Abstract
CD80 and CD86 (B7-1 and B7-2) are the ligands on antigen-presenting cells (APCs) which bind CD28 and deliver the costimulatory signals necessary for T cell activation. The reasons for the existence of two CD28 binding molecules are not well understood. We created a mutant version of CTLA4-Ig that could selectively bind CD80 and block CD28-CD80 interaction but leave CD28-CD86 binding intact. CD80 blockade prevented antigen-induced accumulation of eosinophils and lymphocytes in the lung of immunized mice, but did not block antigen induced systemic blood eosinophilia or IgE antibody production. No preferential expression of CD80 could be demonstrated on a population of lung APC consisting mainly of macrophages. These results indicate that CD80 costimulation is not necessary for the induction of Th2 immune responses but rather for the maintenance or amplification of lung inflammatory responses.
Two APC surface molecules, CD80 and CD86, have been identified as ligands for CD28 and CTLA-4 on T cells. Although CD80 and CD86 only share 26% amino acid homology and bind CD28 utilizing overlapping but distinct binding determinants, they bind with similar avidities and both provide potent costimulatory function for T cells (1–7). It is currently controversial whether the signals provided to the T cells upon interaction of CD80 and CD86 with CD28 are qualitatively different, and lead to the development of functionally distinct types of T cells (8– 12). In this context CD80 and CD86 have quite distinct intracellular domains (1, 2), which raises the possibility that CD80 or CD86 may also deliver different signals to the APC during cognate interaction.
Recent studies offer evidence of differential roles for CD80 and CD86 during in vivo immune responses. Many of these suggest that it is not the ability to mount an immune response which is altered by blockade of either CD80 or CD86, but that instead the outcome of the immune response may differ. Kuchroo et al. (8) found that in a model of experimental allergic encephalomyelitis (EAE), where disease is abrogated by the development of Th2 cells and exacerbated by a Th1 immune response, anti-CD80 treatment reduced disease incidence while anti-CD86 increased disease severity. In apparent contrast to these findings Lenschow et al. (9) found that in a model of autoimmune diabetes (a disease also mediated by Th1 cells) anti-CD80 increased and accelerated disease incidence while antiCD86 blocked the development of disease. However, both studies suggest that CD80 and CD86 may act by influencing the commitment of T cells to a Th1 or Th2 phenotype. Further support of a differential role for CD80 and CD86 comes from studies where treatment of mice with antiCD80 F(ab′) but not anti-CD86 mAb prevented clinical relapse and epitope spreading in EAE (10), and from a model of murine lupus where auto-Ab production was preferentially dependent on CD86 costimulation (11). Some in vitro studies also support a role for CD86 costimulation in the development of Th2 cells (12).
While CD28 is expressed by all murine peripheral T cells, CTLA-4 only appears after T cell activation (13, 14) and binds CD80 and CD86 with much higher avidity than CD28 (7). A soluble form of CTLA-4, CTLA4-Ig, has been developed and found to be a highly effective antagonist of CD28-CD80/CD86 interactions (6, 15, 16). We have used a model of Th2-dependent Ag-induced airway eosinophilia to show that eosinophil recruitment and Ab production are completely abrogated by the expression of transgenic mCTLA4-Hγ1 (16a), thus Th2 cell effector functions are totally dependent on CD28-mediated costimulation in this system. To investigate the role of individual CTLA-4 ligands we created a mutant form of CTLA4-Ig, termed Y100F-Ig, which binds to CD80 but not CD86. We used Y100F-Ig as a selective antagonist to define the role of CD80 costimulation in Ag-induced airway eosinophilia.
Materials and Methods
Reagents.
Y100F-Ig is a mutant human CTLA4-Ig in which tyrosine at position 100 is substituted with phenylalanine. The molecule was constructed by PCR using oligonucleotide-directed mutagenesis (17). CTLA4-Ig and Y100F-Ig were purified from culture media of stably transfected Chinese hamster ovary (CHO) cells.
FACS® Analysis.
