Abstract
Cataract, already a major cause of visual impairment and blindness, is likely to become an increasing problem as the world population ages. In a previous study, we showed that transforming growth factor-β (TGFβ) induces rat lenses in culture to develop opacities and other changes that have many features of human subcapsular cataracts. Here we show that estrogen protects against cataract. Lenses from female rats are more resistant to TGFβ-induced cataract than those from males. Furthermore, lenses from ovariectomized females show increased sensitivity to the damaging effects of TGFβ and estrogen replacement in vivo, or exposure to estrogen in vitro, restores resistance. Sex-dependent and estrogen-related differences in susceptibility to cataract formation, consistent with a protective role for estrogen, have been noted in some epidemiological studies. The present study in the rat indicates that estrogen provides protection against cataract by countering the damaging effects of TGFβ. It also adds to an increasing body of evidence that hormone replacement therapy protects postmenopausal women against various diseases.
The mammalian lens has a highly organized cellular architecture. A monolayer of cuboidal epithelial cells covers the anterior surface of the elongated fiber cells that constitute the bulk of the lens. These cellular elements are enclosed within a thickened basement membrane, the lens capsule. Normally, the lens transmits light and focuses it onto the retina. Cataract, or clouding of the ocular lens, is associated with disruption of normal cellular architecture. This condition is a major cause of visual impairment and is likely to become an even greater public health problem as the world population ages. Predisposing factors include aging, diabetes, UV/sunlight, ocular surgery, and malnutrition (1).
A role for female hormones in protecting against cataract has been suggested by recent epidemiological studies. The prevalence of cataract increases in postmenopausal women (2, 3). Moreover, postmenopausal women on hormone replacement therapy or younger women taking oral contraceptives display a decreased prevalence and severity of cataract (4–7).
We have developed a lens culture system for investigating cataractogenesis and factors that influence it. Using this system, we have shown that transforming growth factor-β (TGFβ) induces responses in lens cells that mimic events in cataractogenesis. Rat lens epithelial explants (8, 9) and lenses (10) cultured with TGFβ undergo molecular and morphological changes that are typically associated with anterior subcapsular cataract and with the subcapsular opacification that often arises from cells left behind after cataract surgery (aftercataract). Some of these changes are also known to be associated with posterior subcapsular cataract. It is significant that TGFβ induces distinct anterior subcapsular opacities in cultured rat lenses (10). Histologically, these correspond with subcapsular plaques of aberrant cells which are virtually indistinguishable from early stage anterior subcapsular cataracts in humans (see reference 11). Two molecular markers for subcapsular cataract, α-smooth muscle actin and type I collagen, are also present. The finding that TGFβ is cataractogenic is consistent with many studies which now indicate that TGFβ is involved in the aetiology of diseases in other organs (for example see references 12 and 13). These diseases are generally associated with fibrotic changes.
In the present study, using our lens culture system, we have shown that lenses from female rats are less susceptible to the cataractogenic effects of TGFβ than those from males. In addition, using whole lenses from ovariectomized rats, we have established that resistance to TGFβ-induced cataract is conferred by estrogen.
Materials and Methods
17-β-estradiol (1,3,5[10]-Estratriene-3, 17β-diol) and progesterone (4-Pregnene-3, 20-dione) were obtained from Sigma Chemical Co. (St. Louis, MO) and human recombinant TGFβ2 from Genzyme Corp. (Cambridge, MA). In some experiments, lenses were derived from normal 6–10-mo-old adult male and female Wistar rats, killed by CO2 asphyxiation before removal of eyes. Alternatively, ovariectomies were performed on 3-mo-old female Wistar rats under anaesthesia as described elsewhere (14). After waiting for 4–5 wk to ensure clearance of residual estrogen and progesterone, ovariectomized rats were given three daily injections of 0.5 μg 17-β-estradiol or 5 mg progesterone, dissolved in benzyl alcohol/peanut oil (1:3, vol/vol). Control rats received vehicle alone. 1 d later, rats were sacrificed by a lethal dose of nembutal (Boehringer Ingelheim, Sydney, Australia) before removal of eyes.
Lens Cultures.
