Abstract
Nonpeptidic compounds stimulate human T cells bearing the TCR-γδ in the absence of major histocompatibility complex restriction. We report that one of these ligands, 2,3-diphosphoglyceric acid (DPG), which induces expansion of Vγ9/Vδ T cells ex vivo, antagonizes the same cell population after repetitive activation. Stimulation with DPG results in partial early protein tyrosine phosphorylation and a prolonged, but reversible, state of unresponsiveness to agonist ligands in Vγ9/Vδ2, but not in other T cells. These findings show that TCR antagonism is a general phenomenon of T cells. However, in contrast to the clonal specificity of altered peptides antagonizing αβ T cells, all the tested Vγ9/Vδ2 polyclonal cell lines and clones become unresponsive, a fact that may be relevant for the regulation of their response in vivo.
Human γδ cells bearing the Vγ9 (TCRGV2S1)/ Vδ2(TCRDV102S1) TCR react to a variety of phosphorylated nonpeptidic ligands (1–4), some of which are natural metabolites (5). The TCR participates in the stimulation of the cells by such ligands as evidenced by reconstitution of reactivity when Vγ9 and Vδ2, but not other Vγ or Vδ genes, are co-transfected into a nonresponder T cell line (6). γδ T cell recognition of phosphorylated nonpeptidic ligands has two remarkable characteristics (5): (a) it is highly specific, since minor modifications in the structure of the ligand abolish recognition, and (b) all Vγ9/Vδ2 cells ex vivo and clones show the same specificity since they are broadly cross-reactive against different metabolites, and all ligands display the same relative potency on all tested T cell clones and polyclonal lines. Without exception, we observe that in vitro cultivated γδ T cells progressively lose their capacity to proliferate in response to the weaker ligands first, and to the stronger ones later on, while maintaining their ability to react to mitogens and to express similar levels of TCR (monitored by flow cytometry analysis using antibodies against CD3ε, Cδ, Vγ9, and Vδ2, data not shown). This consistent loss of proliferative capacity does not necessarily imply a loss of reactivity, but might reflect a change in biological responsiveness. We have investigated the possibility that a ligand which functions as an agonist during the beginning of the immune response, may act as an antagonist on the same cells in later phases.
Materials and Methods
Cells and Cell Culture.
Cells were cultured in RPMI 1640 medium supplemented with 2 mM glutamax I, 100 μg/ml kanamycin, MEM nonessential amino acids, 1 mM Na pyruvate (all from GIBCO BRL, Basel, Switzerland), human serum 5% (Blutspendezentrum, Bern, Switzerland), and 100 U of recombinant human IL-2 (IL-2), unless mentioned as IL-2–free medium. The Vγ9/Vδ2 cell lines were raised by culturing 106 freshly isolated human PBMC with a single dose of the indicated ligand (50 μg/ ml Mycobacterium tuberculosis [M. tub.]1 (Difco Laboratories, Detroit, MI), 10 μl/ml protein-free extract from M. tub. (PFE), 1 mM xylose-1-phosphate (X1P), 1 mM ribose-1-phosphate (R1P), or 100 μM isopentenylpyrophosphate (IPP; Sigma, Buchs, Switzerland). After 72 h, 50 U of IL-2 was added to the cultures. T cell clones were established as reported (7) by limiting dilution using PHA (1 μg/ml; Wellcome, Dartford, UK), IL-2 (100 U/ml), and irradiated PBMC (5 × 105/ml). T cell clones were restimulated periodically following the same protocol (7).
Flow Cytometry.
Vγ9/Vδ2 cells were incubated in the presence of indicated stimuli for 3 h, and then maintained on ice during staining with mAbs against CD3ε (TR66) or Cδ (δ1), and analyzed using a FACScan® flow cytometer. Data analysis was performed using CELLQuest (Becton Dickinson, San Jose, CA).
Proliferation Assay.
Proliferation assays were performed in IL-2– free medium using 105 responder cells/well and 30 Gy-irradiated PBMC (105/well) as APC. After 48 h, 1 μCi/well of [3H]thymidine (TRK120; Amersham Intl., Little Chalfont, England), was added and the cultures were harvested after an additional 18 h. Results are shown as mean cpm ± SD.
TNF Release Assay.
