Abstract
An important checkpoint in early thymocyte development ensures that only thymocytes with an in-frame T cell receptor for antigen β (TCR-β) gene rearrangement will continue to mature. Proper assembly of the TCR-β chain into the pre-TCR complex delivers signals through the src-family protein tyrosine kinase p56lck that stimulate thymocyte proliferation and differentiation to the CD4+CD8+ stage. However, the biochemical mechanisms governing p56lck activation remain poorly understood. In more mature thymocytes, p56lck is associated with the cytoplasmic domain of the TCR coreceptors CD4 and CD8, and cross-linking of CD4 leads to p56lck activation. To study the effect of synchronously inducing p56lck activation in immature CD4−CD8− thymocytes, we generated mice expressing a CD4 transgene in Rag2−/− thymocytes. Remarkably, without further experimental manipulation, the CD4 transgene drives maturation of Rag2−/− thymocytes in vivo. We show that this process is dependent upon the ability of the CD4 transgene to bind Lck and on the expression of MHC class II molecules. Together these results indicate that binding of MHC class II molecules to CD4 can deliver a biologically relevant, Lck-dependent activation signal to thymocytes in the absence of the TCR-α or -β chain.
In mammals, circulating T lymphocytes derive from bone marrow progenitors that mature within the thymus. This maturation process involves both replicative expansion and differentiation, and yields progeny cells expressing a diverse repertoire of clonally-distributed TCRs specific for antigenic peptides bound to MHC molecules. Signals derived from non-receptor protein tyrosine kinases direct the highlyregulated development of thymocyte progenitors (1), which can only take place in close approximation with thymic stromal elements (2).
Immature hematopoietic progenitors arriving in the thymus contain TCR genes in germline configuration, lack both CD4 and CD8 on their cell surfaces, and express only very low levels of the TCR-associated CD3γ, δ, and ε polypeptides. Thereafter, in-frame gene rearrangement at the TCR-β locus leads to expression of a functional TCR-β polypeptide, which is presented at the cell surface in association with the recently described pTα chain (3) and the CD3γ, δ, and ε molecules (4, 5). This complex, known as the pre-T cell receptor (pre-TCR), delivers signals that block further rearrangements at the TCR-β locus, while stimulating both proliferation and the onset of rearrangements at the complementary TCR-α locus (6–8). In addition, the pre-TCR-derived signal leads to simultaneous expression of CD4 and CD8 on the surfaces of developing thymocytes. Subsequent gene rearrangement at the TCR-α locus permits assembly of the mature αβ TCR, which can thereafter deliver signals that trigger the differentiation of functional T lymphocytes, cells which express either CD4 or CD8 in a mutually exclusive fashion (9).
The signaling circuits that link the pre-TCR to appropriate cellular responses remain incompletely elucidated. However, experiments in genetically manipulated mice strongly implicate the src-family protein tyrosine kinase p56lck in the pre-TCR signal transduction pathway (10). In thymocytes from mice lacking expression of CD3ε (11), pTα (12), or TCR-β (7), failure to assemble the pre-TCR results in arrested thymocyte development at a very immature CD4−8− (double-negative or DN1) stage. An analogous phenotype obtains in thymocytes of transgenic mice expressing high levels of a catalytically inactive Lck protein (13) and in mice bearing a targeted disruption of the lck gene (14), although some thymocyte differentiation does occur in the latter, likely reflecting the ability of other, related kinases to substitute in the absence of Lck (15, 16). Importantly, expression of a mutant, activated Lck kinase is sufficient, by itself, to drive both the proliferative expansion and the differentiation of thymocytes made incapable of expressing the pre-TCR. For example, an activated-lck transgene drives thymocyte development in Rag−/−thymocytes, which cannot rearrange their TCR gene loci and therefore fail to make a TCR-β chain (17). Together these experiments strongly support a model in which assembly of the pre-TCR permits, either through binding of an external ligand or through some allosteric alteration, the activation of p56lck, which then directs thymocyte maturation to the CD4+8+ (double-positive or DP) stage.
While the genetic evidence favoring a role for Lck in signaling from the pre-TCR is persuasive, the biochemical effectors upon which Lck acts, as well as the mechanisms of Lck activation, remain unknown. In more mature thymocytes and T cells, p56lck interacts physically and functionally with the TCR coreceptors, CD4 and CD8, by virtue of a sequence motif centered around cysteines 20 and 23 that binds a complementary motif in the cytoplasmic tails of CD4 and CD8 (18). Contemporary models of TCR signaling posit that CD4 and CD8 act to deliver Lck to the antigen receptor complex at the time of ligand binding, permitting activation of Lck, which then acts to phosphorylate specialized tyrosine-containing motifs (ITAMs) in the CD3γ, δ, and ε chains and in the TCR ζ polypeptides, the latter found principally as homodimers associated with the TCR complex (19). This phosphorylation permits binding of the Zap-70 protein tyrosine kinase to the phosphorylated ITAMs, where it too becomes a target for Lck-mediated phosphorylation (19, 20). Interestingly, antibody-mediated cross-linking of CD4 stimulates Lck kinase activity in murine thymocytes (21) and T cell clones (22), suggesting that binding of CD4 to MHC class II polypeptides under physiologic conditions may, by itself, stimulate Lck activity.
Dissection of the biochemical pathways linking Lck to changes in thymocyte maturation requires a means of directly stimulating Lck activity in vivo. Here we report that expression of a CD4 transgene in Rag2−/− mice has this effect, directing thymocyte development in a manner that mimics signaling from the pre-TCR. Remarkably, this process is dependent upon the expression of MHC class II molecules, and hence represents a ligand-stimulated signaling process mediated by CD4. While our experiments leave no doubt as to the ability of CD4 to behave as a receptor in its own right, we also demonstrate that faithful delivery of the CD4/Lck-derived signal requires the simultaneous presence of CD3 components of the pre-TCR complex.
Materials and Methods
Mice.
