Abstract
Due to differential expression of chemokine receptors, the Th1 and Th2 subsets of CD4+ T cells differ in their migratory responses to chemokines. These differences in the migration patterns are likely to play a role in the initiation and regulation of Th1 and Th2 immune responses, inflammatory processes, and T-cell-mediated pathology. In the present study we evaluated the role of activated Th cells as producers of chemokines. Three different sources of murine Th cells were used, i.e., long-term-cultured Th1 and Th2 cell clones, Th1 and Th2 cells differentiated from naïve CD4+ spleen and lymph node cells in vitro, and Th1 and Th2 subsets polarized in vivo using a murine experimental Leishmania major infection model. Following stimulation with anti-CD3, macrophage inflammatory protein 1γ (MIP-1γ) and lymphotactin were produced selectively by Th1 cells but not by Th2 cells. In contrast, only Th2 cells produced MIP-2. The possible biological relevance of these data was substantiated by the finding that in vivo-polarized Th1 cells, but not Th2 cells, produced MIP-1γ and lymphotactin while in vivo-polarized Th2 cells secreted MIP-2. The above data demonstrate that Th1 and Th2 cells differ in their ability to produce chemokines, suggesting that Th1 and Th2 subsets differentially contribute to recruitment of cells into inflammatory foci.
CD4+ Th cells can be classified into functionally distinct subsets based on the profiles of cytokines they produce (30). Th1 cells secrete interleukin-2 (IL-2), gamma interferon (IFN-γ), and lymphotoxin, which predominantly promote cell-mediated immune responses to intracellular pathogens, whereas Th2 cells produce cytokines such as IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13, which are involved in humoral and allergic responses (1, 28). The experimental murine model of Leishmania major infection is a well-characterized system to investigate the in vivo significance of polarized Th1-Th2 responses. In this model the Th1-dominated response leads to healing of the infection in resistant mouse strains such as in C57BL/6 whereas the Th2 type of response leads to severe disease development in susceptible BALB/c mice (44).
The differentiation of Th1 and Th2 cells from naïve precursor CD4+ T cells is dependent on a combination of host genetic factors, the local cytokine milieu, and the type and dose of antigen encountered (32). Cytokines can either positively or negatively regulate the development of Th subsets. IL-12 and IFN-γ are thought to drive the polarization of naïve T cells toward Th1 cells, whereas IL-4 and IL-13 are important for the induction of Th2 responses. Moreover, IL-4 and IL-10 inhibit the differentiation into Th1 cells, whereas IFN-γ acts as a suppressor of Th2 polarization (12, 41).
Chemokines are key regulators in the recruitment of appropriate effector cells to sites of inflammation. There is evidence that Th subsets differ in their migratory response to chemokines (2, 13, 14, 19, 21, 28, 37, 42, 45) due to differential expression of chemokine receptors. Th1 cells preferentially express CC chemokine receptor 5 (CCR5), CCR7, and CXC chemokine receptor 3 (CXCR3) (3, 35, 37), while Th2 cells predominantly express CCR3, CCR4, and CCR8 (3, 36, 37, 48). However, Th cells not only respond to chemotactic factors but also release them, thus contributing to the composition of the cell infiltrates in inflammatory foci (4, 38, 46, 47). Although differential expression of chemokines by Th1 versus Th2 cells has been demonstrated, the chemokine repertoire of the distinct Th-cell populations has not been extensively and comprehensively examined. In previous work, either Th1 and Th2 cell lines (38, 39, 46) or Th cells differentiated in vitro (4, 8, 33, 47) were analyzed. In the present study, the chemokine production of Th-cell clones and Th cells differentiated both in vitro and in vivo was investigated. The gene expression of murine chemokines was investigated by applying the RNase protection assay (RPA) with template sets developed in our laboratory (31). In addition to the in vitro studies, we extended our analyses to Th1 and Th2 cells that were polarized in vivo using the murine experimental L. major infection model. Using all three sources of Th cells, we show that Th1 and Th2 cells exhibit distinct chemokine secretion patterns with selective production of macrophage inflammatory protein 1γ (MIP-1γ) and lymphotactin by Th1 cells and of MIP-2 by Th2 cells.
MATERIALS AND METHODS
Immunologic reagents.
Anti-murine CD3 monoclonal antibody (MAb) was purified from hybridoma 145-2C11 (24) supernatant (SN). Recombinant murine (rm) lymphotactin, rabbit polyclonal immunoglobulin G (IgG) antibody (Ab) to murine MIP-1γ, and biotinylated rabbit polyclonal IgG Ab to murine lymphotactin were obtained from R&D Systems (Wiesbaden, Germany). rm MIP-1γ, rm MIP-2, and biotinylated rabbit polyclonal IgG Abs to murine MIP-1γ and to murine MIP-2 were purchased from PeproTech (Frankfurt, Germany). Rabbit polyclonal IgG Abs to murine lymphotactin and to murine MIP-2 were obtained from DPC Biermann (Bad Nauheim, Germany). For IL-4 and IFN-γ enzyme-linked immunosorbent assay (ELISA) studies, commercial Ab pairs from BD PharMingen were used.
