Abstract
Apoptosis of bystander uninfected CD4+ T lymphocytes by neighboring HIV-infected cells is observed in cell culture and in lymphoid tissue of HIV-infected individuals. This study addresses whether antigen-presenting cells such as human macrophages mediate apoptosis of CD4+ T cells from HIV-infected individuals. Uninfected human macrophages, and to a larger degree, HIV-infected macrophages mediate apoptosis of T cells from HIV-infected, but not from uninfected control individuals. This macrophage-dependent killing targets CD4+, but not CD8+ T lymphocytes from HIV-infected individuals, and direct contact between macrophages and lymphocytes is required. Additional analyses indicated that the apoptosis-inducing ligands, FasL and tumor necrosis factor (TNF), mediate this macrophage-induced apoptosis of CD4+ T cells. These results support a role for macrophage-associated FasL and TNF in the selective depletion of CD4+ T cells in HIV-infected individuals.
The physiologic regulation of peripheral CD4+ T cell homeostasis is influenced by the rate of lymphocyte apoptosis. This form of cell death is tightly regulated and is dependent upon the expression of a family of ligands (Fas ligand [FasL], TNF, tumor necrosis factor–related apoptosisinducing ligand [TRAIL]) and receptors (Fas, TNFR) that mediate apoptosis and upon the induction of susceptibility of these cells to receptor-initiated apoptosis (1–9). Enhanced apoptosis of peripheral uninfected CD4+ T lymphocytes is postulated to contribute to CD4+ T cell depletion in HIVinfected individuals (reviewed in references 10–12). Increased spontaneous and activation-induced apoptosis of peripheral CD4+ T cells from HIV-infected individuals is observed ex vivo (13–15) and in lymph nodes of HIV-infected individuals and SIV-infected macaques (16, 17). Importantly, a correlation between CD4+ T cell apoptosis and CD4+ T cell depletion has been established in different animal models of AIDS (18–20).
Accumulating data indicate that the increased level of CD4+ T cell apoptosis in HIV-infected individuals is due to an aberrant upregulation of the physiological mechanisms controlling peripheral CD4+ T cell apoptosis, namely, the expression level of apoptosis-inducing ligands and receptors and the state of cell susceptibility to such ligand/receptor initiated apoptosis. Fas, FasL, TNF, and TNFR are increased in HIV-infected individuals and inversely correlated with CD4+ T cell numbers (21–28). In addition, peripheral CD4+ T cells from HIV-infected individuals are highly susceptible to FasL/Fas initiated apoptosis (29, 30). The mechanisms whereby the level of apoptosis-inducing receptors/ligands and the state of susceptibility of CD4+ T cells to apoptosis are increased in the context of HIV infection are unknown, although gp120 cross-linking of CD4 and tat and cytokines may be responsible as demonstrated in vitro (31–35).
The circumstances whereby a susceptible CD4+ T cell encounters apoptosis-inducing ligands remains unknown. In vitro culture models demonstrate that uninfected CD4+ T cells undergo apoptosis upon contact by HIV-infected cells (36, 37). Moreover, in lymph nodes from HIV-infected individuals or SIV-infected macaques, the CD4+ T cells that undergo apoptosis are not HIV-infected, but rather in direct contact with neighboring HIV-infected cells (17). These observations support the requirement of direct cell contact between a susceptible CD4+ T cell and a cell that potentially expresses apoptosis-inducing ligands. Antigenpresenting cells such as human macrophages are postulated to play a major role in the physiological deletion/apoptosis of activated, and hence susceptible, peripheral T lymphocytes (38) suggesting that apoptosis-inducing ligands expressed by macrophages can mediate apoptosis of susceptible CD4+ T cells. In fact, differentiated macrophages trigger selective apoptosis of CD4+ T cells that have been rendered susceptible following CD4+ cross-linking (39). These observations, together with the recent identification of FasL expression in human macrophages and its upregulation by HIV infection (40), lead to the hypothesis that antigen-presenting cells such as human macrophages may play a major role both in normal CD4+ T cell homeostasis and in the enhanced CD4+ T cell depletion observed in HIV-infected individuals.
In this study, we have evaluated whether human macrophages lead to the selective apoptosis of CD4+ T cells isolated from HIV-infected individuals. In addition, we have analyzed whether apoptosis-inducing ligands presumably present on human macrophages participate in the apoptosis of CD4+ T cells. Our results demonstrate that human macrophages induce selective apoptosis of CD4+ T lymphocytes from HIV-infected individuals through FasL and TNF.
Materials and Methods
Patient Population and Isolation of Peripheral Blood Mononuclear Cells.
HIV-infected individuals attending the HIV Clinics at Hennepin County Medical Center (Minneapolis, MN) and Mayo Clinic (Rochester, MN) were selected based on CD4+ T lymphocyte counts of >100 cells/dl and absence of concomitant opportunistic infections. After obtaining informed consent, 50 ml of whole blood was obtained and PBMC were isolated by density gradient centrifugation (Ficoll Hypaque; Pharmacia LKB Biotechnology, Inc., Piscataway, NJ). Cells were then cultured in RPMI 1640 supplemented with 10% AB serum (GIBCO BRL, Gaithersburg, MD) and adhered to plastic at 37°C in 5% CO2 for two 1 h periods. The nonadherent cells (PBL) were pelleted and resuspended in RPMI 1640 10% FBS (Hyclone Laboratories Inc., Logan, UT) at a final concentration of 1 × 106 cells/ml.
Monocyte derived macrophages (MDM)1 were obtained from PBMC from HIV seronegative healthy individuals. Briefly, PBMCs were separated from buffy coats by Ficoll Hypaque density gradient centrifugation, and 108 cells were resuspended in 50 ml of RPMI 1640 supplemented with 10% AB serum (GIBCO BRL) and placed horizontally in a 75 cm2 plastic flask (Corning-Costar, Cambridge, MA). 6 d later, nonadherent cells were removed by washing the monolayer three times in RPMI 1640, and adherent cells (MDM) were either HIV- or mock-infected overnight (see below).