All Rag2−/− mice were housed in a specific pathogenfree (SPF) facility and analyzed at 3–6 wk of age. Transgenic lines were derived by microinjection into DBA/2 × C57BL6/J zygotes as described (23). The 727 CD4 transgenic mouse line has previously been described (24). A single line of mice transgenic for tailless CD4 was generated using the previously described construct (25). Transgene-bearing mice for each of these lines were mated with Rag2−/− mice (26), and their progeny intercrossed to obtain transgene expression in homozygous Rag2−/− mice. CD4Tg+(CD4 transgenic) Rag2−/− mice were further crossed with MHC class II−/− mice (27). CD4Tg+ mice were also crossed with CD3ε−/− mice (11). PCR analysis was employed to detect the presence of the transgenes using the following pairs of forward and reverse primers:
CD4 and tailless CD4 (CD4Δ) transgenes:
5′ GTCAGGAGCTTGAATCCCACGATTCGGGGATCATCTAGAC 3′ (specific for the junction of proximal lck promoter sequences with polylinker sequence in the lck-CD4 transgene [24])
5′ CCACAACTCCACCTCCTCTTTCCT 3′ (corresponding to nucleotides 518-541 of the murine CD4 cDNA [28])
Rag2:
5′ CCAGCTGATAACCACCCACAATAA 3′
5′ GTATAGTCGAGGGAAAAGCATGGG 3′
Neomycin cassette in gene targeting constructs:
5′ CAGGATCTCCTGTCATCTCACCTT 3′
5′ TCAGAAGAACTCGTCAAGAAGGCG 3′
I-Ab endogenous locus:
5′ CATTTCGTGTACCAGTTCATGGGC 3′
5′ CTCGTAGTTGTGTCTGCACACCGTGTC 3′
I-Ab gene targeting construct:
5′ TACATCTACAACCGGGAGGAGTAC 3′
Neomycin cassette forward primer
CD3ε:
5′ TCAGGAACCAGTGTAGAGTTGACG 3′
5′ GTCCACCTCCACACAGTACTCACA 3
Flow Cytometric Analysis.
Isolated thymocytes were prepared by disaggregation through a wire mesh, and stained with saturating levels of mAb at 4°C in 1% FCS in PBS. Cells were counted using a hemocytometer and ∼106 thymocytes were stained with combinations of the following murine-specific mAb: Anti-CD4 PE (CT-CD4) and anti-CD8α (CT-CD8α) from Caltag Laboratories (San Francisco, CA); anti-CD25PE (M-A251), anti-CD8α PE (53-6.7), biotinylated anti-I-Ab (AF6-120.1), biotinylated anti-CD3ε (145-2C11), and biotinylated anti-CD69 (H1.2F3) from PharMingen (San Diego, CA). For detection of biotinylated reagents, cells were further stained with tri-color conjugated streptavidin (Caltag Laboratories). After staining, cells were fixed in 2% paraformaldehyde, pH 7.4. 20,000 live lymphocyte-gated events were collected for each analysis in list mode files on a FACScan® flow cytometer (Becton Dickinson) using Cell Quest software, and analyzed using Reproman software (Truefacts Software, Seattle, WA).
Antibody Treatment of Mice.
4-wk-old mice were injected intraperitoneally with 100 μg of anti-CD3ε (145-2C11), or 20-500 μg of anti-CD4 mAb (GK1.5) diluted in 300 μl of PBS. The mAb were affinity-purified on protein A or G Sepharose, respectively. Mice were typically analyzed 4 d after mAb treatment. CD69 induction was measured 20 h after intraperitoneal injection of 150 μg of 145-2C11 or GK1.5 mAb.
Results
CD4 Transgene Promotes Thymocyte Development in Rag2−/− Mice.
To determine whether we could effect CD4-mediated p56lck activation in pre-T cells in vivo, a CD4 minigene under the control of the lck proximal promoter (24) was placed on a Rag2−/− background. By flow cytometry, CD4Tg+ Rag2−/− thymocytes express ∼10-fold higher levels of CD4 on their surfaces as compared with those found on more mature, wild-type CD4+ thymocytes (Fig. 1). An effect of the CD4 transgene was readily apparent: thymuses from the CD4 transgenic animals were larger than those of Rag2−/− littermate controls, and showed a fivefold increase in cell number (∼1 × 107 vs. ∼2 × 106 thymocytes in Rag2−/− mice). Remarkably, the majority of thymocytes in CD4Tg-bearing Rag2−/− mice acquired surface expression of endogenously encoded CD8 (Fig. 1, top). These cells also lost expression of CD25 (Fig. 1, middle), and were smaller in size (Fig. 1, bottom), both phenotypes associated with the DN to DP transition in normal thymocytes (8). Together these results indicate that the simple expression of CD4 (albeit at high levels) in the DN compartment faithfully reproduces signals ordinarily associated with satisfactory assembly of the pre-TCR. This occurred without any measurable change in the surface representation of CD3 components (judged by flow cytometric analysis of CD3ε expression), and hence suggests that the presence of transgene-derived CD4 was directly responsible for this phenomenon.
Figure 1.
