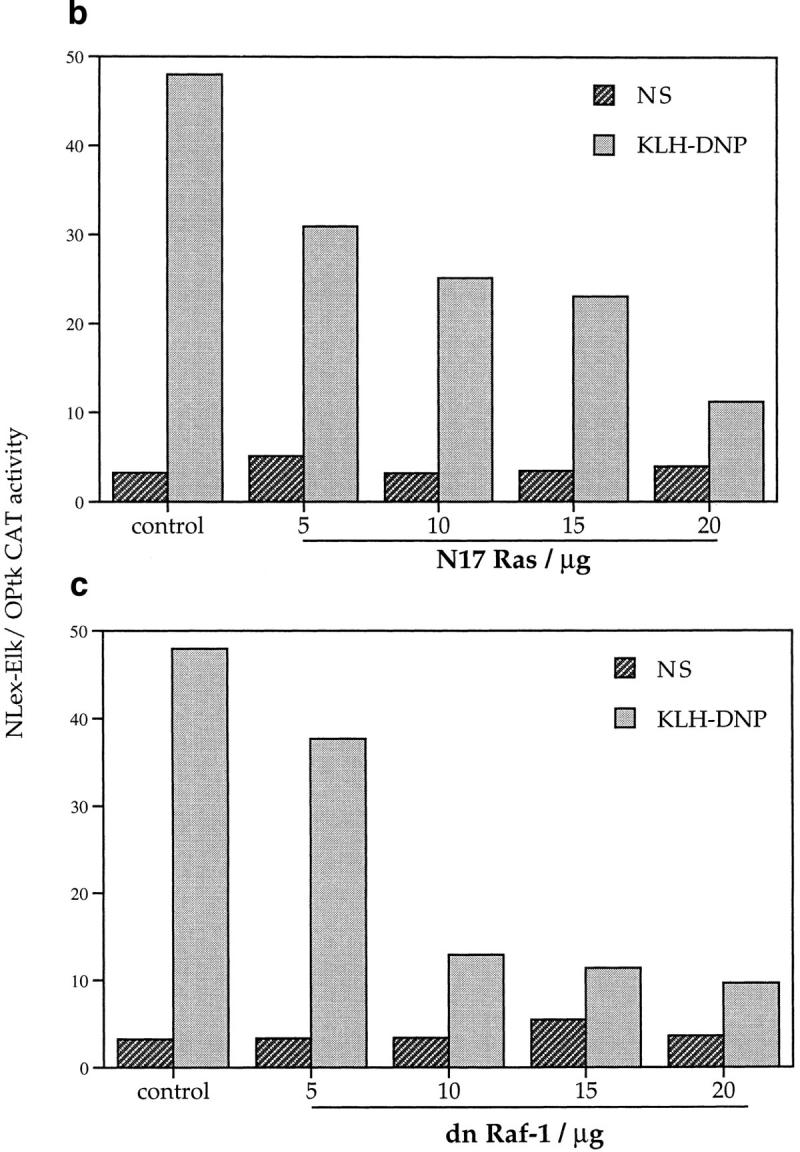
(a) Activated Ras or Raf are sufficient for Elk-1 activation in mast cells. 1 × 107 cells per point were transfected with either the Elk-1 reporter system alone or in combination with 10 μg pEF-V12Ras or 10 μg pEF Raf-CAAX. Cells transfected with activated mutants were left unstimulated, while control cells were exposed to either 50 ng/ml PdBu or 1 μg/ml IgE/500ng/ml KLH-DNP in the concentrations indicated above. (b and c) FcεR1 activation of Elk-1 is dependent on Ras. 1 × 107 cells per point were transfected with either the Elk-1 reporter system alone (control), or in combination with 15 μg of either RSV N17Ras or pEF dn Raf-1. Cells were recovered and left unstimulated (NS) or exposed to IgE/KLH-DNP as described.
In additional experiments we investigated whether FcεR1 induction of Elk-1 is dependent on the activation of a Ras cascade using dominant inhibitory mutants. The N17 mutant of Ras acts to sequester guanine nucleotide exchange factors from endogenous pools of the GTPase, and maintains Ras in its GDP-bound, inactive state. The dominant inhibitory (dn) Raf-1 mutant has the COOH-terminal kinase domain truncated; this mutant binds to activated Ras.GTP and prevents its interaction with effector proteins. Figs. 2 b and c show the effect of dn mutants of Ras and Raf-1 on FcεR1 induction of Elk-1 transactivation. FcεR1 induction of Elk-1 activity is inhibited by expression of either N17Ras or dnRaf-1, in a manner that is dose dependent on the amount of dominant negative plasmid used for cotransfection. At 20 μg cotransfected N17Ras or dnRaf-1, the percentage inhibitions of FcεR1 induction of Elk-1 transactivation were 74 and 79%, respectively. The sensitivity of FcεR1 induction of Elk-1 to dominant inhibitory mutants of both Ras and Raf-1 suggests that the “classical” pathway for MAP kinase stimulation is involved in Elk-1 activation in RBL2H3 cells. In this pathway, Ras.GTP induces membrane targeting and activation of Raf-1, that acts subsequently on Erk members of the MAP kinase family via the Erk-activating kinase MEK. However, the dn Raf-1 mutant not only prevents interactions between activated Ras and endogenous Raf-1, but also blocks Ras binding to other effector molecules. Thus, to explore the role of the MEK/Erk2 pathway in Elk-1 regulation more directly, we used PD098059, an inhibitor of the Erk2 stimulatory kinase, the MAP kinase kinase MEK.