Analysis of CTLA4-Ig and Y100F-Ig binding to CHO cells stably transfected with murine CD80 or CD86 was carried out by incubating cells with CTLA4-Ig or Y100F-Ig for 2 h at 23°C then staining with FITC-conjugated goat anti– human IgG. Binding was analyzed on a FACScan® (Becton Dickinson, Mountain View, CA). Mean fluorescence intensity was determined from data histograms using PC-LYSYS. Analysis of BAL macrophages was carried out by staining cells in 96–well round bottom plates at 105–6/well for 10–15 min on ice using anti-CD80-FITC or anti-CD86-PE (PharMingen, San Diego, CA) appropriately diluted in PBS + 2% FCS. 2.4G2 (10 μg/ml) was used to inhibit FcγRII-mediated uptake. Flow cytometric analysis was performed on a FACSort® (Becton Dickinson) using the CellQuest software. 10,000 live gated cells, as identified by Propidium Iodide dye exclusion, were analyzed and macrophages gated for by size and granularity.
Mice.
C57BL/6J mice were bred and maintained at the Animal Facility of the Wellington School of Medicine. All animal experimental procedures used in this study were approved by the Wellington School of Medicine Animal Ethics Committee, and carried out in accordance with the guidelines of the University of Otago (New Zealand).
OVA-induced Airway Inflammation.
Mice were primed i.p. with 2 μg OVA (Sigma Chem. Co., St. Louis, MO) in 100 μl alum adjuvant (SERVA, Heidelberg, Germany) on day 0 and boosted i.p. with 2 μg OVA/alum at day 10. 4 d after the last i.p. immunization mice were anaesthetized by injection of a mixture of ketamine and xylazine (Phoenix, Auckland, New Zealand), and 100 μg OVA in a 50-μl volume of PBS was administered by intranasal inoculation. 4 d later mice were killed, the trachea cannulated, and a BAL performed by flushing lung and airways three times with 1 ml PBS. BAL cells were counted, spun onto glass slides using a cytospin (Shandon Southern Products Ltd., Astmoor, UK), and stained with Diff-Quik (Dade, Diagnostics, Panmure, Auckland) according to the manufacturer's instructions. Percentages of macrophages, lymphocytes, neutrophils and eosinophils were determined microscopically using standard histological criteria.
ELISA.
Polyvinyl chloride 96-well plates (Nunc, Roskilde, Denmark) were coated overnight at +4°C with OVA (10 μg/ well) and blocked with 10% BSA in PBS for 60 min at room temperature (RT). Twofold dilutions of serum were added and incubated for 2 h at RT. Appropriate dilutions of detecting Ab and then peroxidase-labeled streptavidin (Sigma) were added for 1 h at RT. The isotype-specific anti-mouse IgG1 was from Serotec (Oxford, UK), anti-mouse IgG2a was from PharMingen, the 3-11 monoclonal anti-mouse IgE was generously provided by Dr. Christoph Heusser (Ciba, Basel, Switzerland). 100 μl of freshly made 1 mM ABTS (Sigma) in citrate phosphate buffer, pH 9.2, and 0.03% H2O2 was added to each well to develop the reaction. The reaction was stopped by adding 100 μl 2 mM NaAzide and the plates read at 414 nm using an Anthos Hill (Salzburg, Austria) plate reader. Ab titers are expressed in Units/ ml (reciprocal of 50% Ab titer).
Results
Y100F-Ig Binds to CD80 and not CD86.
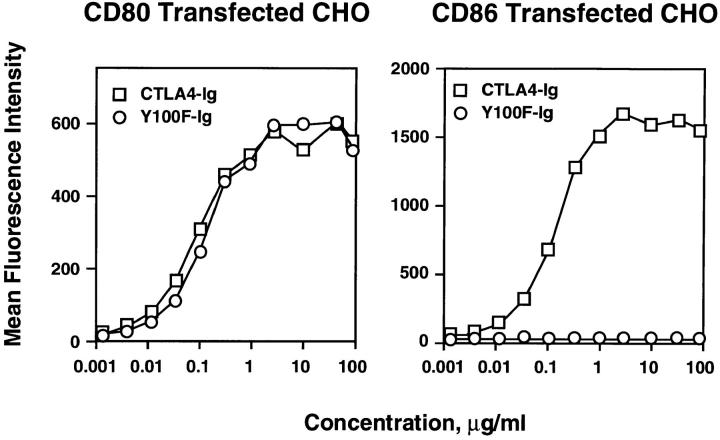
We have previously shown that amino acid residues in the conserved MYPPPY motif of CTLA4-Ig are critical for the binding of CTLA4-Ig to CD80 (17). Mutation of the first tyrosine in this motif (Tyr 100) to alanine resulted in reduced binding to CD80 but abolished binding to CD86 (5). However, mutation of Tyr 100 to phenylalanine resulted in a molecule (Y100F-Ig), which retained wild-type binding to CD80, but with no apparent binding to CD86. As shown in Fig. 1, FACS® analysis demonstrated that Y100F-Ig and CTLA4-Ig bind equally well to CHO cells expressing murine CD80. In contrast, binding of Y100F-Ig to CHO cells expressing murine CD86 was undetectable even at concentrations as high as 100 μg/ml. These data confirm that Y100F-Ig can be used to selectively block CD80-mediated costimulation to T cells.
