Abstract
CD40 ligand (CD40L)-deficient C57BL/6 mice failed to control intracellular Leishmania donovani visceral infection, indicating that acquired resistance involves CD40-CD40L signaling and costimulation. Conversely, in wild-type C57BL/6 and BALB/c mice with established visceral infection, injection of agonist anti-CD40 monoclonal antibody (MAb) induced killing of ∼60% of parasites within liver macrophages, stimulated gamma interferon (IFN-γ) secretion, and enhanced mononuclear cell recruitment and tissue granuloma formation. Comparable parasite killing was also induced by MAb blockade (inhibition) of cytotoxic T lymphocyte antigen-4 (CTLA-4) which downregulates separate CD28-B7 T-cell costimulation. Optimal killing triggered by both anti-CD40 and anti-CTLA-4 required endogenous IFN-γ and involved interleukin 12. CD40L−/− mice also failed to respond to antileishmanial chemotherapy (antimony), while in normal animals, anti-CD40 and anti-CTLA-4 synergistically enhanced antimony-associated killing. CD40L-CD40 signaling regulates outcome and response to treatment of experimental visceral leishmaniasis, and MAb targeting of T-cell costimulatory pathways (CD40L-CD40 and CD28-B7) yields macrophage activation and immunotherapeutic and immunochemotherapeutic activity.
In visceral leishmaniasis, an intracellular protozoan infection which targets macrophages in the liver, spleen, and bone marrow, successful experimental host defense is T-cell (Th1 cell) dependent, requires T-cell- and macrophage-activating cytokines, and is expressed in the tissues by granulomatous inflammation (23, 24). While multiple cytokines enable normal BALB/c and C57BL/6 mice to acquire resistance to visceral Leishmania donovani infection (6, 23, 24, 34), interleukin 12 (IL-12) and gamma interferon (IFN-γ) play particularly prominent roles. IL-12 likely drives the Th1 cell-associated mechanism and induces IFN-γ, both cytokines direct T cells and blood monocytes into granulomas at parasitized tissue foci, and IFN-γ activates effector monocytes and macrophages to kill intracellular parasites (6, 23, 24, 34). Endogenous IL-12 and IFN-γ, as well as other cytokines (1, 29), are also required for the leishmanicidal effect of pentavalent antimony (Sb), used as conventional chemotherapy for visceral leishmaniasis (23, 27, 28).
Efforts to translate the preceding experimental findings into treatment for L. donovani infection have primarily revolved around injecting IFN-γ or IL-12 or other IFN-γ inducers (e.g., IL-2 [23]) or administering agents which reverse suppression of the Th1 cell response (e.g., anti-IL-10 receptor monoclonal antibody [MAb]) (30, 31). These treatments produce leishmanicidal activity by themselves and, when combined with Sb, enhance drug efficacy (23, 30, 31).
An alternative strategy to therapeutically harness the same Th1 cell mechanism has focused on T-cell costimulation (7, 11, 19, 20). Optimal T-cell activation, including induction of the antileishmanial Th1 response with secretion of IL-12, IL-2, and IFN-γ, requires second (costimulatory) signals likely delivered via interaction of surface molecules on T cells and antigen-presenting cells (APC) (7, 11, 19, 20, 39, 41). CD40 ligand [CD40L]-CD40 and CD28-B7 represent two such signal-transducing receptor pathways (38, 40), active in various forms of experimental leishmaniasis (3, 4, 10, 11, 14, 15, 17, 32, 37, 38) and accessible to manipulation by MAb injection (7, 15, 18-20). Depending upon the model, the host, and the timing of MAb administration, receptor manipulation can stimulate Th1 type responses and enhance resistance.
For example, when given prophylactically, injections of agonist anti-CD40 MAb successfully curtail cutaneous L. major infection in susceptible BALB/c mice, an effect mediated by APC-secreted IL-12 and downstream T-cell-derived IFN-γ (7). Similarly, MAb-induced blockade of cytotoxic T lymphocyte antigen-4 (CTLA-4), an inhibitory receptor which limits CD28-B7 costimulation (15, 19, 20, 40), can also enhance IL-12 production and IFN-γ-mediated events (20). While anti-CTLA-4 treatment produces variable effects and can exacerbate cutaneous L. major infection (10, 15), anti-CTLA-4 is active both prophylactically and therapeutically against visceral L. donovani infection (20). In vitro studies with L. chagasi, another agent of visceral leishmaniasis, also support the antileishmanial effect of MAb blockade of either T-cell-expressed CTLA-4 or its B7 ligands on APC (10, 11).
Increased levels of endogenous IL-12 and/or IFN-γ would be expected to enhance antileishmanial defense and strengthen the host response to Sb chemotherapy (20, 23). Therefore, in this study, anti-CD40 was used as an agonist to ligate CD40 and trigger IL-12 secretion (5, 14, 33, 39) while anti-CTLA-4 was used to block negative signaling and maintain CD28-B7-dependent T-cell activation and cytokine secretion (15, 20, 41, 43). We reasoned that in addition to producing activity by itself in the case of established L. donovani infection (20), MAb-induced modulation of T-cell costimulatory mechanisms could also be coupled with Sb in an immunochemotherapeutic regimen.
MATERIALS AND METHODS
Animals.
Twenty- to 30-g female C57BL/6 and BALB/c mice, purchased from the Jackson Laboratory (Bar Harbor, Maine) and Charles River Laboratories (Wilmington, Mass.), respectively, were used as wild-type controls. Pairs of gene-disrupted mice for breeding on a C57BL/6 background were originally obtained from the following sources: CD40L−/−, intracellular adhesion molecule-1 (ICAM-1)-deficient, and IFN-γ−/− mice were from Jackson (22, 27); inducible nitric oxide synthase (iNOS)−/− mice were from C. Nathan, Weill Medical College, New York, N.Y. (26); and respiratory burst (phagocyte oxidase [phox])-deficient gp91 phox−/− mice were from M. Dinauer, Indiana University Medical Center, Indianapolis, Ind. (26). IL-12p35−/− breeders on a BALB/c background were originally provided by J. Sypek (Genetics Institute, Andover, Mass.) (28). Mice were 6 to 12 weeks old when challenged with L. donovani; male and female gene-disrupted mice were used in a random fashion.
Visceral infection and tissue response.