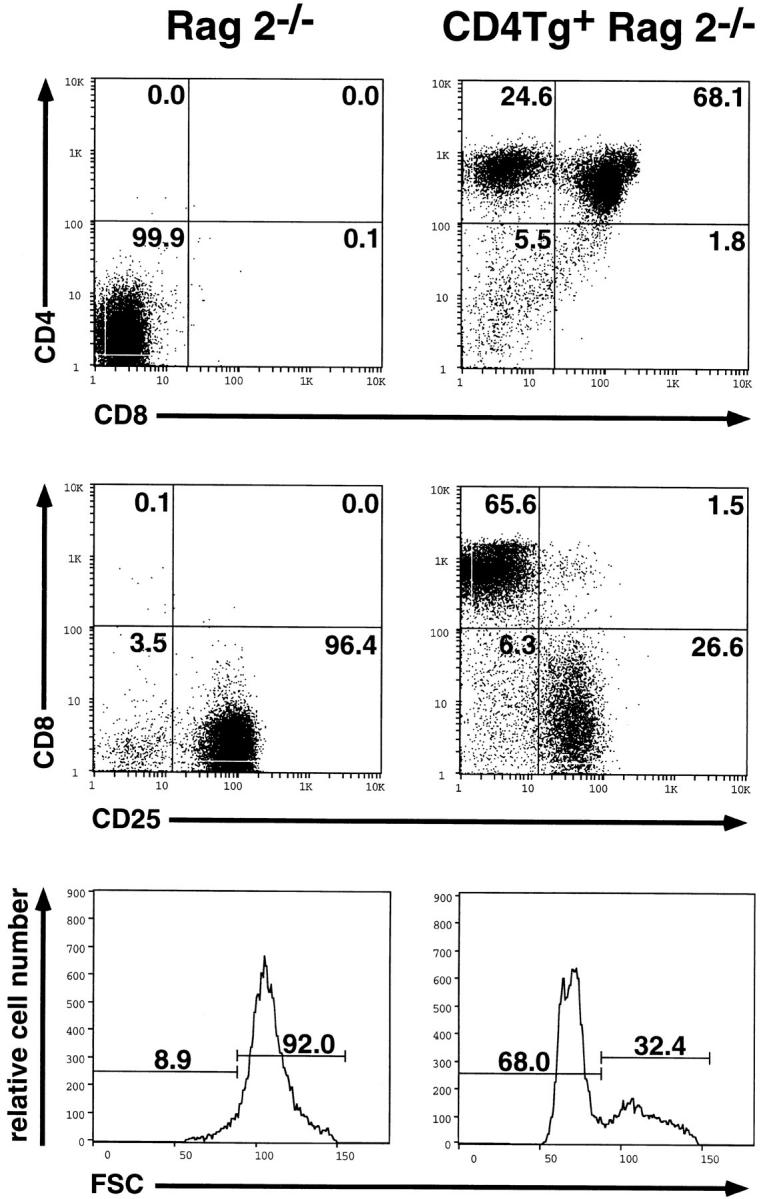
Flow cytometric analysis of thymocytes from Rag2−/− and CD4 transgenic Rag2−/− mice. Two parameter fluorescence histograms of lymphocyte gated events are shown after cell surface staining of thymocytes from age matched mice with anti-CD4-PE and anti-CD8-FITC (top), or anti-CD8-PE and anti-CD25-FITC (middle). Forward light scatter profiles (FSC) are also shown (bottom) as an indication of relative cell size. The percentage of cells in each population is indicated. Under identical staining conditions, wild-type CD4+ thymocytes lie at ∼100 units on the logarithmic scale.
CD4 Transgene-driven Maturation Requires the Lck-Interacting Region.
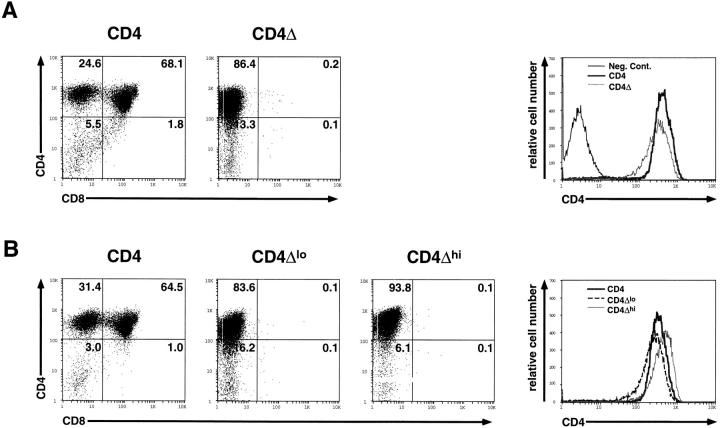
Because activated Lck (containing a phenylalanine-for-tyrosine substitution at position 505) can stimulate the DN to DP transition in Rag−/− thymocytes (17), and because Lck binds directly to CD4 (18), we reasoned that expression of transgene-derived CD4 in Rag2−/− thymocytes had permitted assembly of a signaling complex leading to Lck activation in vivo. This hypothesis predicts that the ability of CD4 to stimulate thymocyte development would depend upon its interaction with Lck. To test this conjecture, we generated an additional line of transgenic animals expressing a mutant CD4 transgene (CD4Δ) in which sequences encoding all but the eight most membrane-proximal cytoplasmic residues were truncated. Prior studies have demonstrated that this truncation yields a molecule that cannot interact with p56lck (25). Fig. 2 A shows that although the CD4Δ transgene was expressed under the lck proximal promoter at levels comparable to those achieved using the wild-type CD4 transgene, Rag2−/− thymocytes were not induced to mature by the presence of this mutant protein. Indeed, even when the CD4Δ transgenic mice were intercrossed in the effort to obtain progeny with increased expression of truncated CD4, no improvement in thymocyte maturation was noted (Fig. 2 B). We conclude that the CD4 cytoplasmic tail, almost certainly reflecting its ability to bind p56lck, is required to permit a CD4-derived signal to drive the DN to DP transition in thymocytes.
Figure 2.
Flow cytometric analysis of thymocytes from wild-type CD4Tg+ Rag2−/− and tailless CD4Δ Rag2−/− transgenic mice. (A) Representative fluorescence staining profiles of CD4 and CD8 are shown for thymocytes from age matched CD4Tg+ Rag2−/− mice (CD4, left panel) or tailless CD4 (CD4Δ, middle panel) Rag2−/− mice (n = 6). A single parameter histogram better shows the relative levels of CD4 expression (right panel) for these mice as compared to a transgene negative littermate control of CD4Δ (Neg. Cont.). (B) A similar immunofluorescense analysis is shown for thymocytes from a CD4Tg+ Rag2−/− mouse (left panel), and two of seven CD4Δ Rag2−/− positive progeny from a CD4Δ × CD4Δ mating (middle two panels). Two parameter fluorescence profiles are shown for wild-type CD4Tg+ and the mice expressing the lowest (CD4Δlo) and highest (CD4Δhi) level of CD4Δ in the litter. The relative levels of CD4 expression are again shown by a single parameter histogram (right panel). The percentage of cells in each population is indicated. While some CD4Δ Rag2−/− mice (7/21 analyzed) contained a very small fraction of CD8+CD25− thymocytes (1–2%), this did not correlate with the level of CD4Δ expression. The relative percentage of CD8+CD25− thymocytes in wild-type CD4 Rag 2−/−/CD4Δ Rag 2−/− mice was >50fold.