Figure 1.
Y100F-Ig binds to murine CD80 but not CD86. Y100F-Ig (circles) and wild-type CTLA4-Ig (squares) were incubated with Chinese Hamster Ovary (CHO) cells stably transfected with murine CD80 or CD86 and binding determined by FACS® analysis.
Effect of Y100F-Ig on Lung and Blood Eosinophilia.
We used Y100F-Ig as a selective antagonist to define the role of CD80 costimulation in a model of Ag-induced airway eosinophilia. In this model mice are immunized twice i.p. with 2 μg OVA in alum adjuvant, and then challenged intranasally with OVA in soluble form. The subsequent Th2dependent lung inflammatory response is characterized by the presence of large numbers of eosinophils and lymphocytes in the BAL fluid, lung and airway tissue. OVA-specific IgE and IgG1 Abs are detected in the serum.
Mice treated with CTLA4-Ig, Y100F-Ig or the control molecule L6-Ig were subjected to the OVA airway immunization protocol. Treatment was continued throughout the experiment with i.p. injections of 400 μg CTLA4-Ig, Y100F-Ig or L6-Ig every 48 h beginning 48 h before the first OVA immunization. The serum levels of CTLA4-Ig and Y100F-Ig at the end of the experiment were 70–75 and 38–40 μg/ml, respectively.
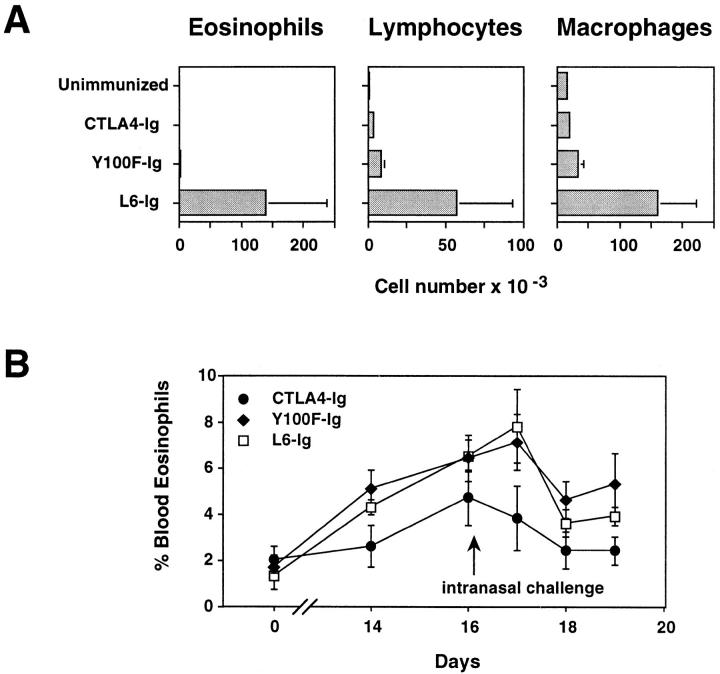
Eosinophil infiltration into the airways of OVA-immunized, airway challenged mice was blocked by treatment with either CTLA4-Ig or Y100F-Ig (Fig. 2 A). The numbers of lymphocytes in the BAL were also strongly diminished in these two groups compared to L6-Ig. Because in vivo injection of anti-CD4 or anti-IL-5 Ab before the intranasal challenge completely abrogates the appearance of eosinophils in the BAL (18), we interpreted the result in Fig. 2 A as suggesting that both CTLA-4 Ig and Y100F-Ig interfere with the generation of IL-5-producing Th2 cells. Indeed, CTLA4-Ig also inhibited the OVA-induced appearance of eosinophils in the blood (Fig. 2 B). This response precedes accumulation of eosinophils in the lung, and is also dependent on IL-5 production (19). Surprisingly, however, Y100F-Ig failed to inhibit blood eosinophilia, with levels being comparable in Y100F-Ig– and L6Ig–treated mice (Fig. 2 B). Thus, CTLA4-Ig and Y100F-Ig have different effects on systemic eosinophilia. CTLA4-Ig most likely acts on IL-5 secretion by T cells, and therefore blocks all eosinophil responses. The effects of Y100F-Ig are more selective in that it prevents the accumulation of eosinophils in the lung without blocking systemic IL-5 secretion.