Groups of four to five mice were injected via the tail vein with 1.5 × 107 hamster spleen-derived L. donovani amastigotes (1 Sudan strain) (30). Visceral infection was monitored microscopically using Giemsa-stained liver imprints in which liver parasite burdens were measured by blinded counting of the number of amastigotes per 500 cell nuclei and multiplication by the liver weight in milligrams (liver parasite burdens are expressed in Leishman-Donovan units [LDU]) (30). The histologic response to infection was assessed microscopically in liver sections stained with hematoxylin and eosin. The numbers of granulomas (infected Kupffer cells which had attracted ≥5 mononuclear cells) were counted in 100 consecutive fields with magnification of ×40, and at 100 parasitized foci, the reaction was scored as (i) none (infected Kupffer cell with no mononuclear cell infiltrate) or (ii) presence of developing or mature granulomas (24, 30). The latter consisted of a core of fused infected Kupffer cells surrounded by numerous mononuclear cells and showed epithelioid-type changes (24, 30).
Treatment with anti-CD40 or anti-CTLA-4 MAb and/or chemotherapy.
Treating infected mice with immunopotentiating agents 2 days prior to injecting Sb optimizes drug efficacy (21, 28, 30). Therefore, since Sb is administered 14 days after L. donovani challenge in this model (28, 30) (see below), MAb was given on day 12 after challenge. Single intraperitoneal (i.p.) injections contained (i) 0.1 to 0.5 mg of rat immunoglobulin G2a (IgG2a) anti-mouse CD40 (FGK45) (18) or purified normal rat IgG (Sigma Chemical Co., St. Louis, Mo.) or (ii) 0.1 to 0.5 mg of hamster anti-mouse CTLA-4 (UC10-4F10-11) provided by J. Bluestone (University of California San Francisco Medical Center, San Francisco) (15) or purified normal hamster IgG (Jackson ImmunoResearch, Baltimore, Md.). To test chemotherapy alone, mice received a single i.p. injection of Sb or three alternate-day i.p. injections of amphotericin B (AmB) (27, 30). Sb (sodium stibogluconate [Pentostam]; Wellcome Foundation Ltd., London, United Kingdom) was given on day 14 at either 500 (optimal dose) or 50 mg/kg of body weight (30); AmB (Gensia Laboratories Ltd., Irvine, Calif.) was used at an optimal dose of 5 mg/kg and given on days 14, 16, and 18 (27, 28, 30). To test chemotherapy in anti-CD40- or anti-CTLA-4-treated animals, mice were injected once with suboptimal MAb or control IgG on day 12 and then once with low-dose Sb (50 mg/kg) on day 14. Day 21 liver parasite burdens (measured in LDU) were compared to day 14 LDU values for mice which had received no treatment to determine percent parasite killing (30); differences between mean values were analyzed by a two-tailed Student t test.
Cytokine assays.
Blood was obtained before and 12 to 21 days after infection. Serum from each mouse was assayed by enzyme-linked immunosorbent assay in duplicate at fourfold dilutions for IL-12p40 and IFN-γ by using antibody pairs from BD Pharmingen (San Diego, Calif.) as described previously (2). Lower limits of detection were 40 pg/ml for IL-12p40 and 6 pg/ml for IFN-γ. Results for MAb- versus control IgG-treated mice were compared using the Mann-Whitney U nonparametric analysis for two independent samples.
RESULTS
CD40L and anti-CD40 in defense against L. donovani.
CD28-B7 signaling has already been shown to be active in experimental L. donovani infection since manipulation of this pathway by blockade of CTLA-4 or one of its B7 ligands increased Th1 type cytokine expression, promoted granuloma formation, and reduced liver parasite burdens (19, 20). To determine whether the CD40L-CD40 mechanism can mediate a similar effect, we first challenged CD40L−/− mice with L. donovani and then treated parasitized normal mice with agonist anti-CD40 MAb. In the latter experiments, anti-CTLA-4 was tested in parallel.
CD40L-deficient mice.
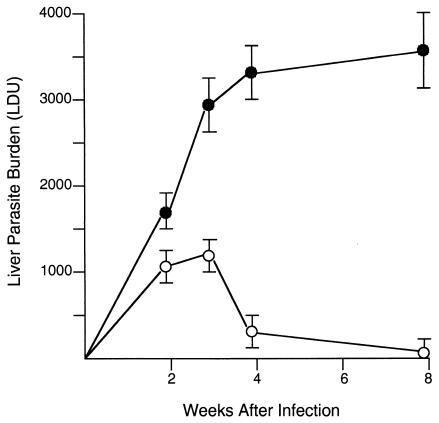
As shown in Fig. 1, wild-type C57BL/6 mice controlled L. donovani infection after week 3, demonstrating a Th1 cell type-dependent response which requires IL-12 and IFN-γ (6, 27, 28, 34, 40). In contrast, CD40L−/− mice developed high-level, nonresolving liver infections. Since CD40L-CD40 interaction is a primary stimulus for IL-12 generation by APC (5, 14, 33, 39, 41), the failure to control L. donovani infection likely involved deficient Th1 cell type responses. In one of the experiments with results shown in Fig. 1, sera (from three to four mice per group) were available for cytokine measurement before (day 0) and on day 21 after infection. In wild-type C57BL/6 mice, levels of IL-12p40 increased from 3.0 ± 0.1 to 8.1 ± 0.4 ng/ml and those of IFN-γ increased from 0.01 ± 0.01 to 0.25 ± 0.11 ng/ml. In CD40L−/− mice, corresponding day 0 and day 21 values for IL-12p40 were 1.9 ± 0.3 and 2.8 ± 0.3 ng/ml and those for IFN-γ were 0 (<6 pg/ml) and 0.01 ± 0.01 ng/ml. Thus, on day 21, when liver parasite burdens in control and deficient mice had clearly diverged (Fig. 1), serum IL-12p40 and IFN-γ levels appeared to be lower in CD40−/− mice. Uncontrolled L. major and L. amazonensis cutaneous infection in CD40L−/− and CD40−/− mice has also been previously linked to defective IL-12 and IFN-γ generation (4, 17, 37).
FIG. 1.
Course of L. donovani infection in livers of wild-type C57BL/6 (open circles) and CD40L−/− (closed circles) mice. Results shown are means ± standard errors of the means (SEM) of values from two experiments with 8 to 10 mice for each time point. P is <0.05 for control versus CD40L−/− mice at weeks 3, 4, and 8.