CD4 Transgene-driven Maturation Requires Expression of MHC Class II Molecules.
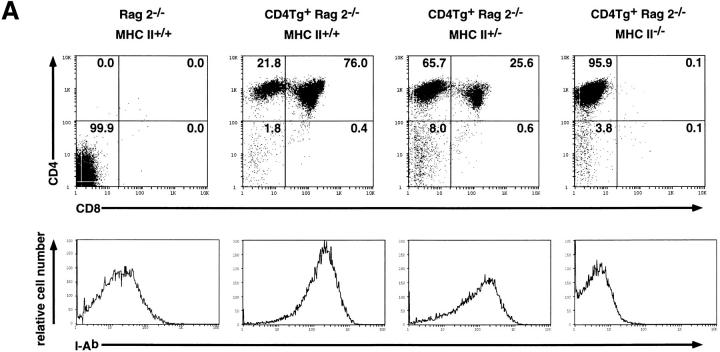
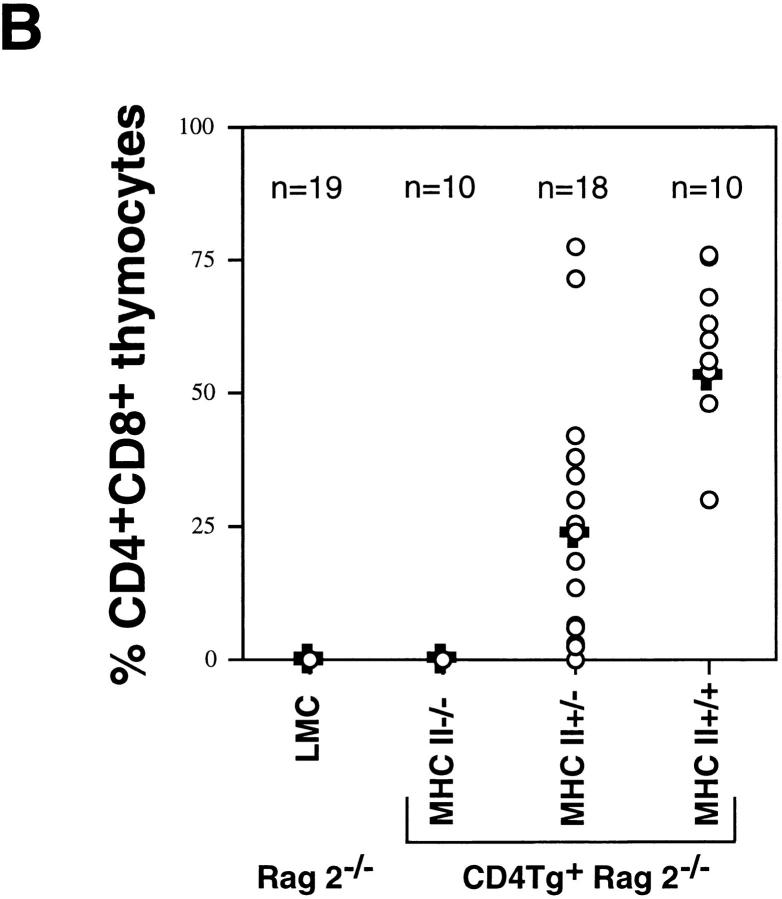
We next asked whether the presence of CD4 in immature thymocytes was by itself sufficient to stimulate maturation, or whether interaction with an MHC class II ligand was required. The extracellular domain of CD4 binds directly to the MHC class II β2 domain (29, 30), and it is well established that CD4 coreceptor function requires MHC class II binding in mature T cells (31). To determine whether CD4 transgene-driven thymocyte maturation in Rag2−/− mice is dependent upon MHC class II molecules, CD4Tg+ Rag2−/− mice were crossed with MHC class II−/− mice (generated through targeted disruption of the I-Aβ locus [27]). Rag2−/−, CD4Tg, and MHC class II−/− mice share the H-2bhaplotype, and therefore lack surface expression of MHC class II I-E proteins due to a pre-existing mutation in the Eα gene promoter (32). As shown in Fig. 3 A, in the absence of MHC class II expression (right panel), the CD4 transgene no longer stimulates the acquisition of CD8 expression in Rag2−/− thymocytes. Indeed, in CD4Tg mice made simultaneously null for Rag2 and heterozygous for the disrupted I-Aβ allele, the representation of DP cells typically reached only 40% of that observed in those bearing two wild-type I-Aβ alleles (Fig. 3 A, middle panels and B). Hence the actual dose of MHC class II molecules available to bind CD4 appears to regulate propagation of a differentiative signal.
Figure 3.
Flow cytometric analysis of thymocytes from CD4Tg+ Rag2−/− mice mated onto an MHC class II−/− background. (A) Fluorescence staining profiles of CD4 and CD8 (top panels) or the MHC class II molecule I-Ab (bottom panels) of thymocytes from a CD4 transgene negative MHC class II wild-type control (Rag2 −/− MHC II +/+) and CD4 transgene positive littermates that are wild-type (CD4Tg + Rag2 −/− MHC II +/+), heterozygous (CD4Tg + Rag2 −/− MHC II +/−) or null (CD4Tg + Rag2 −/− MHC II −/−) for MHC class II expression. The percentage of cells in each population is indicated. Unlike thymi from CD4Tg+ Rag2−/− mice that were first analyzed (Fig. 1), thymi from the crosses of CD4Tg+ Rag2−/− mice with MHC class II−/− mice did not exhibit an increase in cell number relative to CD4 transgene negative littermates. This was observed even in CD4Tg+ Rag2−/− MHC class II+/+ progeny of MHC class II heterozygote crosses. This is likely due to nonspecific changes in genetic background. (B) Scatter graph of the percentage of CD4+CD8+ thymocytes from the progeny of CD4Tg+ Rag2−/− × MHC class II−/− matings. Each circle shows a data point from an individual mouse, with the mean represented by a bold plus sign (+). The number of mice analyzed for each type is indicated across the top of the graph. CD4 transgene negative littermate controls (LMC) included MHC class II null, heterozygous and wild-type mice.