Lenses were carefully dissected free from surrounding ocular tissues in culture medium, as described previously (10), and cultured with TGFβ2 at final concentrations of 0.025–4 ng/ml, which was added immediately unless otherwise indicated. Controls received no TGFβ. Culture medium was renewed every 2 d throughout the culture period, without further addition of TGFβ. Lenses were cultured for 7 d and the anterior surface was photographed daily to record development of opacities. At the end of the culture period, lenses were fixed in Carnoy's fixative (acetic acid/ethanol, 1:3, vol/vol) and embedded in paraffin.
Generally, serum-free medium 199 containing antibiotics and 0.1% bovine serum albumin, as already described (10), was used as culture medium. When testing the effect of estrogen in vitro, medium 199 was replaced by phenol red-free minimum essential medium (Sigma Chemical Co.).
Opacification Index.
TGFβ-induced lens opacification begins as diffuse clouding on the anterior surface of the lens. As the response progresses, these regions condense to form distinct opacities leaving a reduced area of clouding. At low concentrations of TGFβ, few distinct opacities are observed at day 7 of culture, and a large proportion of the lens surface remains cloudy. In contrast, most of the initially cloudy areas condense to form numerous distinct opacities at high concentrations (as in Fig. 1 A). On the basis of these observations, a method for measuring the extent of opacification has been developed (15).
Figure 1.
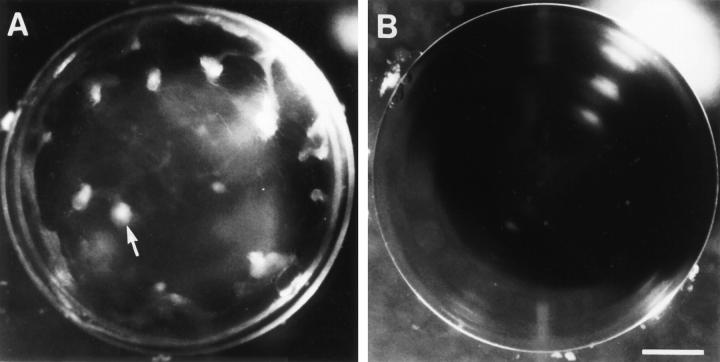
Comparison of response of lenses from male and female rats to TGFβ. Lenses were cultured with 0.15 ng/ml TGFβ2 and photographed after 7 d. Lenses from male rats (A) developed distinct anterior opacities (arrow). Lenses from females remained transparent (B). Some flaring of the light source is evident in the upper righthand quadrant of each lens. Bar, 400 μm.
Micrographs of lenses at day 7 of culture were used to determine lens opacification. Each micrograph was scanned with an x-ray scanner (3CX; XRS Corp., Torrance, CA) using Adobe Photoshop and XRS Omni Media software. A series of measurements was then made using NIH Image v1.52. In some micrographs, flared reflections of the light source made it impossible to assess the extent of opacification in certain regions (for example see Fig. 1 B). Only micrographs in which the assessable area represented >75% of the total area were used. The assessable area (A) and, within this area, the total area of clouding (B), and the total number of distinct opacities (C), were measured. An “opacification index” was then calculated as follows:
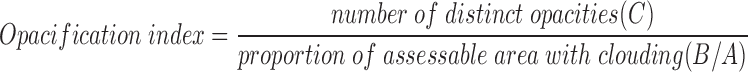
Histology and Immunohistochemistry.
Serial sections of paraffinembedded lenses were processed for routine histology or for immunohistochemical localization of α-smooth muscle actin or type I collagen, as already described (10). Representative lenses from each treatment group were examined by routine histology (all TGFβ2 concentrations) and immunolocalisation (0.15 ng/ml TGFβ2).
Results
Male–Female Difference in Responsiveness of Lenses to TGFβ.
Lenses from male rats developed distinct anterior opacities when cultured with 0.15 ng/ml TGFβ2 (Fig. 1 A). In contrast, lenses from female rats remained transparent under these conditions (Fig. 1 B), as did control lenses from male and female rats cultured without TGFβ (not shown; see reference 10). However, at a higher concentration of TGFβ2 (1 ng/ml), lenses from female rats also developed opacities (Table 1). For both sexes, the response increased significantly with concentration of TGFβ.
Table 1.