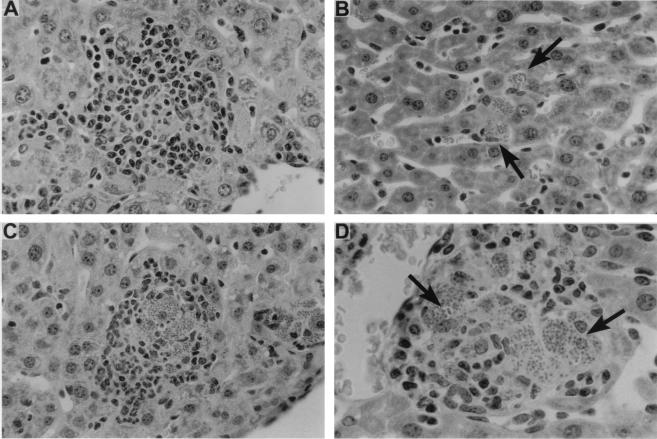
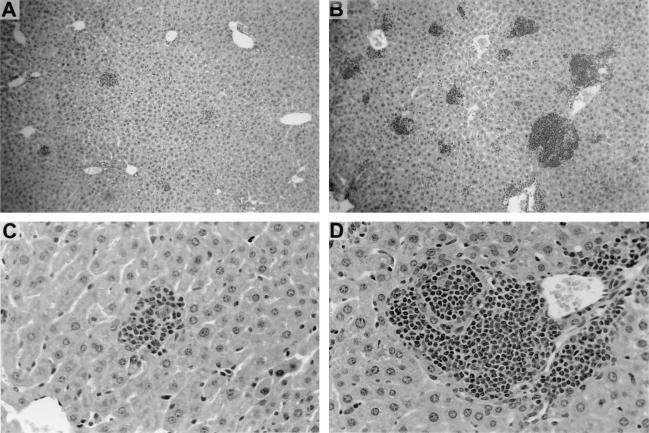
The tissue correlate of the IL-12- and IFN-γ-driven response, granuloma assembly (24), was also impaired in CD40L-deficient mice. In livers of wild-type C57BL/6 mice, granulomas were forming by week 2 at 89% ± 3% of parasitized foci and were developed (mature) at 92% ± 5% of foci by week 4 (Fig. 2A). In CD40−/− mice, this response was essentially absent at week 2 and barely evident at week 4 (Fig. 2B), and then it emerged but remained incomplete. At week 8, 23% ± 6% of infected sites showed no mononuclear cell recruitment; the remainder showed developing (62% ± 7%) or morphologically mature (15% ± 2%) granulomas (Fig. 2C). However, virtually all of the latter contained large numbers of amastigotes (Fig. 2D), indicating that granulomas appearing to be structurally intact were nonfunctional in the absence of CD40L.
FIG. 2.
Histologic response to L. donovani infection in livers of wild-type C57BL/6 and CD40L−/− mice. (A and B) Four weeks after infection, wild-type C57BL/6 mice (A) show formation of mature granulomas while CD40L-deficient mice (B) show no granuloma formation at parasitized foci (arrows). (C and D) At 8 weeks, granulomas have developed in CD40L-deficient mice but remain heavily parasitized (arrows). Panels A, B, and C, magnification of ×284; panel D, magnification of ×450.
Anti-CD40-treated mice.
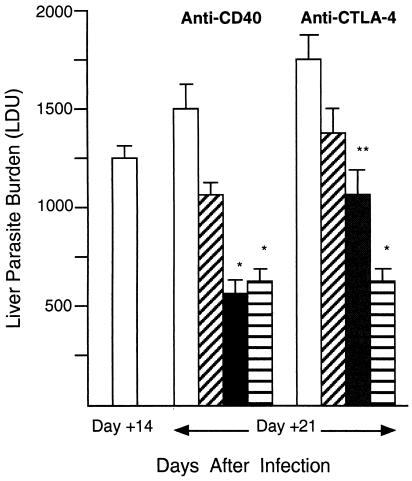
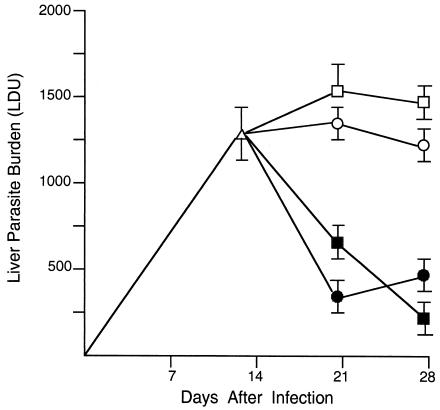
To demonstrate the effect of CD40 ligation under intact conditions, wild-type BALB/c mice with established infection were injected with agonist anti-CD40 on day 12. Measurements made on day 21 showed that treatment with 0.25 to 0.5 mg of MAb induced leishmanicidal activity, with >50% reduction in liver parasite burdens (Fig. 3). Wild-type C57BL/6 mice responded in a similar fashion (Table 1). In addition, the antileishmanial effect of single-dose anti-CD40 persisted beyond day 21 (Fig. 4).
FIG. 3.
Leishmanicidal effect of anti-CD40 and anti-CTLA-4 MAbs in wild-type BALB/c mice. On day 12 after infection, mice were injected once with either control IgG or one of three doses of anti-CD40 or anti-CTLA-4; liver burdens (LDU) were determined on day 21. LDU values for untreated mice on day 14 were used for comparison with day 21 LDU values for treated animals. Treatment of the anti-CD40 group was as follows: rat IgG (0.5 mg; open bar) or MAb at 0.1 mg (diagonally hatched bar), 0.25 mg (closed bar), or 0.5 mg (horizontally hatched bar). Treatment of the anti-CTLA-4 group was as follows: hamster IgG (0.5 mg; open bar) or MAb at 0.1 mg (diagonally hatched bar), 0.3 mg (closed bar), or 0.5 mg (horizontally hatched bar). Results shown are means ± SEM of results from two to three experiments with 7 to 16 mice per group. *, P of <0.05 versus day 14 LDU value and versus day 21 LDU value for IgG-treated controls. **, P of <0.05 versus day 21 LDU value for IgG-treated controls.
TABLE 1.