In accord with this observation, the amount of stainable MHC class II protein on the surfaces of thymocytes was greatly increased by the presence of transgene-derived CD4 (Fig. 3 A, bottom). Thymocytes transgenic for the CD4Δ construct also showed increased staining for I-Ab (data not shown). Passive adsorption of shed class II molecules onto normal thymocytes has been previously demonstrated using allogeneic bone marrow chimeras (33). The increased I-Ab staining on CD4 transgenic thymocytes almost certainly reflects direct binding of shed class II molecules to this transgene-encoded class II receptor, supporting the notion that CD4 delivers an Lck activation signal in Rag2−/− thymocytes in response to binding of the agonist ligand, I-Ab. Murine thymocytes themselves do not synthesize significant amounts of MHC class II molecules (33, 34).
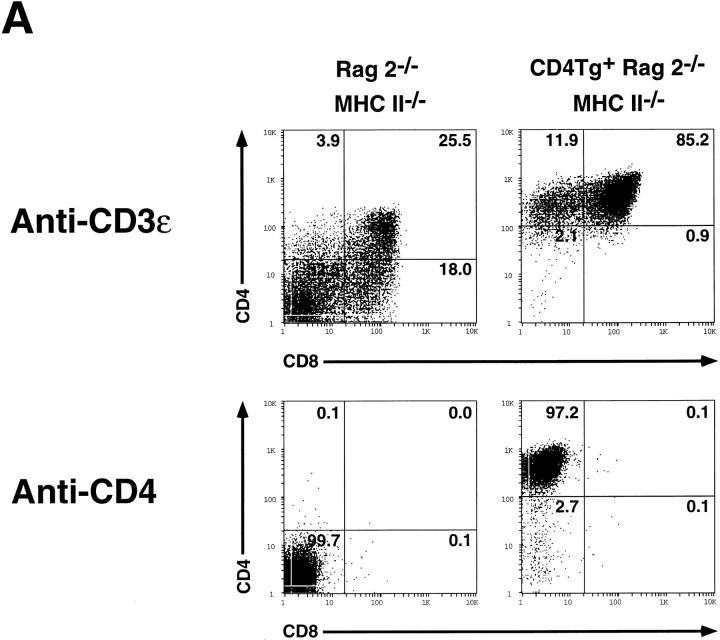
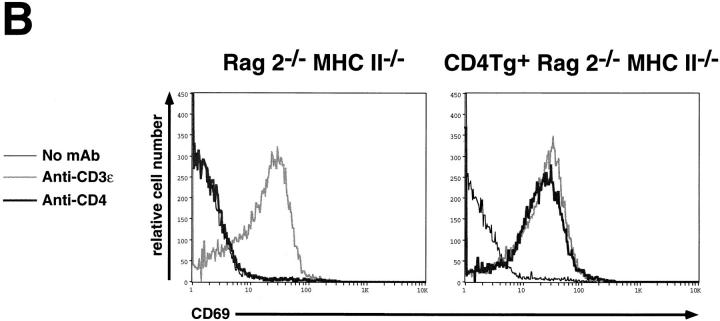
Previous studies demonstrate that administration of antiCD3 antibodies to Rag2−/− mice stimulates the DN to DP transition, an effect which depends upon the presence of Lck (35). Because administration of anti-CD4 antibodies to T cell clones (22) as well as murine thymocytes (21) stimulates Lck activity, and because expression of MHC class II molecules stimulates CD4-driven thymocyte maturation in Rag2−/− mice, we wished to determine whether anti-CD4 mAb treatment would similarly drive maturation of CD4Tg+ Rag2−/−MHC class II−/− thymocytes. Fig. 4 A demonstrates that anti-CD3 treatment effectively stimulates thymocyte maturation in Rag2−/− MHC class II−/− mice, irrespective of the presence of the CD4 transgene. In contrast, administration of the anti-CD4 mAb GK1.5 did not stimulate maturation of CD4Tg-bearing thymocytes. However, all CD4Tg+ Rag2−/− MHC class II−/− thymocytes clearly are capable of receiving a GK1.5 stimulated signal: GK1.5 or anti-CD3 induce comparable surface expression of the activation marker CD69 (36) within 24 h of mAb treatment (Fig. 4 B). CD69 expression is believed to increase after receipt of normal pre-TCR signals (35). Moreover, antiCD3-stimulated CD69 induction is impaired in Lck−/− mice (35). Hence treatment with anti-CD4 mAb delivers a signal to CD4Tg+ Rag2−/− MHC class II−/− thymocytes that recapitulates one feature of the Lck-dependent preTCR signaling process. This signal may differ qualitatively or quantitatively from that which devolves after interaction of CD4 with class II molecules (see below).
Figure 4.
Flow cytometric analysis of thymocytes from control and CD4Tg+ Rag2−/− MHC class II−/− littermates treated with anti-CD3ε or anti-CD4 mAb. (A) 4-wk-old mice were treated intraperitoneally with 100 μg of the anti-CD3ε mAb 2C11 (top panels), or 20 μg of the antiCD4 mAb GK1.5 (bottom panels). After 4 d, thymocytes were stained and analyzed by flow cytometry using anti-CD4-PE and anti-CD8-FITC. Identical results were obtained for CD4Tg+ Rag2−/− MHC II−/− mice treated with 100 μg or 500 μg GK1.5. The percentage of cells in each population is indicated. Total thymocyte numbers were as follows: anti-CD3ε–treated Rag2−/− MHC II−/− (1.2 × 107) or CD4 Rag2−/− MHC II−/− mice (3.2 × 107). (B) Single parameter fluorescence histograms show CD69 expression 20 h after treatment with 150 μg of mAb 2C11 (gray line) or GK1.5 (thick black line) as compared to untreated controls (thin black line).
CD4 Transgene-driven Maturation Requires Expression of CD3 Chains.