Viral Stocks and HIV Infection.
Supernatants from cultures of PHA-IL2–treated PBMC either infected or not with HIV-SF162 (obtained from National Institutes of Health AIDS Reference and Reagent Program) were collected at days 6 through 10 after infection. Clarified and filtered supernatants were analyzed for p24 content using a commercial ELISA kit (Cellular Products Inc., Buffalo, NY), and mycoplasma using a commercial RIA kit (Fisher, Pittsburgh, PA). Supernatants were then aliquoted and stored at −120°C.
HIV infection of MDM was performed by incubating the adherent cell monolayer with 2.5 μg of HIV p24 containing supernatant per T75 flask of MDM during 24 h at 37°C 5% CO2. Mock infection was performed by incubating MDM with an equal volume of mock-infected culture supernatant. After infection, MDM monolayers were washed extensively and cultured in 10% AB serum (GIBCO BRL). At day 7 after infection, HIV- or mock-infected MDM were scraped, counted, and reseeded in 24-well plates (Costar) at cell concentrations indicated in each experiment. 5 d later (12 d after infection), cells were used in experiments. Analysis of p24 content in HIV-infected macrophage supernatants was analyzed by a commercial ELISA kit (Cellular Products Inc.). Flow cytometric evaluation of intracytoplasmic p24 was performed randomly in different batches of HIV-infected MDM by fluorescein isothiocyanate conjugated p24 antibody (Virostat, Portland, ME), as previously described (40, 41), demonstrating specific p24 fluorescein in 15–48% of MDM from a variety of donors.
Antibodies and Fusion Proteins.
Anti-Fas monoclonal antibodies M3 and M33 were used to determine the involvement of Fas/ FasL interaction. M3 and M33 bind to Fas, but M3 and not M33 blocks ligand induced killing (2, 5, 40). Fusion proteins containing the Fc portion of mouse IgG1 and the Fas antigen (Fas-Fc), the TNFR (TNFRFc), or CD40 (CD40-Fc) have been previously described (40, 41).
Determination of Apoptosis in CD4+ or CD8+ T Lymphocytes.
Apoptosis was measured using a flow cytometric method previously described (29, 41). PBL were washed in RPMI 10% AB serum (GIBCO BRL), and resuspended in PBS 0.1% azide. Cells were then incubated for 15 min at 4°C with 1 μg/106 cells of both FITC-labeled anti-CD3 monoclonal antibody (Becton Dickinson, San Jose, CA) and PE-conjugated anti-CD4 monoclonal antibody (CALTAG Labs., South San Francisco, CA) in the presence of 0.01% sodium azide. Hoechst 33342 (2 μg/ml) (Calbiochem Corp., La Jolla, CA) was added, and cells were incubated for an additional 7 min at 4°C. Cells were then washed and resuspended in ice-cold 0.5% paraformaldehyde in PBS. Flow cytometric evaluation was performed using a FACSTAR+® flow cytometer. 30,000 events were recorded and apoptosis was separately quantitated in the CD4+ and CD8+ populations. Apoptotic cells were quantitated by gating on cells with decreased forward angle light scatter and increased Hoechst-specific fluorescence.
Coincubation of Macrophages and PBLs.
Unless stated, 5 × 106 HIV- or mock-infected MDM/well in 24-well plates were washed and incubated for 36 h with 106 PBL from HIV seropositive or seronegative individuals. In experiments addressing cell– cell contact, 6.5 mm transwell porous cell culture inserts (0.4 μm) (Costar) were placed into wells containing HIV- or mock-infected MDM. PBL were added to the top of the transwell.
In experiments addressing the role of autologous killing of PBLs by HIV-infected MDMs, the methodology was as follows. 200 cc of peripheral blood was obtained and MDMs were cultured, HIV infected, and reseeded as above. In the case of the HIV seronegative individual, 9 d after HIV infection of the MDMs, an additional 50 ml of peripheral blood was obtained, and PBLs were isolated and cultured in media containing PHA (Murex Wellcome, Research Triangle Park, NC) at 10 μg/ml and IL-2 (Hoffmann-LaRoche, Nutley, NJ) at 60 IU/ml to generate lymphoblasts. 14 d after HIV infection of MDM, an additional 50 ml of peripheral blood was drawn from the same individual and PBLs were isolated. PHA and IL-2 blasts or freshly isolated PBLs were then incubated with HIV-infected MDMs in the presence or absence of M3 or M33 Fab antibodies for 24 h. PBL blasts, but not freshly isolated PBLs, from the HIV seronegative individual, were shown to be Fas sensitive as demonstrated by the induction of apoptosis by cross-linked agonist anti-Fas antibody (M3), but not by isotype matched control antibody. In experiments addressing syngeneic apoptosis of CD4+ T cells in an HIV-infected individual, MDMs were isolated from 300 cc of peripheral blood from this individual and HIV infected in vitro. 14 d after HIV infection of MDM, PBLs were isolated and coincubated with HIV-MDM in the presence or absence of M3 or M33 Fab antibodies for 24 h.
Statistical Analysis.
Comparison of the amount of apoptosis that was observed with different HIV seropositive or seronegative PBL donors after coincubation with either uninfected or HIV-infected macrophages were made using a one way analysis of variance.
Results
Primary Human MDM Trigger Apoptosis of CD4+ T Cells from HIV-infected Individuals.