Leishmanicidal effects of anti-CD40 and anti-CTLA-4 treatmentsa
| Mouse group | Treatmentb
|
Liver parasite burden (LDU) on day:
|
% Killing | |||
|---|---|---|---|---|---|---|
| IgG | Anti- CD40 | Anti- CTLA-4 | 14 | 21 | ||
| BALB/c | ||||||
| Wild type | + | 1,087 ± 81 | 1,345 ± 99 | 0 | ||
| + | 457 ± 56c | 58 | ||||
| + | 1,833 ± 134 | 0 | ||||
| + | 500 ± 67c | 52 | ||||
| IL-12p35−/− | + | 1,703 ± 141 | 2,004 ± 179 | 0 | ||
| + | 1,513 ± 164 | 11 | ||||
| + | 2,412 ± 201 | 0 | ||||
| + | 1,834 ± 146 | 0 | ||||
| C57BL/6 | ||||||
| Wild-type | + | 898 ± 96 | 1,024 ± 88 | 0 | ||
| + | 341 ± 51c | 62 | ||||
| + | 1,145 ± 141 | 0 | ||||
| + | 458 ± 64c | 49 | ||||
| IFN-γ−/− | + | 2,177 ± 199 | 2,954 ± 290 | 0 | ||
| + | 3,152 ± 241 | 0 | ||||
| + | 2,896 ± 311 | 0 | ||||
| + | 2,579 ± 266 | 0 | ||||
| ICAM-1−/− | + | 1,627 ± 182 | 1,828 ± 124 | 0 | ||
| + | 748 ± 61c | 54 | ||||
| + | 2,635 ± 288 | 0 | ||||
| + | 881 ± 99c | 46 | ||||
| iNOS−/− | + | 2,057 ± 202 | 2,391 ± 226 | 0 | ||
| + | 894 ± 121c | 56 | ||||
| + | 2,501 ± 183 | 0 | ||||
| + | 994 ± 79c | 52 | ||||
| phox−/− | + | 2,116 ± 158 | 2,201 ± 191 | 0 | ||
| + | 773 ± 60c | 63 | ||||
| + | 2,619 ± 246 | 0 | ||||
| + | 903 ± 100c | 57 | ||||
Twelve days after infection, mice received one injection of either 0.25 mg of rat IgG or anti-CD40 or 0.5 mg of hamster IgG or anti-CTLA-4, and LDU values were determined on day 21. Day 14 values indicate LDU values for untreated mice. Results shown are means ± SEM of results from two to three experiments with 7 to 10 mice per group.
+, mice received indicated treatment.
P, <0.05 versus day 14 value.
FIG. 4.
Extended antileishmanial effects of anti-CD40 and anti-CTLA-4 MAb treatment. Wild-type BALB/c mice were injected once 12 days after infection with 0.25 mg of rat IgG (open circles) or anti-CD40 (closed circles) or 0.5 mg of hamster IgG (open squares) or anti-CTLA-4 (closed squares). Results shown are means ± SEM of results from two experiments with seven to eight mice per time point. P is <0.05 for results with anti-CD40 and anti-CTLA-4 at both days 21 and 28 versus the day 12 LDU value.
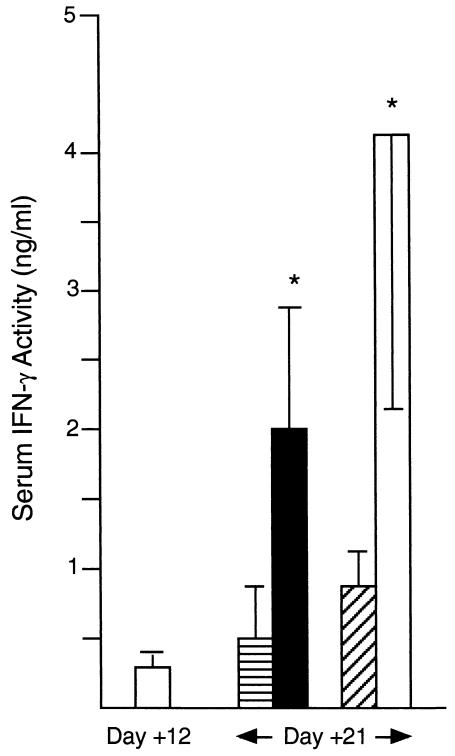
The anti-CD40 effect was also accompanied by increased IFN-γ production, as judged by cytokine levels in the serum of BALB/c mice on day 21 (Fig. 5). In the same mice, IL-12p40 was already readily detectable in sera on day 12, prior to MAb injection (8.1 ± 1.1 ng/ml; n = 10). Serum IL-12p40 levels increased in anti-CD40-treated mice at day 21; however, the day 21 level (11.1 ± 1.4 ng/ml; n = 10) was not significantly different from that in infected controls treated with rat IgG (7.8 ± 0.8 ng/ml; n = 6 [P = 0.11]). Since increases in serum IL-12 levels (as well as IFN-γ levels) appear to peak soon after (38) or within 3 to 5 days (F. Heinzel, unpublished data) of anti-CD40 injection, IL-12p40 levels prior to day 21 may have been higher in our experiments. In addition, it is also possible that examination at the infected tissue site (e.g., at developing liver granulomas) would have better demonstrated an anti-CD40 effect on IL-12 expression.
FIG. 5.
Serum IFN-γ levels on day 21, after MAb treatment on day 12. Wild-type BALB/c mice were injected once 12 days after infection with either 0.25 mg of rat IgG (horizontally hatched bar) or anti-CD40 (closed bar) or 0.5 mg of hamster IgG (diagonally hatched bar) or anti-CTLA-4 (open bar). Results shown are means ± SEM of results from two experiments with 6 to 10 mice. *, P of ≤0.05 versus day 21 value for IgG-treated controls.
Anti-CD40 MAb treatment also induced the remarkable appearance of numerous, often large focal collections of mononuclear cells (primarily lymphocytes, morphologically) in livers of infected BALB/c (Fig. 6 and 7) and C57BL/6 (data not shown) mice. These collections, not present in infected rat IgG-injected controls, were both perivascular and parenchymal and were, in most instances, densely encased parasitized macrophages (Fig. 6D). Anti-CD40 injection also induced some mononuclear cell accumulations in livers of uninfected BALB/c mice (Fig. 7). However, the numbers and sizes of the collections were considerably greater in infected mice (Fig. 7B and D), suggesting that anti-CD40-mobilized cells were attracted to sites of developing inflammation and/or parasite replication. In infected mice examined 2 weeks after anti-CD40 injection on day 12, the histologic reaction had begun to recede (data not shown).
FIG. 6.
