Abstract
We have investigated whether in the human thymus transition of CD4+CD8+ double positive (DP) to CD4+ or CD8+ single positive (SP) cells is sufficient for generation of functional immunocompetent T cells. Using the capacity of thymocytes to expand in vitro in response to PHA and IL-2 as a criterion for functional maturity, we found that functional maturity of both SP and DP thymocytes correlates with downregulation of CD1a. CD1a− cells with a persistent DP phenotype were also found in neonatal cord blood, suggesting that at least a proportion of mature DP cells can emigrate from the thymus. The requirements for generating functional T cells were investigated in a hybrid human/mouse fetal thymic organ culture. MHC class II– positive, but not MHC class II–negative, mouse thymic microenvironments support differentiation of human progenitors into TCRαβ+CD4+ SP cells, indicating that mouse MHC class II can positively select TCRαβ+CD4+ SP human cells. Strikingly, these SP are arrested in the CD1a+ stage and could not be expanded in vitro with PHA and IL-2. CD1a+CD4+ SP thymocytes do not represent an end stage population because purified CD1a+CD4+ SP thymocytes differentiate to expandable CD1a− cells upon cocultivation with human thymic stromal cells. Taken together these data indicate that when CD1a+ DP TCRαβlow cells mature, these cells interact with MHC, but that an additional, apparently species-specific, signal is required for downregulation of CD1a to generate functional mature TCRαβ+ cells.
Tcell progenitors that develop in the thymus to mature T cells are submitted to a series of selective events (reviewed in reference 1), the first of which takes place when immature CD4−CD8−CD3− cells differentiate into CD4+ CD8+ double positive (DP)1 cells. A second selection occurs when DP thymocytes differentiate into CD4+ or CD8+ mature T cells, and is generally referred to as positive selection. It is well established that positive selection involves sustained interactions of the TCR αβ heterodimer with complexes of peptides and MHC antigens on thymic stromal cells (reviewed in references 2–4). During this selection process, either CD4 or CD8 is downregulated. There is current debate over whether downregulation of CD4 or CD8, and thus commitment to CD4+ or CD8+ T cells, is dictated by the MHC specificity of the TCR (instructive model) (5, 6) or whether it occurs in a stochastic fashion independent of TCR/MHC interactions (selective model) (7–9). In the majority of the studies addressing the issue of positive selection, all CD3high thymocytes with a CD4 or CD8 single positive (SP) phenotype were considered to have completed the process of positive selection and to be functionally mature. However, recent studies in the mouse indicate that not all SP thymocytes that have been submitted to positive selection signals are functionally mature. It is known that SP cells are phenotypically heterogeneous with respect to CD24 (heat stable antigen) and CD69 (10, 11). In addition, CD4+ SP thymocytes with intermediate levels of CD24 express very low levels of CD8 when analyzed with a sensitive panning method (11). More recently, it has been demonstrated that although the CD4+CD8low cells had hallmarks of positive selection such as CD69 and high levels of TCR, they were not able to induce a lethal Graft versus host disease upon transfer into irradiated allogeneic recipients and to survive in the periphery (12). The immature CD3highCD4+CD8low cells require the thymic environment to reach the end stage of positive selection (12). These data suggest that when functional immunocompetence of T cells is taken as the end stage of positive selection, this process is not necessarily completed when CD4 or CD8 are downregulated.
Here we report on the identification of downregulation of CD1a as a hallmark for functional maturation, not only of SP human thymocytes, but also of DP cells. To arrive at this model, we made use of the observations that DP cells contain in vitro clonogenic cells both in human (13, 14) and mouse (15). These observations were intriguing because if one accepts that maturity of T cells is appropriately reflected by their capacity to expand in vitro, some DP cells should have been submitted to a maturation signal. The presence of both mature clonogenic DP cells and immature CD4+ SP cells (12) is difficult to reconcile with a linear model of thymocyte differentiation from immature CD3+CD4+CD8+ DP via immature to mature SP cells. A possible explanation for the existence of both in vitro clonogenic mature DP thymocytes and presumably immature SP cells could be that there are bifurcations in the pathway of later stages of T cell development. The data presented here are consistent with this notion, since it was found that acquisition of functional maturity correlates perfectly with downregulation of CD1a and, most importantly, not with downregulation of CD4 or CD8. Moreover, we show here that MHC class II–positive, but not MHC class II–negative, mouse thymic microenvironments can support differentiation of human progenitors into CD3+CD4+ SP cells. However, human TCRαβ+ CD4+ SP cells selected on mouse MHC class II continue to express CD1a and exhibit poor clonogenic potential in vitro, suggesting that a species-specific signal is required for downregulation of CD1a and induction of functional maturity in the CD4 TCRαβ lineage.
Materials and Methods
Preparation and Phenotypic Analysis of Thymocyte Subpopulations.
Thymocyte tissues were obtained from children 3 mo–10 yr of age undergoing median sternotomy and corrective cardiovascular surgery. Suspensions were made by mincing tissue and pressing through a stainless steel mesh. Large aggregates were removed, and the cells were washed once before separating subpopulations.
To prepare CD34+ subpopulations, total thymocytes were first incubated with saturating concentrations of anti-CD4 (RPA-T4), anti-CD8 (RPA-T8) (provided by Dr. G. Aversa, DNAX Research Institute, Palo Alto, CA), anti-CD69 (Leu-23, gift of Dr. J.H. Phillips, DNAX Researach Institute), and anti-CD27 (gift of Dr. R. van Lier, Central Laboratory of the Blood Transfusion Service of the Netherlands Red Cross, Amsterdam, Netherlands). The labeled cells were removed by using magnetic beads coated with sheep anti–mouse immunoglobulins (Dynal Inc., Oslo, Norway) and a samarium cobalt magnet. The cells remaining after the first depletion were labeled with anti-CD56 (L185, from Dr. J.H. Phillips), anti-CD19, and anti-CD14 (CLB CD19 and CLB CD14, respectively, from Dr. R. van Lier) to remove the NK, B, and myeloid cells, and again subjected to depletion with magnetic beads. The enriched cells were incubated with antiCD34 FITC (HPCA-2 from Becton Dickinson, San Jose, CA) and anti-CD1a PE (T6-RD1 from Coulter Corp., Hialeah, FL). CD34+CD1a− cells were sorted with a FACStar plus®. Purity of the cell populations was always >98%.
Three-color analyses were performed with antibodies tagged with FITC, PE, or TriColor (TRC). In some experiments biotinylated antibodies that were revealed with avidin-CyCr were used as third antibody. Cytoplasmic staining with FITC-conjugated anti–Bcl-2 mAb (clone 124; DAKO A/S, Glostrup, Denmark) was performed as described previously (16). Three-color analysis was carried out on the FACScan®.
Limiting Dilution Assays.
CD1a+ and CD1a− DP, CD4+ and CD8+ SP thymocytes were plated under limiting dilution conditions in 96-well round-bottomed wells. The thymocytes were cultured in the presence of 5.104 irradiated (3.103 rad) allogeneic PBMC and 5.103 irradiated (5.103 rad) EBV transformed B cells (JY) per well in 100 μl of culture medium supplemented with 0.1 μg/ml of PHA (Wellcome, Beckenham, Kent, UK) and 30 U/ml of recombinant IL-2 (Chiron Europe, Amsterdam, Netherlands). Culture medium consisted of Yssel's medium (17) supplemented with 2% human serum. After 5 d of culture, 100 μl fresh culture medium with 30 U/ml rIL-2 was added. Wells were screened microscopically for cell growth after 2–4 wk of culture.