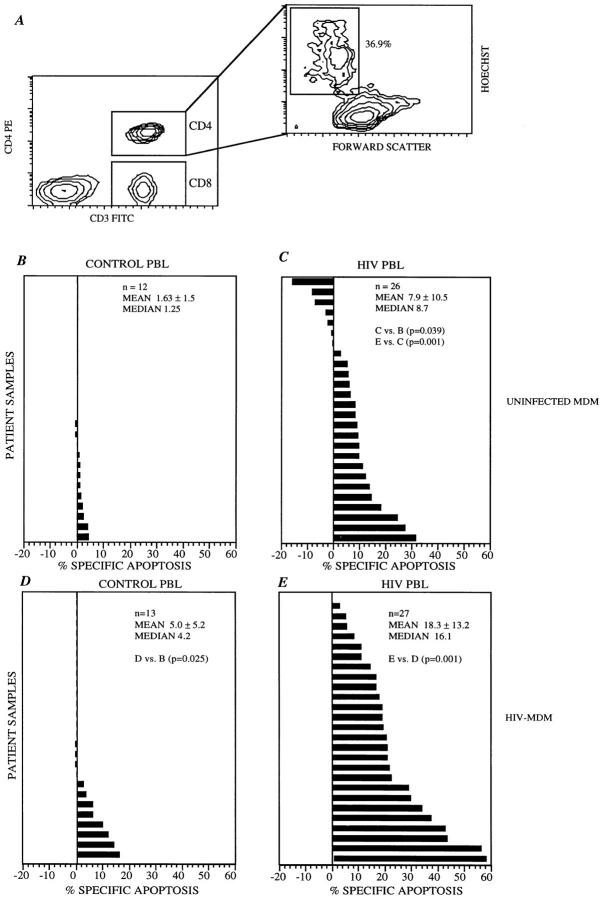
To determine whether MDM trigger apoptosis of CD4+ T cells, 14-d-old MDM from HIV seronegative healthy donors were incubated for 36 h with freshly isolated PBL from HIV-infected individuals or HIV seronegative healthy donors. Thereafter, nonadherent cells were harvested and analyzed using flow cytometry to detect apoptosis within CD4+ or CD8+ T lymphocytes. A representative example of this analysis is depicted in Fig. 1 A. A minimal degree of CD4+ T cell apoptosis (median of 1.25%) is observed in PBL from HIV seronegative healthy control donors (Fig. 1 B). However, when MDM are incubated with PBL from HIV-infected individuals, a larger degree of CD4+ T lymphocyte apoptosis is observed (median of 8.7%, P = 0.039) (Fig. 1 C), suggesting enhanced susceptibility of CD4+ T cells from HIV-infected individuals to MDM-mediated apoptosis.
Figure 1.
Apoptosis of CD4+ T cells from HIV seropositive individuals by uninfected and HIV-infected macrophages. (A) Analysis of apoptosis can be performed separately on CD4+ T lymphocytes (CD3 FITC positive, CD4+ PE positive cells) and on CD8+ lymphocytes (CD3 FITC positive, CD4+ PE negative cells). In the plot depicted, 36.9% of cells have decreased forward angle light scatter and increased Hoechst specific fluorescence, indicating that these cells are apoptotic within the CD4 population (right). (B and C) Uninfected macrophages (UNINFECTED MDM) were used as effector cells at 5:1 effector/target ratio against PBL from either HIV seronegative (B) or infected individuals (C). Mean spontaneous apoptosis was 7.8 ± 4.9% (B), and 23.1 ± 15.1% (C). (D and E) The same experiments repeated with HIV-infected MDM (HIV-MDM) against HIV seronegative (D) or seropositive (E) individuals. Median spontaneous apoptosis was 8.1 ± 4.8% (D), and 23.0 ± 14.8 (E). In B–E, percent specific apoptosis was calculated by subtracting the amount of apoptosis from PBLs cultured in medium alone from the amount of apoptosis when the same PBLs were coincubated with macrophages as indicated.
Previous studies in lymph nodes from HIV-infected individuals indicate that those cells that are undergoing apoptosis are not HIV-infected, but are in direct contact with HIV-infected ones (17). Therefore, we tested whether HIV infection of MDM further enhances the MDM-mediated CD4+ T cell apoptosis in PBL from HIV-infected individuals. Using the same experimental design as described above, PBL from HIV seronegative healthy controls and from HIVinfected individuals were incubated with 14-d-old HIVinfected MDM. A significant increase in CD4+ T cell apoptosis is observed in PBL from HIV-infected individuals incubated with HIV-MDM (median 16.1%) as compared to the degree of CD4+ T cell apoptosis in PBL from HIV seronegative individuals (median 4.2%, compare Fig. 1, E and D, P = 0.001). In addition, HIV-infected MDM, as compared to uninfected ones, further increase the apoptosis of CD4+ T cells from HIV seronegative individuals (compare Fig. 1 D with B, P = 0.025) and HIV-infected individuals (compare Fig. 1, E with C, P = 0.001).
Altogether, these results indicate that uninfected, and to a larger degree, HIV-infected MDM selectively induce apoptosis of CD4+ T cells, and that CD4+ T cells from HIV seropositive individuals are inherently more susceptible to MDM and HIV-MDM–mediated apoptosis.
MDM Trigger Apoptosis of CD4+, but not CD8+, T Lymphocytes.
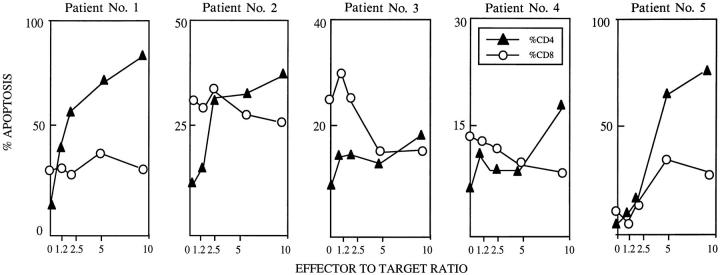
Because CD4+ T lymphocytes are preferentially depleted over CD4+ T cells in HIV-infected individuals, we sought to determine whether MDM induced apoptosis of T lymphocytes is restricted to CD4+ T cells. For this, PBL from five different HIV seropositive individuals (HIV-PBL) were incubated with HIV-infected MDM (HIV-MDM) at different effector (HIV-MDM) to target (HIV-PBL) ratios. After 36 h of coincubation, PBLs were analyzed using flow cytometry to determine the level of apoptosis with CD4+ and CD8+ T cells. Spontaneous apoptosis was higher in CD8+ than CD4+ T cells (Fig. 2). Increasing the effector to target ratio resulted in a progressive increase in the level of CD4+ T cell apoptosis in all patients, an effect that was not observed in CD8+ T lymphocytes from the same HIVinfected individual (Fig. 2). These results indicate that MDM can trigger selective apoptosis of susceptible CD4+ T lymphocytes.