104 responder cells were incubated with the indicated ligand, and culture supernatants were collected after 6 h. 15 × 103 WEHI 164.13 cells were incubated for 18 h with appropriately diluted supernatant in the presence of actinomycin D (2.5 μg/ml). After a further 4 h incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 500 μg/ml), the cells were lysed and the reduced MTT dissolved by adding an equal volume of HCl 0.04 N in isopropanol. OD560–OD650 was determined using a THERMOmax Microplate Reader with SOFTmax (Molecular Devices Corp., Menlo Park, CA), and the released TNF calculated by comparison with the OD in the linear range of a standard curve simultaneously acquired with recombinant human TNF. Results are shown as mean pg/ml ± SD.
Cytotoxicity Assays.
In brief, PHA-stimulated T cell blasts were labeled with 100 μCi51Cr (Amersham Intl.) and 5,000 cells/ well were incubated in round-bottom wells with Vγ9/Vδ2 cells at the indicated effector/target (E/T) ratios. After 6 h, the amount of 51Cr released into the supernatant was measured as cpm and expressed as percent of specific 51Cr release: (effector induced cpm − spontaneous cpm / maximum cpm − spontaneous cpm) × 100. Maximum cpm were obtained by target cell lysis with 1 M HCl.
Analysis of Protein Tyrosine Phosphorylation.
Immunoblot analysis for protein tyrosine phosphorylation was performed with total cell lysates. Briefly, T cells (7.5 × 105 cell equivalents/lane) were lysed with 100 μl/106 cells of lysis buffer containing 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP40, inhibitors of proteases (1 mM PMSF, 4 μg/ml leupeptin, 4 μg/ml aprotinin), and inhibitors of phosphates (10 mM EDTA, 1 mM sodium orthovanadate), for 20 min on ice. After removal of nuclear debris by centrifugation, the supernatant was electrophoresed on 10% SDSPAGE and transferred to nitrocellulose membrane (Hybond-C; Amersham Intl.). Protein tyrosine phosphorylation was detected using the 4G10 mAb (Upstate Biotechnology Inc., Lake Placid, NY). The same results were obtained using another phospho- tyrosine specific mAb (PTyr1; a gift from V. Horejsi). Blots were developed using horseradish peroxidase–conjugated second antibody (sheep anti–mouse Ig; Amersham Intl.) and enhanced chemiluminescence (ECL system; Amersham Intl.).
Results and Discussion