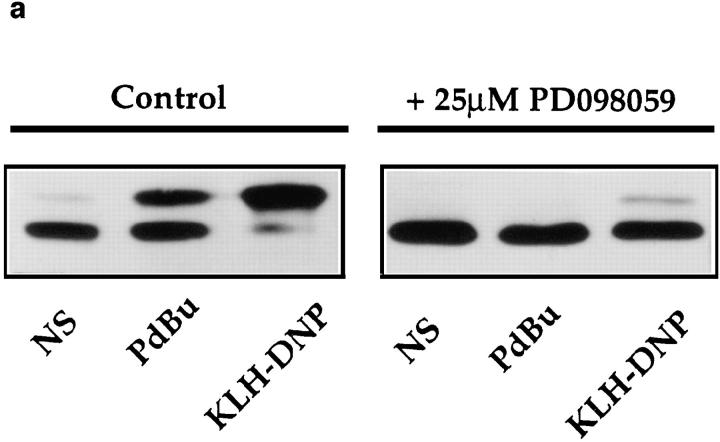
The specificity of PD098059 as a MEK inhibitor has been previously described (33). Nevertheless, in initial experiments we verified that PD098059 was an effective MEK inhibitor in RBL2H3 cells. The phosphorylation of Erk by MEK results in decreased electrophoretic mobility of Erk in SDS-PAGE. Fig. 3 a shows an Erk mobility shift assay visualized by pan-Erk Western blot. In control cells (left), PdBu or FcεR1 stimulation cause the appearance of an hyperphosphorylated Erk population. The application of 25 μM PD098059 (right) efficiently inhibits the activation of Erk by MEK in mast cells. Accordingly, we examined the effect of PD098059 on induction of Elk-1 transactivation in mast cells. As shown in Fig. 3 b, the activation of Elk-1 induced by cotransfection of Raf-CAAX and V12Ras constructs is inhibited by PD098059. Hence, these mutants are indeed reflecting the use of a Raf-1/Erk pathway to target Elk-1. Fig. 3 c shows that the induction of Elk-1 transactivation by both PdBu and FcεR1 cross-linking is inhibited by PD098059 in a dose-dependent manner. These data confirm that the Raf-1/MEK cascade is the critical Ras effector pathway for FcεR1 activation of Elk-1.
Figure 3.
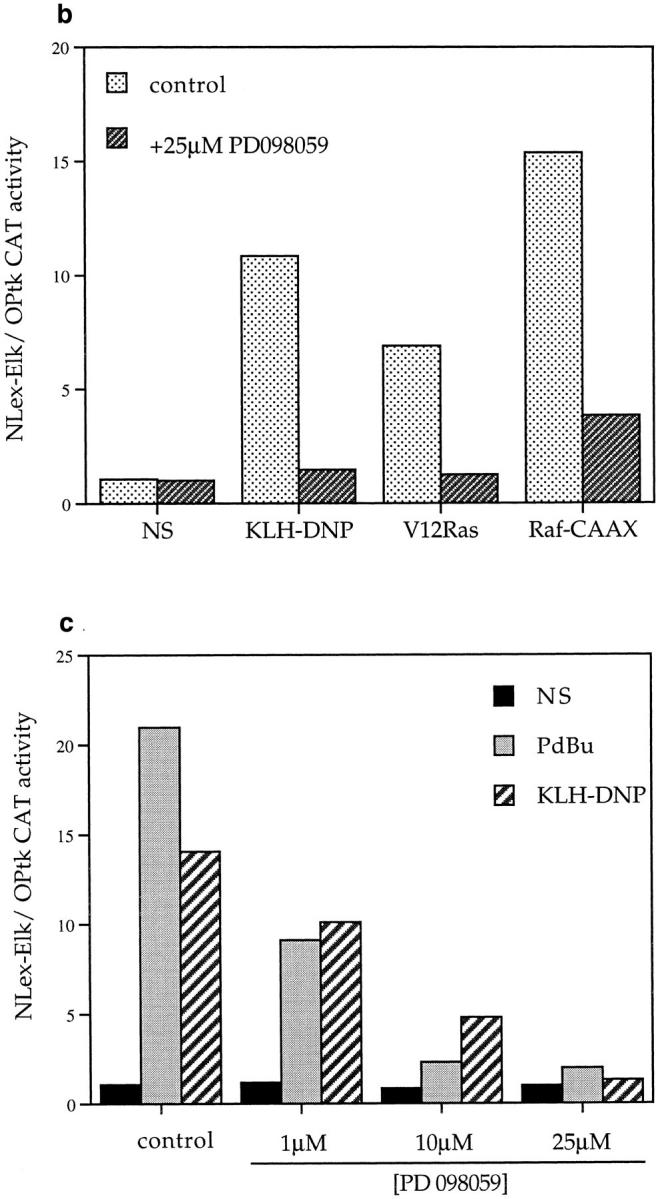
(a) PD098059 inhibits MEK activation of Erk in mast cells. 1 × 106 RBL2H3 per point were incubated for 30 min at 37°C with DMSO (control) or 25 μM PD098059. Stimulations were for 10 min with either 50 ng/ml PdBu or, after priming with IgE anti DNP, 10 min 500 ng/ml KLH-DNP. Western blotting for Erk was carried out as described in Materials and Methods. (b) Induction of Elk-1 via activated Ras and Raf-1 is sensitive to inhibition of MEK. 1 × 107 cells per point were transfected with either the Elk-1 reporter system alone, or in combination with 10 μg pEF V12Ras or 10 μg pEF Raf-CAAX. Control cells were left unstimulated (NS) or exposed to IgE/KLH-DNP. Cotransfected cells were left unstimulated. Preincubation with 25 μM PD098059 or DMSO was for 30 min before stimulation. (c) Induction of Elk-1 activity by FcεR1 or PdBu is sensitive to inhibition of MEK. 1 × 107 cells per point were transfected with the Elk-1 reporter system and recovered for 6 h. Cells were preincubated for 30 min with either DMSO or the indicated concentrations of PD098059. Cells were left unstimulated (NS), exposed to IgE/KLH-DNP, or PdBu as described.
NFAT Is a Target for a Ras Effector Pathways Distinct from Raf-1/MEK in the Mast Cell.