Figure 2.
HIV-infected macrophages induce selective apoptosis of CD4+ T cells from HIV-infected individuals. 106 PBL from HIV-infected and HIV seronegative healthy individuals were coincubated with varying amounts (0, 1, 2, 5, and 10 × 106/well) of HIV-infected macrophages for 36 h, and stained with anti-CD3 FITC, anti-CD4+ PE, and Hoechst 33342. The observed amounts of apoptosis for CD4 lymphocytes (CD3+, CD4+) and CD8 lymphocytes (CD3+, CD4−) are shown. Spontaneous apoptosis is defined as the degree of apoptosis of PBL incubated in the absence of MDM.
Cell Contact Is Required for HIV-MDM–mediated Apoptosis of CD4+ T cells from HIV-infected Individuals.
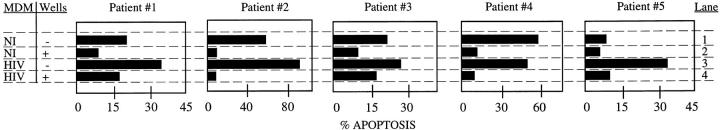
Human macrophages express apoptosis-inducing ligands such as FasL and TNF (40, 42). Because these two apoptosis-inducing ligands may participate in CD4+ T cell apoptosis and are known to be expressed in both cell-associated and soluble form (21), we investigated whether direct cell contact between MDM and PBL is required for CD4+ T cell apoptosis. HIV- or mock-infected MDM were coincubated with PBL from another series of five HIV-infected individuals, in the presence or absence of transwells. Apoptosis of CD4+ T cells was then analyzed by flow cytometry. CD4+ T cell apoptosis mediated by mock-infected MDM (NI) was decreased in the presence of transwells in all patients tested (Fig. 3, compare lanes 1 and 2). Similarly, the higher level of CD4+ T cell apoptosis mediated by HIV-infected MDM (HIV) was also decreased in the presence of transwells (Fig. 3, compare lanes 3 and 4). These results support the requirement for direct contact between MDM and PBL to result in the selective apoptosis of CD4+ T cells from HIVinfected individuals.
Figure 3.
Contact between HIV-MDM and PBL is required to induce apoptosis of T lymphocytes. Macrophages that were either HIV- or mock- infected were coincubated with PBL from HIV seropositive individuals with either the two cell populations in direct contact (−), or with contact interrupted (+) by a semipermeable transwell. The mean spontaneous apoptosis in these experiments was 11.0 ± 3.4%.
Fas/FasL Interactions Participate in the Apoptosis of CD4+ T cells from HIV Seropositive Individuals Triggered by HIVMDM.
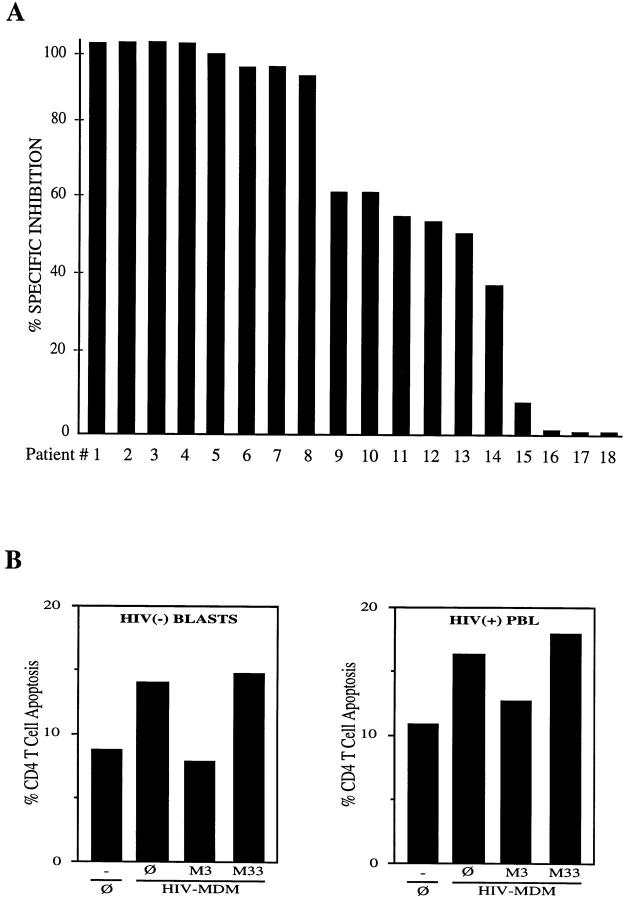
We have previously described that FasL is partly responsible for the HIV-MDM–mediated apoptosis of Fas susceptible targets such as Jurkat T cells and PHA-IL2– treated T cell blasts (40). Based on the above results, we questioned whether HIV-MDM triggered apoptosis of CD4+ T cells from HIV seropositive individuals is mediated through FasL/Fas interactions. PBL from a series of 18 HIV-infected individuals were incubated with HIV-infected MDM (HIVMDM). The role of FasL was analyzed by adding blocking (M3 Fab′′) or nonblocking (M33 Fab′′) anti-Fas antibodies to wells in which MDM-HIV and PBL were coincubated. In a subgroup of patients (patients No. 1–8), Fas blocking antibodies (M3) completely reversed the CD4+ T cell apoptosis triggered by MDM-HIV (Fig. 4 A). However, in additional patients, M3 incompletely reversed CD4+ T cell apoptosis (patients No. 9–14) or had no effect (patients No. 15–18). In those patient samples in which M3 blocked HIV-MDM triggered apoptosis, the nonblocking M33 had no effect. From these results, it is inferred that apoptosis of CD4+ T cells from HIV-infected individuals triggered by HIV-infected MDM is partially mediated by FasL.
Figure 4.