A ligand and intermediate potency, 2,3-diphosphoglyceric acid (DPG), was compared with a ligand with high potency, IPP (4, 5). Fresh γδ T cells fully respond to both compounds. However, γδ T cells in culture gradually lose their proliferative response to DPG without a change in their capacity to proliferate in response to IPP.
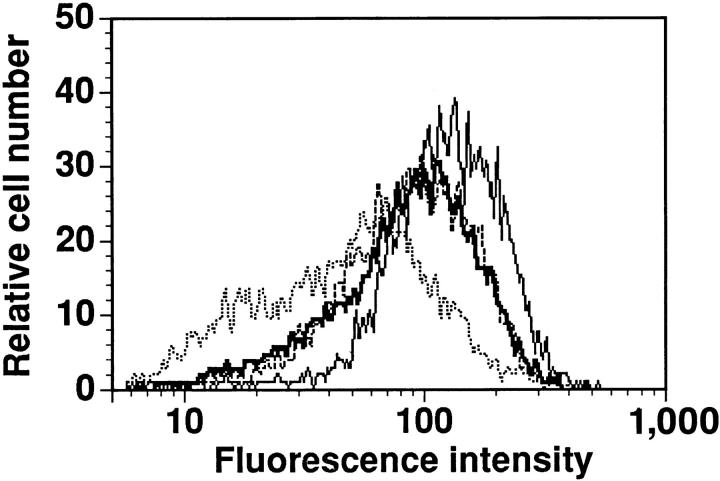
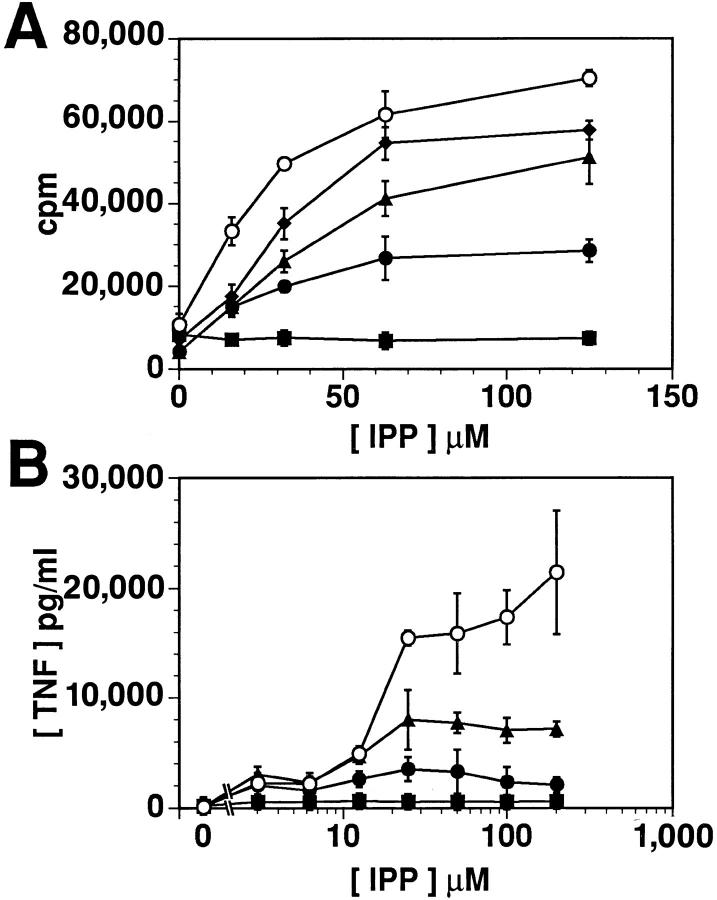
Throughout this study we have sued Vγ9/Vδ2 T cells which proliferate to IPP, but no longer to DPG. Both DPG and IPP induce downmodulation of the TCR γδ on these cells (Fig. 1), confirming that activation by DPG and IPP involves TCR stimulation (8). DPG induces TCR downmodulation without cell proliferation, we investigated whether it might behave as a partial agonist or as an antagonist (9, 10). DPG induces neither release of IFNγ and TNF, nor transcription of IL-1, IL-2, IL-3, IL-4, IL-5, GM-CSF, IFN-γ, and TNF-α genes in Vγ9/Vδ2 cells (assessed by reverse transcriptase-PCR, data not shown), nor killing of target cells by cytotoxic Vγ9/Vδ2 clones (data not shown), which are all effects detected after stimulation with IPP. Therefore, it is unlikely that DPG is a partial agonist. In another series of experiments, γδ T cells were cultured in the presence of both ligands simultaneously (Fig. 2). Although DPG no longer displays its agonistic properties on cultured γδ T cells, it nevertheless has a clear dosedependent effect whereby it inhibits IPP-induced activation in a noncompetitive manner. DPG lowers the efficacy (maximal response) of IPP, but does not alter the potency of IPP (dose for 50% of maximal effect). These results indicate that DPG does not displace IPP from its binding site, but that it may act as a TCR antagonist.
Figure 1.
DPG induces downmodulation of the TCR γδ. Overlaid histograms of immunostaining of δ1 mAB on Vγ9/Vδ2 clone Z1P 101 simulated with medium (—, median fluorescence intensity [MFI] 138, 100%), 1 mM DPG (—, MFI 99, 71%), IPP 100 μM (---, MFI 100, 72%), and PHA 1 μg/ml (\xc9 , MFI 55, 40%). Similar results were obtained with several different Vγ9/Vδ2 cell lines and clones, but not with control clones bearing other types of TCR.
Figure 2.
DPG reduces the efficacy, but not the potency, of IPP. Proliferation (A) and TNF release (B) of the γδ T cell line PG96 coincubated with different concentrations of IPP and DPG (1 mM, ▪; 500 μM, •; 250 μM, ▴; 125 μM, ♦), or medium (○). Comparable dose-response curves were obtained in four independent experiments.