FcεR1 stimulation results in the activation of cytokine genes, the products of which play important roles both locally in inflammation and systemically. Transcription factors of the NFAT family are important for cytokine gene induction in a variety of cells and a well characterized target for NFAT family proteins in an activated mast cell is the gene for IL-4 (25). FcεR1 triggering has been shown to induce NFAT DNA binding activity (26), and we investigated FcεR1 mechanisms for NFAT induction in mast cells using a reporter construct comprising a trimerized NFAT site derived from the murine IL-4 promoter.
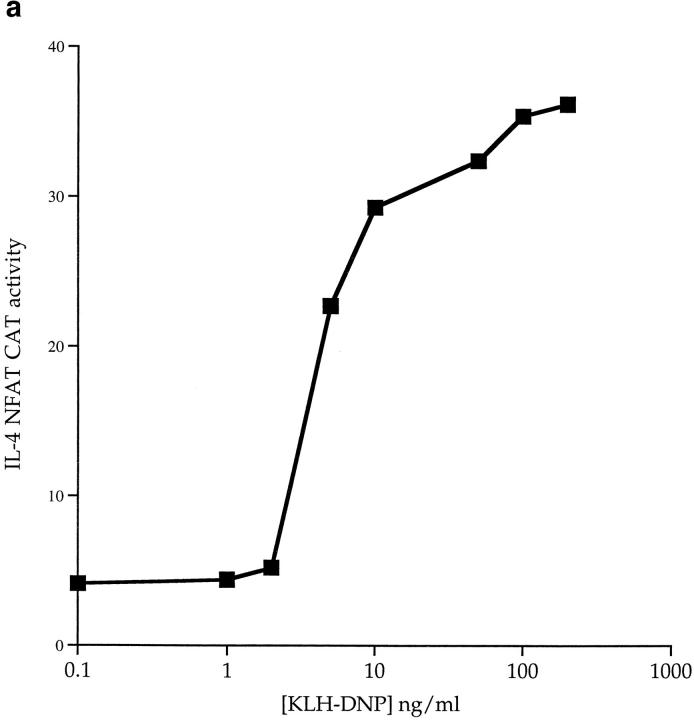
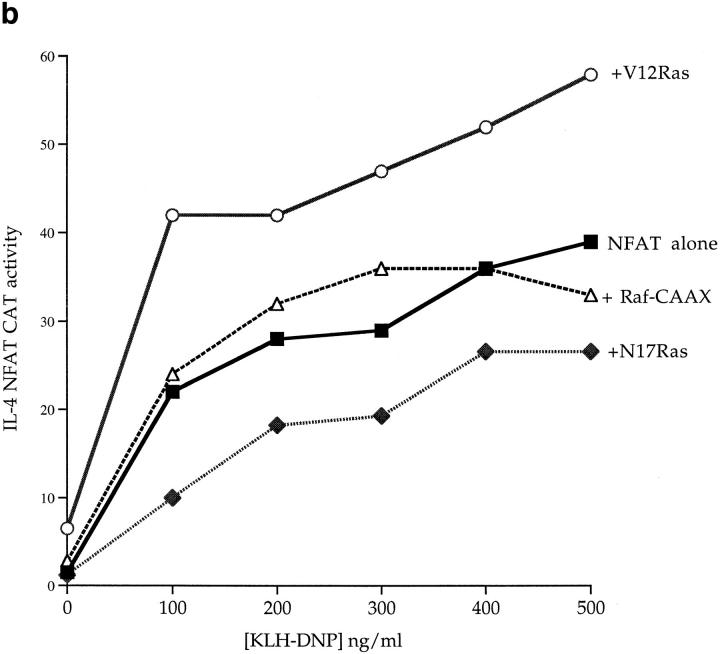
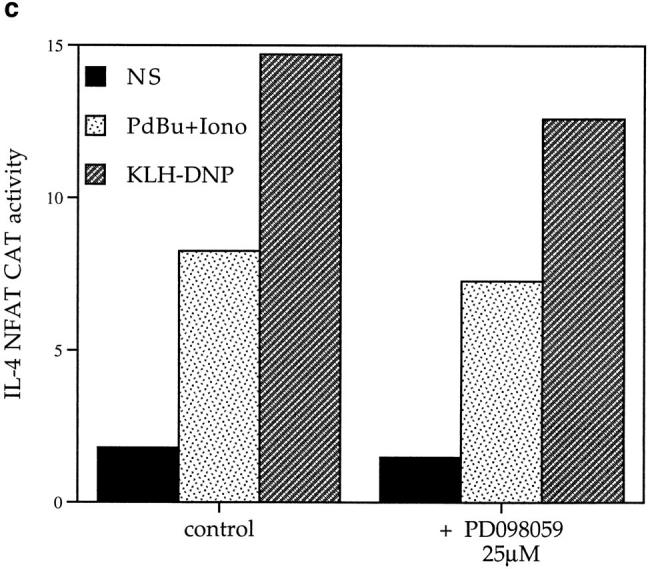
Fig. 4 a shows that FcεR1 cross-linking induces IL-4 NFAT activity in a manner dose dependent on antigen. The PKC inhibitor Ro-318425 did not affect the FcεR1 activation of NFAT (data not shown). To address whether there is a role for Ras in the FcεR1 regulation of NFAT, we cotransfected active and dominant inhibitory Ras mutants with the IL-4 NFAT-CAT reporter gene. Expression of active V12Ras induced a weak increase in the basal activity of the NFAT reporter gene, and robustly potentiated FcεR1 induction of NFAT. Conversely, expression of N17Ras inhibited the NFAT response to FcεR1 (Fig. 4 b). Hence, cotransfected activated Ras (V12Ras) causes a potentiation of FcεR1 activation of IL-4 NFAT that potently increases the sensitivity of NFAT responses to antigen. The presence of N17Ras consistently inhibits the antigen dose response for IL-4 NFAT CAT induction to half-maximal levels, and supresses the antigen sensitivity of the mast cells for NFAT activation.
