Abstract
Human CD34+ multilineage progenitor cells (CD34HPC) from cord blood and bone marrow express CD40, a member of the tumor necrosis factor–receptor family present on various hematopoietic and nonhematopoietic cells. As hyper-IgM patients with mutated CD40 ligand (CD40L) exhibit neutropenia, no B cell memory, and altered T cell functions leading to severe infections, we investigated the potential role of CD40 on CD34HPC development. CD40activated cord blood CD34HPC were found to proliferate and differentiate independently of granulocyte/macrophage colony-stimulating factor, into a cell population with prominent dendritic cell (DC) attributes including priming of allogeneic naive T cells. DC generated via the CD40 pathway displayed strong major histocompatibility complex class II DR but lacked detectable CD1a and CD40 expression. These features were shared by a dendritic population identified in situ in tonsillar T cell areas. Taken together, the present data demonstrate that CD40 is functional on CD34HPC and its cross-linking by CD40L+ cells results in the generation of DC that may prime immune reactions during antigen-driven responses to pathogenic invasion, thus providing a link between hematopoiesis, innate, and adaptive immunity.
The CD34+ compartment of human progenitor cells displays multilineage hematopoietic capacities, including the generation of dendritic cells (DC) (1, 2). Human CD34+ multilineage progenitor cells (CD34HPC)1 express CD40 (3), a member of the TNFR/NGFR family instrumental in the activation, growth, and survival of various hematopoietic and nonhematopoietic cells (4–7). CD40 ligation induces proliferation and survival of B lymphocytes, turns on their isotype-switch machinery, and directs the generation of memory B cells (8–12). CD40 triggering also activates monocytes (13), mature DCs (14), endothelial cells (15, 16), fibroblasts (17), and induces cytokine production by thymic endothelial cells (18). However, in addition to mature B cells, only progenitor B cells (19) and fibroblasts have been shown to proliferate under CD40 stimulation.
The importance of CD40–CD40 ligand (CD40L) interactions in vivo has been revealed in hyper-IgM (HIM) patients bearing a mutated CD40L on T cells (20–24). As a consequence, patients suffer from various bacterial and viral infections as well as from Cryptosporidial diarrhea and Pneumocystis carinii pneumonia. These infections may be the consequence of several primary immunodeficiencies, including defective B cell memory (excess of IgM and low or absent secondary Ab responses), altered T cell responses, and neutropenia (25). Because of the prominent link of CD40 with cell proliferation and differentiation, and because of the defective humoral, cell-mediated immunity and granulopoietic responses observed in HIM patients, we engaged into the analysis of CD40 function on CD34HPC. The data show that CD40 ligation induces CD34HPC to proliferate and differentiate into cells with dendritic cell attributes.
Materials and Methods
Cell Cultures.
Mouse fibroblastic Ltk− cells obtained from American Type Culture Collection (Rockville, MD) and transfected with either CD40L (CD40L cells/CD40L system) or with CD32 as control (CD32L cells), were prepared as previously described (10, 14). Cultured fibroblastic cells were detached, washed, irradiated (7,000 rads), and counted to seed 5,000 cells in 100 μl of culture medium (CM) per well in flat-bottomed 96well culture plates. Human CD34+ progenitor cells were purified from cord blood of normal full-term deliveries according to institutional guidelines. In brief, mononuclear cells obtained by centrifugation on Ficoll–HyPaque were sequentially labeled with a sterile endotoxin-free murine mAb to CD34 (IOM34; Immunotech, Marseille, France) and with filtered magnetic microbeads (MiniMacs; Miltenyi Biotec, Bergish-Gladbach, Germany). Labeled cells were selected by two passages through a magnetic separation column (MiniMacs Separation Column; Miltenyi Biotec), and then thoroughly washed and seeded for culture. Yielding of CD34+ cells ranged from 0.3% to 1.3% of the total mononuclear cell initial population and final purity was ⩾98% as confirmed by flow cytometry and immunocytochemistry performed on cytospins and BioRad slides (BioRad, Munich, Germany). CD34+ selected cells thus scored negative for lineage-restricted markers (CD3, CD4, CD14, CD15, CD16, CD19, CD20, CD23, CD33) and were homogeneous small mononuclear cells with scant cytoplasm. 5 × 103 CD34+ cells were seeded in 100 μl/well onto equal amounts of irradiated murine fibroblastic L cells transfected either with CD40L or with CD32 as control, or in CM alone prepared with RPMI-1640 supplemented with 10% heat-inactivated FCS plus antibiotics. To estimate CD34HPC proliferation after defined time periods, cultured cells were either thoroughly resuspended to quantitate the number of viable cells by exclusion of Trypan blue, or pulsed overnight with1 μCi of [3H]thymidine and harvested in triplicates. To assess the production of DC, 105 CD34HPC were plated onto 5 × 104 irradiated L cells (CD40L L cells or CD32 L cells as controls), in 48-well culture plates, in a final volume of 500 μl of culture medium. At the time periods indicated, cultured cells were resuspended to score the proportion of DC. To assess the specificity of the system, neutralizing antibodies to CD40 (mAb 89), CD40L (LL48) (both from our laboratory), to granulocyte/macrophage colony-stimulating factor (GM–CSF) (mAb 23B6, provided by Dr. J. Abrams, DNAX, Palo Alto, CA), or isotype control antibodies (Sigma Immunochemicals, St. Louis, MO) were added to the cultures. The presence of DC was assessed by four well-established criteria: morphology, mobility, phenotype, and function. DC were then scored on BioRad adhesion slides following staining with anti-MHC class II DR antibody. In addition, an evaluation was performed on May– Grünwald-Giemsa stained cytospin preparations. Criteria for DC identification included both clearly defined filiform cytoplasmic projections and strong class II DR positivity. Cells showing only pseudopod-like appearance were not considered as DC.