Figure 2.
Treatment with Y100F-Ig inhibits lung (A) but not blood (B) eosinophilia in OVA-immunized, airway-challenged mice. C57BL/6 mice were primed twice i.p. with 2 μg OVA in alum adjuvant on day 0 and day 10 then given an intranasal challenge with 100 μg OVA in PBS 4 d after the last i.p. immunization. BAL fluid was collected 4 d after intranasal challenge. Differential cell counts were made on BAL cytospins and blood smears stained with Diff-Quik. Mice were treated i.p. with 400 μg of either CTLA4-Ig, Y100F-Ig or L6-Ig every 48 h. Values represent the mean ± SE for groups of 5–7 mice. Results shown are representative of four (A) and two (B) experiments.
Effect of Y100F-Ig on Ab Production.
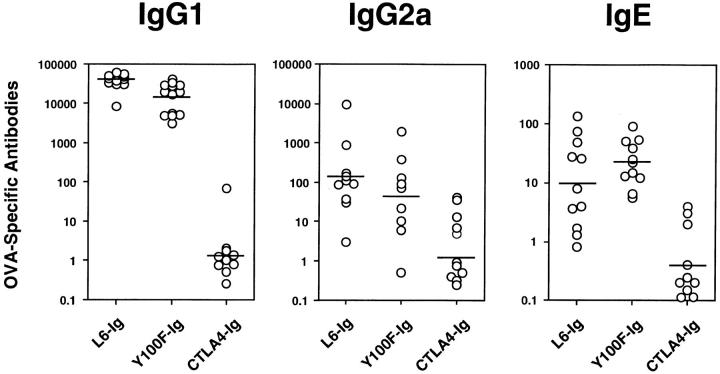
To determine the effect of CD80 costimulation on Ab production OVA-specific IgG and IgE titers were measured in CTLA4-Ig–, Y100F-Ig–, and L6-Ig–treated mice. As expected all Ab isotypes were lowered by CTLA4-Ig treatment (Fig. 3). Levels of IgG1, IgG2a and IgE were similar in Y100F-Ig– and L6-Ig–treated mice (Fig. 3). This result indicates that OVA-specific T cells that can deliver help to B cells have been generated in Y100F-Ig–treated mice. These T cells must be capable of IL-4 secretion in vivo since the development of an Ag-specific IgE response has been shown to be entirely dependent upon production of IL-4 (20, 21). The high levels of IgG1 and IgE, and low levels of IgG2a in Y100F-Ig– and L6-Ig–treated mice (Fig. 3) indicated that both groups generated a good Th2 response but little or no Th1 response.
Figure 3.
Treatment with CTLA4-Ig, but not Y100F-Ig, inhibits the production of OVAspecific IgG1, IgG2a and IgE. Mice were treated with CTLA4Ig, Y100F-Ig or L6-Ig and subjected to a protocol of OVA immunization and airway challenge as detailed in Fig. 2. Serum was collected at the time of death and OVA-specific antibody levels determined by ELISA. Values represent serum antibody titers for individual mice. Shown are the results of two pooled experiments.
Expression of CD80 and CD86 in the Lung.