Mice.
Female C57BL/6 and BALB/c mice were purchased from Charles River Breeding Laboratories (Sulzfeld, Germany) and maintained within the University of Lübeck animal care facility. The mice were used at 8 to 12 weeks of age.
Th-cell clones.
The Th1-cell clones used were B10BI, derived from a C57BL/6 mouse and specific for bovine insulin (26); LNC-2, derived from a BALB/c mouse and specific for Mycobacterium tuberculosis purified protein derivative (26); AgB4, derived from a C57BL/6 mouse and specific for an L. major antigen preparation; and COH-2, derived from a BALB/c mouse and specific for ovalbumin. The Th2-cell clones used were D10.G4.1, derived from an AKR/J mouse and specific for conalbumin, and L1/1, established from an L. major-infected BALB/c mouse and specific for an L. major antigen preparation (26). The T-cell clones were propagated as described previously (26). Briefly, 5 × 105 cells/well were restimulated every 4 weeks in 12-well tissue culture plates (Costar, Cambridge, Mass.) with syngeneic irradiated (25 Gy) spleen cells (5 × 106/well) and antigen in a total volume of 3 ml of Clicks' RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 2 mM l-glutamine, 100 U of penicillin per ml, 10 mM HEPES, 50 μM 2-mercaptoethanol, and 10% fetal calf serum (Sigma, Deisenhofen, Germany). At 48 h after restimulation, the wells were split and SN (4%) of the cell line X63Ag8-653/IL-2 (20) was added as a source of IL-2. Thereafter, IL-2 was added once per week. Cells were routinely used for the experiments 4 weeks after their final antigenic restimulation and 2 weeks after the final addition of IL-2.
For in vitro restimulation, T cells were cultured (4 × 106/well in 4 ml of medium) in six-well plates (Greiner, Frickenhausen, Germany) with immobilized anti-CD3 MAb (coating concentration, 1 μg/ml). Total RNA was isolated after 20 h of incubation and subjected to an RPA as described below. Lymphokine and chemokine production in SN were determined using commercial ELISA kits (BD PharMingen) and home-made ELISA kits as described below, respectively.
T-cell differentiation in vitro.
Naïve CD4+ T cells were positively enriched from spleens and mesenteric lymph nodes of C57BL/6 mice by using the MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously (27). The resulting cell population was >96% CD4+ CD62L+ as determined by fluorescence-activated cell sorter analysis. These cells were differentiated into Th1 or Th2 cells by stimulation with immobilized anti-CD3 MAb and either 1 ng of rm IL-12 per ml plus anti-IL-4 MAb (Th1) or IL-4 (Th2) as described previously (27). After 4 days, the cells were expanded for 48 h in medium containing IL-2 SN. Subsequently, the cells were harvested, washed, and restimulated with immobilized anti-CD3 MAb (coating concentration, 5 μg/ml) for 24 h. Total RNA was isolated and analyzed using RPA. Aliquots of SN were assayed for lymphokine and chemokine release.
In vivo polarization into Th1 and Th2 subsets.
Stationary-phase L. major (MHOM/IL/81/FEBNI) promastigotes were obtained from cultures in biphasic Novy-McNeal-Nicolle blood agar medium (43). For in vivo induction of Th1 and Th2 cells, C57BL/6 and BALB/c mice were infected subcutaneously into both hind footpads with 5 × 106 stationary-phase L. major promastigotes as described previously (22, 43). At 4 weeks later, CD4+ T cells were positively selected from lymph node (LN) single-cell suspensions by the MACS system, using anti-CD4 MAb conjugated to magnetic beads (Miltenyi Biotec). The purified cells contained >97% CD4+ cells as determined by fluorescence-activated cell sorter analysis. For restimulation, 106 CD4+ cells were cultured in 24-well plates (Nunc, Wiesbaden, Germany) with immobilized anti-CD3 MAb (coating concentration, 1 μg/ml). After 48 h, SN was harvested and tested for lymphokine and chemokine release.
Analysis of chemokine mRNA expression by RPA.