(A) MDM induced apoptosis of CD4+ T lymphocytes is partially mediated by FasL. PBL from HIV-infected individuals were incubated alone or with HIV-infected macrophages at an effector/target ratio of 5:1, either in the absence or presence of blocking (Fab′ M3) or nonblocking (Fab′ M33) anti-Fas antibodies (10 μg/well). In all cases, Fab′ M33 did not significantly alter the level of apoptosis seen in the PBL coincubated with macrophages without antibody. In order to determine the contribution of FasL to MDM killing of PBLs, percent specific inhibition was calculated as follows: ([apoptosis of PBL coincubated with MDM − apoptosis of PBL coincubated with Fab′3 and MDM] [apoptosis of PBL coincubated with MDM − apoptosis of PBL incubated alone]). The mean spontaneous apoptosis was 20.7 ± 13.7%. (B) Syngeneic apoptosis of susceptible CD4+ T cells by HIV-infected MDM. (Left) Lymphocyte blasts from an HIV seronegative individual (HIV(−) BLASTS) were incubated with 3 × 106 syngeneic HIV-infected MDM (HIV-MDM) either alone (∅) or in the presence of blocking (Fab′ M3) or nonblocking (Fab′ M33) antibodies (10 μg/well). Spontaneous apoptosis was calculated by incubating PBL blasts without macrophages (∅). Incubation with crosslinked agonistic M3 anti-Fas antibody lead to 27% CD4+ T cell apoptosis. Freshly isolated PBLs had a spontaneous level of CD4+ T cell apoptosis of 2% which was not modified (3%) upon coincubation with HIV-infected MDM. (Right) Freshly isolated PBLs from an HIV-infected individual (HIV(+) PBL) were incubated with syngeneic HIV-infected MDMs (3 × 106) in the absence or presence of Fab M3 or M33 (10 μg/ml) antibodies. Cross-linked M3 antibodies induced 15% of CD4+ T cell apoptosis.
The above experiments were performed under allogeneic conditions in which MDM, obtained from healthy HIV seronegative individuals and infected in vitro with HIV, were coincubated with freshly isolated allogeneic PBLs from a series of HIV-infected individuals. While FasL/Fas interactions are presumed to be unaffected by allo-antigens, we performed additional experiments to address this issue. First, we determined whether lymphocyte blasts that were generated by incubating PBL from an HIV seronegative individual with PHA and IL-2, would become apoptotic upon incubation with syngeneic HIV-MDM. It has been previously demonstrated that PHA–IL-2–treated lymphocytes become susceptible to FasL-dependent apoptosis (40). In addition, we tested whether FasL/Fas interactions participated in the MDM-HIV–triggered apoptosis of syngeneic lymphocyte blasts. As shown in Fig. 4 B (left), HIVMDMs increased the spontaneous level of apoptosis of syngeneic CD4+ T cell blasts, an effect that was reversed by anti-Fas blocking (M3) but not nonblocking (M33) antibodies. The susceptibility of lymphocyte blasts to FasL was confirmed by their coincubation with cross-linked anti-Fas antibodies (see legend Fig. 4 B). This suggests that the HIV-MDM–mediated, FasL-dependent, apoptosis of susceptible CD4+ T cells can also be observed in a syngeneic model. These observations were confirmed using MDM and PBL obtained from an HIV seropositive individual. MDM were HIV infected in vitro and coincubated 14 d later with freshly isolated PBL. As shown in Fig. 4 B (right), HIV-infected MDMs enhanced the level of spontaneous apoptosis of freshly isolated CD4+ T lymphocytes, an effect that was reduced by anti-Fas blocking (M3), but not by nonblocking (M33) Fab antibodies. These results suggest that the enhanced FasL-dependent apoptosis of CD4+ T cells of this HIV-infected individual is also observed using syngeneic HIV-infected MDM.
TNF Participates in the MDM-triggered Apoptosis of CD4+ T cells from HIV Seropositive Individuals.
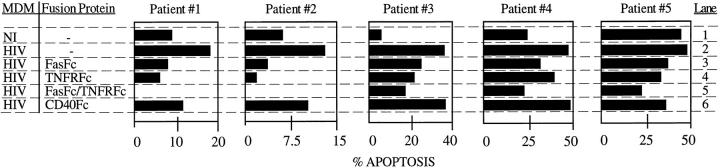
TNF is present as a soluble or cell membrane bound form (6), is produced by macrophages (42), and, as for FasL, can cause apoptosis of activated T cells (8, 9). To determine whether TNF participates in the apoptosis of CD4+ T lymphocytes mediated by MDM, PBLs from five HIV-infected individuals were coincubated with uninfected and HIV-infected MDM for 48 h in the presence or absence of the human TNFRFc fusion protein. This incubation period was selected to allow for possible differences in the timing of induction of T lymphocyte apoptosis by FasL and TNF (9). In parallel, we tested possible additive or synergistic effects between TNF and FasL using FasFc fusion proteins. As shown in Fig. 5, FasFc inhibited the HIV-MDM induced apoptosis of CD4+ T lymphocytes from the five HIV-infected individuals (compare lanes 2 and 3). However, TNFRFc also reduced CD4+ T cell apoptosis from the same individuals to a similar degree to that observed with FasFc (compare lanes 2–4). Interestingly, combining of TNFRFc and FasFc resulted in an additive inhibition of HIV-MDM mediated apoptosis of CD4+ T cells (lane 5). CD40Fc, included to control for the specificity of fusion protein dependent inhibition, reduced the MDM-mediated apoptosis of CD4+ T cells in three patients, albeit to a lower degree than FasFc or TNFRFc (lane 6). Altogether, these results indicate that FasL and TNF participate in the selective apoptosis of CD4+ T cells from HIV-infected individuals mediated by human macrophages.
Figure 5.