Hybrid Human/Mouse Fetal Thymic Organ Cultures.
The in vitro development of human T and NK cells from CD34+ thymocytes was studied using the hybrid human/mouse fetal thymic organ culture (FTOC) in which human progenitor cells were cocultured with murine fetal thymuses (18). These thymuses were obtained from embryos of recombination activating gene (RAG)-1–deficient mice on days 15–16 of gestation. To investigate the role of murine MHC class II antigens in development of human cells, FTOC were set up with thymuses of MHC class II–deficient mice (19), provided by Dr. L. Glimcher (Harvard School of Public Health, Boston, MA).
The fetal thymuses were first precultured for 5 d in the presence of 1.35 mM 2-deoxyguanosine to remove endogenous thymocytes. Next, the thymic lobes were cocultured for 2 d in hanging drops in Terasaki wells with FACS® sorted human progenitor cells, transferred to nucleopore filters which were layered over gelfoam rafts in 6-well plates, and cultured for the indicated number of days. Culture medium consisted of Yssel's medium supplemented with 2% normal human serum and 5% fetal calf serum. To analyze differentiation of human cells, the mouse thymuses were dispersed into single cell suspensions and stained with mAbs specific for human cell surface antigens.
Isolation of Stromal Cells.
Heterogeneous cell cultures of thymic stroma were obtained for mechanic disruption of the thymic parenchyma and enzymatic treatment with collagenase and lipase and enrichment for large adherent cells (20, 21). Adherent cells were cultured in RPMI-1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FCS. The cultures were washed each day during the first days of culture to remove any remaining thymocytes. Stromal cells were used after two or three passages.
RNA Isolation and cDNA Preparation.
Total RNA was isolated from sorted cells using the guanidine thiocyanate method (22). Glycogen (20 μg; Boehringer Mannheim, Indianapolis, IN) was added to each sample to facilitate precipitation of the RNA. The cDNA was prepared with oligo(dT)15 (PharMingen, San Diego, CA) and reversed transcribed with 200 U M-MLV reverse transcriptase (GIBCO BRL). Dilutions of the cDNA in water (5 ngeq RNA/μl) were used in PCR amplification reactions.
Semi-quantitative PCR.
A semi-quantitative PCR method (23, 24) was used to compare the expression of RAG-1 in thymocyte subpopulations. Synthetic oligonucleotides (Pharmacia LKB Biotechnology Inc., Piscataway, NJ) used as primers are as follows: 5′-TATGGACAGGACTGAACGTCTTGC-3′ (hypoxanthine phosphoribosyl transferase [HPRT] sense), 5′-GACACAAACATGATTCAAATCCCTGA-3′ (HPRT antisense), 5′-GAACACACTTTGCCTTCTCTTTGG-3′ (RAG1 sense), 5′-CGCTTTGCCTCTTGCTTTCTCGTT-3′ (RAG1 antisense). Standard curves for HPRT and RAG-1 were set up using serial dilutions of cDNA prepared from RNA isolated from total thymus. Dilutions of cDNA samples in water were made starting with a concentration of 0.5 ngeq RNA/μl to determine HPRT expression and 5 ngeq RNA/μl to determine RAG-1 expression. PCR was carried out in a total volume of 50 μl consisting of 1 μM of each primer set, 200 μM each dNTP (Pharmacia LKB Biotechnology Inc.), 2.5 mM MgCl2, 1× PCR buffer, 1 U Taq DNA polymerase (GIBCO BRL), and 10 μl of the cDNA. Samples were covered with 50 μl paraffin oil and heated to 94°C for 5 min and then amplified for 30 cycles of 1 min at 94°C, 1 min at 65°C, and 2 min at 72°C. After the last cycle, a final extension step at 72°C for 10 min was done. 10 μl of each PCR reaction was dot blotted on a nylon filter (Hybond N+; Amersham Intl. Buckinghamshire, UK). Filters were prehybridized at 55°C for at least 2 h (6× SSC, 0.5% SDS, 5× Denhardt's, and 100 mg herring sperm DNA per liter), and hybridized overnight with an oligoprobe recognizing specifically the HPRT or RAG-1 PCR product internal to the PCR primers. Oligoprobes were 32P labeled according to the manufacturer's recommendations (Boehringer Mannheim). Sequences of the probe are as follows: 5′-GTCCCCTGTTGACTGGTCATTACAAT-3′ (HPRT probe), 5′-TCCTTTGAAAAGACACCTGAAGAAGC-3′ (RAG1 probe). To remove any nonspecifically bound probe, the filters were washed with excess amount of 2× SSC; 0.1% SDS at 50°C. Quantitation of the PCR products was done with a phosphoimager (Fujix Bas 2000; Fuji Photo Film Co., Ltd., Tokyo, Japan) and analyzed with the supplied software. Finally, the ratio of RAG-1/HPRT was calculated to compare the expression of the RAG-1 mRNA in the different samples.
Results
Identification of Clonogenic CD4+CD8+ DP and CD4+ or CD8+ SP Thymocytes in the Human Thymus.
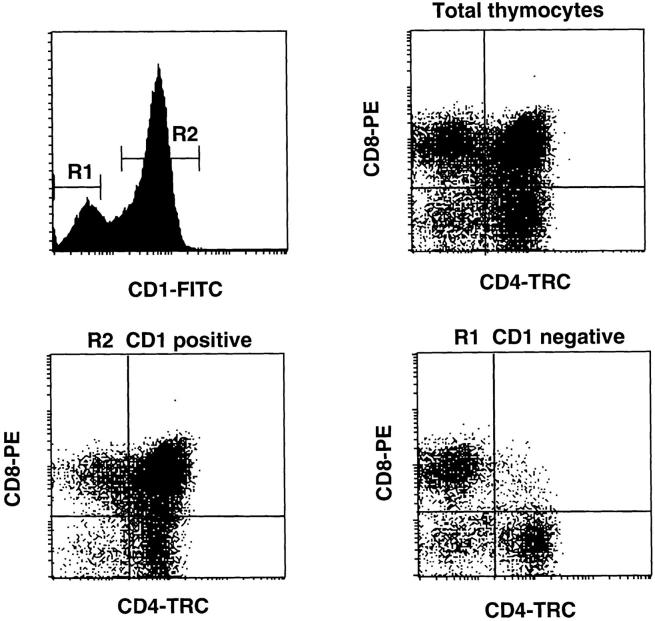
CD1a is a marker that is expressed on the great majority of DP thymocytes and part of the SP cells (25). Since this marker is not present on mature peripheral T cells, it is generally assumed that thymic emigrants are CD1a−, and that therefore, CD1a+ thymocytes are immature. Since a proportion of the SP cells is CD1a+, a linear model of differentiation predicts that virtually all DP cells would be CD1a+. A nonlinear differentiation model, however, would predict existence of CD1a− cells among both DP and SP thymic populations. To examine this issue, we performed three parameter flow cytometric analysis of CD1a, CD4, and CD8, which confirmed earlier data that the vast majority of DP cells, and around 40% of the SP cells, express CD1a (Fig. 1). A very small percentage of the DP cells, however, is clearly negative for CD1a. Interestingly, the levels of CD4 and CD8 on the CD1a− DP cells are lower than on total DP thymocytes (Fig. 1), suggesting that downregulation of either one of these coreceptors had already been initiated before completion of CD1a downregulation. To address whether the CD1a− cells are functionally mature, we performed a limiting dilution of CD1a− and CD1a+ cells. Table 1 shows that the cloning efficiencies of CD1a− DP, CD4+, and CD8+ SP thymocytes in one representative experiment were 24, 41, and 34%, respectively. In sharp contrast, virtually none of the CD1a+ DP or CD1a+ SP cells could be cloned. The lack of clonogenic potential of CD1a+ subsets was not due to an inhibiting effect of the anti-CD1a antibody, since cloning efficiencies of unseparated SP cells plated in presence or absence of anti-CD1a mAb were virtually identical (results not shown). The in vitro expandable DP thymocytes could be cloned, and the majority of the DP clones maintained their DP phenotype for a prolonged period of time (results not shown) which is consistent with data published previously (13, 14). These data conclusively demonstrate that the clonogenic potential of SP and DP thymocytes resides exclusively in the CD1a− subset, and that functional maturation, as defined by the ability to clonally expand, can already be manifested at the DP stage of thymocyte maturation.