Immunostaining.
Cultured cells resuspended and washed three times in PBS were left 15 min at 20°C (104 cells in PBS on prewashed BioRad adhesion slides). The following primary antibodies were used for 30 min at 20°C: CD1a (OKT6) from Ortho Diagnostics (Raritan, NJ), CD1a (IOM6), CD4, CD13, CD19, CD23, CD32, CD33, CD34, CD38, CD40 and CD44 from Immunotech; CD26, CD59, and CD90 from Serotec (Oxford, England); CD32 from Medarex, (West Lebanon, NH); CD14, CD15, CD16, CD20, CD21, CD80 (B7-1), anti-MHC class II DR, and isotype control antibodies from Becton-Dickinson (Mountain View, CA); CD43 and CD68 from Dakopatts (Glostrup, Denmark); CD74 from The Binding Site (Birmingham, England); CD86 (B7-2) from PharMingen (San Diego, CA); antiMHC class I from SeraLab (Sussex, England); anti-relB from Santa Cruz Biotechnology (Santa Cruz, CA); recombinant HIVgp120 from Neosystem (Strasbourg, France); anti-NGFR from Boehringer-Mannheim (Federal Republic of Germany). CD45, CD60, anti-pan cadherin, and anti-heat shock protein (HSP60) antibodies were from Sigma Immunochemicals. Secondary step antibodies included biotinylated sheep anti–mouse IgG, IgA, and IgM (The Binding Site), biotinylated goat anti–rabbit (Dako) and streptavidin–alkaline phosphatase from Tago-Immunologicals (Camarillo, CA). For assessing HIV binding to DC, recombinant HIVgp120-FITC (Neosystem) was used, followed by a biotinylated mouse mAb to FITC (Sigma) and streptavidin–alkaline phosphatase (Tago). For intracytoplasmic staining, saponin at 0.33% in PBS was used throughout the staining protocol. Color was developed using a Fast Red developing System (Dako). For in situ analysis, immunohistochemical staining of acetone-fixed cryostat sections (8 μm) was performed by incubation with primary antibodies to CD1a (IgG1, Immunotech), CD40 (IgG1, mAb 89), followed by anti–mouse IgG1 and mouse APAAP or to MHC class II DR (IgG2b), a biotinylated anti-mouse IgG2b mAb, and streptavidin peroxidase. The alkaline phosphatase was revealed using Fast Blue as a chromogen (blue color) and the peroxidase was developed by 3-amino-9-ethyl carbazole (red color).
Cell Cycle Analysis of CD34HPC.
Freshly prepared CD34HPC or CD34HPC cultured for 3 d onto CD32 or CD40L-transfected fibroblasts were gently resuspended, washed and incubated with 5 μg/ml of Hoechst 33342 (Calbiochem Novabiochem, La Jolla, CA) during 45 min at room temperature to quantitate cycling cells. Hoechst-stained cells were subsequently labeled with a MoAb to CD34, washed, and analyzed in a FACSTAR® flow cytometer (Becton-Dickinson).
MLR.
To test their allostimulatory properties, CD40-derived DC obtained at day 14 as previously described were irradiated (6,000 rads), seeded at different concentrations, and incubated with 2 × 104 cord blood–derived CD4+ T cells obtained from a different donor. CD4+ T cells were prepared from the mononuclear cell fraction by negative selection using a cocktail of antibodies to CD8, CD14, CD16, CD19, CD20, CD40, and MHC class II DR. Proliferation of naive T cells is expressed as incorporation of tritiated thymidine at day 5.
Limiting Dilution Assay.
CD34HPC were seeded at a density of 1, 10, or 100 cells/well in round-bottomed microtiter plates at a final volume of 100 μl either onto irradiated CD40L+ L cells or with GM–CSF (100 ng/ml)/TNF (2.5 ng/ml). After 12 d of culture, the number of wells with cell growth and the wells that contained cells with recognizable DC morphology, respectively, were scored. For each condition, a total number of 60 wells were seeded with 100 CD34HPC/well and 600 wells were seeded with 10 or 1 CD34HPC/well. One plate containing CD40L− fibroblastic cells was seeded with 100 CD34HPC/well and used as a negative control.
Results
CD40 Ligation Induces Proliferation of CD34HPC.