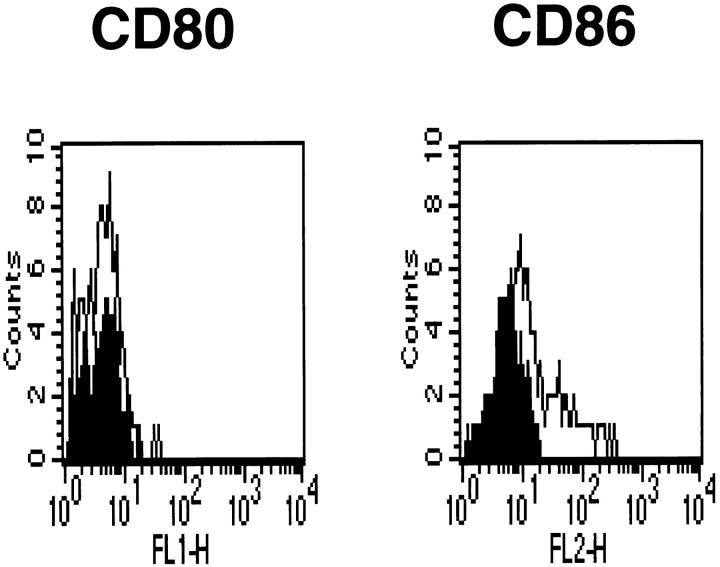
It was possible that T cells capable of secreting both IL-4 and IL-5 could accumulate in the lung of Y100F-Ig–treated mice, but that once in the lung their further activation was blocked because of the preferential expression of CD80 ligands by local APC. This could lead to termination of the response in the lung due to lack of production of T cell– derived cytokines and chemotactic factors necessary for the amplification of the response. To address this we stained lung macrophages, isolated from OVA-immunized and airway challenged mice, with Abs to CD80 and CD86. Although CD80 was not detectable by FACS analysis, staining for CD86 was observed (Fig. 4). Other lung resident APC, such as dendritic cells, are also reported to express both CD80 and CD86 (22). Together, these results rule out the possibility that Y100F-Ig treatment can selectively block the activation of T cell responses because of selective expression of CD80 ligands on local APC. This system differs from another disease model in which CD80 was the predominant costimulatory molecule expressed at the site of inflammation and in which CD80 blockade was effective (10).
Figure 4.
CD86 is expressed on lung macrophages from OVA-immunized and airway-challenged mice. BAL cells were collected from mice subjected to a protocol of OVA immunization and airway challenge as detailed in Fig. 2 and CD80/CD86 expression determined by FACS® analysis. Filled histogram, non-stained samples; empty histogram, samples stained as indicated.
Discussion
The simultaneous blockade of CD80 and CD86 causes complete inhibition of most T cell responses, either by preventing T cell activation altogether, or by inhibiting T cell–dependent recruitment of effector immune responses (15, 16, 23–25). Individually, the two molecules appear to play largely redundant roles (25–27) although the blockade of either CD80 or CD86 has been reported to cause immune deviation in some experimental systems (8, 9, 11, 12). With the present study we propose that additional mechanisms exist by which the blockade of CD80/CD86mediated costimulation causes inhibition of immune responses. Using an OVA airway immunization protocol, we observed that selective CD80 blockade had no effect on the induction of systemic Th2-type immune responses but strikingly inhibited the infiltration of eosinophils, and to a lesser extent lymphocytes, into the lungs and airways of intranasally challenged mice. This was not due to induction of immune deviation by Y100F-Ig treatment, as other Th2-type responses, e.g., blood eosinophilia and IgE production, were not affected by Y100F-Ig treatment. Evidence of Th1 responses could not be found in Y100FIg–treated mice as titers of OVA-specific IgG2a were not increased in comparison to L6-Ig–treated controls (Fig. 3).