Figure 4.
(a) FcεR1 cross-linking induces IL-4 NFAT CAT activity in a dose-dependent manner. 1 × 107 cells per point were transfected with 15 μg IL-4 NFAT CAT reporter. Cells were recovered for 6 h before IgE priming and stimulation with the indicated concentrations of KLHDNP. (b) FcεR1 stimulation of IL-4 NFAT is potentiated by active V12Ras, but not Raf-CAAX, and is inhibited by the presence of dominant negative N17Ras. 1 × 107 cells per point were transfected with 15 μg IL-4 NFAT CAT reporter alone (solid line, filled squares), or in combination with 15 μg active V12Ras (solid line, open circles), 15 μg dominant inhibitory N17Ras (broken line, filled diamonds), or 15 μg Raf-CAAX (broken line, open triangles). Cells were recovered for 6 h before IgE priming, and then stimulation with the indicated concentrations of KLH-DNP. (c) FcεR1 stimulation of IL-4 NFAT CAT activity is insensitive to PD098059. 1 × 107 cells per point were transfected with 15 μg IL-4 NFAT CAT reporter alone, and recovered for 6 h. Cells were preincubated for 30 min with either DMSO or the indicated concentrations of PD098059. Cells were left unstimulated (NS), exposed to IgE/KLH-DNP, or treated with 50 ng/ml PdBu and 500 ng/ml ionomycin.
Raf-1 was a key effector for activation of Elk-1 in mast cells and expression of membrane targeted, and hence active, Raf-1 (Raf-CAAX) could mimic the effects of V12Ras and potently stimulate Elk-1 transcriptional activity. However, Fig. 4 b shows that expression of Raf-CAAX cannot substitute for V12Ras to induce NFAT transcriptional activity. These data prompted us to investigate the effect of inhibiting the Raf-1/MEK pathway using PD098059 on NFAT transcriptional activity. Fig. 4 c shows that the activation of IL-4 NFAT CAT by the FcεR1 is insensitive to the application of PD098059. Hence, in contrast to Elk-1, the critical Ras effector pathway for NFAT regulation in mast cells cannot be Raf-1/Erk.
The Rho Family GTPase Rac-1 Regulates the NFAT Response to FcεR1 Stimulation in Mast Cells.
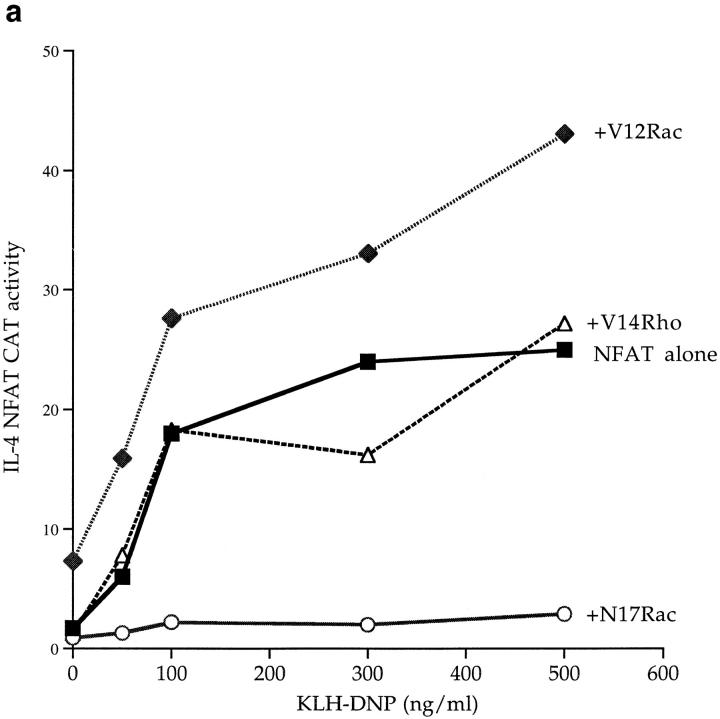
The Rho family GTPase Rac-1 has been shown to play a role in Ras regulation of fibroblast transformation (19) and in T cell antigen receptor regulation of NFAT (34). We therefore investigated the effects of Rho family GTPases in FcεR1 signaling to NFAT. Active mutants of Rac-1 and Rho (V12Rac and V14Rho, respectively) were used to assay whether these GTPases can regulate IL-4 NFAT activation in the mast cell. Fig. 5 a shows that expression of the active V12Rac mutant induced an increase in the basal activity of the NFAT reporter gene, and highly potentiated the FcεR1 activation of IL-4 NFAT. To demonstrate the specificity of this effect, we showed that an activated V14Rho had no discernable potentiating effect on IL-4 NFAT induction (Fig. 5 a).
Figure 5.