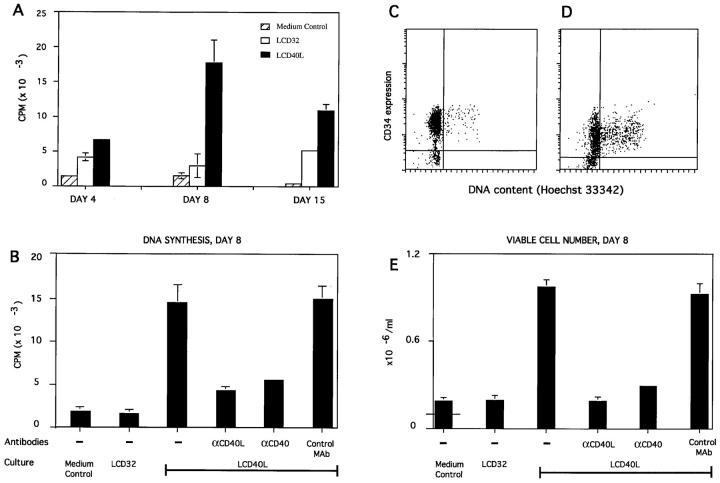
To investigate the potential role of the CD40 molecule on CD34HPC, progenitor cells were cultured over CD40Ltransfected fibroblasts (CD40L cells/CD40L system), (10, 14) or on CD32-transfected cells as control. CD40L-activated CD34HPC entered into DNA synthesis, as measured by tritiated thymidine incorporation that peaked around day 8 and declined by day 15 (Fig. 1 A). CD40-triggered proliferation of progenitor cells was specific, because it was prevented by antibodies to CD40 and CD40L but not by isotype-matched control antibodies (Fig. 1 B and E ). The observed DNA synthesis reflected actual cell proliferation (Fig. 1 E) with a mean 10-fold increase in viable cells observed at day 8 (four independent experiments). In contrast, there was no or only marginal increase in proliferation of CD34HPC cultured over control CD32-transfected cells (CD32 L cells; Fig. 1 A, B, E). Double labeling with Hoechst 33342 and CD34 MoAb to assess the cell cycle of CD34HPC confirmed the quiescent status of these cells when freshly isolated (data not shown). After 3 d of culture over control CD32 L cells, few CD34HPC were cycling (Fig. 1 C ). By contrast, a significant proportion of CD34HPC were found in cycle when stimulated for 3 d by CD40L (Fig. 1 D). However, at 24 h (data not shown), the percentage of cycling cells in CD40L cultures was only marginally higher than that of control cultures, indicating that CD40L probably recruits a small proportion of CD34+ cells whose cycling progeny accumulates with time.
Figure 1.
CD40 ligation induces proliferation of human CD34+ progenitor cells. (A) Kinetics, (B, E) specificity, (C, D) cell cycle analysis. CD34HPC cultured as described in Materials and Methods were either pulsed overnight with 1 μCi of [3H]thymidine (A, B) or counted by Trypan blue exclusion (E). Experiments shown are representative of three experiments and data are presented as mean cpm ± SD (A and B) and cell numbers per ml ± SD (E), as determined in triplicate wells. For quantitating recovery of viable cells, 105 progenitor cells (indicated by horizontal line in medium control bar) were seeded in 1.0 ml of culture medium onto 105 CD40L+ cells or control CD32 cells. Results were evaluated at days 4, 8, and 15 (A) or at day 8 only (B, E). Where indicated, cultures were supplemented with mAbs to CD40 (mAb 89), CD40L (mAb LL48), or isotype-matched control antibodies. (C, D) Cell cycle analysis. CD34HPC freshly isolated (data not shown) or cultured either on CD32L (C) or CD40L-transfected fibroblasts (D) for 3 d were resuspended and incubated with Hoechst 33342. To quantitate specifically CD34HPC entering into cycle, Hoechst-stained cells were subsequently labeled with a mAb to CD34, washed, and analyzed with a FACSTAR+® flow cytometer.
CD40 Ligation Induces CD34HPC to Differentiate into DC.
8-d cultures of CD34HPC on CD32L cells yielded small round mononuclear cells morphologically comparable to fresh CD34HPC (Fig. 2 a), whereas CD34HPC cultured over CD40L L cells revealed small clusters of 10–50 cells displaying long cytoplasmic projections (Fig. 2 b). These clusters grew progressively in size releasing individual cells (Fig. 2, c and d ) that, by day 14, exhibited long delicate and highly motile cytoplasmic processes (Fig. 2, c and d, 15-s interval). Cytocentrifugation and Giemsa staining of CD40L-cultured CD34HPC revealed that a proportion of cells displayed numerous and long dendrites (Fig. 2 e). A detailed immunophenotypic analysis of CD40-generated DC at day 14 showed high levels of MHC class II DR antigen (Fig. 2 i), and the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) (Table 1). At variance with GM–CSF/TNF-derived DC, CD40-derived DC were negative for CD1a and CD40 (Table 1). Interestingly, CD40generated DC expressed high levels of the transactivating factor rel-B (Fig. 2 h), associated with mature DC but absent from dendritic Langerhans cells (26). A small proportion of CD40-derived DC could bind recombinant HIVgp120 (Fig. 2 f ) and expressed CD26 (Fig. 2 g). Occasional DC exhibited beaded dendrites reminiscent of those observed on follicular dendritic cells associated with iccosome release (Fig. 2 g) (27). The nondendritic population from CD40L cultures were nongranulocytic monocyte-like cells as determined by May–Grünwald–Giemsa staining (data not shown). Independent analysis of five different CD34HPC samples cultured 14 d over CD40L cells demonstrated 29.9% ± 1.4% motile cells by phase contrast microscopy, 37.4% ± 3.7% cells with dendrites by Giemsa staining, and 39.6% ± 3.6% DC with strong HLA DR expression (data not shown). Taken together, these results demonstrate that a population of CD34HPC can differentiate into DC after CD40 triggering.