Figure 1.
Expression of CD1a on CD4+CD8+DP, CD4+SP, and CD8+SP postnatal thymocyte populations. Total postnatal thymocytes were stained with CD1a FITC, CD8 PE, and CD4 TRC. The dot plots show the pattern of CD4 against CD8 staining of total thymocytes gated on CD1a− (R1) and CD1a+ (R2) thymocytes.
Table 1.
Plating Efficiencies of Subsets of CD1a+ and CD1a− Thymocytes
| Thymocytes | Number of positive wells | |||||
|---|---|---|---|---|---|---|
| 1 cell/well | 5 cells/well | 25 cells/well | ||||
| Exp. 1 | ||||||
| CD1+DP | 0/144 | 0/24 | 0/12 | |||
| CD1−DP | 34/144 (24%) | 15/24 (63%) | 12/12 (100%) | |||
| CD1+CD4SP | 1/144 (1%) | 0/24 | 0/12 | |||
| CD1−CD4SP | 59/144 (41%) | 23/24 (96%) | 12/12 (100%) | |||
| CD1+CD8SP | 0/144 | 0/24 | 1/12 (8%) | |||
| CD1−CD8SP | 49/144 (34%) | 24/24 (100%) | 12/12 (100%) | |||
| Exp. 2 | ||||||
| CD1+CD4SP | 0/60 | 0/24 | NT | |||
| CD1−CD4SP | 15/60 (25%) | 22/24 (92%) | 6/6 (100%) | |||
| CD1+CD8SP | 0/60 | 0/24 | 0/6 | |||
| CD1−CD8SP | 16/60 (27%) | 20/24 (83%) | 6/6 (100%) | |||
Characterization of Immature CD1a+ SP Thymocytes.
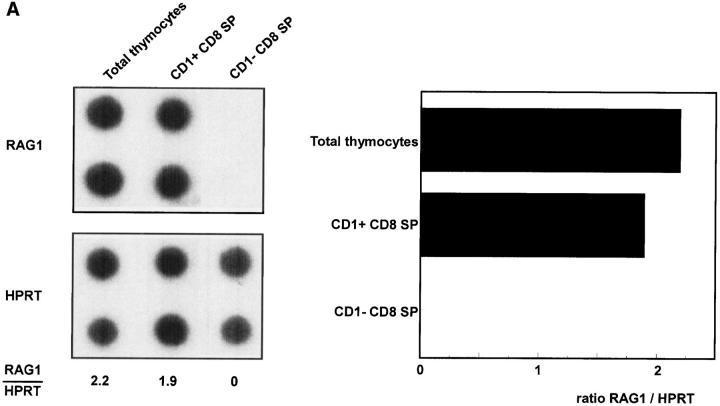
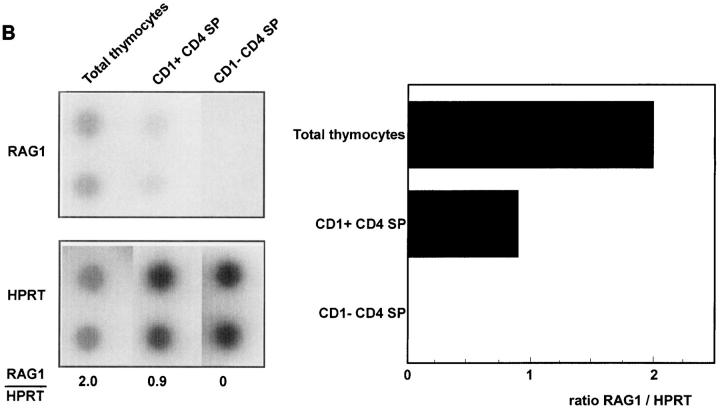
The results of the limiting dilution experiments indicate that CD1a+CD3high SP thymocytes are functionally immature. The observation that the great majority of the CD3high cells in the thymus express the activation marker CD69, which is induced after positive selection (26–28) suggests, however, that positive selection signals have been delivered to a significant proportion of the CD1a+ SP cells. To further substantiate whether the CD1a+ SP cells have been submitted to selection signals, we investigated not only expression of CD69, but also Bcl-2 which is associated with positive selection as well (29, 30). In addition, expression of CD27 was analyzed. CD27 is expressed on most CD3high human thymocytes, and may also be associated with positive selection (31). Three parameter analysis of CD1a+ SP cells confirms that the majority of these cells express CD69, Bcl-2, and CD27 (Table 2). These data are consistent with expression profiles of CD69 (25), Bcl-2 (32), and CD27 (31) on total human CD3high thymocytes published previously. Besides upregulation of CD69, positive selection also results in downregulation of RAG-1 (28, 33). A semi-quantitative reverse transcriptase-PCR of the CD1a+ and CD1a− SP populations revealed that CD8+CD1a+ cells still express levels of RAG-1 which are comparable to that of total thymocytes (Fig. 2 A). The levels of RAG mRNA in CD1a+ CD4+ SP cells, however, are much lower than that of total cells (Fig. 2 B). Taken together, these data can be interpreted to indicate that CD1a+ SP cells express some, but not all, features of cells that have received a TCR-mediated positive selection signal.
Table 2.
Expression of CD27, CD69, and Bcl-2 on CD1a+ versus CD1a− CD4 and CD8 SP Postnatal Thymocytes
| Percentage of thymocytes expressing | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD27 | CD69 | Bcl-2 | ||||||
| % | % | % | ||||||
| CD3CD4SP | CD1+ | 62 | 95 | 99 | ||||
| CD1− | 100 | 67 | 100 | |||||
| CD3CD8SP | CD1+ | 88 | 100 | 96 | ||||
| CD1− | 100 | 72 | 100 | |||||
Thymocytes were stained with CD4TRC/CD8PE or CD8TRC/CD4 PE and FACStar® sorted for TRC+PE− CD4 and CD8 SP cells. Sorted cells were stained with CD27 FITC, CD69 FITC, Bcl-2 FITC, and CD3 FITC against CD1a PE or with control antibodies. The percentages of CD1 positive and negative cells CD4 and CD8 SP thymocytes that express the above markers were determined by FACScan® analysis. The indicated percentages are those on CD3highSP cells.