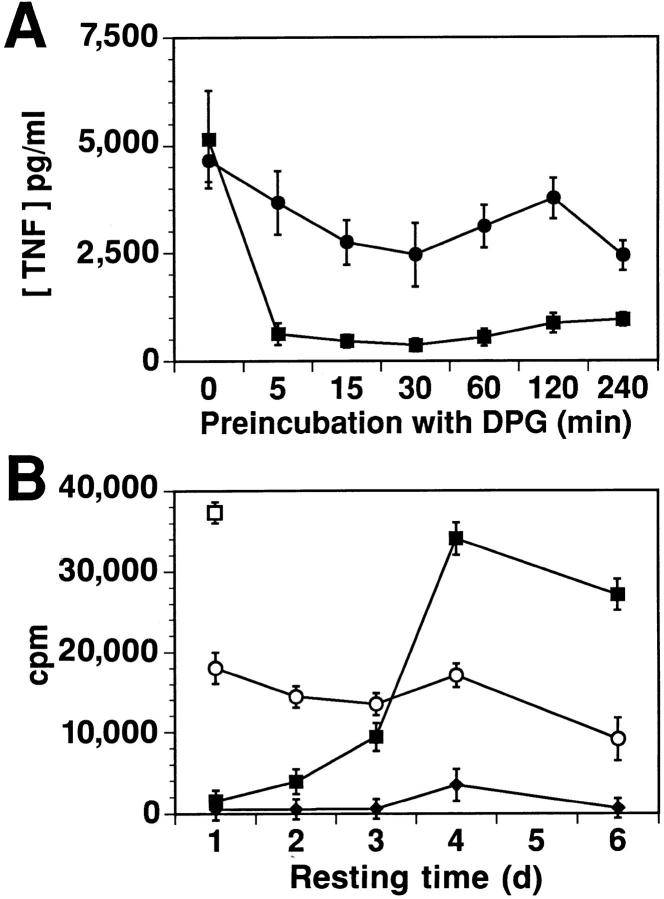
To investigate whether stimulation by DPG could result in a state of altered responsiveness, Vγ9/Vδ2 T cells were preincubated with DPG, and then washed and challenged with IPP. Preincubation with DPG blocks the subsequent ability of IPP to stimulate TNF release and cell proliferation (Fig. 3) indicating that DPG induces a state of unresponsiveness which persists after removal of the ligand. This prolonged unresponsiveness is not due to inadequate washout of the antagonist, since control cells are fully reactive in the presence of equal numbers of DPG-preincubated cells (data not shown). Incubation with DPG for only 5 min is sufficient to block IPP-mediated TNF release (Fig. 3 A). The rapidity with which DPG exerts its inhibitory effect is consistent with observations that phosphorylated nonpeptidic ligands induce Ca2+ fluxes in Vγ9/Vδ2 T cells within 2 min (11, 12). The IPP-unresponsiveness induced by DPG is not a consequence of cell death since the number of viable cells recovered is the same (70–100%) as controls, and the cells remain responsive to low doses (20 U/ml) of IL-2 (Fig. 3 B). Furthermore, once a state of unresponsiveness is induced, it lasts for 1–5 d (using different cell lines and clones), after which the cells regain their ability to respond to the agonist (Fig. 3 B). The induction of unresponsiveness by DPG differs from the induction of T cell refractoriness by repetitive stimulation with agonist ligands. Indeed, (a) γδ cells stimulated by DPG become unresponsive without induction of effector functions, and (b) the induction of unresponsiveness by DPG has a dominant effect even over simultaneous stimulation by IPP (Fig. 2).
Figure 3.
DPG rapidly induces a prolonged, but reversible, state of unresponsiveness to IPP. (A) The Vγ9/Vδ2 cell line BCI 31 was incubated with DPG for the time indicated on the x-axis. After extensive washing, the TNF release stimulated by 50 μM IPP (▪) or 1 μg/ml PHA (•) was measured. (B) Vγ9/Vδ2 clone BCI 49 (2 × 106 cells/ml) was incubated in IL-2–free medium with 1 mM DPG overnight and washed extensively. The cells were then rested in medium for the time indicated on the x-axis. Proliferative responses to medium (♦), 100 μM IPP (▪), or 20 U/ml IL-2, (○) are shown. □ indicates control proliferation to IPP (100 μM) of the same cells not preincubated with DPG. Results obtained in four independent experiments using different Vγ9/Vδ2 clones showed recovery of responsiveness after 1 to 5 d. Preincubation with DPG did not affect the responsiveness of CD4+ TCR αβ cells to their specific peptide (data not shown).
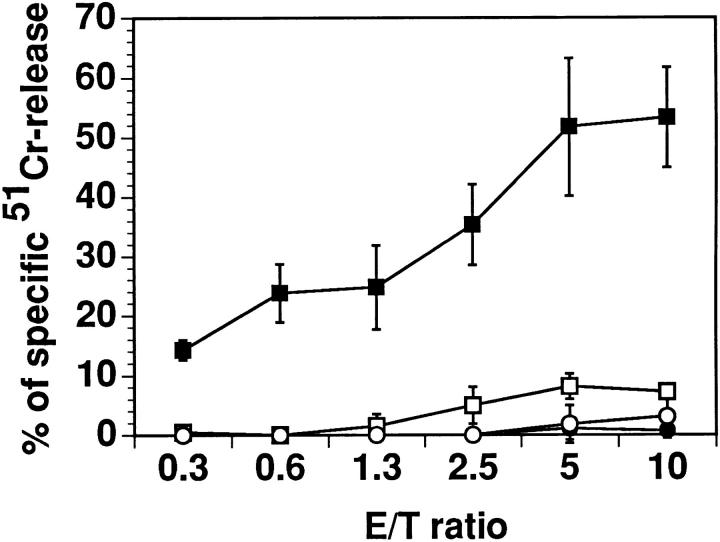
Proliferation and lymphokine release both require nuclear gene activation. To study whether effects induced by earlier signaling events are altered by DPG, we performed cytotoxicity assays. IPP stimulates cytotoxic Vγ9/Vδ2 cells to kill target cells at low E/T ratios; however, IPP does not trigger killing by the same cells preincubated with DPG (Fig. 4). These results suggest that in unresponsive cells, not only late events, but also earlier signaling events leading to cytotoxic activity are affected.