TGFβ-induced Opacification in Lenses from Adult Rats
| TGFβ | Opacification index | |||
|---|---|---|---|---|
| Male | Female | |||
| ng/ml | ||||
| 0 | 0 | 0 | ||
| 0.025 | 13 ± 0.3 | 0 | ||
| 0.15 | 57 ± 5 | 0 | ||
| 1 | 69 ± 9 | 54 ± 6 | ||
| 4 | 187 ± 19 | 118 ± 10* | ||
Lenses from adult male and female rats were cultured for 7 d. TGFβ2 was used at the concentrations indicated. The opacification index was calculated as described in Materials and Methods. Values represent the mean ± SEM of determinations for four individual lenses.
This value is significantly lower than the corresponding value for lenses from male rats (P <0.05 Student's t test).
Histological examination of lenses cultured with 0.15 ng/ml TGFβ2 revealed distinct subcapsular plaques, containing spindle-shaped cells and extracellular matrix (see reference 10) in the lenses from males (not shown). In contrast, the lenses from females and controls retained normal lens architecture, with a monolayer of epithelial cells overlying the fiber mass. Immunolocalization of α-smooth muscle actin and type I collagen showed that, for males, strong reactivity for both of these cataract markers was present in plaques induced by culturing with 0.15 ng/ml TGFβ2, whereas corresponding lenses from females showed no reactivity for α-smooth muscle actin and only very weak reactivity for type I collagen in a few cells in the epithelium (not shown). These markers were not detectable in sections of control lenses from males or females cultured for ⩽7 d without TGFβ (not shown).
Effect of Hormone Replacement after Ovariectomy on TGFβinduced Cataract.
Having established that lenses from female rats showed more resistance to the cataractogenic effects of TGFβ than those from males, ovariectomized rats were used to assess the contribution of ovarian hormones to this phenomenon. Lenses from ovariectomized rats (without hormone replacement) developed opacities when cultured with 0.15 ng/ml TGFβ (Table 2; Fig. 2 A), a concentration shown to have negligible effect on lenses from normal female rats (Table 1; Fig. 1 B). Lenses from ovariectomized rats which received estrogen, however, did not develop opacities under these conditions (Table 2; Fig. 2 B), while the response of lenses from rats treated with progesterone was similar to that of lenses from vehicle-treated rats (Table 2; Fig. 2 C).
Table 2.
TGFβ-induced Opacification in Lenses from Ovariectomised Rats on Various Hormone Replacement Regimes
| Hormone regime | Opacification index | |
|---|---|---|
| Vehicle alone | 240 ± 18 | |
| Estrogen | 0 | |
| Progesterone | 210 ± 19* |
TGFβ2 (0.15 ng/ml) was used and lenses were cultured for 7 d. The opacification index was calculated as described in Materials and Methods. Values represent the mean ± SEM of determinations for six individual lenses.
This value is not significantly different from the value for controls (vehicle alone).
Figure 2.
Influence of ovarian hormones on induction of cataract by TGFβ. Ovariectomized rats received vehicle alone (A), estrogen replacement (B), or progesterone replacement (C). Lenses were cultured with 0.15 ng/ml TGFβ2 and photographed after 7 d. Lenses from rats that received vehicle alone or progesterone developed distinct opacities (A and C), whereas lenses from rats that received estrogen remained transparent (B). Bar, 400 μm.
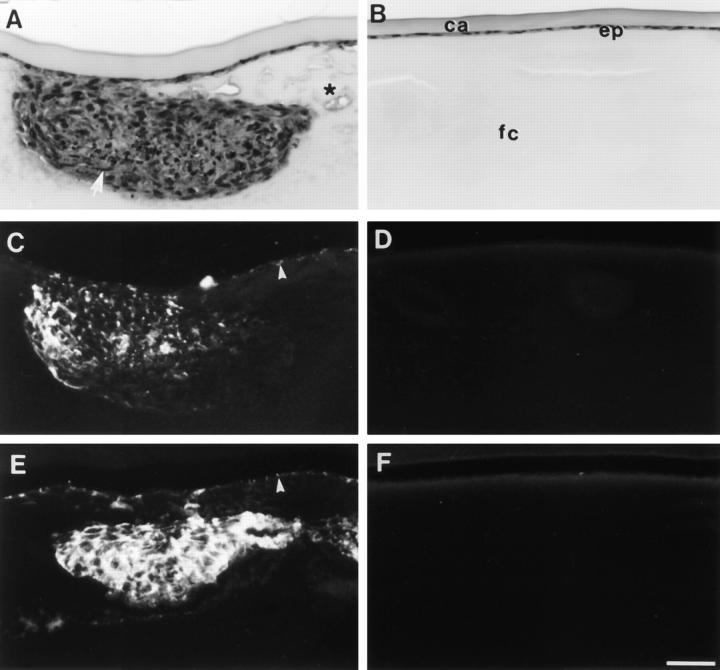
Histologically, the opacities observed in ovariectomized rats that only received vehicle corresponded with subcapsular plaques or clumps of abnormal cells (Fig. 3 A). Reactivity for the cataract markers α-smooth muscle actin and type I collagen was observed predominantly within the subcapsular plaques (Fig. 3, C and E). In contrast, lenses from estrogen-treated rats retained normal cellular morphology (Fig. 3 B), and no reactivity for α-smooth muscle actin or type I collagen was detected (Fig. 3, D and F). In all these respects, lenses from rats that received progesterone replacement were indistinguishable from lenses from rats that received vehicle alone.