Total-cell RNA from Th cells was isolated as described previously (31). RPA was used for the assessment of chemokine gene expression. This method enables the quantitative determination of several chemokine mRNA species simultaneously. Two multiprobe RPA template sets were established in our laboratory for the mRNA analysis of the murine chemokines stromal cell-derived factor 1 (SDF-1), lymphotactin, exodus-1, thymus-expressed chemokine (TECK), fractalkine, KC, macrophage-derived chemokine (MDC), monokine induced by gamma interferon (MIG), MIP-1γ, B-lymphocyte chemoattractant (BLC), monocyte chemoattractant protein 5 (MCP-5), and EBI-1 ligand chemokine (ELC) (31). In addition, a commercially available RPA template set (mCK-5b; BD PharMingen) detecting the murine chemokines regulated on activation normal T-cell expressed and secreted (RANTES), eotaxin, MIP-1α, MIP-1β, MIP-2, T-cell-attracting chemokine 3 (TCA-3), and MCP-1 was applied.
RPAs were carried out as described previously (31) using [α-33P]UTP. For autoradiography, dried gels were exposed to XAR film (Kodak, Rochester, N.Y.) at room temperature (RT). For quantitation of the radiation intensity of the RNA bands, gels were placed on imaging plates (Raytest, Straubenhardt, Germany) and images were analyzed using a phosphorimager (BAS 2000; Fuji Photo Film Co., Tokyo, Japan) and TINA 2.0 software (Raytest). Chemokine mRNA levels were expressed as relative units with respect to the expression value of the housekeeping gene L32, which was set as 1 relative unit.
Chemokine ELISAs.
The levels of lymphotactin, MIP-1γ, and MIP-2 in SN were determined by sandwich ELISA. Ninety-six-well microtiter plates (Nunc) were coated overnight at 4°C at 100 μl/well with carbonate buffer (pH 9.6) containing rabbit polyclonal IgG Abs directed against lymphotactin (2 μg/ml), MIP-1γ (0.5 μg/ml), or MIP-2 (0.5 μg/ml). The wells were washed with phosphate-buffered saline containing 0.05% Tween 20 and blocked by incubation for 2 h at RT with phosphate-buffered saline containing 1% bovine serum albumin (Sigma). After the wells were washed, SN were added and incubated either for 90 min at 37°C or overnight at 4°C. The wells were again washed, and biotinylated rabbit polyclonal IgG Ab directed against lymphotactin (0.1 μg/ml), MIP-1γ (0.1 μg/ml), or MIP-2 (0.1 μg/ml) was added. After a 2-h incubation at RT, the wells were washed, 100 μl of a 1/1,000 dilution of streptavidin peroxidase conjugate (DAKO, Hamburg, Germany) per well was added, and the wells were incubated for 1 h at RT. They were washed again, substrate was added, and the wells were incubated at RT. The absorbance was read at 405 nm. The detection limits were 30 pg/ml for lymphotactin, 40 pg/ml for MIP-1γ, and 7.8 pg/ml for MIP-2. ELISA data are presented as mean and standard deviation (SD) for triplicate assays.
RESULTS
Lymphotactin and MIP-1γ are produced by activated Th1-cell clones, whereas Th2-cell clones release MIP-2.
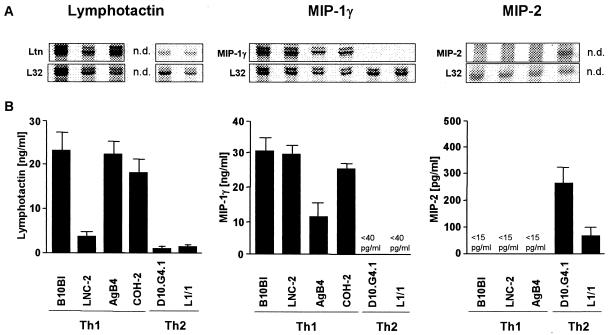
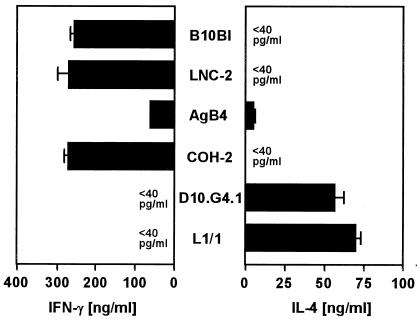
The murine Th1-cell clones B10BI, LNC-2, AgB4, and COH-2 and the Th2-cell clones D10.G4.1 and L1/1 were stimulated with immobilized anti-CD3 MAb for 20 h and analyzed for chemokine mRNA expression. Both lymphotactin and MIP-1γ mRNA were strongly expressed by all of the tested Th1 cell clones (Fig. 1A). In contrast, the Th2-cell clones expressed lymphotactin mRNA only at a minimal level and MIP-1γ mRNA not at all (Fig. 1A). Moreover, it was observed that the activated Th2-cell clone D10.G4.1 selectively expressed MIP-2 mRNA (Fig. 1A). The preferential expression of these chemokines by Th1 and Th2 cells was confirmed by ELISA showing the production of lymphotactin and MIP-1γ by Th1 cell clones and of MIP-2 by Th2 cell clones (Fig. 1B). To confirm that the production of lymphotactin and MIP-1γ correlated with the typical cytokine pattern of Th1 cells, IFN-γ and IL-4 protein levels were measured in the same SN of activated Th-cell clones. All activated Th1 cell clones produced high levels of IFN-γ (Fig. 2). With the exception of AgB4, which produced low levels of IL-4, no secretion of IL-4 was detected (Fig. 2). As expected, both Th2-cell clones released high levels of IL-4 but no IFN-γ (Fig. 2).