Figure 2.
RAG-1 expression of total unseparated thymocytes and CD1a− and CD1a+ CD8 (A) and CD4 SP (B) postnatal thymocytes. Thymocytes were depleted with magnetic beads for >97% of CD4 or CD8 positive cells. CD4− cells were labeled with CD8 FITC, CD1a PE, and CD3 TRC and sorted from CD1a+ and CD1a− CD3+CD8+ SP cells (A). Likewise, CD1a+ and CD1a− CD4+ SP cells were sorted from CD8− cells stained with CD4 FITC, CD1a PE, and CD3 TRC (B).
Development of Human CD4+ SP Cells in a Mouse Fetal Thymus Requires Mouse MHC Class II Antigens, but the Mouse Thymus Is Inefficient at Inducing Maturation of Human CD4+ SP T Cells.
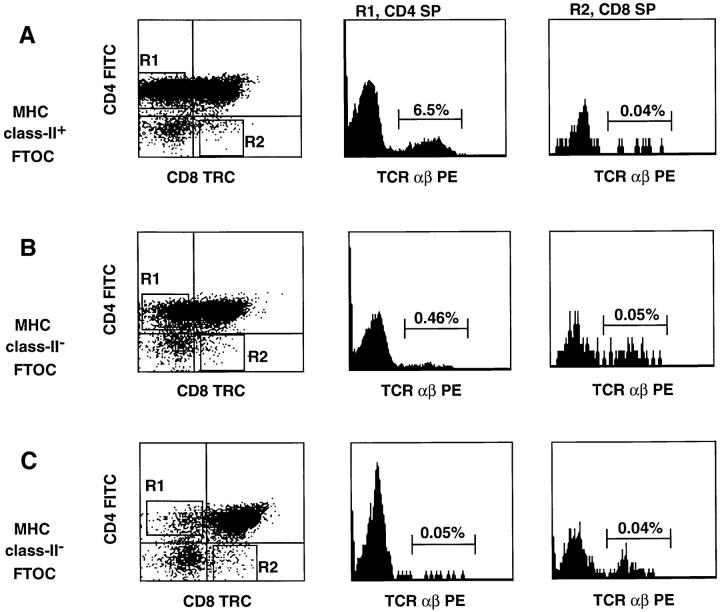
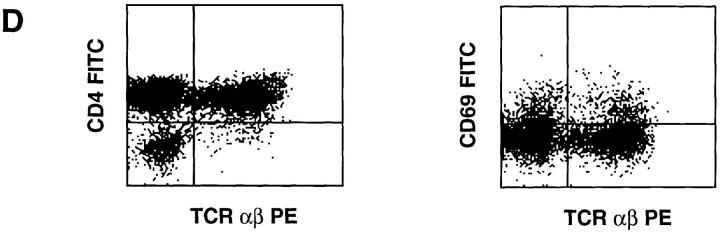
Recently it was demonstrated that human progenitor cells can develop in mouse thymic organs in a FTOC (18, 34–37). Human progenitor cells developed into SP cells, but human stromal cells were not detectable in such cultures (36). Human CD4 can replace mouse CD4 in development of mouse MHC class II–restricted T cells (38). To address the question of whether interaction of human CD4 with mouse MHC class II can select human CD4+ T cells, FTOC were performed with thymi from MHC class II–positive and MHC class II–deficient mice. The mouse thymi were reconstituted with CD34+CD1a− postnatal thymocytes that include the most primitive thymic T cell precursors (39, 40). After incubation in a MHC class II–positive murine thymic microenvironment, 6.5% of the harvested cells were TCR αβ+ CD4+ SP (Fig. 3 A). By contrast, the number of TCRαβ+CD4+ SP T cells recovered from thymi of MHC class II–deficient mice was reduced considerably to 0.46% in experiment 1 (Fig. 3 B) and 0.05% in experiment 2 (Fig. 3 C), compared to that recovered from thymi of MHC class II–positive mice (6–10% in four independent experiments). A significant portion of the TCRαβ+ cells that developed in a class II MHC–positive thymic environment expressed CD69 (Fig. 3 D), indicating that some cells were activated, presumably as a consequence of selection via the TCR. These findings indicate that mouse MHC class II antigens can positively select human CD4+ cells. It is noteworthy that very few human CD8+TCRαβ+ SP cells could be recovered from the FTOC with human CD34+CD1a− cells and the mouse thymi. There were more CD3+CD8+ SP cells present and >90% of those cells express TCR γδ (results not shown). One possible reason for this is that mouse MHC class I does not efficiently select human CD8+ T cells, despite the fact that human CD8 is able to functionally interact with the α3 domain of mouse H2Kb (41). Another explanation is that in addition to class I MHC, other signals are required for selection of CD8+ T cells.
Figure 3.
Multiparameter analysis of cells harvested from one FTOC with thymi of MHC class II–positive mice (A and D) and two FTOC with MHC class II–deficient mice (B and C). FTOC were set up as indicated in Materials and Methods with 25,000 CD34+CD1a− postnatal thymocytes per lobe and incubated for 4 wk. Cell numbers harvested from the FTOC cultures were 150,000 cells per lobe in the culture with MHC class II–positive thymic lobes (A and D), and 175,000 (B) and 100,000 cells (C) per lobe in the cultures with MHC class II–negative thymic lobes. Cells harvested from the FTOC cultures were stained for threecolor analysis with CD4 FITC, TCR αβ PE, and CD8 TRC. Human cells were gated on the basis of forward and side scatter profile; all cells within this gate were positive for human CD45. CD4 against CD8 staining is indicated as dot plots. The histograms show the expression of TCR αβ on the CD4+CD8− and CD8+CD4− thymocytes. Numbers in histograms represent the percentages of CD4+ or CD8+ TCR αβ+ SP cells in the total population of human cells derived from the FTOC.