Figure 3.
Histology and immunolocalization of cataract markers. Lenses from ovariectomized rats that received vehicle alone (A, C, and E) or estrogen replacement (B, D, and F) were cultured with 0.15 ng/ml TGFβ2 and fixed at the end of a 7 d culture period. Serial sections were stained with haematoxylin and eosin (A and B), or used for localization of α-smooth muscle actin (C and D) and type I collagen (E and F). Lenses from rats that received vehicle alone developed large anterior subcapsular plaques (A), which contained spindle-shaped cells (arrow) and many condensed nuclei. In addition, the fiber cells around the plaques appeared swollen and vacuoles were commonly present (asterisk). α-Smooth muscle actin (C) and type I collagen (E) were localized within the TGFβinduced subcapsular plaques and also in some of the cells that remained attached to the capsule (arrowheads). Lenses from rats that received estrogen retained normal lens morphology (B) with a monolayer of epithelial cells (ep) adjacent to the lens capsule (ca) and overlying the fibre cells (fc). These lenses showed no reactivity for either α-smooth muscle actin (D) or type I collagen (F). Bar, 40 μm.
A variety of more subtle histological changes were observed in lenses from ovariectomized rats that did not receive estrogen. Swelling of cortical fiber cells with evidence of degeneration, reminiscent of cortical cataract (11), was commonly observed (for example see Fig. 3 A), generally in the region of the lens anterior to the equator. In addition, nucleated cells were observed migrating along the posterior capsule towards the posterior pole (Fig. 4 A); these cells showed reactivity for type I collagen (Fig. 4 C), but not for α-smooth muscle actin. None of these changes were observed in lenses from estrogen-treated ovariectomized rats, which remained transparent (Fig. 4, B and D). In lenses from normal male and female rats (not shown), concentrations of TGFβ >0.15 ng/ml were required to induce changes as pronounced as those in Figs. 3 A and 4 A. Thus, lenses from normal rats of either sex seemed to be more resistant to the effects of TGFβ than those from ovariectomized rats that did not receive estrogen.
Figure 4.

Posterior migration of cells associated with induction of cataract by TGFβ. Lenses from ovariectomized rats that received vehicle alone (A and C) or estrogen replacement (B and D) were cultured with 0.15 ng/ml TGFβ2 and fixed at the end of the 7 d culture period. Serial sections were stained for routine histology with haematoxylin and eosin (A and B) or used for immunofluorescent localization of type I collagen (C and D). The lens equator is positioned at the top of each micrograph. In lenses from rats that received vehicle alone (A), nucleated cells were observed migrating along the lens capsule toward the posterior pole (A, arrowheads). Strong reactivity for type I collagen was associated with these posteriorly migrating cells (C, arrowheads). In contrast, lenses from rats that received estrogen replacement maintained a normal lens morphology with no abnormal migration of cells below the lens equator (B). No reactivity for type I collagen was observed (D). Bar, 40 μm.
Estrogen administered in vivo as in the above experiments may exert its effects on lens cells directly or indirectly. To determine whether estrogen is capable of influencing lens cells directly, lenses from ovariectomized rats were treated with estrogen in vitro before and during culture with TGFβ (Table 3). Estrogen-treated lenses showed negligible opacification in response to TGFβ. While a small region of haziness was observed at the center of some of these lenses within 6 d of adding TGFβ, no tendency to condense into discrete opacities with time was noted (not shown). Numerous distinct opacities developed in corresponding lenses cultured in parallel without the addition of estrogen, which were thus highly susceptible to the cataractogenic effects of TGFβ under these conditions (Table 3).