Failure to observe BAL eosinophilia in Y100F-Ig–treated mice was not due to differences in the kinetics of the response, as different time points after intranasal inoculation were analyzed, nor to the use of a limiting amount of antigen in the intranasal challenge. Previous studies (Harris, N., unpublished observation) had shown that intranasal inoculation of 100-fold less OVA than used in this study was still sufficient to induce a maximum eosinophil response. Also, intranasal Ag challenge was followed by comparable increases in blood eosinophilia in Y100F-Ig– and L6-Ig–treated mice, again suggesting that a sufficient amount of Ag was used. It is possible that CD80 blockade prevents acquisition by T cells of the adhesion molecules involved in recirculation through tissues, or the upregulation of their ligands on endothelium (28). We think this is rather unlikely, as T cells could be recovered from the lung of Y100F-Ig–treated mice, and these cells did produce IL-5 upon in vitro restimulation with anti-CD3 (data not shown). A perhaps more likely possibility is that in nonlymphoid tissues the amount of T cell costimulatory signals in the form of CD86 may be limiting, leading to the need for further signaling provided by CD80. The later appearance of CD80 would serve to prolong or increase the intensity of costimulatory signals delivered by the APC to the T cell, leading to the maintenance and amplification of the local inflammatory response. In this regard, a higher costimulatory threshold may be preferred in tissues to prevent injury caused by inappropriate immune activation.
Migration of different cell populations to the lung is under the control of a number of factors (29–31) whose regulation and function are still largely undetermined. Definition of the precise consequences of CD80 blockade in this context is therefore not possible at this stage. However, it is clear from the present study how the blockade of individual T cell costimulatory signals can have complex effects on T cell responses, and may lead to striking differences in responses in tissues.
Acknowledgments
We are grateful to the Personnel of the Animal Facility of the Wellington School of Medicine for Animal Husbandry, and to Julie Rodgers for her excellent technical assistance.
Footnotes
This work was supported by Grants from the Wellington Medical Research Foundation, the New Zealand Lottery Board, the Wellcome Trust and the Health Research Council of New Zealand. N. Harris is recipient of an Otago University Ph.D. Scholarship. G. Le Gros is a recipient of a Wellcome Trust Senior Research Fellowship.
The first two authors contributed equally to this work.
References
- 1.Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature (Lond) 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo V, Jr, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science (Wash DC) 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 3.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G, McKnight AJ, Kim J, Du L, Lombard DB, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science (Wash DC) 1993;262:907–909. doi: 10.1126/science.7694362. [DOI] [PubMed] [Google Scholar]
- 4.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science (Wash DC) 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 8.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 9.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dalcanto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima A, Azuma M, Kodera S, Nuriya S, Terashi A, Abe M, Hirose S, Shirai T, Yagita H, Okumura K. Preferential dependence of autoantibody production in murine lupus on CD86 costimulatory molecule. Eur J Immunol. 1995;25:3060–3069. doi: 10.1002/eji.1830251112. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 13.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 14.Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science (Wash DC) 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 16.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science (Wash DC) 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 16a.Harris, N., C. Campbell, G. Le Gros, and F. Ronchese. 1996. Blockade of CD28/B7 co-stimulation by mCTLA4Hγ1 inhibits antigen-induced lung eosinophilia but not Th2 cell development or recruitment in the lung. Eur. J. Immunol. In press. [DOI] [PubMed]
- 17.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima H, Iwamoto I, Tomoe S, Matsumura R, Tomioka H, Takatsu K, Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992;144:374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 19.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Airways hyperreactivity and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn R, Rajewsky K, Muller W. Generation and analysis of IL-4 deficient mice. Science (Wash DC) 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Le Gros G, Bachmann M, Lamers MC, Blüthmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (Lond) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 22.Masten, B.J., J.L. Yates, A.M. Pollard, and M.F. Lipscomb. 1995. Characterization of accessory molecules important for lung dendritic cell function: evidence for a third CTLA4 ligand. Proc. Int. Congr. Immunol. 389(Abstr.).
- 23.Ronchese F, Hausmann B, Hubele S, Lane P. Mice transgenic for a soluble form of murine CTLA-4 show enhanced expansion of antigen-specific CD4+ T cells and defective antibody production in vivo. J Exp Med. 1994;179:809–817. doi: 10.1084/jem.179.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science (Wash DC) 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 25.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 26.Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 28.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baggiolini M, Dahinden C. CC chemochines in allergic inflammation. Immunol Today. 1994;15:127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 30.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 31.Gonzalo J-A, Jia G-Q, Aguirre V, Friend D, Coyle AJ, Jenkins NA, Lin G-S, Katz H, Lichtman A, Copeland N, et al. Mouse eotaxin expression parallels eosinophil accumulation during lung allergic inflammation but it is not restricted to a Th2-type response. Immunity. 1996;4:1–14. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]