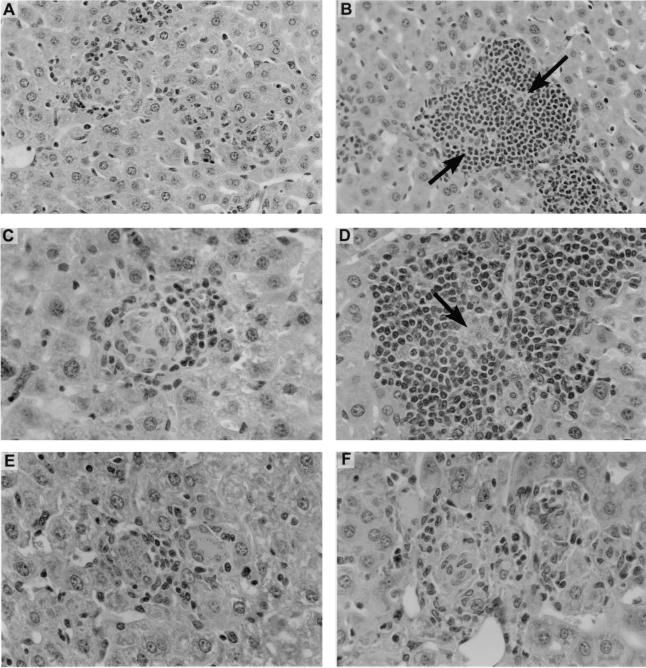
Liver histologic responses to anti-CD40 and anti-CTLA-4 MAbs in infected wild-type BALB/c mice. Mice were injected on day 12 with 0.25 mg of rat IgG or anti-CD40 or 0.5 mg of hamster IgG or anti-CTLA-4, and sections were prepared on day 21. (A and C) Infected foci in rat IgG-treated mice show early, developing granulomas. (B and D) In contrast, in anti-CD40-treated mice, parasitized foci are encased by large accumulations of mononuclear cells (arrows). (E and F) Developing granulomas in hamster IgG-treated liver (E) versus more-mature-appearing granulomas in anti-CTLA-4-injected animal (F). Panels A and B, magnification of ×180; panels C to F, magnification of ×284.
FIG. 7.
Histologic reactions in livers of uninfected and infected wild-type BALB/c mice 9 days after a single injection of 0.25 mg of anti-CD40 MAb. Tissue response in uninfected mice (A and C) is modest compared to the pronounced reaction in infected mice (B and D) which were injected on day 12 as described in the legend to Fig. 6. Panels A and B, magnification of ×45; panels C and D, magnification of ×180.
Effect of anti-CTLA-4 MAb versus that of anti-CD40 MAb.
In the experiments described above, wild-type BALB/c mice were also injected once on day 12 with anti-CTLA-4 MAb. Effects on day 21 mirrored those previously reported for anti-CTLA-4 (20): (i) leishmanicidal activity, which also persisted for ≥2 weeks (Figs. 3 and 4), (ii) increased IFN-γ secretion (Fig. 5), and (iii) some increase in granuloma numbers (1.6-fold increase) and maturity as judged by enhanced epithelioid changes (Fig. 6E and F). Serum IL-12p40 levels on day 21 were similar in MAb-treated (9.8 ± 1.7 ng/ml; n = 10) and hamster IgG-injected (10.6 ± 2.1 ng/ml; n = 6) BALB/c mice and not significantly different from pretreatment levels (8.1 ± 1.1 ng/ml). Anti-CTLA-4 was comparably active in wild-type C57BL/6 mice (Table 1) and did not induce the mononuclear cell collections observed in livers of anti-CD40-treated mice.
Treatment with anti-CD40 plus anti-CTLA-4.
CD40L-CD40 and CD28-B7, linked by induction of B7 expression by CD40 stimulation (5, 8, 13, 39, 41), also converge functionally since both support Th1 cell-associated responses and stimulate IFN-γ secretion downstream (39, 41). Since the initial activating cytokines primarily induced are thought to differ (i.e., IL-12 via CD40 versus IL-2 via CD28) (39, 41, 43), we tested whether leishmanicidal activity could be increased by triggering both pathways. Wild-type BALB/c mice were injected on day 12 with anti-CD40 (0.25 mg) and 2 h later with anti-CTLA-4 (0.5 mg). Combined treatment did not, however, increase parasite killing on day 21 nor yield additional histologic changes other than those induced by anti-CD40 alone (data not shown).
Host mechanisms in response to anti-CD40 and anti-CTLA-4.
In normal BALB/c and C57BL/6 mice, the IL-12-driven Th1 cell response activates macrophages for intracellular L. donovani killing within encircling granulomas (23, 24). The leishmanicidal mechanism requires IFN-γ induced by IL-12 and/or IL-2, influxing T cells and blood monocytes, and mononuclear phagocyte secretion of toxic products, largely iNOS-derived reactive nitrogen intermediates (6, 20, 22, 23, 24, 26, 28, 34, 40). To characterize these host components in responses to anti-CD40 and anti-CTLA-4, we tested mice deficient in cytokine production (IL-12p35−/− and IFN-γ−/−), monocyte influx (ICAM-1 deficient), and reactive nitrogen intermediate secretion (iNOS−/−) (22, 26, 28, 40).
Leishmanicidal responses.
Killing induced by both anti-CD40 and anti-CTLA-4 required endogenous IFN-γ (Table 1). Optimal killing also involved IL-12, although both MAbs induced a limited effect in IL-12p35−/− mice. In contrast, anti-CD40 and anti-CTLA-4 were fully active in mice deficient in ICAM-1, and killing was also retained in iNOS−/− animals. Mice deficient in the mononuclear phagocyte's auxiliary leishmanicidal mechanism, respiratory burst (phox)-generated toxic oxygen intermediates (26), were tested next. Phox-deficient mice also showed intact responses to both MAbs (Table 1), leaving open the question of what macrophage leishmanicidal pathway is triggered by MAb-induced CD40 ligation or CTLA-4 blockade.
Tissue granulomatous responses.
Expression of ICAM-1, an endothelial adhesion molecule, is required early on in L. donovani infection (weeks 1 to 4) for monocyte entry into and formation of liver granulomas (22). Nevertheless, ICAM-1-deficient mice treated on day 12 readily responded to both anti-CD40 and anti-CTLA-4 with mononuclear cell recruitment and assembly (Fig. 8A and B and data not shown). On day 21, the percentage of infected foci which showed mature granulomas increased from <10% in rat and hamster IgG-treated mice to 81% ± 4% and 84% ± 6%, respectively. This ICAM-1-independent tissue response may reflect activation of a compensatory mechanism previously demonstrated in these same ICAM-1-deficient mice (22).
FIG. 8.