FIG. 1.
Differential production of lymphotactin, MIP-1γ, and MIP-2 by Th1- and Th2-cell clones.The Th1-cell clones B10BI, LNC-2, AgB4, and COH-2 and the Th2-cell clones D10.G4.1 and L1/1 were stimulated with immobilized anti-CD3 MAb for 20 h. Total-cell RNA was subjected to RPA, and chemokine release was measured by ELISA. (A) RPA autoradiograms showing the mRNA expression of lymphotactin, MIP-1γ, and MIP-2 and of the corresponding housekeeping gene L32. (B) Lymphotactin, MIP-1γ, and MIP-2 content in SN of activated Th-cell clones. The results are the mean and SD of one representative experiment of three performed. n.d., not done.
FIG. 2.
IFN-γ and IL-4 production by Th1- and Th2-cell clones. The Th1 cell clones B10BI, LNC-2, AgB4, and COH-2 and the Th2-cell clones D10.G4.1 and L1/1 were activated with immobilized anti-CD3 MAb for 20 h. SN were assayed for IFN-γ and IL-4 release by ELISA. The results are the mean and SD of one representative experiment of three performed.
Expression of mRNA for the chemokines SDF-1, exodus-1, TECK, fractalkine, KC, MIG, BLC, MCP-5, ELC, eotaxin, and MCP-1 was not detectable in activated Th1 or Th2 cell clones (data not shown). None of the Th-cell clones expressed chemokine mRNA in the resting state, indicating the lack of constitutive chemokine gene expression (data not shown).
Differential chemokine gene expression by activated naïve T cells differentiated into Th1 or Th2 phenotypes in vitro.
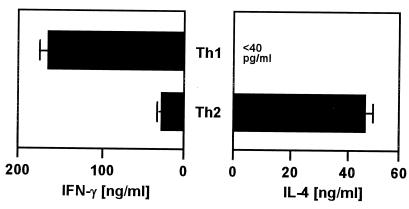
Next, we investigated whether the differences observed with the long-term-cultured Th1- and Th2-cell clones also apply to naïve CD4+ T cells that were shifted in vitro to Th1 or Th2 subsets and restimulated with anti-CD3 for 24 h. As expected, the Th1 cells produced high levels of IFN-γ but no IL-4 whereas the Th2 cells synthesized IL-4 but only a little IFN-γ (Fig. 3) demonstrating the generation of Th1- and Th2-cell populations, respectively.
FIG. 3.
IFN-γ and IL-4 release by Th1 and Th2 cells differentiated in vitro. Naïve CD4+ T cells were purified from spleens and lymph nodes of C57BL/6 mice and differentiated to Th1 or Th2 phenotypes in vitro as described in Materials and Methods. The cells were restimulated with immobilized anti-CD3 MAb for 24 h, and SN were assayed for IFN-γ and IL-4 using ELISA. The results are the mean and SD of two experiments.
RPA revealed that the Th1 cells expressed lymphotactin and MIP-1γ mRNA at high levels whereas the mRNA expression of these chemokines in Th2 cells was very low (Fig. 4A). The selective lymphotactin and MIP-1γ mRNA expression patterns were reflected by the respective protein secretion as determined by ELISA (Fig. 4B). In vitro-differentiated Th2 cells preferentially produced MIP-2 (Fig. 4B).
FIG. 4.
CD4+ Th cells polarized in vitro differentially release lymphotactin, MIP-1γ, and MIP-2. Naïve CD4+ T cells differentiated to either Th1 or Th2 cells in vitro were restimulated with anti-CD3 MAb for 24 h as described in Materials and Methods. Total-cell RNA was analyzed for chemokine mRNA expression by RPA. (A) RPA autoradiograms showing the determination of mRNA expression of lymphotactin and MIP-1γ as well as of the corresponding housekeeping gene L32. (B) The SN were tested for lymphotactin, MIP-1γ, and MIP-2 production by ELISA. The results are the mean and SD of two experiments.
The data derived from analysis of in vitro-differentiated Th1 and Th2 cells were in line with the results obtained with the Th1- and Th2-cell clones.
Th1 and Th2 subsets generated from L. major-infected mice differentially produce chemokines.