Figure 4.
Early signaling events, necessary for cytolytic function, are altered in cells made unresponsive by DPG. The figure shows results of killing of PHA-blasts by Vγ9/Vδ2 clone RNM.t.73 preincubated overnight with medium (solid symbols) or DPG 1 mM (open symbols) in the presence (squares) or absence (circles) of 100 μM IPP, at the indicated E/T ratios.
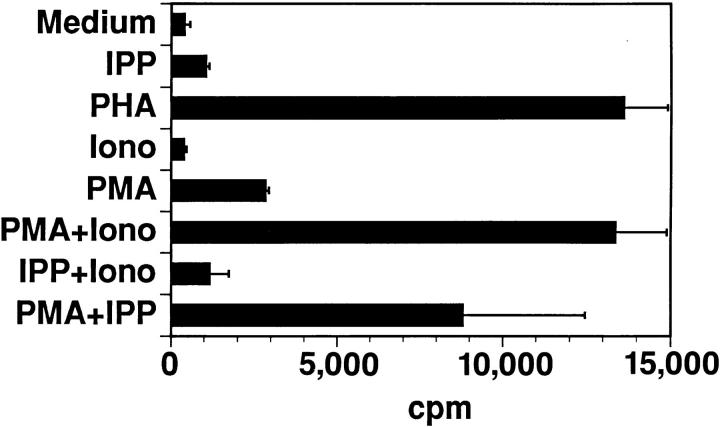
To determine which signal transduction pathways might be affected, we applied various procedures described to prevent anergy induction or restore responsiveness. While cyclosporin A completely prevents anergy with αβ T cells (13, 14), it has only a partial effect on γδ T cells (data not shown). This suggests that both the late signals blocked by cyclosporin A, as well as other signals, participate in establishing unresponsiveness. IPP-unresponsive cells could be stimulated by PHA or a combination of PMA and Ca2+ ionophore (Fig. 5). Importantly, reactivity to IPP could be restored by PMA, which was not stimulatory by itself. Thus, IPP can apparently still trigger unresponsive cells with a signal that is mimicked by Ca2+ ionophore. In addition, the ability of PMA to overcome unresponsiveness indicates that the blockade of signaling in unresponsive cells most likely occurs between the TCR and P21ras. Blockade of proximal signaling has been recently shown in two αβ T cell anergy models (15, 16).
Figure 5.
Unresponsiveness induced by DPG is the consequence of the alteration of TCR proximal signals. Vγ9/Vδ2 clone BCI 49 (106 cells/ ml) was incubated overnight in IL-2–free medium with 1 mM DPG and washed extensively. Proliferative responses to medium, 100 μM IPP, 1 μg/ml PHA, 500 ng/ml Ca2+ Ionophore A23187 (Iono) 50 ng/ml PMA, or the indicated combinations were measured.
Alterations of the TCR proximal signals have been previously described for modified peptide ligands which induce unresponsiveness in TCR αβ clones (17, 18). To investigate whether early signals are also altered in γδ T cells, the IPP- and DPG-induced protein tyrosine phosphorylation patterns were compared. A similar spectrum of phosphorylated proteins was detected (Fig. 6). However, the overall level of phosphorylation induced by DPG was quantitatively less than that induced by IPP.
Figure 6.