Figure 2.
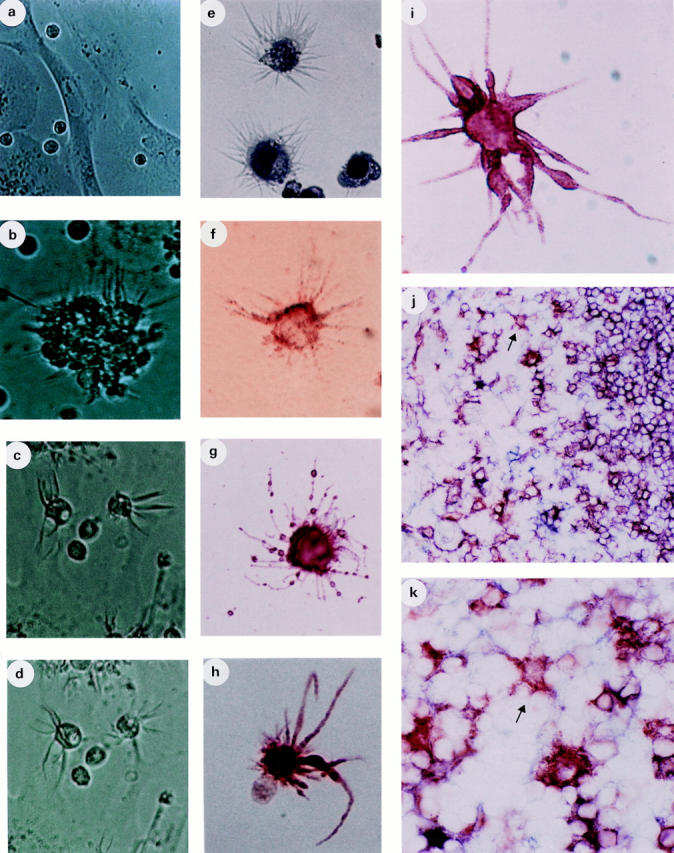
Morphology and phenotype of CD40-activated CD34+HPC and in situ localization of CD1a−/CD40−, DR+ DC. CD34+ HPC prepared as described were seeded onto control (CD32L cells) cultures (a) or onto CD40L (CD40L-L cells) cultures (b–i) and evaluated at days 8 (a and b) and days 14 (c–i). Photomicrographs were taken from cells in culture wells (a–d) at ×200 (a and b) and ×400 (c and d) magnification, and from cytospin preparations (e) or from BioRad slides ( f–i ) at ×1,000 magnification. In (c) and (d), the same field was taken at a 15-s interval to show dendrite motility. (e) May– Grünwald staining of DC. ( f ) HIV–gp120 binding. (g) shows a CD26+ DC. (h) A rel-B+ DC together with a rel-B−, nondendritic cell. (i) MHC class II DR+ DC. ( j and k) Tonsil sections stained for CD1a and CD40 (both in blue) and MHC class II DR (red) showing single DR+, CD1a− and CD40− DC scattered in the T cell area. ( j ) ×400 magnification and (k) ×1,000 magnification.
Table 1.
Comparative Phenotype of CD40 versus GM–CSF/ TNF-derived DC
| CD40–DC | GM–CSF/TNF–DC | |||
|---|---|---|---|---|
| CD1a | − | ++ | ||
| CD2 | − | − | ||
| CD4 | ± (sb) | ++ | ||
| CD14 | − | − | ||
| CD15 | − | − | ||
| CD16 | − | − | ||
| CD19 | − | − | ||
| CD20 | − | − | ||
| CD21 | − | − | ||
| CD23 | − | − | ||
| CD26 | +++ | ND | ||
| CD32 | − | ++ | ||
| CD34 | − | − | ||
| CD40 | − | ++ | ||
| CD43 | +++ | ++ | ||
| CD44 | − | ++ | ||
| CD45 | +++ | ++ | ||
| CD59 | − | ++ | ||
| CD68 | − | + (sb) | ||
| CD74 | ++ | ++ | ||
| CD80 | + | ++ | ||
| CD86 | ++ | ++ | ||
| CD90 | − | − | ||
| Pan-Cadherin | + | ND | ||
| NGFR | +++ | − | ||
| HSP60 | ++ | ND | ||
| MHC class II | +++ | +++ | ||
| MHC class I | +++ | +++ | ||
| rel-B | +++ | + | ||
| Phenotype of CD40-derived DC, comparison to GM–CSF/TNFderived DC. 104 DC harvested at day 14 were deposited onto BioRad slides to perform immunostaining. Culture conditions, preparation of cells, and staining procedures as described in Fig. 2. For intracytoplasmic staining, saponin at 0.33% in PBS was used throughout the staining protocol. Cells were scored as positive when a clearer and stronger staining than that of control antibodies was observed. Reactivity with the different antibodies tested was scored as (−) negative, (±) weak, (+) modest, (++) intermediate, to strong (+++), based on staining intensity. In addition, (sb) indicates reactivity only with a subset (<50%) of the DC. | ||||
Immunohistochemical analysis of secondary lymphoid tissue (tonsils) revealed the presence of MHC class II DR+, CD1a− and CD40− cells, with clear dendritic morphology, scattered through the T cell areas where interdigitating cells congregate (Fig. 2, j and k). These findings identify a correlate population in situ, precisely in the area where mature DC localize to interact with low frequency Ag-specific T cells in vivo.