The data presented so far clearly demonstrate a differential chemokine expression pattern by Th1 and Th2 cells in vitro. Next, we aimed to verify that our findings also apply to in vivo-differentiated CD4+ Th cells. Resistent C57BL/6 and susceptible BALB/c mice were infected with the protozoan parasite L. major to induce the in vivo differentiation of Th1 and Th2 cells, respectively.
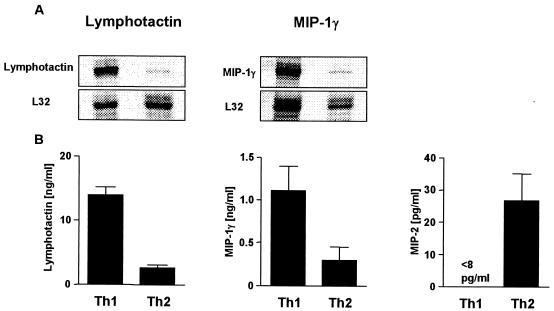
At 4 weeks after infection, CD4+ T cells that were enriched from single-cell suspensions from the popliteal LN of infected mice were restimulated with anti-CD3 for 48 h. The IFN-γ and IL-4 contents in SN were measured by ELISA to demonstrate the polarized differentiation into Th1 or Th2 cells, respectively. CD4+ T cells from resistant C57BL/6 mice produced high levels of IFN-γ but no IL-4. In contrast, SN of CD4+ T cells from susceptible BALB/c mice contained high levels of IL-4 and lower levels of IFN-γ (Fig. 5A).
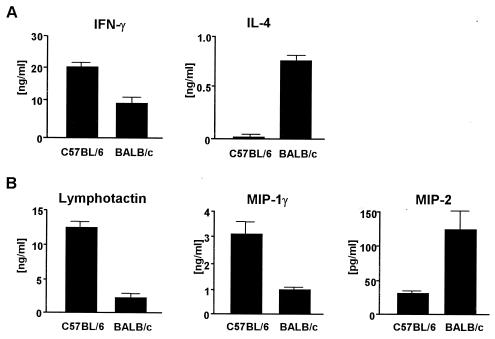
FIG. 5.
In vivo-differentiated Th1 cells secrete lymphotactin and MIP-1γ, whereas Th2 cells produce MIP-2. Resistent C57BL/6 and susceptible BALB/c mice were infected with L. major promastigotes in the hind footpads. At 4 weeks after infection, CD4+ T cells were purified from draining LN and restimulated with anti-CD3 MAb for 48 h. SN were assayed for IFN-γ and IL-4 (A) and for lymphotactin, MIP-1γ, and MIP-2 (B) using ELISA. The results are the mean and SD of one representative experiment of three performed.
Next, the SN were assayed for the presence of lymphotactin, MIP-1γ, or MIP-2 by ELISA. CD4+ T cells from C57BL/6 mice secreted high levels of lymphotactin and MIP-1γ (Fig. 5B). The SN of CD4+ T cells from infected BALB/c mice contained low levels of lymphotactin and MIP-1γ (Fig. 5B). In contrast, MIP-2 protein was preferentially secreted by CD4+ T cells from infected BALB/c mice (Fig. 5B).
These data clearly demonstrate that polarized Th-cell responses in vivo lead not only to differential expression of lymphokines like IFN-γ and IL-4 but also to the same differential chemokine profiles that we had observed for in vitro-polarized Th cells.
DISCUSSION
In the present study we have demonstrated that in addition to producing specific lymphokines, murine Th1 and Th2 cells exhibit distinct chemokine secretion patterns. Th1 cells, but not Th2 cells, produced lymphotactin and MIP-1γ, while only Th2 cells secreted MIP-2. To prove that the observed Th subset-specific chemokine production is a general characteristic of murine CD4+ T cells, different sources of Th1 and Th2 cells were used, i.e., long-term-cultured Th1 and Th2 cell clones and Th1 and Th2 cells differentiated in vitro from naïve CD4+ T cells. Moreover, polarized Th cells were obtained from mice infected with L. major. In line with the in vitro data, in vivo-differentiated Th1 cells from infected C57BL/6 mice produced lymphotactin and MIP-1γ whereas Th2 cells from infected BALB/c mice released MIP-2.