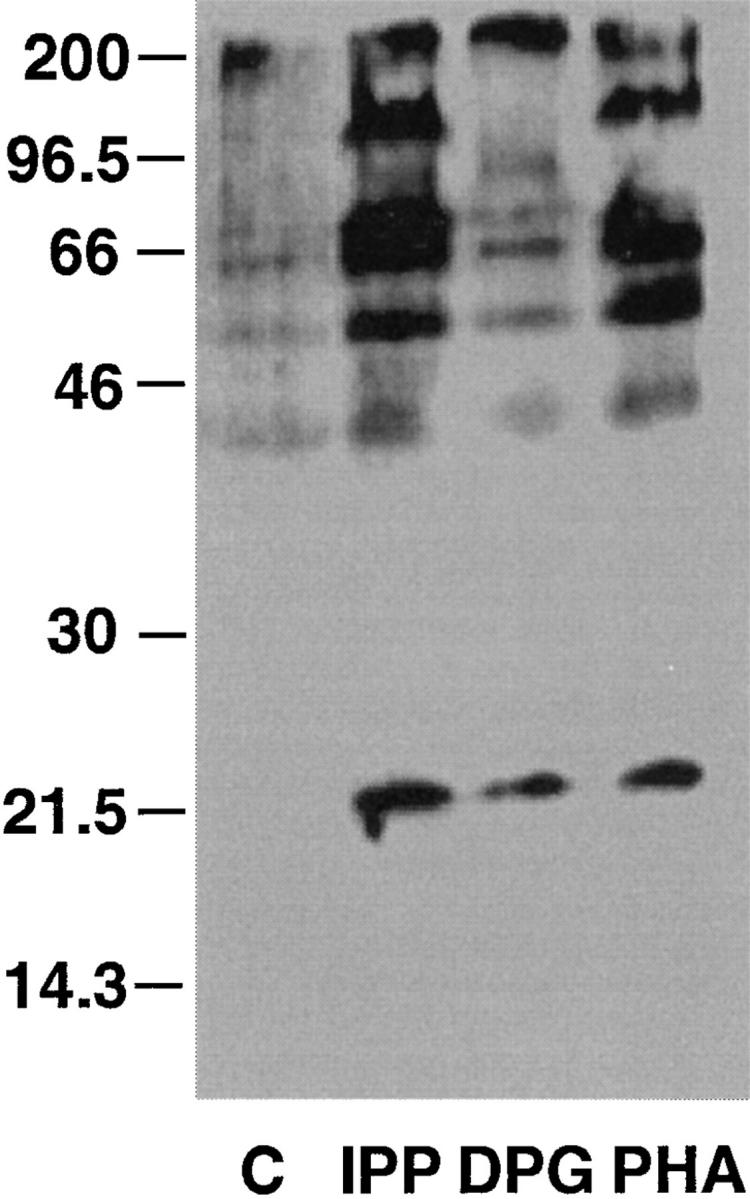
Upon DPG treatment, tyrosine phosphorylation of TCR-associated proteins is reduced when compared to IPP stimulation. Vγ9/Vδ2 T cells were incubated for 10 min with either medium (C), 100 μM IPP, 1 mM DPG, or 1 μg/ml PHA. Protein tyrosine phosphorylation is visualized by immunoblotting of total cell lysates. Similar results were obtained in four independent experiments using Vγ9/Vδ2 cell lines and clones. IPP and DPG did not induce any protein tyrosine phosphorylation in TCR αβ cells (data not shown).
The presented data show that TCR γδ antagonism has several characteristics in common with TCR αβ antagonism induced by altered peptide ligands (9, 19, 20). However, there are also important differences. In Vγ9/Vδ2 T cells, the antagonistic effects of DPG occur only after cell activation and extensive proliferation. Since the effects of DPG are observed without changes in its structure, the change in response must be due to a change occurring in the T cells. Cultured γδ T cells, on which DPG has lost its agonistic effects, express normal levels of TCR, which can transduce a full signal after stimulation with IPP. Therefore, it is likely that molecules other than the TCR are involved in the altered responsiveness to DPG. In our case, modified expression of costimulatory molecules seems unlikely, because cells which have lost the capacity to proliferate with DPG still proliferate with IPP, implying adequate costimulation. Yet, triggering the TCR with various stimuli in the absence of costimulation always leads to αβ T cell anergy (21, 22). The finding that cytotoxicity, which is not so dependent on costimulation (23), is lost in unresponsive cells also supports this conclusion.
The hypophosphorylation observed after DPG stimulation indicates that the signal transduction cascade is affected, and this might be due to loss of molecules important in the signaling pathway. Interestingly, the potency of DPG as agonist is the same as its potency as antagonist (ED50 250 μM), thus implying that DPG interacts with its receptor with the same affinity on both fresh and expanded γδ cells and that the nonproliferating γδ cells have not modified the receptor for DPG. We hypothesize that responsiveness of Vγ9/Vδ2 cells is modulated by the expression levels of a (unknown) molecule with a coreceptor-like function similar to that described for CD4 and CD8 coreceptors on αβ cells (24–29). Based on the presented data, our hypothesis predicts that stimulation of γδ T cells with strong ligands (e.g., IPP) is largely independent of coreceptor density, while stimulation with weak ligands (e.g., DPG) would require a high density. If the coreceptor density is reduced, weak ligands switch their properties from being agonists to antagonists. A progressive loss of the coreceptor might allow a given ligand to function as a full agonist in the early phase of the immune response, and subsequently acquire antagonistic properties and regulatory functions.