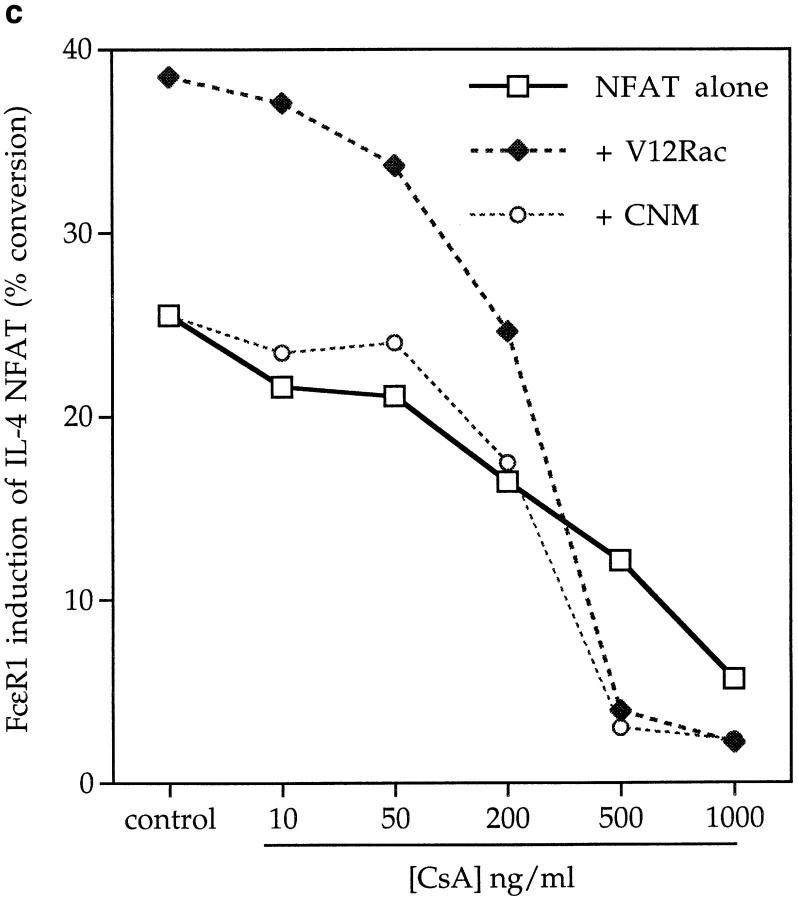
(a) FcεR1 stimulation of IL-4 NFAT is potentiated by active V12Rac, and is inhibited by the presence of dominant negative N17Rac. 1 × 107 cells per point were transfected with 15 μg IL-4 NFAT CAT reporter alone (solid line, filled squares), or in combination with 15 μg active V12Rac (broken line, filled diamonds), 15 μg dominant inhibitory N17Rac (solid line, open circles), or 15 μg active V14Rho (broken line, open triangles). Cells were recovered for 6 h before IgE priming and stimulation with the indicated concentrations of KLH-DNP. (b) FcεR1 stimulation of Elk-1 activity, a Raf-1/MEK-dependent process, is insensitive to N17Rac expression. 1 × 107 cells per point were transfected with either the Elk-1 reporter system alone (control), or in combination with 15 μg of pEFN17Rac. Cells were recovered and left unstimulated (NS), or exposed to IgE/ KLH-DNP as described. (c) Stimulation of NFAT by the FcεR1 alone or in concert with V12Rac is sensitive to CsA. 1 × 107 cells per point were transfected with 15 μg IL-4 NFAT CAT reporter alone (solid line, open squares), or in combination with 15 μg active V12Rac (broken line, filled diamonds). Cells were recovered, primed with IgE anti-DNP, and preincubated for 30 min with either vehicle (control) or the indicated dose of CsA before FcεR1 stimulation as described.
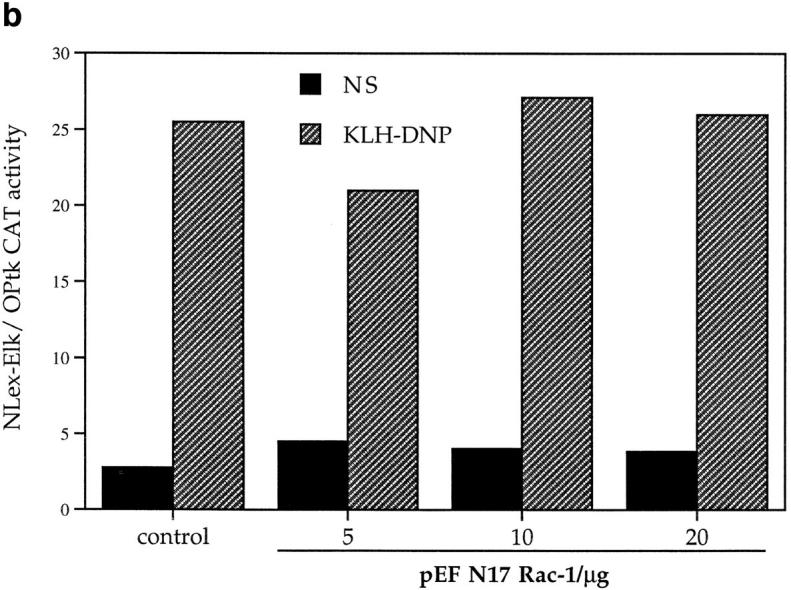
The ability of active Rac-1 to potentiate FcεR1/NFAT responses shows that this GTPase has the potential to regulate NFAT activity in mast cells. To examine whether Rac-1 actually plays a role in the NFAT response to FcεR1 stimulation, we cotransfected the NFAT reporter gene with an inhibitory mutant of Rac-1, N17Rac. The data in Fig. 5 a show that expression of N17Rac had a marked inhibitory effect on FcεR1 induction of NFAT. As a specificity control, we examined the effect of N17Rac on FcεR1 activation of Elk-1 transcription. Fig. 5 b shows that FcεR1 induction of Elk-1 is insensitive to the presence of N17Rac; therefore, we have demonstrated a specific requirement for Rac-1 activity in FcεR1 regulation of NFAT.
Induction of NFAT trancriptional activity in mast cells is dependent on the calcium/calcineurin pathway, and is therefore sensitive to inhibition via the immunosuppressive drug CsA (25). The data in Fig. 5 c show that the FcεR1 induction of NFAT is also sensitive to the action of CsA in a dose-dependent manner. Expression of the activated V12Rac mutant, which potentiates FcεR1 induction of NFAT, was unable to reverse the inhibition of the response observed with CsA. These data indicate that FcεR1 induction of NFAT requires at least the convergent action of Rac and CN pathways.