Kinetics and Specificity of CD40-dependent Generation of DC.
Kinetic followup of CD40 stimulated CD34HPC indicated early emergence of DC in cultures with 6% identifiable DC at day 4, 16% at day 8, and a maximum 36% by day 14 (Fig. 3 A). As observed for proliferation, DC generation was specific for CD40 engagement, because antibodies to either CD40 or CD40L efficiently blocked their appearance, whereas isotype-matched control antibodies were ineffective (Fig. 3 B). Of note is the fact that a soluble fusion protein constructed with CD40L and CD8 (sCD40L) was also capable of inducing development of DC. However, the soluble product was less effective than polyvalently immobilized CD40L, especially regarding dendrite development (data not shown). Interestingly, neutralizing anti-GM–CSF antibodies did not affect the CD40L-dependent generation of DC (Fig. 3 B), which contrasts with previous studies in vitro where DC generation was strictly dependent on addition of GM–CSF (1, 28–31).
Figure 3.
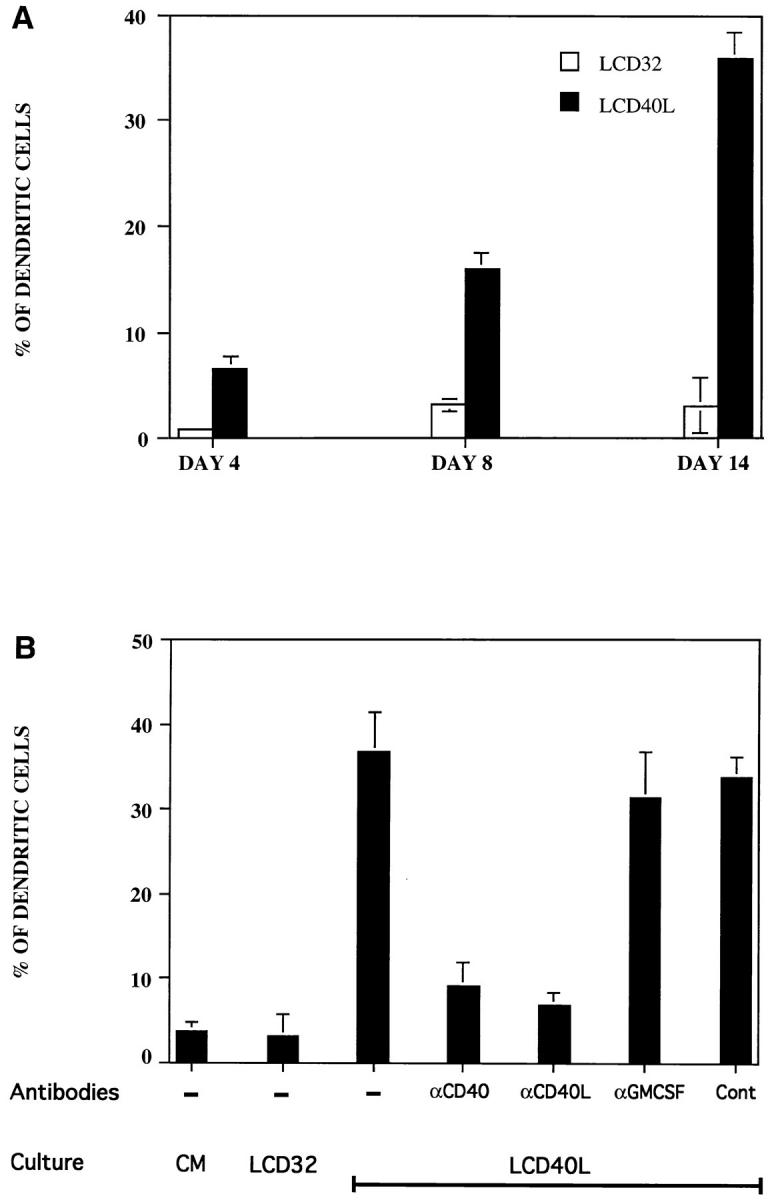
CD40 triggering of CD34+ progenitors specifically induces the generation of DC that accumulate with time. (A) Human CD34+ progenitor cells seeded in CD40L (LCD40L cells) or in control cultures (LCD32 cells) were evaluated for DC generation at different times. (B) The effect of neutralizing antibodies to CD40, CD40L, and GM–CSF, or an irrelevant control Ab was also determined at day 14. Results are expressed as percentage DC ± SD evaluated in triplicates. Cultures and evaluation of DC production were performed as described in Materials and Methods.
Clonal Analysis of CD34HPC Stimulated Through CD40.
Limiting dilution assays scored with an inverted microscope after 12 d of culturing CD34HPC first confirmed that control CD32-transfected fibroblasts alone do not promote generation of DC, even at the highest number of progenitor cells (100) plated per well (Table 2). By contrast, cultures over CD40L L cells induced the generation of clones containing DC (as determined by typical morphology) in 3% of the wells seeded at 1 cell/well, a proportion that increased to 10% and 36% when 10 and 100 CD34HPC were seeded per well, respectively. Parallel experiments with GM–CSF-treated CD34HPC showed that this cytokine was at least twice as efficient as CD40L in generating DC in vitro (Table 2).