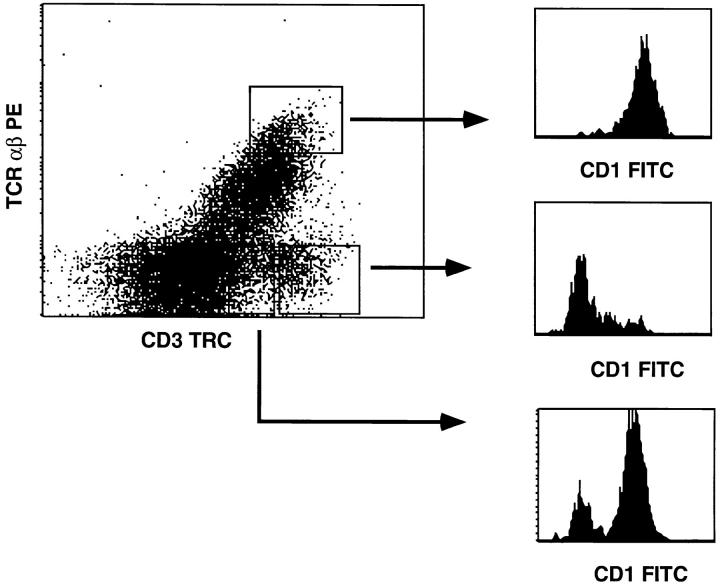
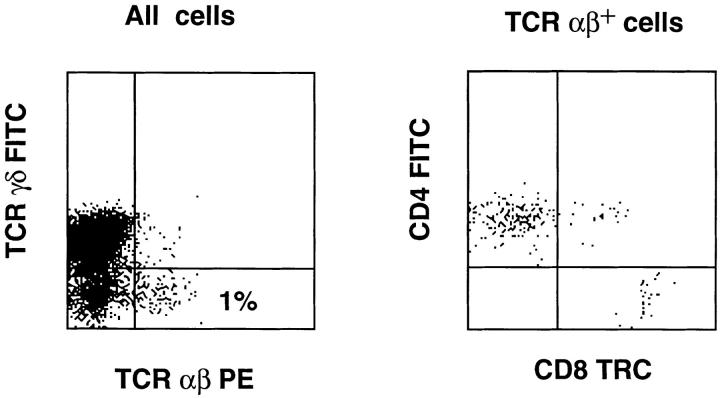
Having established that MHC class II antigens can support development of human CD4+ SP T cells, we next investigated whether the mouse thymic environment can induce downregulation of CD1a and functional maturation. Early thymic CD34+CD1a− progenitors were isolated and cultured in FTOC for 4 wk. Analysis of the cells harvested from the FTOC revealed the presence of TCR αβ and TCR γδhigh cells. Almost all TCR αβhigh cells expressed CD1a, while most TCR γδhigh cells lacked CD1a (Fig. 4). Immature TCR γδdim cells mostly expressed CD1a (Fig. 4). Stimulation of the cells harvested from the FTOC with a feeder cell mixture, PHA, and IL-2 resulted in expansion mostly of TCR γδ+ cells and few TCR αβ+ cells (Fig. 5). Most of those TCR αβ+ cells that were expanded expressed CD4 (Fig. 5). These data demonstrate that although the mouse MHC class II–positive mouse thymic environment can support development of CD4+ SP thymocytes, it is very inefficient in induction of functional maturation of these cells. By contrast, the mouse thymic microenvironment efficiently induces maturation of TCR γδ+ cells. Thus, failure of the mouse MHC class II–positive environment to induce functional maturation of TCR αβ+ cells is not due to a intrinsic incapability to support maturation of human T cells.
Figure 4.
CD1a expression on CD3+TCR αβ+ and CD3+TCR αβ− (TCR γδ+) cells, harvested from an FTOC incubated for 4 wk with CD34+CD1a− postnatal thymocytes. FTOC-derived cells were stained with CD1a FITC, TCR αβ PE, and CD3 TRC. The dot plot demonstrates the pattern of TCR αβ PE versus CD3 TRC staining. The histograms indicate CD1a FITC expression of the CD3high cells that express TCR αβ, and the CD3int and CD3high TCR αβ− cells which represent the immature and mature γδ T cells, respectively.
Figure 5.
Phenotype of T cells expanded from the FTOC (the same one as indicated in Fig. 4) with a feeder cell mixture, PHA, and IL-2. Expanded cells were stained with TRC γδ FITC and TCR αβ PE or with CD4 FITC, TCR αβ PE, and CD8 TRC. The dot plots show the expression of TCR γδ versus TCR αβ of all cells and the expression of CD4 against CD8 of gated TCR αβ+ cells.
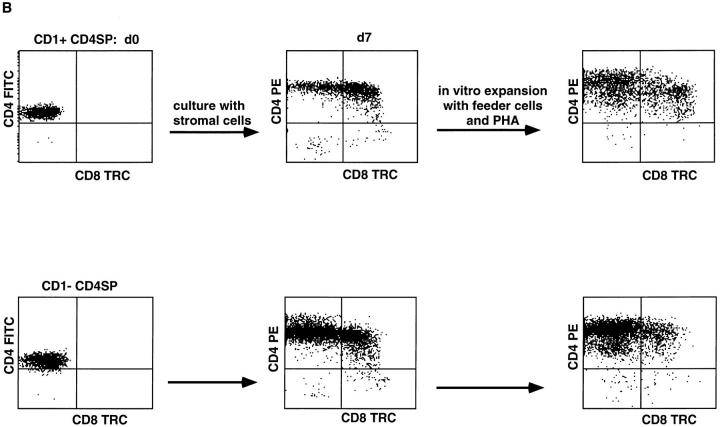
Differentiation of CD1a+ to CD1a− CD4+ SP Cells.
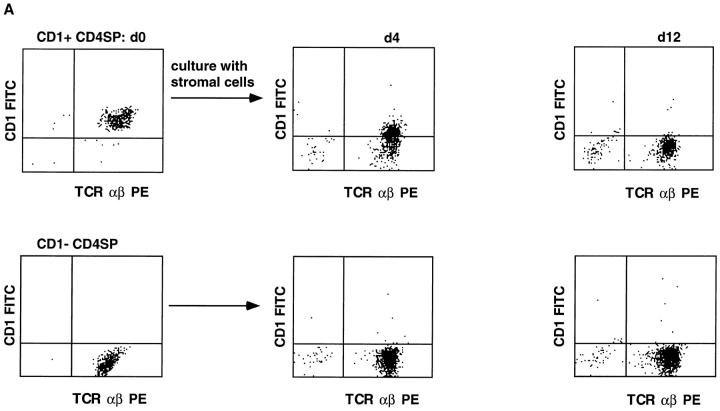
The presence of CD1a+ and CD1a− SP thymocytes raises the question whether CD1a+ SP cells are the direct precursors of CD1a− SP cells. An alternative possibility would be that the CD1a− SP cells are derived from the CD1a− DP cells and that CD1a+ SP cells represent a dead-end lineage. To investigate this, we cocultured purified CD1a+CD4+ SP cells with short term cultured human thymic stromal cells. This coculture resulted in a gradual downregulation of CD1a which was completed on day 12 (Fig. 6 A). Phenotypic analysis of these cells reveals that they express high levels of TCR αβ and CD4. Unexpectedly, many of these cells also express CD8α (Fig. 6 B). These differentiated CD1a− cells could be expanded in vitro and the phenotype did not alter upon expansion (Fig. 6 B). These data indicate that CD1a+ CD4+ SP cells can differentiate to expandable CD1a−CD4+ SP cells and also to expandable CD1a− CD4+CD8α+ cells.
Figure 6.
CD1a+CD4+ SP thymocytes differentiate into CD1a− cells upon coculture with thymic stromal cells (A), and part of these cells upregulate CD8 expression (B). Thymocytes were labeled with CD1a FITC, CD4 PE, and CD8 TRC. CD1a+ and CD1a− CD4high SP cells were sorted and part of the cells were used to check CD3 expression by staining with CD3 TRC (all CD1a− and >99% of CD1+ CD4 SP thymocytes were CD3+). In experiment A, 2 × 105 CD1a+CD4 SP (>98% purified) and 2 × 105 CD1a−CD4 SP cells were cultured on a monolayer of human thymic stromal cells. After 4 and 12 d, cells were tested for CD1a expression. The cell numbers of wells started with CD1a+ and CD1a−CD4 SP cells were both reduced to 8 × 104 after 4 d, whereas at day 12, 2.5 × 104, and 7.0 × 104 (CD1a−) cells were recovered from the cultures started with CD1a+ and CD1a−CD4 SP thymocytes, respectively. In experiment B, sorted CD1a+ and CD1a− CD4 SP thymocytes were cultured for 7 d on a monolayer of thymic stromal cells, assayed for CD4 and CD8 expression, and expanded in vitro with feeder cells, PHA, and IL-2. Expanded cells were also analysed for CD4 against CD8 expression.