A second important difference is that while most of the TCR αβ antagonists are highly T cell clone specific, DPG is broadly active on human Vγ9/Vδ2 T cells. When many different γδ T cell lines and clones from various donors are tested, all the Vγ9/Vδ2 cells become unresponsive, irrespective of the ligand used to expand the original cell lines (Table 1). This is observed with assays detecting either proliferation or TNF release.
Table 1.
Unresponsiveness of Vγ9/Vδ2 Polyclonal Lines and Clones After Overnight Incubation with DPG
| Response to: | Medium | IPP | IL-2 | PHA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preincubation with DPG: | No | Yes | No | Yes | No | Yes | No | Yes | ||||||||||
| Donor | Raised against | Proliferation | ||||||||||||||||
| cpm | ||||||||||||||||||
| OP | M. tub. | 2,039 | 1,280 | 15,175 | 1,160 | 11,785 | 10,584 | 13,961 | 15,927 | |||||||||
| VJ | M. tub. | 6,353 | 3,366 | 12,061 | 3,087 | 12,827 | 13,003 | 10,488 | 14,474 | |||||||||
| GDL | M. tub. | 1,320 | 5,075 | 27,385 | 6,899 | 31,537 | 36,617 | 16,890 | 23,029 | |||||||||
| GDL | R1P | 628 | 1,360 | 19,198 | 1,870 | 12,086 | 16,345 | 15,123 | 17,415 | |||||||||
| GDL | X1P | 7,324 | 5,757 | 12,104 | 6,171 | 17,094 | 16,486 | 23,042 | 22,941 | |||||||||
| GDL | PFE | 10,607 | 8,178 | 70,393 | 7,326 | ND | ND | ND | ND | |||||||||
| BCI 49* | IPP | 381 | 706 | 37,324 | 1,767 | 44,938 | 50,429 | 19,102 | 19,514 | |||||||||
| BCI 5* | IPP | 411 | 323 | 24,857 | 1,080 | ND | ND | 6,350 | 8,388 | |||||||||
| TNF release | ||||||||||||||||||
| pg/ml | ||||||||||||||||||
| GDL | M. tub. | ND | 744 | 14,746 | 2,295 | ND | ND | ND | ND | |||||||||
| GDL | IPP | ND | 101 | 5,147 | 956 | ND | ND | 4,658 | 3,102 | |||||||||
| BCI 13* | IPP | ND | 475 | 7,585 | 1,736 | ND | ND | ND | ND | |||||||||
| G2C25* | PHA | <15 | <15 | 2,369 | 155 | ND | ND | ND | 1,649 | |||||||||
Vγ9/Vδ2 T cell clone. PFE, protein-free extract from M. tub.; R1P, ribose-1-phosphate; X1P, xylose-1-phosphate. Each line or clone was tested two to four times.
The induction of a state of unresponsiveness in a whole lymphocyte subpopulation may have important consequences for the immune response of γδ T cells. It has been proposed that γδ T cells provide an efficient first line of defense against infectious agents (30, 31). This property may derive from their ability to broadly cross-react with many ligands (5) and with diverse microorganisms (7, 32–36). However, this promiscuous ligand recognition might lead to an uncontrolled expansion of Vγ9/Vδ2 T cells with harmful effects for the organism. The presently described regulatory mechanism could control the whole Vγ9/Vδ2 T cell population in a late phase of the immune response. A paradigmatic example might be malaria infection where either plasmodium ligands or DPG, which is present inside red blood cells at a physiological concentration of ∼5 mM, are released after massive erythrocyte rupture. In patients with acute malaria, a synchronized erythrocyte rupture is followed by hyperactivation of the Vγ9/Vδ2 population (37, 38) and by a massive release of TNF resulting in a severe clinical picture (39). The recurrent release of nonpeptidic ligands from ruptured red blood cells and the successive activation and expansion of γδ T cells could change γδ T cell reactivity, and thereby result in a beneficial unresponsiveness. Indeed, patients with the chronic form of malaria experience neither Vγ9/Vδ2 cell expansion nor raised TNF serum levels and have an asymptomatic clinical course (38).