Discussion
The data herein show that the FcεR1 activation of Ras and its effector pathways allow receptor regulation of the transcription factors Elk-1 and NFAT. The diversity of its effector pathways enables Ras to act in a critical position to direct activation of various nuclear targets. We have shown that Ras signals are thus necessary and sufficient for FcεR1 transcriptional activation of Elk-1, a transcription factor important in the context of the serum response element which is a regulatory component of immediate early gene promoters (31). Ras signals also play a role in FcεR1 regulation of NFAT complexes, although they are not sufficent for this response. In this report we have also identified NFAT as a target for Rac-1 signaling pathways in mast cells. Expression of active Rac-1 protein dramatically potentiates FcεR1 induction of NFAT, whereas expression of an inhibitory Rac-1 mutant severely abrogates the NFAT response to FcεR1 stimulation. These data place the GTPases Ras and Rac-1 in a critical position regulating the nuclear component of mast cell end function.
The SRE is responsible for regulation of immediate early genes such as c-fos and egr-1, and has been shown to be a convergence point for varied signaling pathways including the Ras/MEK pathway in fibroblasts (35, 28). Extensive studies have shown that transcriptional activation of Elk-1 is dependent on its COOH-terminal phosphorylation by members of the MAP kinase family such as Erk-1/2, the Jun (stress-activated) kinase JNK, and the p38 kinase (28, 36). Despite the potential for Elk-1 to be targeted by multiple MAP kinases, the data herein show that the Ras effector pathway involved in FcεR1 regulation of Elk-1 absolutely requires the activity of the Erk-activating kinase MEK, acting downstream of Ras and its effector Raf-1. These data show that the Ras/Raf-1/MEK pathway has a dominant role in FcεR1 regulation of Elk-1, reflecting clear parallels between Elk-1 activation following antigen receptor ligation and growth factor stimulation of fibroblasts.
NFAT family members form regulatory complexes which bind cytokine gene promoters (37). Initially described as critical for IL-2 production in T cells, NFAT has also been implicated in transcriptional activation of the genes for IL-4, GM-CSF, and TNFα. NFAT comprises a cytoplasmic component which translocates to the nuclei of stimulated cells and complexes with an inducible nuclear component. The former component is encoded by an extensive gene family including NFATp, NFATc, NFAT-3, and NFAT-4/x. The latter is composed of AP-1 family members, e.g., Fos/Jun. NFATp is a crucial transcription factor for regulation of the IL-4 gene in the T cell (38), and is implicated in transcriptional activation of the IL-4 gene in mast cells (25). IL-4 produced by mast cells after FcεR1 cross-linking is thought to play an important role in the generation of a sustained inflammatory response. Locally, a pulse of mast cell IL-4 stimulates T cells into sustained IL-4 production and subsequent inflammatory cell infiltration. By feeding back on B cells to promote antigen specific IgE production, mast cell– derived IL-4 contributes to the sustained responsiveness of mast cells themselves to antigen/allergen.
Previous studies in T cells have shown that transcriptional activity of the NFAT complex required for IL-2 gene induction is dependent on the concerted action of Ras and calcium/calcineurin signaling pathways (39). Moreover, previous data from our laboratory has shown that multiple Ras effector pathways converge on NFAT in the context of the IL-2 promoter (34). The Raf-1/MEK pathway is one component of TCR induction of NFAT, but there is also a contribution from signals regulated by the GTPase Rac-1 (34). In the context of the activated mast cell, one target for NFAT is the IL-4 gene promoter. We have found that Ras signals are an absolute requirement for FcεR1 regulation of a reporter construct driven by a region of the IL-4 promoter 270 base pairs upstream of the transcriptional start site (Turner, H., and D.A. Cantrell, unpublished observation). The clear implication of this finding is that the IL-4 promoter contains targets for Ras signaling pathways, one of which we establish as NFAT.
Calcium/calcineurin signals are known to be critical for NFAT responses; IL-4 NFAT CAT activity can be strongly induced by an activated mutant of calcineurin or by ionophore treatment (Turner, H., and D.A. Cantrell, unpublished observations). The role of calcium signals in NFAT regulation in the T cell is to induce nuclear translocation of NFAT protein (37, 40, 41). The present data show that in addition to a requirement for calcium/calcineurin, Ras and Rac-1 signaling pathways are important for FcεR1 induction of IL-4 NFAT. While expression of activated Ras could potentiate FcεR1 induction of NFAT, this effect could not be mimicked by stimulation of the Raf-1/MEK pathway using Raf-CAAX. Furthermore, inhibition of MEK/Erk2 activity does not affect FcεR1 activation of NFAT. Hence, we are able to exclude the “classical” Ras/ Raf-1/MEK cascade. In comparison with the data in the T cell system, we also observe in mast cells that NFAT integrates multiple signals. In the mast cell, as in the T cell, there is a requirement for both calcium/calcineurin and Rac-1 signals in antigen receptor regulation of NFAT. However, the nature of the Ras signal, which is also required, differs between the two systems. In the mast cell, there is no requirement for Raf-1/MEK activity, while in the T cell, NFAT is regulated by this and another uncharacterized Ras effector pathway (34).
It is recognized that certain cellular responses to Ras require the activity of other GTPases; notably, Ras mediated transformation of fibroblasts requires signaling pathways mediated by Rac-1 and RhoA (18, 19). The data herein show that IL-4 NFAT activation in mast cells requires the function of Rac-1. The recognition that Rac-1 may play a pivotal role in NFAT responses raises the issue of the identity of Rac-1 effector pathways in mast cells. Proteins that can bind directly to GTP-bound active Rac-1 include members of the p21-associated kinase serine/threonine kinase family (42) and the ribosomal p70S6 kinase (43). Rac-1 has also been shown to activate the MAP kinase family members JNK-1 and p38 (44), making these candidate kinases for transduction of the Rac-1 signal leading to NFAT activation in mast cells.