FasL and TNF participate in MDM-induced apoptosis of CD4+ T cells from HIV-infected individuals. 5 × 106 uninfected (NI) or HIV- infected (HIV) macrophages were incubated with 106 PBL from five different HIV-infected individuals in the presence or absence of 20 μg FasFc, TNFRFc, or CD40Fc or 10 μg of each of TNFRFc and FasFc for 48 h. Analysis of CD4+ T cell apoptosis was done as described in Fig. 1 A. Observed amounts of apoptosis for CD4 T cells are shown. Mean spontaneous apoptosis was 23.5 ± 9.8%.
Discussion
This report provides evidence of a cellular interaction that selectively mediates CD4+ T cell apoptosis of HIVinfected, but not uninfected, individuals. This finding is noteworthy in several regards. First, macrophage-induced apoptosis is selective for CD4+ T lymphocytes. Second, HIV infection of macrophages further enhances CD4+ T cell apoptosis from HIV-infected individuals. Third, CD4+ T cells from HIV-infected individuals are inherently more susceptible to macrophage-derived apoptosis-triggering ligands, including FasL and TNF.
A large number of studies addressing apoptosis of PBL from HIV-infected individuals have demonstrated that ex vivo activation of PBL from HIV-infected individuals results in enhanced CD4+, but also CD8+ T cell apoptosis (14, 15, 18, 32, 43), hence raising doubts about the relevance of this observation to the in vivo CD4+ T cell depletion observed in HIV-infected individuals. Our results indicate that macrophage-PBL interactions do not further enhance the spontaneous apoptosis of CD8+ T cells, but selectively increases CD4+ T cell apoptosis. This in vitro finding may be of relevance to the pathogenesis of HIV where selective CD4+ T cell depletion is observed, and which correlates with animal models of AIDS where ex vivo apoptosis of PBL is primarily enhanced within CD4+ T cells (18). It is possible that differences in susceptibility to macrophage-bearing apoptosis-inducing ligands (FasL and TNF) between CD4+ and CD8+ T cells account for such selectivity. Alternatively, the frequency or affinity of the interaction between macrophages and CD4+ T cells is higher than between macrophages and CD8+.
If interactions between macrophages and susceptible CD4+ T cells participate in CD4+ T cell depletion in HIV-infected individuals, uninfected MDM may suffice to mediate apoptosis of susceptible CD4+ T cells, and thus not depend solely on the apoptosis-inducing capabilities of HIVinfected macrophages. Alveolar macrophages from HIVinfected individuals are highly activated (potentially expressing apoptosis-inducing ligands) (44–48). While the role of these macrophages in AIDS pathogenesis is unknown, their presence correlates with decreased CD4+ T cells in alveolar fluid (49), thus suggesting that organs with high lymphocyte toxicity such as the lung may be involved in CD4+ T cell depletion via macrophage–lymphocyte interactions.
In other organs such as lymphoid tissue, uninfected antigen-presenting cells but also HIV-infected macrophages, can participate in further enhancing apoptosis of susceptible CD4+ T cells. Studies from lymph nodes indicate a bystander effect in the apoptosis of uninfected cells by HIVinfected neighboring cells (17); thus, future studies should characterize the type of HIV-infected cell that interacts with susceptible bystander CD4+ T cells. The key role of HIV-infected macrophages in causing CD4+ T cell depletion is inferred from hu-SCID HIV mouse models. Mosier et al. demonstrated that monocytotropic viruses resulted in enhanced and accelerated apoptosis of the repopulated CD4+ T cell population, suggesting that macrophages might play an important role in CD4+ T cell depletion upon HIV infection (50). The enhanced FasL-dependent apoptosis of freshly isolated CD4+ T lymphocytes from HIV-infected individuals triggered by HIV-infected MDM is most likely a result of enhanced FasL expression or synthesis. However, the possibility that CD4+ T cells become infected during the 24–36-h incubation period with HIV-MDM cannot be completely excluded. The fact that uninfected MDM also induce FasL-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals, albeit to a lower degree than HIV-infected MDMs, points to macrophage–related mechanisms. Moreover, HIV infection of MDM increases the RNA steady state levels of FasL, and this correlates with increased apoptosis of susceptible target cells through FasL-dependent mechanisms (40). Lastly, fixing of HIVinfected MDMs does not abrogate the selective apoptosis of CD4+ susceptible T lymphocytes (40). The percentage of HIV-infected MDM, as detected by intracytoplasmic p24 (which may overestimate the number of HIV-infected cells by taking into account that MDM can phagocytize p24), was variable in each experiment, reflecting donor variability and macrophage susceptibility to HIV infection. This variability of HIV infection may have impacted the range of apoptosis observed. Additional studies are in progress to determine whether HIV-infected macrophages and/or uninfected macrophages present in the HIV-infected culture is/are responsible for the enhanced apoptosis of susceptible CD4+ T lymphocytes by FasL or TNF. Additional studies should also focus on determining which of the different subpopulations present within MDM cultures express functional apoptotic-inducing ligands.
Macrophages are thought to play a role in the physiological CD4+ T cell depletion in healthy individuals (38). Our results support this observation, and point to macrophages as having a major role in FasL-dependent apoptosis of susceptible CD4+ T cells from HIV-infected patients (29, 30). The state of activation, and thus of susceptibility, of the CD4+ T cell, and not the state of differentiation or HIV infection of the macrophage, may be the critical factor regulating CD4+ T cell apoptosis. Abundant data supports that CD4+ T cells from HIV-infected individuals, even at early stages of the disease, are highly activated (51, 52). Although the mechanism(s) leading to aberrant T cell activation in HIV-infected individuals remain unknown, they may be related to the degree of viral load. Decreases in viral load correlates with decreased CD4+ T cell depletion (53, 54). If HIV-dependent mechanisms result in CD4+ T cell activation and potential susceptibility to apoptosis, reductions of viral load should decrease the state of T cell activation and hence susceptibility to apoptosis.