Presence of DP Cells in Neonatal Cord Blood.
As indicated in Fig. 1, the thymus contains expandable CD1a DP cells. Although not shown here, we were able to clone DP cells and these clones maintained a persistent DP phenotype upon long-term culture in accord with data published (13, 14). The presence of DP cells, expressing several characteristics of maturity, raises the question whether these cells are able to migrate from the thymus. DP cells could be observed in T cell samples from neonatal cord blood (Fig. 7) in percentages ranging from 0.5 to 3% of the total number of CD3+ T cells (n = 4). All DP cord blood cells express CD3 and CD27, and they lack CD1a or the activation antigen CD69 (Fig. 7). Further analysis of these cells indicate that the majority of these cells express CD45RA, and are negative for CD45RO and Fas (Fig. 7) suggesting that these DP cells represent naive, not memory, cells. Moreover, like in the thymus (42), both CD8α+β−CD4+ and CD4+CD8α+β+ cells could be observed. These observations are compatible with the notion that DP cells can migrate out of the thymus.
Figure 7.
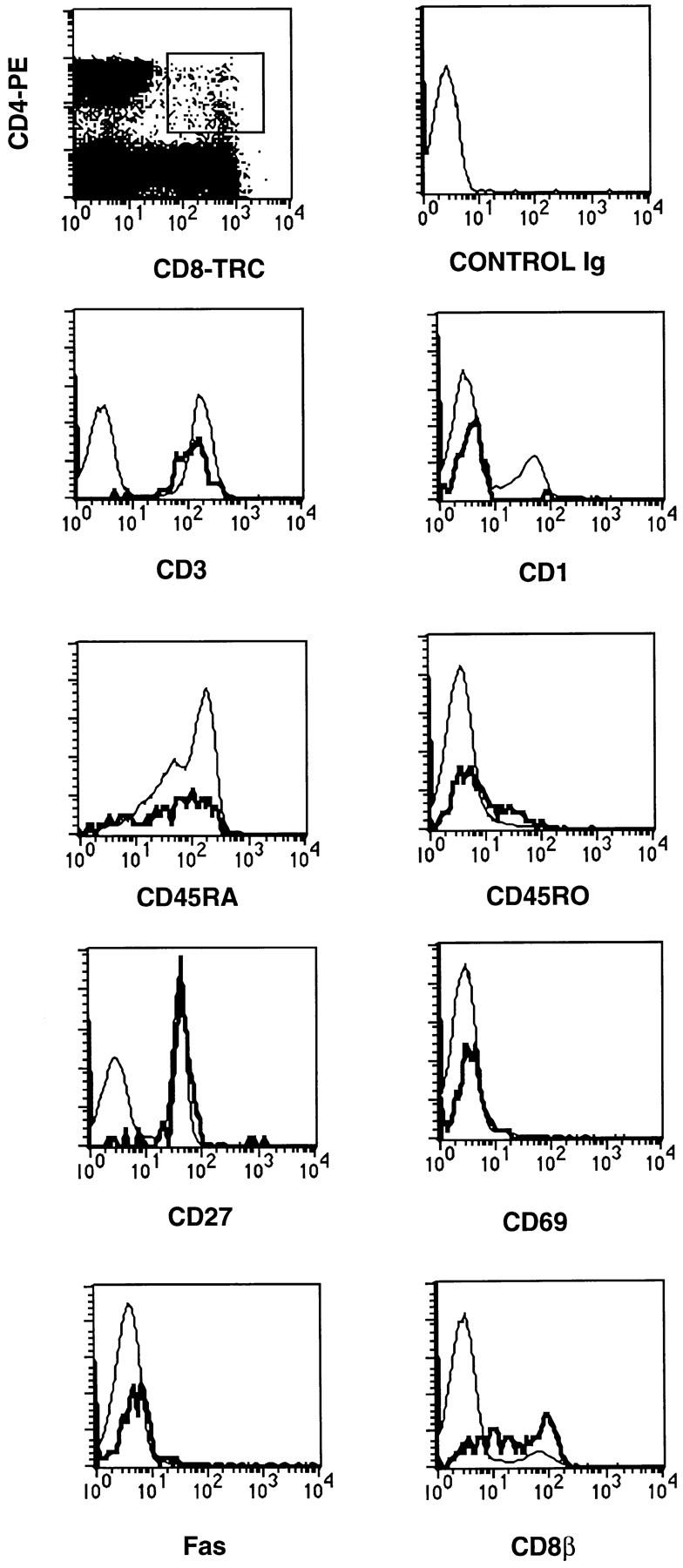
Three parameter analysis of neonatal cord blood cells. Cord blood lymphocytes were obtained by centrifugation over Lymphoprep (Nycomed Pharma, Oslo, Norway). Monocytes/macrophages and contaminating erythrocytes were depleted with goat anti–mouse IgG coated magnetic beads (Dynal Inc.) using monoclonal antibodies against CD14 and glycophorin. The remaining cells were stained with CD4 PE and CD8 TRC against the indicated FITC conjugated antibodies.
Discussion
In this paper we have investigated acquisition of functional maturity by human thymocytes. The hypothesis that forms the basis of this study is that maturity of T cells is appropriately and faithfully reflected by their capacity to expand in vitro. We think that this is the case because in vitro expandability is a general property of mature peripheral T cells. Moreover, T cell clones derived from mature thymocytes can mediate cytotoxic activities and produce cytokines upon stimulation in vitro (data not shown). Accepting our premise, the data argue that some DP are mature, while a considerable proportion of the SP cells in the human thymus are functionally immature. Most importantly, acquisition of functional maturity correlates with downregulation of CD1a. The cognizance that CD1a marks immature cells within thymocyte subpopulations allowed a meaningful and detailed analysis of these cells and a comparison with functionally mature thymocytes.
Our findings that CD1a+ SP cells are not clonogenic in vitro confirm and extend findings of Vanhecke et al. who investigated the clonogenic potential of CD4+ SP human thymocyte subsets (42). The CD1a+CD4+ SP thymocytes acquire the capacity to expand in vitro after cocultivation with short term cultured thymic stromal cells. This acquisition paralleled downregulation of CD1a. The observations identifying functionally immature CD1a+CD4+ SP cells are compatible with recent findings in the mouse by Dyall et al. (12) who demonstrated that a proportion of CD4+ SP murine thymocytes are functionally immature by several criteria. In many respects, the immature CD1a+CD3+ CD4+ SP thymocytes in the human thymus are similar to the functionally immature CD3+CD4+ SP in the mouse thymus (12). The immature mouse CD4+ SP thymocytes can be distinguished from mature cells by virtue of their expression of CD24 and high levels of CD69 (12). CD8 was not detectable by fluorimetric analysis, but the fact that the immature CD4+ SP mouse cells can be retained on antiCD8 immobilized on plastic indicates that low levels of CD8 are present on the immature CD4+ SP cells (12). Similar to the immature CD3+CD4+ SP population in the mouse, the human CD1a+CD4+ cells express CD69 and Bcl-2, indicative for cells that have been submitted to a positive selection signal (26–30). In addition, most CD1a+CD4+ SP cells express CD27, which may be another marker that is induced by positive selection (31, 42, 43). Human CD1a+CD3+ CD4+ SP cells expressed RAG-1, though in lower levels than total thymocytes. However, our inability to analyse RAG expression in individual cells precludes consideration of the possibility that a proportion of CD4+ CD1a+ SP cells are negative for RAG-1. Also, the CD8+ SP population in the human thymus contains functionally immature CD1a+ cells. The observation that heat stable antigen+CD8+ SP cells are present in the mouse thymus (10) suggested that there are immature cells also within the CD8+ SP thymic population, but the functional activity of those cells was not tested. The immature human CD1a+ CD8+ SP cells are similar to the CD1a+CD4+ SP cells in that the majority expresses CD69, Bcl-2, and CD27, but differ in expression levels of RAG-1, which are much higher than on CD1a+ CD4+ SP cells.