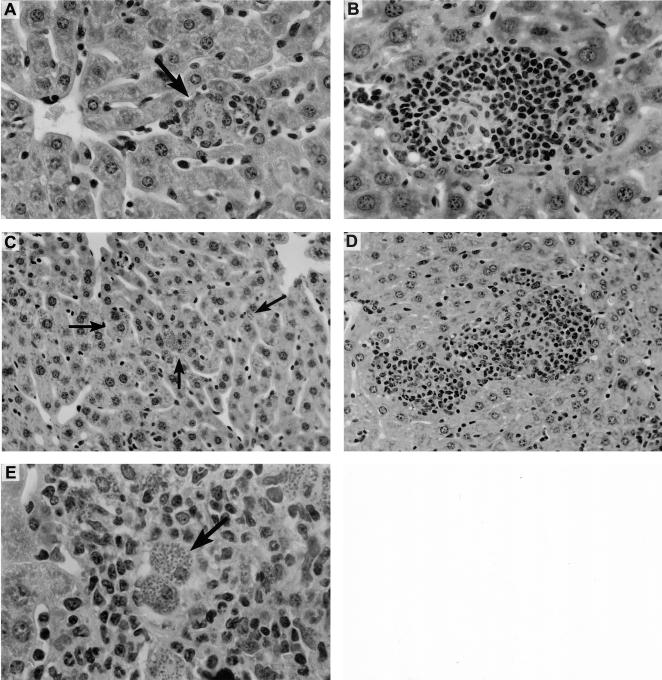
Liver histologic response to anti-CD40 on day 21 in granuloma-deficient ICAM-1−/− and IFN-γ−/− mice. Mice were injected on day 12 after infection with 0.25 mg of rat IgG or anti-CD40. (A and B) In ICAM-1−/− mice, IgG had no effect on deficient granuloma formation (arrow) (A), while anti-CD40 induced a granulomatous reaction at parasitized foci (B). (C to E) Rat IgG-treated IFN-γ-deficient mice show no tissue inflammatory response at infected foci (arrows) (C), while anti-CD40-induced granulomas (D) remain heavily parasitized (arrow) (E). Panels A and B, magnification of ×290; panels C and D, magnification of ×184; panel E, magnification of ×460.
Histologic inspection of livers from cytokine-deficient mice yielded additional information about the actions of anti-CTLA-4 and anti-CD40 and the ways in which their effects differ. In livers of IFN-γ−/− and IL-12p35−/− mice, which fail to show granulomas or any tissue inflammatory reaction in response to L. donovani (28, 34, 39), anti-CTLA-4 treatment on day 12 had no effect (data not shown). In contrast, in the same IFN-γ−/− mice, anti-CD40 induced both the mononuclear cell accumulations seen in normal mice and developing granulomas at 79% ± 9% of infected liver foci on day 21 (Fig. 8C and D). Thus, while IFN-γ is strictly required for granuloma assembly in intact mice (40), CD40 ligation (but not CTLA-4 blockade) activated an apparently quiescent mechanism for mononuclear cell recruitment in IFN-γ−/− mice. IL-12 is one inducer of such a compensatory, IFN-γ-independent granuloma-forming pathway (40). In IL-12p35−/− mice, anti-CD40 treatment had no effect on the absent histologic response (data not shown), suggesting that IL-12 may participate in the anti-CD40-induced IFN-γ-independent effect. Not surprisingly, however, in the absence of IFN-γ and macrophage activation (23, 24), granulomas induced by anti-CD40 in IFN-γ−/− mice remained heavily parasitized (Fig. 8E).
Responses to antileishmanial chemotherapy.
In the case of L. donovani infection, the leishmanicidal efficacy of Sb chemotherapy also requires host T cells and an intact Th1 cell type response with IL-12 and IFN-γ secretion (23, 27, 28). Sb's efficacy can be enhanced by IFN-γ either by coadministration in exogenous form or by injection of IL-12 to increase endogenous IFN-γ production (21, 25, 28). Therefore, we hypothesized that Sb's effect would be (i) diminished in Th1 cell cytokine-deficient CD40L-deficient mice and (ii) enhanced in normal animals first treated with anti-CD40 or anti-CTLA-4.
The results presented in Table 2 confirm the first hypothesis. Wild-type C57BL/6 animals responded to optimal-dose Sb (500 mg/kg) with killing of 88% of liver amastigotes; identically treated CD40L−/− mice failed to show leishmanicidal activity. To demonstrate responsiveness to directly acting chemotherapy which does not require the host Th1 cell response (23, 27, 28), animals were also treated with AmB. CD40L knockout mice responded normally to this agent (Table 2).
TABLE 2.
Response of CD40L knockout mice to chemotherapya
| Mouse group | Treatment | Liver parasite burden (LDU) on day:
|
% Killing | |
|---|---|---|---|---|
| 14 | 21 | |||
| C57BL/6 | None | 1,168 ± 122 | 1,315 ± 92 | 0 |
| Sb | 140 ± 37b | 88 | ||
| AmB | 91 ± 16b | 92 | ||
| CD40L−/− | None | 1,785 ± 201 | 2,911 ± 270 | 0 |
| Sb | 1,948 ± 194 | 0 | ||
| AmB | 52 ± 33b | 97 | ||
Two weeks after infection (day 14), C57BL/6 wild-type and CD40L−/− mice received no treatment, a single injection of 500 mg of Sb/kg, or three every-second-day injections of 5 mg of (AmB/kg). Results shown are means ± SEM of results from two experiments with six to seven mice per group.
P, <0.05 versus day 14 value.
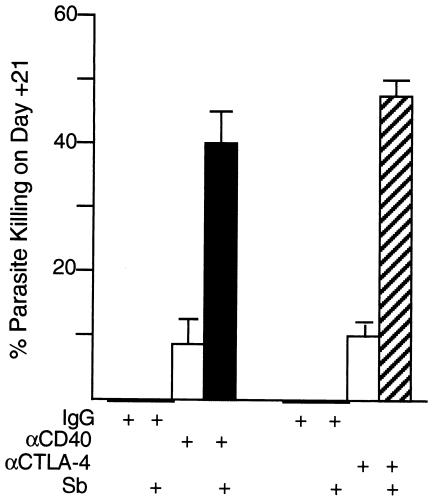
To test the second hypothesis and complete this analysis, we used wild-type BALB/c mice and injected suboptimal doses of MAb on day 12 and low-dose Sb on day 14. Injections were separated by 48 h since Sb's efficacy appears to be enhanced if administered after cytokine effects are established (21, 28, 30). Results on day 21 indicated no parasite killing by low-dose Sb in animals pretreated with either rat or hamster IgG (Fig. 9). In contrast, in anti-CD40- or anti-CTLA-4-treated animals, Sb was significantly more active (P < 0.05) and induced killing of 40 to 50% of amastigotes. A portion of the effect (∼10%) was due to MAb by itself (Fig. 9). However, four- to fivefold increases in killing induced by combination treatment indicated clearly enhanced responsiveness to Sb in the presence of either CD40 ligation or MAb-induced blockade of CTLA-4.
FIG. 9.