Table 2.
Limiting Dilution Analysis of CD40-stimulated CD34 HPC
| Stimuli | Nbr CD34 HPC seeded/well* | Percent wells with growth | Percent wells containing DC | |||
|---|---|---|---|---|---|---|
| CD40L− L-cells | 100 | 1 | 0 | |||
| CD40L+ L-cells | 100 | 84 | 36 | |||
| 10 | 27 | 10 | ||||
| 1 | 6 | 3 | ||||
| GM–CSF/TNF | 100 | 100 | 100 | |||
| 10 | 38 | 27 | ||||
| 1 | 16 | 12 | ||||
| Limiting dilution assay. CD34HPC were seeded at a density of 1, 10, or 100 cells/well in round-bottomed microtiter plates at a final volume of 100 μ1 on irradiated CD40L+ cells or with GM–CSF (100 ng/ml)/ TNF (2.5 ng/ml). After 12 d of culture, the percentage of total wells with cell growth and the percentage of total wells containing DC (as determined from morphology in culture plates examined with an inverted microscope) were scored. For each condition, a total number of 60 wells were seeded with 100 CD34HPC/well and 600 wells were seeded with 10 or 1 CD34HPC/well. One plate containing CD40L negative fibroblastic L cells was seeded with 100 CD34HPC/well and used as a negative control. *For 1 and 10 cells/well, 600 wells were seeded. For 100 cells/wells, 60 wells were seeded. | ||||||
DC Generated after CD40 Ligation of CD34HPC Stimulate T Cell Alloreaction.
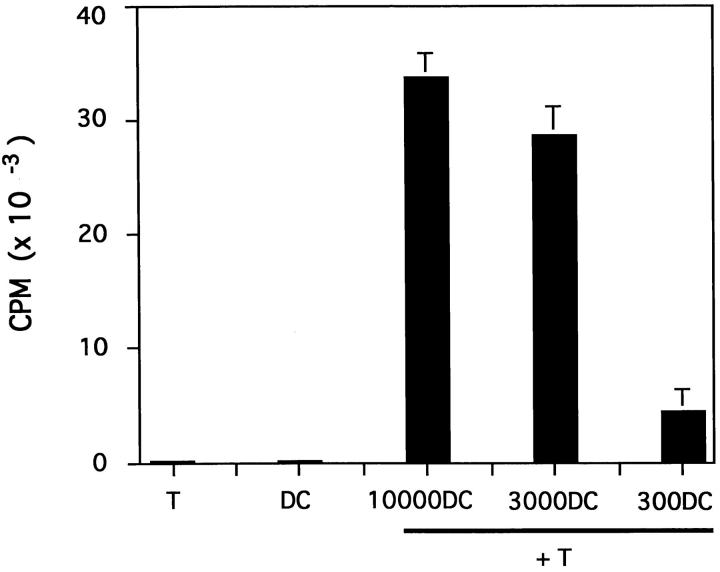
DC, unlike monocytes or lymphocytes, display the unique property of vigorously stimulating the proliferation of naive T cells (28). As few as 300 CD40L-cultured CD34HPC were able to induce naive CD4+ cord blood T cells (ratio 1/66) to enter into DNA synthesis and 3,000 CD40L-derived DC (ratio 1/6.6) increased T cell thymidine incorporation by 250-fold (Fig. 4). In contrast, freshly isolated or IL-3-cultured CD34HPC are unable to induce naive T cell proliferation (1). Thus, in addition to their morphology, motility and phenotype, cells generated by CD40 ligation of CD34HPC possess the antigen-presenting capacity of DC.
Figure 4.
DC generated in the CD40L system stimulate primary T cell responses. The functional capacity of CD40-generated DC was determined in a primary MLR culturing varying amounts of irradiated DC with CD4+ cord blood T cells. DC obtained at day 14 as described were irradiated and plated at different concentrations with 2 × 104 cord blood– derived CD4+ T cells prepared from a different donor by negative selection (see Materials and Methods). Stimulation of naive T cells is presented as mean [3H]thymidine incorporation ± SD measured in triplicate wells at day 5. Data are representative of three different experiments.
Discussion
The present study demonstrates that CD40 ligation of cord blood CD34HPC results in their proliferation and subsequent differentiation into DC.
DC generation was not dependent on the fibroblasts themselves, because CD40L− Ltk− cells transfected with CD32 were uneffective. This was further corroborated by using a soluble CD40L–CD8 fusion protein that also permitted the production of DC (data not shown), though, as observed for B cell functions (our unpublished observations), this reagent was less efficient than cell-bound CD40L, especially for dendrite development.
CD34HPC were observed to be cycling in response to CD40L triggering at day 3 of culture, but not at day 1. This suggests that only a minor proportion of CD34HPC may be driven into cycle and that the cycling population identified by day 3 results from accumulation of the initially recruited progenitors. The low clone numbers obtained by limiting dilution in response to CD40L is consistent with the observed cell cycle kinetics. Limiting dilution analysis also indicates that CD40L by itself is less efficient than the combination of GM–CSF/TNF to generate DC.