Characterization of the molecules responsible for inducing apoptosis of CD4+ T cells from HIV-infected individuals is important to advance our knowledge of the cause of CD4+ T cell depletion. The participation of FasL in mediating the apoptosis triggered by MDM was not unexpected based on the recent identification of FasL in MDM (40) and the observed enhanced susceptibility to FasL in PBL from HIV-infected individuals (29). However, it was surprising to observe that TNF could also play a role in inducing apoptosis of CD4+ T lymphocytes from HIV-infected individuals. Recent studies indicate that Fas ligand triggers apoptosis of T lymphocytes earlier than TNF (9). Although we did not address this time sequence in our experimental design, MDMs and CD4+ T lymphocytes were coincubated for 48 h so that the potential effect of both apoptotic inducing ligands could be detected. The role of TNF as an apoptotic inducing ligand in macrophages may be relevant to HIV pathogenesis. Increased TNF and TNFR levels are observed in HIV-infected individuals and correlate with CD4+ T cell depletion (26, 27), and our results suggest that this receptor/ligand family may play a key role in directly causing CD4+ T cell apoptosis. Such prediction is supported by the recent observation that TNF, aside from FasL, is partially responsible for activation-induced T lymphocyte apoptosis in animal models (9, 29), and that antigen-induced apoptosis of CD4+ T cells from HIV-infected individuals may be mediated by TNF (55). Our results also indicate that while Fas ligand and TNF appear to be the major mediators of apoptosis of CD4+ T lymphocytes by MDMs, in a small number of patients, additional MDMmediated apoptosis independent from TNF and/or Fas ligand was observed. This suggests that other ligands known to induce apoptosis may play a role. TRAIL (Apo-2L) is a new member of the TNF family with apoptotic inducing capability (7, 55). Likewise, lymphotoxin has also been recently suggested to play a role in the apoptosis of CD4+ T lymphocytes from HIV-infected individuals (56). Based on this, additional experiments should determine whether any of these two ligands participate in MDM-induced apoptosis of CD4+ T lymphocytes from HIV-infected individuals.
Although our study was aimed at identifying basic mechanisms that participate in the enhanced apoptosis of CD4+ T cells, their relevance to HIV pathogenesis needs to be validated in clinical studies. Correlation of viral load and CD4+ T cell number with susceptibility of CD4+ T cells to FasL and TNF needs to be established. Level and localization of the expression of these ligands in lymphoid tissue and organs from HIV-infected individuals also needs to be addressed. Characterization of the mechanisms that lead to enhanced susceptibility to lymphocyte apoptosis and the abnormal regulation of apoptosis-inducing ligands in HIVinfected patients would allow future therapeutical interventions to reduce or control virus-dependent enhanced CD4+ T cell depletion.
Footnotes
We would like to acknowledge the excellent secretarial assistance of Mr. Douglas Hauschild.
Drs. Badley and Dockrell contributed equally to this work.
1 Abbreviation used in this paper: MDM, monocyte derived macrophage.
References
- 1.Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin K-M. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;6:279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 2.Alderson MR, Tough TW, Braddy S, Davis T, Smith, Roux E, Schooley K, Miller RE, Lynch DH. Regulation of apoptosis and T cell activation by Fas-specific mAb. Int Immunol. 1994;6:1799–1806. doi: 10.1093/intimm/6.11.1799. [DOI] [PubMed] [Google Scholar]
- 3.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature (Lond) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 4.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fasligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 5.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activationinduced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang C-P, Nicholl JK, Sutherland GR, Smith TD, Rauch D, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Sytwu H-K, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 9.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature (Lond) 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 10.Finkel TH, Banda NK. Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells. Curr Opin Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 11.Ameisen JC. Programmed cell death (apoptosis) and cell survival regulation: relevance to AIDS and cancer. AIDS (Phila) 1994;8:1197–1213. doi: 10.1097/00002030-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Oyaizu N, Pahwa S. Role of apoptosis in HIV disease pathogenesis. J Clin Immunol. 1995;15:217–231. doi: 10.1007/BF01540879. [DOI] [PubMed] [Google Scholar]
- 13.Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infection. AIDS Res Hum Retroviruses. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 14.Groux H, Torpier G, Monté D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+T cells from human immunodeficiency virus– infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RPM, Miedema F. Programmed death of T cells in HIV-1 infection. Science (Wash DC) 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 16.Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV–infected persons. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 17.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIVinfected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 18.Estaquier J, Idziorek T, De Bels F, Barré-Sinoussi F, Hurtrel B, Aubertin A-M, Venet A, Mehtali M, Muchmore E, Michel P, et al. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson BK, Stone GA, Godec MS, Asher DM, Gajdusek DC, Gibbs CJ., Jr Long-term observations of human immunodeficiency virus–infected chimpanzees. AIDS Res Hum Retroviruses. 1993;9:375–378. doi: 10.1089/aid.1993.9.375. [DOI] [PubMed] [Google Scholar]
- 20.Heeney J, Jonker R, Koornstra W, Dubbes R, Niphuis N, Di Rienzo AM, Gougeon ML, Montagnier L. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 21.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aries SP, Schaaf B, Müller C, Dennin RH, Dalhoff K. Fas (CD95) expression on CD4+T cells from HIV-infected patients increases with disease progression. J Mol Med. 1995;73:591–593. doi: 10.1007/BF00196352. [DOI] [PubMed] [Google Scholar]
- 23.Silvestris F, Cafforio P, Frassanito MA, Tucci M, Romito A, Nagata S, Dammacco F. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS (Phila) 1996;10:131–141. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Gehri R, Hahn S, Rothen M, Steuerwald M, Nuesch R, Erb P. The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS (Phila) 1996;10:9–16. [PubMed] [Google Scholar]