Although the expression of CD27, CD69, Bcl-2, and high levels of CD3 on part of the immature CD1a+ DP and almost all CD1a+ SP thymocytes indicates that these CD1a+ cells have been submitted to a positive selection signal, it is clear that a transition to CD1a− cells is required to confer functional maturity to these thymocytes. Two possible mechanisms for the discrepancy between upregulation of CD27, CD69, and Bcl-2, and the CD1a+ to CD1a− switch can be put forward. One is that downregulation of CD1a and acquisition of functional competence requires a much greater sustained MHC/TCR interaction than induction of CD69. This idea would take into consideration data from mouse studies indicating that consecutive, or perhaps even continual, TCR/MHC interactions are required to complete positive selection (44–46). A second possibility is that MHC/TCR interactions are sufficient for upregulation of CD69, but that an additional signal is required for downregulation of CD1a and acquisition of functional maturity. Our experiments with the hybrid human/mouse FTOC system provides support for the notion that two signals are required for induction of the functional program in immature thymocytes. We observed that although interactions of mouse MHC class II antigens with human CD4 and TCR drive generation of CD4+ SP cells, the mouse thymic microenvironment is very inefficient in downregulating CD1a and inducing functional maturation of CD4+ TCR αβ T cells. The inefficiency of mouse thymic microenvironment to induce functional maturity in CD4+ TCRαβ+ T cells is not due to, for example, tissue culture conditions since TCR γδ cells mature efficiently in the mouse FTOC. Moreover, we observed that cocultivation of CD1a+ CD4+ SP cells with human thymic stromal cells results in differentiation to CD1a− cells. Taken together, these observations raise the possibility that species-specific activating or costimulatory molecules are required for efficient maturation of human CD4+ T cells.
In this paper, we confirm and extend earlier findings (13, 14) that the human thymus contains in vitro expandable DP cells. In vitro expandable DP thymocytes have also been found in the mouse (15). In vitro expandability of human DP thymocytes correlates perfectly with completion of downregulation of CD1a, as was also the case for SP cells. Our observations that DP cells are present in the periphery of neonates suggest that some mature CD1a− DP cells may migrate out of the thymus. Most of these peripheral DP cells have characteristics of naive cells in that they express CD45RA and are negative for CD45RO and Fas, which are selectively expressed on memory cells. As was also found within the mature DP thymocytes, cord blood DP T cells lack CD1a and a proportion lacks CD8β as well. The characteristics of these DP cord blood cells make it unlikely that they are derived from peripheral SP cells that have upregulated CD4 or CD8 due to activation. It seems, therefore, that the CD4CD8 phenotype becomes stable once the DP cells have been submitted to a maturation signal. The fact that cloned lines of DP thymocytes with sustained CD4CD8 phenotype can be established is consistent with this notion. It is relevant to note that cloned lines of DP T cells have been established from peripheral T cells (47). It is possible that those clones originated from cells that emigrated from the thymus as DP cells.
The presence of both mature clonogenic DP cells and immature SP cells is difficult to reconcile with a generalized linear model of thymocyte differentiation from immature TCRαβ+CD4+CD8+ DP cells via immature to mature SP cells. It is possible that for some thymocytes, completion of positive selection and acquisition of functional competence can occur at the DP stage, while for others this could happen at the SP stage. However, at least part of the CD1a− DP cells could be derived not from CD1a+ DP cells but from CD1a+CD4+ SP cells. This is suggested by the experiments in which CD1a+CD4+ SP thymocytes were cocultured with short term cultures of postnatal stromal cells. The cells harvested from such cocultures lacked CD1a and expressed CD3 and CD4, but a large proportion of these cells coexpress CD8α. This phenotype persisted after expansion of these cells. It is also possible that some of the CD1a− DP cells are derived from CD1a− SP cells as suggested by the experiments depicted in Fig. 6. Finally, we have considered the possibility that the CD1a− DP cells are derived from cells that never expressed CD1. This cannot be excluded; however, we consider this unlikely since virtually all CD3−CD4+ immature SP cells considered to be the precursors of the DP cells, express CD1a (39). Further experiments are needed to elucidate the differentiation patterns of thymocytes after being submitted to positive selection mediated by the MHC/TCR interaction.
Acknowledgments
We thank F. Couwenberg for technical assistance, Drs. L. Lanier, J.H. Phillips, E. Reinherz, R. van Lier, and G. Aversa for their gifts of mAbs and Drs. L. Glimcher and A. Kruisbeek for making available the MHC class II KO mice. We confer our thanks to Dr. A. Kruisbeek for a critical review of the manuscript.
Footnotes
This work was supported by a grant from the Dutch Cancer Foundation (No. NKI 95-960).
1 Abbreviations used in this paper: DP, double positive; FTOC, fetal thymic organ culture; HPRT, hypoxanthine phosphoribosyl transferase; RAG, recombination activating gene; SP, single positive; TRC, TriColor.