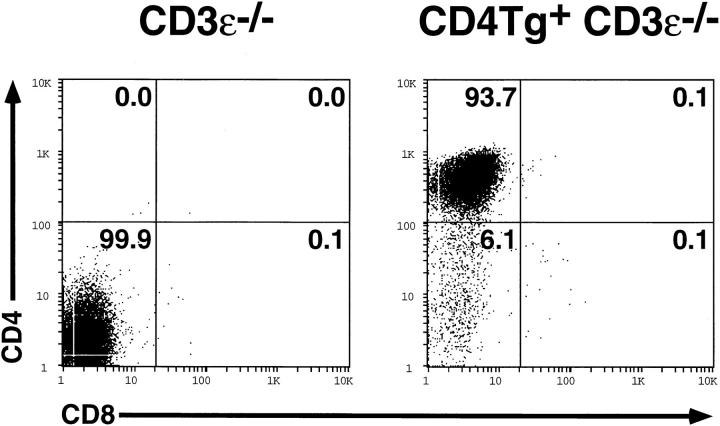
Maturation of DN thymocytes ordinarily requires assembly of the pre-TCR complex (6), which is composed of the CD3 chains, the pTα polypeptide, and the TCR-β chain (5, 7, 10–12). Although the CD4/class II interaction in our transgenic animals mimics pre-TCR signaling in Rag2−/− thymocytes (in the absence of a TCR-β chain), it remained plausible that other pre-TCR components, notably the CD3 polypeptides, might be necessary to permit delivery of an Lck-derived signal. To investigate the signaling requirements in this system, we introduced the CD4 transgene into a CD3ε−/− background. As in Rag2−/− mice, thymocytes of CD3ε−/− mice do not mature beyond the DN stage (11). These thymocytes also contain greatly reduced levels of the CD3γ and CD3δ transcripts, an effect ascribed to the presence of the neomycin phosphotransferase cassette within the closely integrated CD3γδε gene cluster (11). Despite high-level expression of the CD4 transgene in CD3ε−/− thymocytes, no appreciable induction of maturation was observed (Fig. 5). We conclude that although CD4 expression can substitute for the TCR-β chain in promoting thymocyte development, this effect requires expression of CD3 polypeptides.
Figure 5.
Flow cytometric analysis of thymocytes from CD3ε−/− (left panel) or CD4 transgene-bearing CD3ε−/− (right panel) littermates. The percentage of cells in each population is indicated.
Discussion
Experiments in genetically manipulated mice support a generally accepted model for pre-TCR function in which productive rearrangement of a TCR-β chain in immature DN thymocytes leads to formation of a functional preTCR complex composed of the TCR-β chain, the nonpolymorphic pTα-chain, and CD3 signaling molecules. Formation of the pre-TCR complex subsequently results in the activation of p56lck, leading to both proliferation and maturation to the DP stage of thymocyte development (6, 10, 37). As Lck is a pivotal component of the TCR signaling apparatus (19), it might be expected to function similarly in pre-TCR and mature TCR signaling.
Animals lacking either of the recombinase activating genes (Rag1 and Rag2) have provided a convenient experimental platform for identifying signaling components that regulate early thymocyte maturation (26, 38). For example, the introduction of transgenes encoding a functional TCR-β chain, or an activated version of p56lck (7, 17), into Rag−/− mice stimulates maturation of DN thymocytes. In addition, some functional pre-TCR components are expressed at the cell surface on Rag−/− thymocytes, because treatment of these cells with anti-CD3ε can induce both thymocyte proliferation and differentiation (35) in an Lck-dependent manner. However, CD3ε treatment almost certainly activates several signaling pathways, including some that may not involve p56lck. Interestingly, a chimeric transgene encoding an IL-2 receptor α chain (CD25) extracellular domain linked to intracellular sequences derived from CD3ε or the ζ chain can also stimulate pre-T cell development when cross-linked with anti-CD25 antibodies in vivo (39). This experiment, analogous to those performed using cultured T cell lines, further reinforces the view that preTCR signal transduction involves similar biochemical mechanisms to those that mediate TCR signaling in mature T cells.
By expressing CD4 in the DN thymocytes of Rag2−/− mice, we sought to develop a system wherein a defined, extracellularly-imposed signal would stimulate thymocyte maturation through activation of p56lck. The remarkable changes provoked by CD4 expression—an increase in cell number, activation of the CD8 gene, extinction of CD25 synthesis, and a decrease in cell size—mimic those changes imposed after pre-TCR stimulation. Propagation of these effects requires sequences in the CD4 cytoplasmic domain known to couple CD4 to p56lck, as well as the expression of MHC class II molecules and CD3 proteins. As murine T-lineage progenitors do not themselves express significant levels of MHC class II molecules (33, 34), these results demonstrate that CD4 can couple to endogenous Lck in such a way that interaction with an extracellular ligand, in this case class II molecules, stimulates changes in gene expression.
Numerous previous studies have documented the ability of the CD4 coreceptor to behave as a ligand for MHC class II molecules and to augment the response of class II– restricted T cells to appropriate antigen-presenting cells. Though the ability of CD4 to associate physically with Lck (18) and to stimulate Lck activity when experimentally oligomerized (21, 22) has long been recognized, the mechanism by which CD4 functions in cell signaling remains unclear. For example, in T cell hybridomas known to require CD4 for antigen recognition, a chimeric CD4 protein covalently linked to a catalytically inactive Lck molecule proved equipotent to wild-type CD4 (40). This result was observed even when the kinase domain of Lck was deleted entirely. Similarly, a tailless CD4 transgene (a construct identical to the CD4Δ construct which we have employed) was capable of directing substantial maturation of class II– restricted cells from the DP to the SP stage (41). These observations prompted the development of models in which Lck might act to regulate CD4 adhesion in a kinase-independent manner, rather than to deliver CD4-derived signals through tyrosine phosphate transfer (19).
Our experiments show that CD4, by binding class II, can stimulate an Lck-dependent process. Prior studies reveal that disruption of the lck gene substantially blocks thymocyte development at the DN to DP transition (14). Moreover, high level expression of a catalytically inactive version of Lck can completely block thymocyte development at the DN stage (13). These experiments demonstrate that the catalytic activity of Lck is required for satisfactory thymocyte maturation, a conclusion supported by the ability of activated Lck to stimulate the same maturational events (17). In a sense, the DN to DP transition serves as an extremely sensitive in vivo bioassay for Lck function, which permitted us to demonstrate that the binding of CD4 to class II molecules can stimulate signal transduction. This result in turn may serve to explain why ζ ITAM motifs are constitutively phosphorylated in wild-type DP thymocytes (42): the binding of class II to CD4 triggers an increase in Lck kinase activity, resulting in phosphorylation of antigen receptor components. By inference, the presence of a constitutive Lck-activating stimulus can therefore be considered a normal feature of cortical thymic environments.