In previous studies, lymphotactin mRNA expression was found in human and murine Th1 cell clones and in murine Th1 cells differentiated in vitro (4, 16, 33, 47). Recently, secretion of lymphotactin protein by polyclonally activated in vitro-polarized murine Th1 cells was shown (8). Here we demonstrate for the first time that Th1 cells differentiated in vivo are also producers of lymphotactin. Although the in vivo relevance of Th1-cell-derived lymphotactin is not clear, the available data suggest that this chemokine is a strong regulator of the cellular immune response. Several studies have proposed that lymphotactin can function as an immunological adjuvant, promoting antitumor immunity through the enhancement of CD8+ T-cell-mediated cytotoxic activity, CD4+ T-cell proliferation, and cytokine production after systemic administration (7, 25). In addition, it was shown that lymphotactin can augment CD8+ T-cell proliferation and IL-2 secretion responses (6, 7). These findings may explain why the numbers of CD8+ cells are also expanded in the cutaneous lesion of resistant L. major-infected mice (18). Immunization with LACK (the Leishmania protein homologue of receptor for activated C kinase) was found to induce antigen-specific CD8+ IFN-γ-producing T cells which mediate long-term immunity to L. major (15). Thus, lymphotactin may function to recruite CD8+ cytotoxic T cells not only into tumors but also into the inflamed tissues of mice after infection.
In contrast to these considerations, evidence was recently presented that lymphotactin can act as a negative regulator of human CD4+ T-cell activation by inhibiting the production of the Th1 cytokine IL-2 (6). It is tempting to speculate that Th1-cell-derived lymphotactin may function as negative regulator of Th1 responses and thereby participates in the mechanisms preventing Th1-mediated pathology. The mRNA expression of the lymphotactin receptor XCR1 was also demonstrated on B cells (17). Therefore, lymphotactin may also participate in the local recruitment of B cells. This is in line with the finding that although the resistance to L. major depends on a Th1-dominated cellular immune response, B cells contribute substantially to healing of the infection (40).
The present study provides the first evidence that CD4+ T cells have the capacity to produce MIP-1γ. Our data clearly showed that MIP-1γ is produced selectively by Th1 but not by Th2 cells. MIP-1γ was shown to mediate the migration of CD4+ and CD8+ T cells (29). Plasmacytoid dendritic cells play a major role in the induction of Th1 responses. Recently, these cells were shown to preferentially produce CCR1 ligands that mostly recruit effector T cells and, in particular, Th1-type cells (34). Our present finding that Th1 cells secrete the CCR1 ligand MIP-1γ suggests that Th1 cells can maintain and/or amplify an ongoing Th1 response via the release of chemokines such as MIP-1γ. In addition, based on the chemotactic effect of MIP-1γ on CD8+ T cells, the data suggest that MIP-1γ recruits CD8+ cytotoxic T cells into inflamed or infected tissues.
Importantly the CCR1 ligand MIP-1α was shown to enhance nitric oxide production in macrophages infected with Leishmania (5). Since MIP-1α shares the CCR1 receptor with MIP-1γ (23), it is likely that Th1-cell-derived MIP-1γ exerts similar activity and therefore that MIP-1γ production by Th1 cells may contribute to parasite clearance.
In the present study we demonstrated a clear preference of Th2 cell clones to express MIP-2 mRNA and to produce MIP-2. Th2 cells differentiated both in vitro and in vivo were also found to secrete MIP-2. MIP-2 has been shown to be instrumental for the development of an early inflammatory process due to its function as a potent recruitment and activation factor for neutrophil granulocytes and NK-T cells (9, 11). In a previous study, the functional relevance of MIP-2 was analyzed by applying MIP-2 topically as adjuvant in a mouse model of herpes simplex virus mucosal infection and was found to enhance the Th1-type CD4+ T-cell-mediated adaptive immunity by increasing IFN-γ secretion from activated NK cells (10). Thus, early MIP-2 may be involved in the development of the Th1-type immune response. However, the role of Th2-cell-derived MIP-2 in the course of the ongoing polarized Th2 response remains to be clarified.
Taken together, our data demonstrate that Th1 and Th2 cells produce distinct sets of chemokines, suggesting that Th1 and Th2 cells differentially contribute to selective recruitment of appropriate effector cells to sites of inflammation or infection. The selective chemokine production by Th1 and Th2 cells thus plays an important role in the regulation of immune responses.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (grants SFB 367/B10 and LA1267/1-1).