There is an inhibitor of the p38 kinase available SB203580 (45) which does not modulate NFAT responses to FcεR1 activation (Turner, H., and D.A. Cantrell, unpublished observations). The immunosupressant Rapamycin which inhibits p70S6 kinase also does not inhibit NFAT activation, excluding a role for this kinase in NFAT responses. Hence, it is unlikely that these enzymes are responsible for Rac-1 activation of NFAT. We have observed in preliminary experiments that the FcεR1 activates the MAP kinase JNK-1 (Turner, H., and D.A. Cantrell, unpublished observation, and P. Bauer, personal communication). However, there are no small molecule inhibitors of JNK-1 pathways analogous to the PD098059 MEK inhibitor or the SB203580 p38 inhibitor that can be used to probe the cellular role of JNK-1 in NFAT responses. Therefore, the role of Rac-1 in coupling the FcεR1 to JNK-1 has yet to be examined. Moreover, JNK-1 is by no means the sole remaining candidate for a Rac-1 effector after the elimination of ERK, p38 and p70S6 kinase. There is now a burgeoning body of work on novel Rac-1 effectors including members of the p21-associated kinase family and the cytoskeletal regulatory protein POR-1 (42, 46).
The requirement for both Ras and Rac-1 function for FcεR1 activation of NFAT in mast cells is consistent with a model where Rac-1 is part of a separate FcεR1 signaling pathway that converges with Ras signals on IL-4 NFAT to fully activate the transcription factor complex. Nevertheless, it is equally possible to envisage a mechanism in which Rac-1 acts as a downstream effector for Ras. For example, Vav is a putative Rac-1/RhoA guanine nucleotide exchange protein (21) that is tyrosine phosphorylated in response to FcεR1 ligation (20), and is able to stimulate Rac-1–mediated JNK-1 activation in fibroblasts (22). Vav would thus be a candidate molecule for coupling the FcεR1 to Rac-1 responses. This model is analogous to the links between Ras and Rac-1 that have been documented in fibroblasts where Rac-1 couples Ras to the signaling pathways that control rearrangements of the actin cytoskeleton (47).
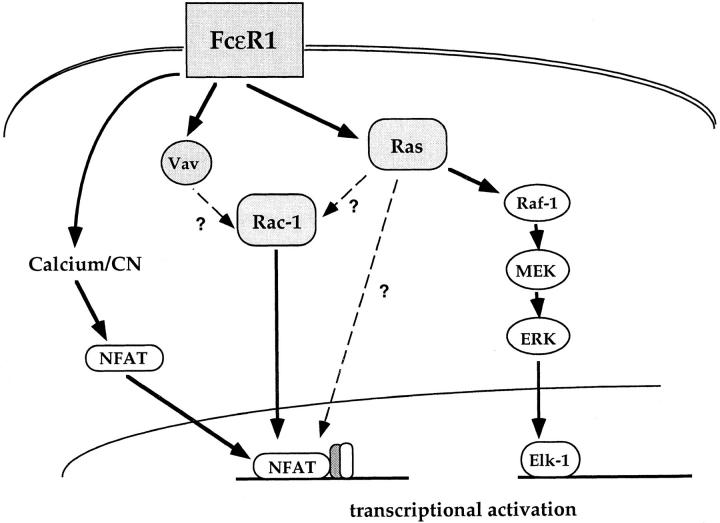
It is difficult to resolve these various possibilities (summarized in Fig. 6), and they are not mutually exclusive; Ras and Rac-1 may integrate signals from multiple inputs in the mast cell. This may be true both in terms of multiple intracellular signals generated by antigen receptor ligation, and in terms of reactivity to different extracellular stimuli. Certainly, Ras is not only activated by antigen receptor triggering in mast cells, but can be activated by cytokines such as IL-3 and GM-CSF (48). Activation of Ras-stimulatory pathways by integrins, which are likely to be involved in mast cell adherence to a tissue substratum, has also been documented (49). It is reasonable to expect that during the early course of an inflammatory response, mast cells would be exposed to Ras-activating cytokines such as IL-3 and GM-CSF produced by antigen activated T cells. The effect of Ras activation on IL-4 NFAT activity is essentially that of altering the antigen receptor dose response threshold. Hence, exposure of mast cells to Ras-activating cytokines can be viewed as an important priming step which increases the responsiveness of mast cells to a given antigen dose.
Figure 6.
Putative mechanism for FcεR1 action on transcription factor targets in mast cells. Ras signals through the Raf-1/MEK pathway are necessary and sufficient for Elk-1 activation. In contrast, the NFAT complex integrates multiple signals from the FcεR1. Calcium signals induce nuclear translocation of NFAT protein. Ras and Rac-1 GTPases are also implicated in FcεR1 activation of NFAT, acting either in parallel or in series.
Footnotes
For gifts of constructs we thank E. Serfling for the IL-4 NFAT reporter and Dr. Richard Treisman for the Elk-1 reporter system. Also, we thank members of the Lymphocyte Activation Laboratory for valuable advice and discussions of the manuscript.
H. Turner is supported by Glaxo-Wellcome (Stevenage, UK).
1 Abbreviations used in this paper: CAT, chloramphenicol acetyl transferase; CN, calcineurin; CsA, cyclosporin A; dn, dominant inhibitory; MAP, mitogen-activated protein; MEK, Erk-activating kinase; NFAT, nuclear factor of activated T cells; PKC, protein kinase C; PTK, protein tyrosine kinase.