- 25.Mitra D, Stainer M, Lynch DH, Staiano-Coico L, Laurence J. HIV-1 upregulates Fas ligand on CD4+ T cells in vitro and in vivo: association with Fas-mediated apoptosis. Immunology. 1996;87:581–585. doi: 10.1046/j.1365-2567.1996.510589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilello JA, Stellrecht K, Drusano GL, Stein DS. Soluble tumor necrosis factor–α receptor type II (sTNFαRII) correlates with human immunodeficiency virus (HIV) RNA copy number in HIV-infected patients. J Infect Dis. 1996;173:464–467. doi: 10.1093/infdis/173.2.464. [DOI] [PubMed] [Google Scholar]
- 27.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Membrane tumour necrosis factor–α is involved in the polyclonal B cell activation induced by HIV-infected human T cells. Nature (Lond) 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 28.Debatin K-M, Fahrig-Faissner A. High expression of APO-1 (CD95) on T lymphocytes from human immunodeficiency virus-1–infected children. Blood. 1994;83:3101–3103. [PubMed] [Google Scholar]
- 29.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estaquier J, Idziorek T, Zou W, Emilie D, Farber C-M, Bourez J-M, Ameisen JC. T helper type1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO1)-mediated apoptosis of CD4+T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly R-P, Finkel TH. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood. 1993;82:3392–3400. [PubMed] [Google Scholar]
- 33.Cohen DI, Tani Y, Tian H, Boone E, Samelson LE, Lane C. Participation of tyrosine phosphorylation in the cytopathic effect of human immunodeficiency virus-1. Science (Wash DC) 1992;256:542–545. doi: 10.1126/science.1570514. [DOI] [PubMed] [Google Scholar]
- 34.Laurent-Crawford AG, Krust B, Riviére Y, Desgranges C, Muller S, Kieny MP, Hauguet C, Hovanessian AG. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 35.Westendorp MO, Frank R, Ochsenbauer O, Strickler K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 tat and gp120. Nature (Lond) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 36.Heinkelein M, Sopper S, Jassoy C. Contact of human immunodeficiency virus type 1–infected and uninfected CD4+T lymphocytes is highly cytolytic for both cells. J Virol. 1995;69:6925–6931. doi: 10.1128/jvi.69.11.6925-6931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nardelli B, Gonzalez CJ, Schechter M, Valentine FT. CD4+blood lymphocytes are rapidly killed in vitro by contact with autologous human immunodeficiency virus–infected cells. Proc Natl Acad Sci USA. 1995;92:7312–7316. doi: 10.1073/pnas.92.16.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. J Immunol. 1996;156:523–532. [PubMed] [Google Scholar]
- 39.Wu MX, Daley JF, Rasmussen RA, Schlossman SF. Monocytes are required to prime peripheral blood T cells to undergo apoptosis. Proc Natl Acad Sci USA. 1995;92:1525–1529. doi: 10.1073/pnas.92.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV. Upregulation of Fas ligand expression by HIV in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McElhinny JA, MacMorran WS, Bren GD, Ten RM, Israel A, Paya CV. Regulation of IkBα and p105 in monocytes and macrophages persistently infected with human immunodeficiency virus. J Virol. 1995;69:1500–1509. doi: 10.1128/jvi.69.3.1500-1509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gendelman HE, Orenstein JM, Baca LM, Weiser B, Burger H, Kalter DC, Meltzer MS. The macrophage in the persistence and pathogenesis of HIV infection. AIDS (Phila) 1989;3:475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Gougeon M-L, Garcia S, Heeney J, Tschopp R, Lecoeur H, Guetard D, Rame V, Dauguet C, Montagnier L. Programmed cell death in AIDS-related HIV and SIV infection. AIDS Res Hum Retroviruses. 1993;9:553–563. doi: 10.1089/aid.1993.9.553. [DOI] [PubMed] [Google Scholar]
- 44.Agostini C, Zambello R, Trentin L, Garbisa S, Di Celle PF, Bulian P, Onisto M, Poletti V, Spiga L, Raise E, et al. Alveolar macrophages from patients with AIDS and AIDS-related complex constitutively synthesize and release tumor necrosis factor alpha. Am Rev Respir Dis. 1991;144:195–201. doi: 10.1164/ajrccm/144.1.195. [DOI] [PubMed] [Google Scholar]
- 45.Israël-Biet D, Cadranel J, Beldjord K, Andrieu J-M, Jeffrey A, Even P. Tumor necrosis factor production in HIV-seropositive subjects. J Immunol. 1991;147:490–494. [PubMed] [Google Scholar]
- 46.Trentin L, Garbisa S, Zambello R, Agostini C, Caenazzo C, Di Francesco C, Cipriani A, Francavilla E, Semenzato G. Spontaneous production of interleukin-6 by alveolar macrophages from human immunodeficiency virus type 1–infected patients. J Infect Dis. 1992;166:731–737. doi: 10.1093/infdis/166.4.731. [DOI] [PubMed] [Google Scholar]
- 47.Twigg HL, III, Iwamoto GK, Soliman DM. Role of cytokines in alveolar macrophage accessory cell function in HIV-infected individuals. J Immunol. 1992;149:1462–1469. [PubMed] [Google Scholar]
- 48.Buhl R, Jaffe HA, Holroyd KJ, Borok Z, Roum JH, Mastrangeli A, Wells FB, Kirby M, Saltini C, Crystal RG. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993;150:1019–1028. [PubMed] [Google Scholar]
- 49.Wasserman K, Subklewe M, Pothoff G, Banik N, Schell-Frederick E. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV infection as detected by flow cytometry. Chest. 1994;105:1324–1334. doi: 10.1378/chest.105.5.1324. [DOI] [PubMed] [Google Scholar]
- 50.Mosier DE, Gulizia RJ, MacIsaac A, Torbett BE, Levy JA. Rapid loss of CD4+T cells in human-PBLSCID mice by noncytopathic HIV isolates. Science (Wash DC) 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 51.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science (Wash DC) 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 52.Ascher MS, Sheppard HW. AIDS as immune system activation. II. The panergic imnesia hypothesis. J Acquired Immune Defic Syndr. 1990;3:177–191. [PubMed] [Google Scholar]
- 53.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature (Lond) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 54.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature (Lond) 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 55.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by APO-2 ligand. A new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 56.Clerici M, Sarin A, Berzofsky JA, Landay AL, Kessler HA, Hashemi F, Hendrix CW, Blatt SP, Rusnak J, Dolan MJ, et al. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS (Phila) 1996;10:603–611. doi: 10.1097/00002030-199606000-00005. [DOI] [PubMed] [Google Scholar]