In accordance with the latter finding, a comparative analysis of bulk cultures of cord blood CD34+ progenitors showed that GM–CSF/TNF results in a considerably higher cell expansion (34.0 ± 16.0-fold; n = 10) than does CD40 triggering (8.3 ± 2.7 fold; n = 5). In keeping with differences observed between the two in vitro derived DC populations, the presently described DC, although clearly able to stimulate allogeneic naive T cells, appear less potent stimulators on a cell-to-cell basis than the DC obtained in GM–CSF/TNF cultures (Caux, C., and J. Banchereau, unpublished data). This observation may be related to the lack of detectable CD40 on the CD40-derived DC as compared with GM–CSF/TNF-derived cells (Table 1).
Although DC migratory routes and tissue residence are relatively well known (32, 33), their precise ontogeny is poorly understood. GM–CSF is considered an essential requirement for generation of DC, whether from CD34HPC (1, 29) or from monocytic cells (30, 31, 34). However, our findings suggest an alternative GM–CSF-independent pathway of DC ontogeny, the existence of which was indeed revealed by the normal DC status of mice with inactivated GM–CSF or GM–CSF-receptor genes (35, 36). However, at this stage, the possibility that CD40 stimulation may trigger the production of other endogenous cytokines relevant for inducing DC differentiation from progenitor cells (i.e., kit–ligand/TNF), cannot be ruled out.
Interestingly, in a recent immunohistological study of two HIM patients, interdigitating cells (IDCs) (referred to as S100+ cells) in lymph node paracortex are reported as being normal in one case, but as being rare in the second patient (37). Although the topographic distribution and exact phenotype of DC subsets in HIM patients requires further analysis, it is possible that CD40-derived DC represent a distinct subpopulation of mature DC with a putative particular function. The notion that the DC lineage is constituted of discrete subsets (28, 38–41) may be in accordance with our in vivo finding of a DC population that phenotypically mirrors the CD40-derived DC. Moreover, the in situ localization of these cells in the T cell/IDC-rich area would also be consistent with a more mature status of the CD40-derived DC, as suggested by lack of CD1a and the strong expression of rel-B.
The altered CD40L from HIM patients and from genetargeted mice results in disrupted interactions between T cells and DC that may be the cause of deficient T cell priming (42), explaining perturbed T cell responses such as frequent Cryptosporidium and Pneumocystis carinii infections (43, 44) and susceptibility to Leishmania (45–47). Furthermore, unproductive CD40L interactions with CD40 on monocytes (13) or stromal cells (18) may result in decreased production of hematopoietic growth factors, which thus may explain the frequent neutropenia associated with HIM syndrome.
The existence of a CD40L-dependent pathway of DC development calls for the identification of the CD40L+ partner. CD40L+-activated T cells represent an obvious candidate, a possibility that would be consistent with the T cell secretion of hematopoietic growth factors (including the T cell–specific IL-3), the presence of T cells in the bone marrow (48–53), and the various hematopoietic abnormalities observed in T cell–deficient animals and humans (51). Recirculating CD34HPC may also encounter CD40L+ mast cells (54) in peripheral tissues, thereby contributing to extramedullary DC production. In keeping with this, DC may also be generated by contact with CD40L+ T cells outside bone marrow, e.g., at the sites of inflammation where chemokines are released and may attract the progenitors.
Taken together, our findings indicate that a novel CD40dependent pathway of DC generation may be operating when activated CD40L+ T cells encounter CD34HPC. This would provide an alternative and probably a necessarily redundant DC pathway to satisfy the increased DC demands required for the appropriate priming to immune responses during pathogenic invasion. CD40–CD40L interactions, thus, may represent an important link between hematopoiesis, innate, and adaptive immunity.
Acknowledgments
The authors thank S. Ait-Yahia and I. Durand for excellent technical assistance, Dr. Y.-J. Liu and Dr. C. Caux for helpful discussions, Dr. K. Bacon and Dr. J. Chiller for critically reading the manuscript, and S. Bonnet-Arnaud and M. Vatan for their editorial assistance.
Footnotes
1 Abbreviations used in this paper: CD34HPC, human CD34 multilineage progenitor cells; CM, culture medium; DC, dendritic cells; HIM, hyper IgM; IDCs, interdigitating cells; NGFR, nerve growth-factor receptor.