Treatment with anti-CD40 or anti-CTLA-4 enhances effect of antimony (Sb) in wild-type BALB/c mice. Liver parasite burdens were determined 12 days after infection, and mice were then injected once with either control rat or hamster IgG or suboptimal anti-CD40 (0.1 mg) or anti-CTLA-4 (0.3 mg). Mice either were not treated further or received low-dose Sb on day 14. Liver burdens on day 21 were compared to day 14 levels (1,202 ± 58 LDU; n = 12) to determine percent parasite killing. Results are from two to three experiments with 7 to 12 mice per group. P is <0.05 for results with both anti-CD40 plus Sb and anti-CTLA-4 plus Sb versus (i) day 14 LDU value for untreated mice and (ii) day 21 results with all other indicated treatments.
DISCUSSION
The findings in this report extend prior studies of the role and effect of the CD40-CD40L pathway in cutaneous L. major and L. amazonensis infection (3, 4, 7, 10, 14, 15, 17, 32, 37, 38) and of CTLA-4 blockade in L. major and L. donovani infection (10, 15, 20). Together, our results (i) demonstrate that CD40L is required for control of L. donovani infection, appropriate generation of IL-12p40 and especially IFN-γ, development of functional tissue granulomas, and response to Sb chemotherapy; (ii) identify mechanisms of the therapeutic effect of anti-CD40 MAb in the case of established visceral infection and show that anti-CD40 enhances IFN-γ and modifies mononuclear cell recruitment to parasitized sites; and (iii) confirm the antileishmanial action of CTLA-4 blockade in the case of L. donovani infection (20). In addition, our results indicate that both anti-CD40 and anti-CTLA-4 increase the efficacy of chemotherapy (Sb), consistent with effects on the Th1 type cytokine (e.g., IFN-γ) response which primarily regulates Sb's leishmanicidal activity (1, 23, 27). In the assays we employed, the only meaningful difference between the actions of the two MAbs was the capacity of anti-CD40 to stimulate focal recruitment of inflammatory mononuclear cells and to accomplish this effect in an IFN-γ-independent fashion.
Although induction of granuloma assembly in IFN-γ−/− mice demonstrated an IFN-γ-independent action for anti-CD40, its leishmanicidal activity and that of anti-CTLA-4 required endogenous IFN-γ and, to a lesser extent, its primary inducer, IL-12 (28). While anti-CD40 and anti-CTLA-4 both stimulated IFN-γ production, likely enhancing downstream effector cell mechanisms, parasite killing triggered by these MAbs was nevertheless preserved in mice deficient in either iNOS or phox. This finding was unexpected since anti-CD40 induces macrophage iNOS (42) and iNOS in turn regulates the key leishmanicidal pathway in the IFN-γ-activated macrophage (26). While a prior study indicated that the phox mechanism by itself was not sufficient to control L. donovani in otherwise untreated iNOS−/− mice (26), gene-deficient animals, including iNOS−/− and phox−/− mice, may express heightened compensatory responses (29, 30, 31). Thus, it is possible that the efficacy of phox in iNOS−/− mice was enhanced by anti-CD40 or anti-CTLA-4 treatment leading to respiratory burst-mediated parasite killing. However, we have not tested anti-CD40 or anti-CTLA-4 in mice deficient in both iNOS and phox (36); therefore, it also remains possible that a recently identified but undefined macrophage mechanism independent of both pathways may be involved (36). Given the intracellular location of L. donovani, the likelihood that anti-CD40 or anti-CTLA-4 stimulated an altogether separate, IFN-γ-dependent mechanism unrelated to macrophage activation seems low.
Anti-CD40 was used here to ligate CD40 and primarily trigger IL-12 secretion by APC, a well-defined effect of signaling through CD40 in dendritic cells (5, 14, 33, 39). Anti-CTLA-4 was employed to block negative signaling and maintain CD28-B7-dependent T-cell activation and cytokine (e.g., IL-2) secretion (15, 20, 41, 42). Despite differing modes of action, both MAb-directed immunointerventions would be expected to enhance IFN-γ production and generally raise the level of T-cell reactivity (9, 15, 20, 34, 37, 38, 40, 42). Such reactivity, expressed by increased Th1 cell type cytokine activity, regulates the outcome of visceral L. donovani infection and in vivo responsiveness to Sb chemotherapy (6, 23, 24, 27, 28, 30, 34, 40). Thus, in the case of established visceral infection, manipulation of CD40L-CD40 or CD23-B7 costimulatory mechanisms, conveniently achieved by a single injection of MAb, appears to represent a viable immunotherapeutic or immunochemotherapeutic strategy. Triggering other costimulatory pathways may also have similar treatment potential (8, 12, 32, 35, 43).
Since the preceding experiments were carried out in normal animals in the midst of developing a Th1 cell response, the therapeutic usefulness of anti-CD40 or anti-CTLA-4 is likely predicated on host capacity to satisfactorily produce IL-12 and IFN-γ. In addition, it is also worth noting that CD40 activation and blockade of CTLA-4 have the potential to induce the release of multiple proinflammatory cytokines (7, 16, 39, 41); thus, although not seen in our model, MAb treatment, especially if given repeatedly (16), could induce an undesirable inflammatory cytokine cascade. Nonetheless, from the perspective of treatment intended to activate macrophages, modulation of Th1 cell responses via costimulation pathways may well be an alternative to the administration of exogenous cytokines (23).
Acknowledgments
This work was supported by NIH grants AI 16393 (H.W.M.) and AI 35979 and AI 45602 (F.P.H.).