Editor: J. M. Mansfield
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Andrew, D. P., M. S. Chang, J. McNinch, S. T. Wathen, M. Rihanek, J. Tseng, J. P. Spellberg, and C. G. Elias III. 1998. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 161:5027-5038. [PubMed] [Google Scholar]
- 3.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, L. M., V. C. Asensio, L.-K. Schioetz, J. Harbertson, T. Krahl, G. Patstone, N. Woolf, I. L. Campbell, and N. Sarvetnick. 1999. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J. Immunol. 162:2511-2520. [PubMed] [Google Scholar]
- 5.Brandonisio, O., M. A. Panaro, I. Fumarola, M. Sisto, D. Leogrande, A. Acquafredda, R. Spinelli, and V. Mitolo. 2002. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1 alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin. Exp. Med. 2:125-129. [DOI] [PubMed] [Google Scholar]
- 6.Cerdan, C., E. Serfling, and D. Olive. 2000. The C-class chemokine, lymphotactin, impairs the induction of Th1-type lymphokines in human CD4(+) T cells. Blood 96:420-428. [PubMed] [Google Scholar]
- 7.Dilloo, D., K. Bacon, W. Holden, W. Zhong, S. Burdach, A. Zlotnik, and M. Brenner. 1996. Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nat. Med. 2:1090-1095. [DOI] [PubMed] [Google Scholar]
- 8.Dorner, B. G., A. Scheffold, M. S. Rolph, M. B. Hüser, S. H. E. Kaufmann, A. Radbruch, I. E. A. Flesch, and R. A. Kroczek. 2002. MIP-1α, MIP-1β, RANTES, and ATAC/lymphotactin function together with IFN-γ as type 1 cytokines. Proc. Natl. Acad. Sci. USA 99:6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll K. E. 1994. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp. Lung Res. 20:473-490. [DOI] [PubMed] [Google Scholar]
- 10.Eo, S. K., S. Lee, S. Chun, and B. T. Rouse. 2001. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J. Virol. 75:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faunce, D. E., K. H. Sonoda, and J. Stein-Streilein. 2001. MIP-2 recruits NKT cells to the spleen during tolerance induction. J. Immunol. 166:313-321. [DOI] [PubMed] [Google Scholar]
- 12.Fitch, F. W., M. D. McKisic, D. W. Lancki, and T. F. Gajewski. 1993. Differential regulation of murine T lymphocyte subsets. Annu. Rev. Immunol. 11:29-48. [DOI] [PubMed] [Google Scholar]
- 13.Gangur, V., F. E. Simons, and K. T. Hayglass. 1998. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J. 12:705-713. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalo, J. A., Y. Pan, C. M. Lloyd, G. Q. Jia, G. Yu, B. Dussault, C. A. Powers, A. E. Proudfoot, A. J. Coyle, D. Gearing, and J. C. Gutierrez-Ramos. 1999. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163:403-411. [PubMed] [Google Scholar]
- 15.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 16.Hautamaa, D., R. Merica, Z. Chen, and M. K. Jenkins. 1997. Murine lymphotactin: gene structure, post-translational modification and inhibition of expression by CD28 costimulation. Cytokine 9:375-382. [DOI] [PubMed] [Google Scholar]
- 17.Huang, H., F. Li, C. M. Cairns, J. R. Gordon, and J. Xiang. 2001. Neutrophils and B cells express XCR1 receptor and chemotactically respond to lymphotactin. Biochem. Biophys. Res. Commun. 281:378-382. [DOI] [PubMed] [Google Scholar]
- 18.Huber, M., E. Timms, T. W. Mak, M. Röllinghoff, and M. Lohoff. 1998. Effective and long-lasting immunity against the parasite Leishmania major in CD8-deficient mice. Infect. Immun. 66:3968-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai, T., M. Baba, M. Nishimura, M. Kakizaki, S. Takagi, and O. Yoshie. 1997. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 272:15036-15042. [DOI] [PubMed] [Google Scholar]
- 20.Karasuyama, H., and F. Melchers. 1988. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur. J. Immunol. 18:97-104. [DOI] [PubMed] [Google Scholar]
- 21.Karpus, W. J., N. W. Lukacs, K. J. Kennedy, W. S. Smith, S. D. Hurst, and T. A. Barrett. 1997. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158:4129-4136. [PubMed] [Google Scholar]
- 22.Laskay, T., I. Wittmann, A. Diefenbach, M. Röllinghoff, and W. Solbach. 1997. Control of Leishmania major infection in BALB/c mice by inhibition of early lymphocyte entry into peripheral lymph nodes. J. Immunol. 158:1246-1253. [PubMed] [Google Scholar]
- 23.Lean, J. M., C. Murphy, K. Fuller, and T. J. Chambers. 2002. CCL9/MIP-1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J. Cell. Biochem. 87:386-393. [DOI] [PubMed] [Google Scholar]
- 24.Leo, O., M. Foo, D. H. Sachs, L. E. Samelson, and J. A. Bluestone. 1987. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA 84:1374-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillard, J. W., Jr., P. N. Boyaka, J. A. Hedrick, A. Zlotnik, and J. R. McGhee. 1999. Lymphotactin acts as an innate mucosal adjuvant. J. Immunol. 162:1959-1965. [PubMed] [Google Scholar]