In summary, the presented data provide a rationale for manipulating the reactivity of the whole Vγ9/Vδ2 T cell population and offer a new model with which to assess whether TCR antagonism is an important principle of T cell regulation.
Acknowledgments
We thank A. Lanzavecchia, R. Nisini, and T. J. Resink for reading the manuscript.
Footnotes
This work was supported by the Swiss National Fund grants No. 31-36450-92 and No. 31-045518.95 to G. De Libero and the Marie-Heim Vögtlin grant No. 32-38848-93 to L. Mori.
1 Abbreviations used in this paper: DPG, 2,3-diphosphoglyceric acid; E/T, effector/target; IPP, isopentenylpyrophosphate; MFI, median fluorescence intensity; M. tub., Mycobacterium tuberculosis.
References
- 1.Pfeffer K, Schoel B, Gulle H, Kaufmann SH, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of γδ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 2.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science (Wash DC) 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita C. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature (Lond) 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 5.Bürk MR, Mori L, De Libero G. Human Vγ9/ Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 7.De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γδ T cells. J Exp Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padvoan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor α chains: dual receptor T cells. Science (Wash DC) 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 9.Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 10.Kersch GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature (Lond) 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 11.Lang F, Peyrat MA, Constant P, Davodeau F, David AJ, Poquet Y, Vie H, Fournie JJ, Bonneville M. Early activation of human Vγ9Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 12.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins MK, Ashwell JD, Schwartz RH. Allogenic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 14.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature (Lond) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Whaly CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+T cells. Science (Wash DC) 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 16.Fields PE, Gajewski TF, Fitch FW. Blocked ras activation in anergic CD4+T cells. Science (Wash DC) 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 17.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 18.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science (Wash DC) 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 19.Germain, R.N., E.H. Levine, and J. Madrenas. 1995. The T-cell receptor as a diverse signal transduction machine. The Immunologist. 3/4:113–121.
- 20.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and a role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins MJ, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–309. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science (Wash DC) 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 23.Otten GR, Germain RN. Split anergy in a CD8+T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science (Wash DC) 1991;251:1228–1231. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 24.Alexander J, Snoke K, Ruppert J, Sidney J, Wall M, Southwood S, Oseroff C, Arrhenius T, Gaeta FCA, Colon SM, et al. Functional consequences of engagement of the T cell receptor by low affinity ligands. J Immunol. 1993;150:1–7. [PubMed] [Google Scholar]
- 25.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature (Lond) 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignali DAA, Stromiger JL. Amino acid residues that flank core peptide epitopes and the extracellular domains of CD4 modulate differential signaling through the T cell receptor. J Exp Med. 1994;179:1945–1956. doi: 10.1084/jem.179.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature (Lond) 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 28.Mannie MD, Rosser JM, White GA. Autologous rat myelin basic protein is a partial agonist that is converted into a full antagonist upon blockade of CD4. J Immunol. 1995;154:2642–2654. [PubMed] [Google Scholar]
- 29.Vidal K, Hsu BL, Williams CB, Allen PM. Endogenous altered peptide ligands can affect peripheral T cell responses. J Exp Med. 1996;183:1311–1321. doi: 10.1084/jem.183.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway CR., Jr Frontiers in the immune system. Nature (Lond) 1988;333:804–806. doi: 10.1038/333804a0. [DOI] [PubMed] [Google Scholar]
- 31.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy+dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 32.Holoshitz J, Koning F, Coligan JE, De Bruyn J, Strober S. Isolation of CD4− CD8−mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature (Lond) 1989;339:226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- 33.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein BS, Voss SD, Morrissey LW, DeMars R, Welch WJ, et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science (Wash DC) 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 34.Munk ME, Gatrill AJ, Kaufmann SH. Target cell lysis and IL-2 secretion by γδ T lymphocytes after activation with bacteria. J Immunol. 1990;145:2434–2439. [PubMed] [Google Scholar]
- 35.Roussilhon C, Agrapart M, Ballet JJ, Bensussan A. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparummalaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 36.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, Nomoto K. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho M, Tongtawe P, Kriangkum J, Wimonwattrawatee T, Pattanapanyasat K, Bryant L, Shafiq J, Suntharsamai P, Looareesuwan S, Webster HK, Elliott JF. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparummalaria. Infect Immun. 1994;62:855–862. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating γδ T cells during Plasmodium vivaxmalarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karunaweera ND, Grau GE, Gamage PE, Carter R, Mendis KN. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivaxmalaria. Proc Natl Acad Sci USA. 1992;89:3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]