References
- 1.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM–CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature (Lond) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 2.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 3.Saeland S, Duvert V, Caux C, Pandrau D, Favre C, Vallé A, Durand I, Charbord P, de Vries JE, Banchereau J. Distribution of surface-membrane molecules on bone marrow and cord blood CD34+hematopoietic cells. Exp Hematol. 1992;20:24–33. [PubMed] [Google Scholar]
- 4.van Kooten C, Banchereau J. CD40–CD40 ligand, a multifunctional receptor–ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 6.Hollenbaugh D, Ochs HD, Noelle RJ, Ledbetter JA, Aruffo A. The role of CD40 and its ligand in the regulation of the immune response. Immunol Rev. 1994;138:23–37. doi: 10.1111/j.1600-065x.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 7.Noelle RJ. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 8.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan ICM. Mechanisms of antigendriven selection in germinal centers. Nature (Lond) 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 9.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpin C, Déchanet J, van Kooten C, Merville P, Grouard G, Brière F, Banchereau J, Liu Y-J. Generation of memory B cells and plasma cells in vitro. Science (Wash DC) 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, de Paoli P, Vallé A, Garcia E, Rousset F. Long term human B cell lines dependent on interleukin 4 and antibody to CD40. Science (Wash DC) 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 12.Rousset F, Garcia E, Banchereau J. Cytokineinduced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: Regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Vanbervliet B, Dubois B, van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karmann K, Hughes CCW, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci USA. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollenbaugh D, Mischel-Petty N, Edwards C, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yellin MJ, Winikoff S, Fortune SM, Baum D, Crow MK, Lederman S, Chess L. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J Leukocyte Biol. 1995;58:209–216. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Galy AHM, Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992;149:775–782. [PubMed] [Google Scholar]
- 19.Saeland S, Duvert V, Moreau I, Banchereau J. Human B cell precursors proliferate and express CD23 after CD40 ligation. J Exp Med. 1993;178:113–120. doi: 10.1084/jem.178.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuleihan R, Ramesh N, Loh R, Jabara H, Rosen FS, Chatila T, Fu S-M, Stamenkovic I, Geha RS. Defective expression of the CD40 ligand in X chromosome– linked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint G, Basile CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature (Lond) 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 22.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 23.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, Bedell MA, Edelhoff S, Disteche CM, Simoneaux DK, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science (Wash DC) 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 24.Korthäuer U, Graf D, Mages HW, Brière F, Padayachee M, Malcolm S, Ugazio AG, Notarangelo LD, Levinsky RJ, Kroczek RA. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature (Lond) 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 25.Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM) Immunodef Rev. 1992;3:101–122. [PubMed] [Google Scholar]
- 26.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature (Lond) 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 27.Szakal AK. Microanatomy of lymphoid tissue during humoral immune responses : structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 29.Santiago-Schwarz F, Belilos E, Diamond B, Carsons SE. TNF in combination with GM–CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukocyte Biol. 1992;52:274–281. [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell–dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L-J, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, Azuma S, Yoshida S, Toyoda Y, Arai K-I, et al. Mice deficient for the IL-3/GM–CSF/IL-5 βc receptor exhibit lung pathology and impaired immune response, while βIL3receptor–deficient mice are normal. Immunity. 1995;2:211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC. Involvement of granulocyte–macrophage colony-stimulating factor in pulmonary homeostasis. Science (Wash DC) 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 37.Facchetti F, Appiani C, Salvi L, Levy J, Notarangelo LD. Immunohistologic analysis of ineffective CD40–CD40 ligand interaction in lymphoid tissues from patients with X-linked immunodeficiency with hyper-IgM. Abortive germinal center cell reaction and severe depletion of follicular dendritic cells. J Immunol. 1995;154:6624–6633. [PubMed] [Google Scholar]
- 38.Caux, C., B. Vanbervliet, C. Massacrier, B. Dubois, C. Dezutter-Dambuyant, D. Schmitt, and J. Banchereau. 1995. Characterization of human CD34+ derived dendritic/Langerhans cells (D-Lc). In Dendritic Cells in Fundamental and Clinical Immunology. J. Banchereau and D. Schmitt, editos. Plenum Press, London. 1–5.
- 39.Knight SC, Stagg AJ. Antigen-presenting cell types. Curr Opin Immunol. 1993;5:374–382. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- 40.O'Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder TF, Baseler M, Fauci AS. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature (Lond) 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 43.Renshaw BR, Fanslow WC, III, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand–deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 45.Kamanaka M, Yu P, Yasui T, Yosha K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 46.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 47.Soong L, Xu J-C, Grewal IS, Kima P, Sun J, Longley BJJ, Ruddle NH, McMahon-Pratt D, Flavell RA. Disruption of CD40–CD40 ligand interactions results in an enhanced susceptibility to Leschmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 48.Janossy G, Tidman N, Papageorgiou ES, Kung PC, Goldstein G. Distribution of T lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981;126:1608–1613. [PubMed] [Google Scholar]
- 49.Fauci A. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975;56:98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahidi, N.T., and W.B. Ershler. 1989. Bone marrow microenvironment: clinical observations. In Handbook of the Hemopoietic Microenvironment. M. Tavassoli, editor. Humana Press, Clifton, NJ. 433–444.
- 51.Garland, J.M. 1990. Lymphocytes, lymphokines, and hematopoiesis. In Colony Stimulating Factors, Molecular and Cellular Biology. T.M. Dexter, J.M. Garland and N.G. Testa, editors. Marcel Dekker. New York. 297–328.
- 52.Shin SS, Sheibani K, Kezirian J, Nademanee A, Forman SJ, Lee SK, Winberg CD. Immunoarchitecture of normal human bone marrow: a study of frozen and fixed tissue sections. Hum Pathol. 1992;23:686–694. doi: 10.1016/0046-8177(92)90326-x. [DOI] [PubMed] [Google Scholar]
- 53.Nakao S, Takamatsu H, Yachie A, Itoh T, Yamaguchi M, Ueda M, Shiobara S, Matsuda T. Establishment of a CD4+T cell clone recognizing autologous hematopoietic progenitor cells from a patient with immune-mediated aplastic anemia. Exp Hematol. 1995;23:433–438. [PubMed] [Google Scholar]
- 54.Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature (Lond) 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]