- 26.Lohoff, M., E. Schmitt, A. B. Reske Kunz, and M. Röllinghoff. 1990. Different response of Th1 cells for stimulation with anti-CD3 antibodies. Eur. J. Immunol. 20:653-658. [DOI] [PubMed] [Google Scholar]
- 27.Lohoff, M., G. S. Duncan, D. Ferrick, H. W. Mittrucker, S. Bischof, S. Prechtl, M. Röllinghoff, E. Schmitt, A. Pahl, and T. W. Mak. 2000. Deficiency in the transcription factor interferon regulatory factor (IRF)-2 leads to severely compromised development of natural killer and T helper type 1 cells. J. Exp. Med. 192:325-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs, N. W., S. W. Chensue, W. J. Karpus, P. Lincoln, C. Keefer, R. M. Strieter, and S. L. Kunkel. 1997. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am. J. Pathol. 150:1861-1868. [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamadzadeh, M., A. N. Poltorak, P. R. Bergstressor, B. Beutler, and A. Takashima. 1996. Dendritic cells produce macrophage inflammatory protein-1 gamma, a new member of the CC chemokine family. J. Immunol. 156:3102-3106. [PubMed] [Google Scholar]
- 30.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 31.Müller, K., S. Ehlers, W. Solbach, and T. Laskay. 2001. Novel multi-probe RNase protection assay (RPA) sets for the detection of murine chemokine gene expression, J. Immunol. Methods 249:155-165. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, K. M., W. Ouyang, J. D. Farrar, J. Yang, S. Ranganath, H. Asnagli, M. Afkarian, and T. L. Murphy. 2000. Signaling and transcription in T helper development. Annu. Rev. Immunol. 18:451-494. [DOI] [PubMed] [Google Scholar]
- 33.Nagai, S., S. Hashimoto, T. Yamashita, N. Toyoda, T. Satoh, T. Suzuki, and K. Matsushima. 2001. Comprehensive gene expression profile of human activated T(h)1- and T(h)2-polarized cells. Int. Immunol. 13:367-376. [DOI] [PubMed] [Google Scholar]
- 34.Penna, G., M. Vulcano, A. Roncari, F. Facchetti, S. Sozzani, and L. Adorini. 2002. Cutting edge: differential chemokine production by myeloid and plasmacytoid dendritic cells. J. Immunol. 169:6673-6676. [DOI] [PubMed] [Google Scholar]
- 35.Randolph, D. A., G. Huang, C. J. Carruthers, L. E. Bromley, and D. D. Chaplin. 1997. The role of CCR7 in Th1 and Th2 cell localization and delivery of B cell help in vivo. Science 286:2159-2162. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 1997. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 277:2005-2007. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto, F., E. Kremmer, B. Palermo, A. Hoy, P. Ponath, S. Qin, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 29:2037-2045. [DOI] [PubMed] [Google Scholar]
- 39.Schrum, S., P. Probst, B. Fleischer, and P. F. Zipfel. 1996. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J. Immunol. 157:3598-3604. [PubMed] [Google Scholar]
- 40.Scott, P., P. Natovitz, and A. Sher. 1986. B lymphocytes are required for the generation of T cells that mediate healing of cutaneous leishmaniasis. J. Immunol. 137:1017-1021. [PubMed] [Google Scholar]
- 41.Sher, A., and R. L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385-409. [DOI] [PubMed] [Google Scholar]
- 42.Siveke, J. T., and A. Hamann. 1998. T helper 1 and T helper 2 cells respond differentially to chemokines. J. Immunol. 160:550-554. [PubMed] [Google Scholar]
- 43.Solbach, W., K. Forberg, E. Kammerer, C. Bogdan, and M. Röllinghoff. 1986. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J. Immunol. 137:702-707. [PubMed] [Google Scholar]
- 44.Solbach, W., and T. Laskay. 2000. The host response to Leishmania infection. Adv. Immunol. 74:275-317. [DOI] [PubMed] [Google Scholar]
- 45.Soto, H., W. Wang, R. M. Strieter, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, J. Hedrick, and A. Zlotnik. 1998. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc. Natl. Acad. Sci. USA 95:8205-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, S. D., P. R. Burd, P. R. Billings, C. A. Martin, and M. E. Dorf. 1988. The expression and regulation of a potential lymphokine gene (TCA3) in CD4 and CD8 T cell clones. J. Immunol. 141:1563-1570. [PubMed] [Google Scholar]
- 47.Zhang, S., N. W. Lukacs, V. A. Lawless, S. L. Kunkel, and M. H. Kaplan. 2000. Differential expression of chemokines in Th1 and Th2 cells is dependent on Stat6 but not Stat4. J. Immunol. 165:10-14. [DOI] [PubMed] [Google Scholar]
- 48.Zingoni, A., H. Soto, J. A. Hedrick, A. Stoppacciaro, C. T. Storlazzi, F. Sinigaglia, D. D'Ambrosio, A. O'Garra, D. Robinson, M. Rocchi, A. Santoni, A. Zlotnik, and M. Napolitano. 1998. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J. Immunol. 161:547-551. [PubMed] [Google Scholar]