References
- 1.Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 2.Plaut M, Pierce JH, Watson CJ, Hanley J, Hyde, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature (Lond) 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 3.Jouvin MH, Numerof RP, Kinet JP. Signal transduction through the conserved motifs of the high affinity IgE receptor Fc epsilon RI. Semin Immunol. 1995;7:29–35. doi: 10.1016/1044-5323(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 4.Eiseman E, Bolen JB. Engagement of the high affinity IgE receptor activates src protein related tyrosine kinases. Nature (Lond) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 5.Park DJ, Min HK, Rhee SG. IgE induced tyrosine phosphorylation of phospholipase C-γ 1 in rat basophilic leukaemiacells. J Biol Chem. 1991;266:24237–24240. [PubMed] [Google Scholar]
- 6.Li W, Deanin GG, Margolis B, Schlessinger J, Oliver JM. FcεR1 mediated tyrosine phosphorylation of multiple proteins including phopsholipase Cγ1 and the receptor βγ2 complex, in rat basophilic leukemia cells. Mol Cell Biol. 1992;12:3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozawa K, Szallis Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca dependent and independent isozymes of protein kinase C mediate exocytosis in antigen stimulated rat basophilic RBL2H3 cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 8.Buccione R, Di Tullio G, Caretta M, Marinetti M, Bizzarri C, Francavilla S, Luini A, De Matteis MA. Analysis of protein kinase C requirement for exocytosis in permeabilised rat basophilic leukemia RBL2H3 cells: a GTP binding protein(s) as a potential target for protein kinase C. Biochem J. 1994;298:149–156. doi: 10.1042/bj2980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wodnar Filipowicz, A., and C. Moroni. Regulation of interleukin 3 mRNA expression in mast cells occurs at the posttranscriptional level and is mediated by calcium ions. Proc Natl Acad Sci USA. 1990;87:777–781. doi: 10.1073/pnas.87.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner H, Reif K, Rivera J, Cantrell DA. Regulation of the adapter molecule Grb2 by the FceR1 in the mast cell line RBL2H3. J Biol Chem. 1995;270:9500–9506. doi: 10.1074/jbc.270.16.9500. [DOI] [PubMed] [Google Scholar]
- 11.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature (Lond) 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 12.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase, and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 13.White M, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 14.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature (Lond) 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 15.Downward J. Regulation of p21rasby GAPs and guanine nucleotide exchange proteins in normal and oncogenic cells. Curr Opin Genet Dev. 1992;2:13–18. doi: 10.1016/s0959-437x(05)80315-6. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesbroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature (Lond) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 17.Hofer F, Fields S, Schneider C, Martin GS. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prendergast GC, Khosravi R, Far, Solski PA, Kurzawa H, Lebowitz PF, Der CJ. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- 19.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature (Lond) 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 20.Margolis B, Hu P, Katzav S, Li W, Oliver JM, Ullrich A, Weiss A, Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature (Lond) 1992;356:71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- 21.Quilliam LA, Khosravi R, Far, Huff SY, Der CJ. Guanine nucleotide exchange factors: activators of the Ras superfamily of proteins. Bioessays. 1995;17:395–404. doi: 10.1002/bies.950170507. [DOI] [PubMed] [Google Scholar]
- 22.Crespo P, Bustelo X, Aaronsen D, Coso O, Barahona M, Barbacid M, Gutkind J. Rac-1 dependent stimulation of the JNK/SAPK signaling pathway by Vav. Oncogene. 1996;13:455–460. [PubMed] [Google Scholar]
- 23.Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 24.Kaye R, Fruman D, Bierer B, Albers M, Zydowski L, Ho S, Jin Y, Castells M, Schreiber S, Walsh C, Burakoff S, Austen K, Katz H. Effects of Cyclosporin A and FK506 on FcεR1 initiated increases in cytokine mRNA in mouse bone marrow derived mast cells. Proc Natl Acad Sci USA. 1992;89:8542–8546. doi: 10.1073/pnas.89.18.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss D, Hural J, Tara D, Timmerman L, Henkel G, Brown M. Nuclear factor of activated T cells is associated with a mast cell interleukin 4 transcription complex. Mol Cell Biol. 1996;16:228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson L, McCloskey M. Fcε R1 mediated induction of nuclear factor of activated T cells. J Biol Chem. 1995;270:16333–16338. doi: 10.1074/jbc.270.27.16333. [DOI] [PubMed] [Google Scholar]
- 27.Prieschl E, Pendl G, Harrer N, Baumruker T. p21Ras links FcεR1 to NF-AT family member in mast cells: the AP-3 like factor in these cells is an NFAT family member. J Immunol. 1995;155:4963–4970. [PubMed] [Google Scholar]
- 28.Price M, Rogers A, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO (Eur Mol Biol Organ) J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobes C, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibres, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 30.Williams DH, Woodrow M, Cantrell AD, Murray JE. Protein kinase C is not a downstream effector of p21ras in activated T cell. Eur J Immunol. 1995;25:42–47. doi: 10.1002/eji.1830250109. [DOI] [PubMed] [Google Scholar]
- 31.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 32.Leevers S, Paterson H, Marshall C. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature (Lond) 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 33.Alessi D, Cuenda A, Cohen P, Dudlet D, Saltiel A. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 34.Genot E, Cleverley S, Henning S, Cantrell DA. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO (Eur Mol Biol Organ) J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 35.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1 and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 36.Whitmarsh A, Shore P, Sharrocks A, Davis R. Integration of MAP kinase signal transduction pathways at the serum response element. Science (Wash DC) 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 37.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 38.Hodge M, Ranger A, de la Brousse FC, Hoey T, Grusby M, Glimcher L. Hyperproliferation and dysregulation of IL-4 expression in NFATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 39.Woodrow M, Clipstone NA, Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993;178:1517–1522. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Northrop J, Ho S, Chen L, Thomas D, Timmerman L, Nolan G, Admon A, Crabtree G. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature (Lond) 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 41.Hoey T, Sun Y, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 42.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature (Lond) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 43.Chou M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G Proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 44.Symons M. Rho family GTPases: the cytoskeleton and beyond. TIBS (Trends Biochem Sci) 1996;21:178–181. [PubMed] [Google Scholar]
- 45.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 46.Van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac-1 interacting protein involved in membrane ruffling. EMBO (Eur Mol Biol Organ) J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- 47.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Ann Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 48.Satoh T, Nakafuku M, Miyajima A, Kaziro Y. Involvement of p21ras in signal transduction pathways from interleukin 2, interleukin 3 and granulocyte/macrophage colony stimulating factor, but not from interleukin 4. Proc Natl Acad Sci USA. 1991;88:3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature (Lond) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]