Efficiency of CD4 Transgene-driven Thymocyte Maturation and Proliferation.
An activated p56lck transgene stimulates thymocyte development in Rag1−/− mice (17), and augmented expression of wild-type p56lck triggers allelic exclusion at the TCR β-chain locus (43). The results presented here show that activation of endogenous Lck, using a transgenic wild-type CD4 molecule as a signal sensor, promotes the DN to DP transition. These observations are consistent with experiments demonstrating that ionizing radiation (known to activate src-family PTKs) promotes thymocyte development in an lck-dependent manner (44).
However, the CD4 transgene induced only a modest increase in total thymocyte number in Rag2−/− mice, much less than that observed when a TCR-β (17) or activated lck transgene (17) is used. This difference suggests that CD4 behaves less efficiently on a per cell basis in promoting the DN to DP transition. Alternatively CD4 may deliver a qualitatively different signal to developing thymocytes than does the normal pre-TCR complex. Using anti-CD4 mAb treatment, we observed that all DN, transgene-bearing thymocytes can respond to CD4-derived signals, as judged by CD69 induction (Fig. 4 B). However, inasmuch as even a 50% reduction in class II expression dramatically decreases the efficiency of CD4-stimulated thymocyte maturation (Fig. 3), satisfactory stimulation of the DN to DP transition by transgenic CD4 appears to be exquisitely sensitive to the presence of an appropriate ligand. This limiting effect of class II abundance suggests that the CD4-derived signal may differ only quantitatively from that which is entrained by pre-TCR stimulation. As thymocytes are thought to undergo at least seven rounds of replication in developing from the DN to the DP stage (13, 45), CD4 transgenemediated activation of a small number of pre-T cells could produce thymi in which the majority of cells exhibit a more mature phenotype, without greatly increasing the total number of thymocytes. We cannot, however, exclude the possibility that CD4-derived signals differ qualitatively from pre-TCR signals, perhaps by activating only a subset of Lck-dependent signal transduction cascades.
Must p56lck Interact with Pre-TCR Components to Drive Development?
Unlike Rag2−/− mice, expression of the CD4 transgene in CD3ε−/− mice failed to induce thymocyte development (Fig. 5). These results echo those obtained in the Jurkat transformed T cell line, where coupling of p56lck to CD3 signaling components is important for the transmission of intracellular signals (46). Viewed from one perspective, the inability of the CD4/Lck signal to mediate development in the absence of CD3 indicates that the CD3 components function downstream of Lck, especially because CD3 ITAM motifs are good substrates for the Lck kinase (47). However it remains equally plausible that CD4/Lck complexes must colocalize with the pre-TCR as a means of approximating Lck with another positive regulator, e.g., CD45. Prior studies implicate the CD45 phosphatase in the dephosphorylation of a negative regulatory site (tyrosine 505) in Lck (48).
Although we cannot yet identify the targets of Lckmediated catalysis, it seems certain that simple activation of the Lck kinase cannot serve by itself to stimulate thymocyte development. We conclude that Lck must be juxtaposed with its intracellular targets to permit satisfactory signal transduction. The localization of these proteins therefore could serve as another means of regulating thymocyte maturation.
Finally, it bears mention that the expression of CD4 varies in a peculiar and characteristic way during thymocyte maturation. Very immature hematopoietic progenitors express CD4, which disappears shortly after arrival in the thymus (49). CD4 reappears in all thymocytes after preTCR-mediated stimulation, and then is extinguished in cells that express class I–restricted receptors. Suppression of CD4 transcription requires the activity of a dedicated silencer, a conserved cis-acting element (50). Because CD4 expression can, as we demonstrate, drive thymocyte maturation even in the absence of TCR-β chain expression, our results suggest that extinction of CD4 expression in very immature thymocytes is required to permit proper sensing of TCR-β chain gene assembly, and the consequent regulation of thymocyte maturation.
Acknowledgments
We thank Dr. S. Anderson for the suggestion to generate CD4Tg+ Rag2−/− mice, Dr. F.W. Alt for Rag2−/− mice, and Dr. P. Fink for the class II−/− mice. We also thank S. Chien and R. Peet for technical assistance, K. Prewitt for secretarial assistance, and our colleagues for helpful discussions.
Footnotes
A.M. Norment is supported by a National Cancer Institute KO8 Clinical Investigator Award (no. K08 CA64448) and R.M. Perlmutter is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by a grant from the National Institutes of Health (no. CA45682) to R.M. Perlmutter.
1 Abbreviations used in this paper: DN, double-negative; DP, double-positive; ITAM, immunoreceptor tyrosine-based activation motifs.