References
- 1.Von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CAJ. Thymic selection: two pathways to life and death. Immunity. 1994;1:3–6. doi: 10.1016/1074-7613(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 3.Hogquist KA, Jameson SC, Bevan MJ. The ligand for positive selection of T lymphocytes in the thymus. Curr Opin Immunol. 1994;6:273–278. doi: 10.1016/0952-7915(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 4.Allan PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 5.Robey EA, Fowlkes BJ, Gordon JW, Kioussis D, von Boehmer H, Ramsdell F, Axel R. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 1991;64:99–107. doi: 10.1016/0092-8674(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 6.Borgulya P, Kishi H, Muller U, Kirberg J, von Boehmer H. Development of the CD4 and CD8 lineage of T cells: instruction versus selection. EMBO (Eur Mol Biol Organ) J. 1991;10:913–918. doi: 10.1002/j.1460-2075.1991.tb08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SH, Benoist C, Mathis D. A challenge to the instructive model of positive selection. Immunol Rev. 1993;135:119–131. doi: 10.1111/j.1600-065x.1993.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis CB, Killeen N, Crooks ME, Raulet D, Littman DR. Evidence for a stochastic mechanism in the differentiation of mature subsets of T lymphocytes. Cell. 1993;73:237–247. doi: 10.1016/0092-8674(93)90226-g. [DOI] [PubMed] [Google Scholar]
- 9.Itano A, Cado D, Chan FKM, Robey E. A role for the cytoplasmic tail of the β chain of CD8 in thymic selection. Immunity. 1994;1:287–290. doi: 10.1016/1074-7613(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, Day LM, Scollay R, Shortman K. Subpopulations of mature murine thymocytes: properties of CD4−CD8+ and CD4+CD8− thymocytes lacking the heat stable antigen. Cell Immunol. 1988;117:312–316. doi: 10.1016/0008-8749(88)90121-9. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic-Zugic J, Bevan MJ. Functional and phenotypic delineation of two subsets of CD4 single positive cells in the thymus. Int Immunol. 1990;2:135–141. doi: 10.1093/intimm/2.2.135. [DOI] [PubMed] [Google Scholar]
- 12.Dyall R, Nikolic-Zugic J. The majority of postselection CD4+ single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Libero G, Lanzavecchia A. Establishment of human double-positive thymocyte clones. J Exp Med. 1989;170:303–308. doi: 10.1084/jem.170.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boumsell L, Schmid M, Dastot H, Gouttefangeas C, Mathieu-Mahul D, Bensussan A. In vitro differentiation from a pluripotent human CD4+CD8+ thymic cloned cell into four phenotypically distinct subsets. J Immunol. 1990;145:2797–2802. [PubMed] [Google Scholar]
- 15.Howe RC, MacDonald HR. Clonogenic potential of murine CD4+8+ thymocytes. Direct demonstration using a V beta 6–specific proliferative stimulus in Mlsa mice. J Immunol. 1989;143:793–797. [PubMed] [Google Scholar]
- 16.Phillips JH, Hori T, Nagler A, Bhat N, Spits H, Ranier LL. Ontogeny of human natural killer (NK) cells: fetal NK cells mediate cytolytic function and express cytoplasmic CD εδ proteins. J Exp Med. 1992;175:1055–1066. doi: 10.1084/jem.175.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yssel H, De Vries J E, Koken M, van Blitterswijk W, Spits H. Serum-free medium for the generation and the propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 18.Res P, Martínez E, Cáceres, Jaleco AC, Noteboom E, Weijer K, Spits H. CD34+CD38dimcells in the human thymus can differentiate into T, natural killer and dendritic cells but are distinct from stem cells. Blood. 1996;87:5196–5206. [PubMed] [Google Scholar]
- 19.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II–deficient mice. Science (Wash DC) 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 20.Galy AH, Spits H. IL-1, IL-4, and IFN-gamma differentially regulate cytokine production and cell surface molecule expression in cultured human thymic epithelial cells. J Immunol. 1991;147:3823–3830. [PubMed] [Google Scholar]
- 21.Galy AH, Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992;149:775–782. [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.O'Garra A, Viera P. Polymerase chain reaction for detection of cytokine gene expression. Curr Opin Immunol. 1992;4:211–215. doi: 10.1016/0952-7915(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 24.Ranes-Goldberg MG, Hori T, Mohan-Peterson S, Spits H. Identification of human pre-T/NK cell–associated genes. J Immunol. 1993;151:5810–5821. [PubMed] [Google Scholar]
- 25.Lanier LL, Allison JP, Phillips JH. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986;137:2501–2507. [PubMed] [Google Scholar]
- 26.Yamashita I, Nagata T, Tada T, Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor–mediated positive selection. Int Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- 27.Lucas B, Vasseur F, Penit C. Production, selection, and maturation of thymocytes with high surface density of TCR. J Immunol. 1994;153:53–62. [PubMed] [Google Scholar]
- 28.Brändle D, Müller S, Müller C, Hengartner H, Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur J Immunol. 1994;24:145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- 29.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+CD8+ stage during positive selection and promotes thymocytes differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 30.Gratton-Deans J, Merino R, Nunez G, Turka LA. Bcl-2 expression during T cell development: early loss and late return occur at specific stages of commitment to differentiation and survival. Proc Natl Acad Sci USA. 1994;91:10685–10689. doi: 10.1073/pnas.91.22.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martorell J, Roho I, Vilella R, Martinez-Caceres E, Vives J. CD27 induction on thymocytes. J Immunol. 1990;145:1356–1363. [PubMed] [Google Scholar]
- 32.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 33.Brändle D, Muller C, Rulicke T, Hengartner H, Pircher H. Engagement of the T-cell receptor during positive selection in the thymus down-regulates RAG-1 expression. Proc Natl Acad Sci USA. 1992;89:9529–9533. doi: 10.1073/pnas.89.20.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher AG, Larson L, Goff LK, Restall DE, Happerfield L, Merkenschlager M. Human thymocyte development in mouse organ cultures. Int Immunol. 1990;2:571–578. doi: 10.1093/intimm/2.6.571. [DOI] [PubMed] [Google Scholar]
- 35.Yeoman H, Gress RE, Bare CE, Leary AG, Boyse EA, Bard J, Shultz LD, Harris DT, DeLuca D. Human bone marrow and umbilical cord blood cells generate CD4+ and CD8+ single-positive T cells in murine fetal thymus organ culture. Proc Natl Acad Sci USA. 1993;90:10778–10782. doi: 10.1073/pnas.90.22.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plum J, De Smedt M, Defresne M-P, Leclercq G, Vanderkerckhove B. Human CD34+ fetal stem liver cells differentiate into T cells in a mouse thymic microenvironment. Blood. 1994;84:1587–1594. [PubMed] [Google Scholar]
- 37.Sánchez MJ, Spits H, Lanier LI, Philips JH. Human natural killer cell committed thymocytes and their relationship to the T cell lineage. J Exp Med. 1993;178:1857–1866. doi: 10.1084/jem.178.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killeen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO (Eur Mol Biol Organ) J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galy A, Verma S, Barcena A, Spits H. Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymic development. J Exp Med. 1993;178:391–401. doi: 10.1084/jem.178.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Márquez C, Trigueros C, Fernández E, Toribio ML. The development of T and non-T cell lineages from CD34+human thymic precursors can be traced by the differential expression of CD44. J Exp Med. 1995;181:475–483. doi: 10.1084/jem.181.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moots RJ, Samberg NL, Pazmany L, Frelinger JA, McMichael AJ, Strauss HJ. A cross-species functional interaction between the murine major histocompatibility complex class I α3 domain and human CD8 revealed by peptide-specific cytotoxic T lymphocytes. Eur J Immunol. 1992;22:1643–1646. doi: 10.1002/eji.1830220645. [DOI] [PubMed] [Google Scholar]
- 42.Vanhecke D, Verhasselt B, Debacker V, Leclercq G, Plum J, Vandekerckhove B. Differentiation to T helper cells in the thymus: gradual acquisition of T helper cell function by CD3+CD4+ cells. J Immunol. 1995;155:4711–4718. [PubMed] [Google Scholar]
- 43.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during differentiation of human CD69+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–1872. [PubMed] [Google Scholar]
- 44.Pircher H, Ohashi PS, Boyd RL, Hengartner H, Brduscha K. Evidence for a selective and multi-step model of T cell differentiation: CD4+CD8low thymocytes selected by a transgenic T cell receptor on major histocompatibility complex class I molecules. Eur J Immunol. 1994;24:1982–1987. doi: 10.1002/eji.1830240907. [DOI] [PubMed] [Google Scholar]
- 45.Kisielow P, Miazek A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;1815:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson RW, Anderson G, Owen J, Jenkinson EJ. Positive selection of thymocytes involves sustained interactions with the thymic microenvironment. J Immunol. 1995;155:5234–5240. [PubMed] [Google Scholar]
- 47.Moebius U, Kober G, Griscelli AL, Hercend T, Meuer SC. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]