Editor: J. M. Mansfield
REFERENCES
- 1.Alexander, J., K. C. Carter, N. Al-Fasi, A. Satoskar, and F. Brombacher. 2000. Endogenous-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur. J. Immunol. 30:2935-2943. [DOI] [PubMed] [Google Scholar]
- 2.Balkhy, H. H., and F. P. Heinzel. 1999. Endotoxin fails to induce interferon-γ in endotoxin tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J. Immunol. 162:3633-3638. [PubMed] [Google Scholar]
- 3.Belkaid, Y. S., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a profound “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-976. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, K. A., P. J. Ovendale, M. K. Kennedy, W. C. Fanslow, S. G. Reed, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 5.Cella, M., D. Scheidegger, L. K. Palmer, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engwerda, C. R., M. L. Murphy, S. E. Cotterell, S. C. Smelt, and P. M. Kaye. 1998. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28:669-680. [DOI] [PubMed] [Google Scholar]
- 7.Ferlin, W. G., T. von der Weid, F. Cottrez, D. A. Ferrick, R. L. Coffman, and M. C. Howard. 1998. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur. J. Immunol. 28:525-531. [DOI] [PubMed] [Google Scholar]
- 8.Frauwirth, K. A., and C. B. Thompson. 2002. Activation and inhibition of lymphocytes by costimulation. J. Clin. Investig. 109:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French, R. R., H. T. C. Chan, A. L. Tutt, and M. J. Glennie. 1999. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548-553. [DOI] [PubMed] [Google Scholar]
- 10.Gomes, N. A. 2001. The dual role of CTLA-4 in Leishmania infection. Trends Parasitol. 17:487-491. [DOI] [PubMed] [Google Scholar]
- 11.Gomes, N. A., V. Barretto-de-Souza, M. E. Wilson, and G. A. DosReis. 1998. Unresponsive CD4+ T lymphocytes from Leishmania chagasi-infected mice increase cytokine production and mediate parasite killing after blockade of B7-1/CTLA-4 molecular pathway. J. Infect. Dis. 178:1847-1851. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald, R. J., A. J. McAdam, D. Van der Woude, A. B. Saroskar, and A. H. Sharpe. 2002. Cutting edge: inducible costimulator protein regulates both Th1 and Th2 responses to cutaneous leishmaniasis. J. Immunol. 168:991-995. [DOI] [PubMed] [Google Scholar]
- 13.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 14.Heinzel, F. P., R. M. Rerko, and A. M. Hujer. 1998. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell. Immunol. 184:129-142. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel, F. P., and R. A. Maier. 1999. Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA-4 antibody. Infect. Immun. 67:6454-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hixon, J. A., B. R. Blazar, M. R. Anver, R. H. Wiltrout, and W. J. Murphy. 2001. Antibodies to CD40 induce a lethal cytokine cascade after syngeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 7:136-143. [DOI] [PubMed] [Google Scholar]
- 17.Kamanaka, M., P. Yu, T. Yasui, K. Yoshide, T. Kawabe, T. Horii, T. Kishimoto, and H. Kikutanti. 1996. Protective role of CD40 in Leishmania major infection at distinct phases of cell-mediated immunity. Immunity 4:275-281. [DOI] [PubMed] [Google Scholar]
- 18.Martin, D. L., C. L. King, E. Pearlman, E. Strine, and F. P. Heinzel. 2000. IFN-γ is necessary but not sufficient for anti-CD40 antibody-mediated inhibition of the Th2 response to Schistosoma mansoni eggs. J. Immunol. 64:779-785. [DOI] [PubMed] [Google Scholar]
- 19.Murphy, M. L., C. R. Engwerda, P. M. A. Gorak, and P. M. Kaye. 1997. B7-2 blockade enhances T cell responses to Leishmania donovani. J. Immunol. 159:4460-4466. [PubMed] [Google Scholar]
- 20.Murphy, M. L., S. Cotterell, M. Gorak, C. R. Engwerda, and P. M. Kaye. 1998. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J. Immunol. 161:4153-4160. [PubMed] [Google Scholar]
- 21.Murray, H. W. 1990. Effect of continuous administration of interferon-gamma in experimental visceral leishmaniasis. J. Infect. Dis. 161:992-994. [DOI] [PubMed] [Google Scholar]
- 22.Murray, H. W. 2000. Mononuclear cell recruitment, granuloma assembly, and response to treatment in experimental visceral leishmaniasis: intracellular adhesion molecule 1-dependent and -independent regulation. Infect. Immun. 68:6294-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray, H. W. 2001. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 45:2185-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, H. W. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 82:249-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, H. W., J. D. Berman, and S. D. Wright. 1988. Immunochemotherapy for intracellular Leishmania donovani infection: interferon-gamma plus pentavalent antimony. J. Infect. Dis. 157:973-978. [DOI] [PubMed] [Google Scholar]
- 26.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen vs. oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray, H. W., and S. Delph-Etienne. 2000. Role of endogenous gamma interferon and macrophage microbicidal mechanisms in host response to chemotherapy in experimental visceral leishmaniasis. Infect. Immun. 68:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, H. W., C. Montelibano, R. Peterson, and J. P. Sypek. 2000. Interleukin 12 regulates the response to chemotherapy in experimental visceral leishmaniasis. J. Infect. Dis. 182:1497-1502. [DOI] [PubMed] [Google Scholar]
- 29.Murray, H. W., A. Jungbluth, E. Ritter, C. Montelibano, and M. W. Marino. 2000. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect. Immun. 68:6289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray, H. W., C. M. Lu, S. Mauze, S. Freeman, A. L. Moreira, G. Kapla, and R. L. Coffman. 2002. Interleukin 10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 70:6284-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, H. W., A. L. Moreira, C. M. Lu, J. L. DeVecchio, M. Matsuhashi, X. Ma, and F. P. Heinzel. Determinants of response to interleukin 10 receptor blockade immunotherapy in experimental visceral leishmaniasis. J. Infect. Dis.188:458-464. [DOI] [PubMed]
- 32.Padigel, U. M., P. J. Perrin, and J. P. Farrell. 2001. The development of a Th1-type response and resistance to Leishmania major infection in the absence of CD40-CD40L costimulation. J. Immunol. 167:5874-5879. [DOI] [PubMed] [Google Scholar]
- 33.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 34.Satoskar, A. R., S. Rodig, S. R. Telford, A. A. Satoskar, S. K. Ghosh, F. von Lichtenberg, and J. R. David. 2000. IL-12 gene-deficient C57BL/6 mice are susceptible to Leishmania donovani but have diminished hepatic pathology. Eur. J. Immunol. 30:834-839. [DOI] [PubMed] [Google Scholar]
- 35.Schultze, J., and P. Johnson. 1999. A stimulating new target for cancer immunotherapy. Lancet 354:1225-1226. [DOI] [PubMed] [Google Scholar]
- 36.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 37.Soong, L., J.-C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 38.Speirs, K., J. Caamano, M. H. Golschmidt, C. A. Hunter, and P. Scott. 2002. NF-κB2 is required for optimal CD40-induced IL-12 production but dispensable for Th1 cell differentiation. J. Immunol. 168:4406-4413. [DOI] [PubMed] [Google Scholar]
- 39.Stout, R. D., and J. Suttles. 1996. The many roles of CD40 in cell-mediated inflammatory responses. Immunol. Today 17:487-492. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, A., and H. W. Murray. 1997. Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin 12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J. Exp. Med. 185:1231-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, C. B., and J. P. Allison. 1997. The emerging role of CTLA-4 as an immune attenuator. Immunity 7:445-450. [DOI] [PubMed] [Google Scholar]
- 42.Tian, L., R. J. Noelle, and D. A. Lawrence. 1995. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur. J. Immunol. 25:306-309. [DOI] [PubMed] [Google Scholar]
- 43.Watts, T. H., and M. A. DeBenedette. 1999. T cell co-stimulatory molecules other than CD28. Curr. Opin. Immunol. 11:286-293. [DOI] [PubMed] [Google Scholar]