References
- 1.Perlmutter RM, Levin SD, Appleby MW, Anderson S J, Alberola-Ila J. Regulation of lymphocyte function by protein phosphorylation. Ann Rev Immunol. 1993;11:451–499. doi: 10.1146/annurev.iy.11.040193.002315. [DOI] [PubMed] [Google Scholar]
- 2.Jenkinson EJ, Owen JJ. T-cell differentiation in thymus organ cultures. Semin Immunol. 1990;2:51–58. [PubMed] [Google Scholar]
- 3.Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling HJ, von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science (Wash DC) 1994;266:1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- 4.Levelt CN, Carsetti R, Eichmann K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor β CD3ε and maturation to the CD4+8+stage are highly correlated in individual thymocytes. J Exp Med. 1993;178:1867–1875. doi: 10.1084/jem.178.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groettrup M, Baron A, Griffiths G, Palacios R, von Boehmer H. T cell receptor (TCR) β chain homodimers on the surface of immature but not mature α, γ, δ chain deficient T cell lines. EMBO (Eur Mol Biol Organ) J. 1992;11:2735–2746. doi: 10.1002/j.1460-2075.1992.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in the T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature (Lond) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 8.Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3ε. Proc Natl Acad Sci USA. 1993;90:11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SJ, Perlmutter RM. A signaling pathway governing early thymocyte maturation. Immunol Today. 1995;16:99–105. doi: 10.1016/0167-5699(95)80096-4. [DOI] [PubMed] [Google Scholar]
- 11.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3ε gene. EMBO (Eur Mol Biol Organ) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature (Lond) 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 13.Levin SD, Anderson SJ, Forbush KA, Perlmutter RM. A dominant-negative transgene defines a role for p56lckin thymopoiesis. EMBO (Eur Mol Biol Organ) J. 1993;4:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck . Nature (Lond) 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 15.Groves T, Smiley P, Cooke MP, Forbush KA, Perlmutter RM, Guidos CJ. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–528. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 16.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 18.Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of the tyrosine kinase p56lckwith the cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 19.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 20.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science (Wash DC) 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 21.Veillette A, Zúñiga-Pfücker JC, Bolen JB, Kruisbeek AM. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. J Exp Med. 1989;170:1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veillette A, Bookman MA, Horak EM, Samelson LE, Bolen JB. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck . Nature (Lond) 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 23.Garvin AM, Abraham KM, Forbush KA, Farr AG, Davison BL, Perlmutter RM. Disruption of thymocyte development and lymphomagenesis induced by SV40 T-antigen. Int Immunol. 1990;2:173–180. doi: 10.1093/intimm/2.2.173. [DOI] [PubMed] [Google Scholar]
- 24.Teh H, Garvin AM, Forbush KA, Carlow DA, Davis CB, Littman DR, Perlmutter RM. Participation of CD4 coreceptor molecules in T-cell repertoire selection. Nature (Lond) 1991;349:241–243. doi: 10.1038/349241a0. [DOI] [PubMed] [Google Scholar]
- 25.van Oers NSC, Garvin AM, Davis CB, Forbush KA, Carlow DA, Littman DR, Perlmutter RM, Teh HS. Disruption of CD8-dependent negative and positive selection of thymocytes is correlated with a decreased association between CD8 and the protein tyrosine kinase, p56lck . Eur J Immunol. 1992;22:735–743. doi: 10.1002/eji.1830220317. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai Y, Rathbun G, Lam K, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 27.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC class II molecules. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 28.Littman DR, Gettner SN. Unusual intron in the immunoglobulin domain of the newly isolated murine CD4 (L3T4) gene. Nature (Lond) 1987;325:453–455. doi: 10.1038/325453a0. [DOI] [PubMed] [Google Scholar]
- 29.König R, Huang L, Germain RN. MHC class interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature (Lond) 1992;356:796–798. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 30.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, Guardiola J, Sinigaglia F. Identification of a CD4 binding site on the β2domain of HLA-DR molecules. Nature (Lond) 1992;356:799–801. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 31.Janeway CA. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 32.Mathis DJ, Benoist C, Williams VE, II, Kanter M, McDevitt HO. Several mechanisms can account for defective Eα gene expression in different mouse haplotypes. Proc Natl Acad Sci USA. 1983;86:273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharrow SO, Mathieson BJ, Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981;126:1327–1335. [PubMed] [Google Scholar]
- 34.Schwartz BD, Kask AM, Sharrow SO, David CS, Schwartz RH. Partial chemical characterization of Ia antigens derived from murine thymocytes. Proc Natl Acad Sci USA. 1977;74:1195–1199. doi: 10.1073/pnas.74.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levelt CN, Mombaerts P, Wang B, Kohler H, Tonegawa S, Eichmann K, Terhorst C. Regulation of thymocyte development through CD3: Functional dissociation between p56lckand CD3ζ in early and late thymic selection. Immunity. 1993;3:215–222. doi: 10.1016/1074-7613(95)90091-8. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama WM, Koning F, Kehn PJ, Pereira GMB, Stingl G, Coligan JE, Shevach EM. Characterization of a cell surface-expressed disulfide-linked dimer involved in murine T cell activation. J Immunol. 1988;141:369–376. [PubMed] [Google Scholar]
- 37.Owen MJ, Venkitaraman AR. Signalling in lymphocyte development. Curr Opin Immunol. 1996;8:191–198. doi: 10.1016/s0952-7915(96)80057-4. [DOI] [PubMed] [Google Scholar]
- 38.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai Y, Ma A, Cheng A, Alt FW. CD3ε and CD3ζ cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995;2:401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Littman DR. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell. 1993;74:633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- 41.Killeen N, Littman DR. Helper T-cell development in the absence of CD4-p56lckassociation. Nature (Lond) 1993;364:729–732. doi: 10.1038/364729a0. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T, Singer A, Hsi ED, Samelson LE. Intrathymic signalling in immature CD4+CD8+thymocytes results in tyrosine phosphorylation of the T cell receptor ζ chain. Nature (Lond) 1989;341:651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- 43.Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck . EMBO (Eur Mol Biol Organ) J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu G, Danska JS, Guidos CJ. Lck-dependence of signaling pathways activated by γ-irradiation and CD3ε engagement of immature RAG-deficient thymocytes. Int Immunol. 1996;8:1159–1164. doi: 10.1093/intimm/8.7.1159. [DOI] [PubMed] [Google Scholar]
- 45.Baron C, Penit C. Study of the thymocyte cell cycle by bivariate analysis of incorporated bromodeoxyuridine and DNA content. Eur J Immunol. 1990;20:1231–1236. doi: 10.1002/eji.1830200606. [DOI] [PubMed] [Google Scholar]
- 46.Straus DB, Weiss A. Genetic evidence for the involvement of the lcktyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 47.Watts JD, Wilson GM, Ettehadieh E, Clark-Lewis I, Kubanek C, Astell CR, Marth JD, Aebersold R. Purification and initial characterization of the lymphocytespecific protein-tyrosyl kinase p56lckfrom a Baculovirus expression system. J Biol Chem. 1992;267:901–907. [PubMed] [Google Scholar]
- 48.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Scollay R, Egerton M, Pearse M, Spangrude GJ, Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature (Lond) 1991;349:71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- 50.Sawada S, Scarborough JD, Killeen N, Littman DR. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]