Table 3.
Effect of Estrogen Exposure In Vitro on TGFβ-induced Opacification of Lenses from Ovariectomized Rats
| Treatment | Opacification index | |
|---|---|---|
| 17-β-estradiol | 0 | |
| No estradiol | 151 ± 19 |
Lenses from ovariectomized rats were precultured for 2 d with or without 10−10 M 17-β-estradiol, as indicated. Medium was then replaced (with or without estradiol, as before) and TGFβ2 (0.15 ng/ml) was added immediately. After a further 7 d culture, the opacification index was determined as described in Materials and Methods. Values represent the mean ± SEM of determinations for three individual lenses.
Discussion
The present study grew out of our findings that TGFβ induces lens epithelial explants and cultured lenses to undergo molecular and morphological changes that are typically associated with human subcapsular cataracts (8–10, 16). Both TGFβ1 and TGFβ2 induce cataractous changes, TGFβ2 being the more potent isoform (16). The cataractogenic effects of TGFβ are blocked by a pan-specific antibody against TGFβ (17), and are not simply a “growth factor” effect. Weanling lenses cultured with another growth factor, FGF-2 (or FGF-1), at concentrations known to induce lens cell proliferation and/or fiber differentiation (18) remain transparent (Hales, A., C. Chamberlain, J. McAvoy, unpublished data).
In cultured lenses from weanling rats, opacities induced by TGFβ correspond with subcapsular plaques of aberrant cells including spindle-shaped cells which are often associated with wrinkling of the lens capsule. Abnormal extracellular matrix deposition also occurs, mainly in the plaques. All these changes are typical of anterior and posterior subcapsular cataract and aftercataract (19–22). In addition, TGFβ induces the accumulation of α-smooth muscle actin and type I collagen in both explants and cultured lenses (9, 10). These proteins, which are not generally found in the lens, are present in human anterior subcapsular cataract and in aftercataract (23–26). The present study shows that in lenses from adult rats, as for weanlings, TGFβ induces opacities with morphological and molecular features of cataract. In each case, the plaques associated with the opacities are morphologically indistinguishable from early stage human anterior subcapsular cataracts (11).
Major findings of the present study are that the ovarian hormone, estrogen, protects rat lenses against TGFβ-induced cataract, and that susceptibility to the cataractous changes induced by TGFβ is sex dependent. Culturing lenses from ovariectomized females with TGFβ resulted in marked opacification of the lens. Estrogen replacement in vivo prevented this response, but progesterone replacement did not (Table 2; Fig. 2). Furthermore, lenses from male rats were found to be more susceptible to the cataractogenic effects of TGFβ than those from normal females (Table 1; Fig. 1), and lenses from normal rats of either sex seemed more resistant to the effects of TGFβ than those from ovariectomized rats (Tables 1 and 2). The latter results are also consistent with a protective role for estrogen since circulating estrogens are present in male rats, albeit at much lower levels than in normal females (27), but not in ovariectomized females (28). Further support comes from a recent study involving transgenic mice. Females expressing a mutant, “nonresponsive” estrogen receptor progressively develop cortical and nuclear cataracts (29). It seems unlikely that the greater susceptibility of male rats to the cataractogenic effects of TGFβ is due to the presence of testosterone since, in aging males, cataract increases (5) while testosterone decreases (30).
Sex-dependent and estrogen-related differences in susceptibility to cataract formation, consistent with a protective role for estrogen, have been noted in epidemiological studies. After menopause, the prevalence of cortical cataracts in females suddenly increases relative to males of equivalent age (2, 3). Before menopause, the prevalence of cataract seems to be similar in males and females (3, 31). In addition, the prevalence and severity of certain forms of cataract is lower in postmenopausal women on hormone replacement therapy involving administration of estrogen with or without progesterone, than in those who are not undergoing hormone replacement. This is true whether menopause occurs naturally or as a result of hysterectomy (5–7). Delayed menopause also seems to protect against cataract (5–7). Cernea et al. have suggested a possible link between presenile cataract development and ovarian hormone insufficiency (32). Furthermore, analysis of the age of patients who undergo cataract surgery indicates that men tend to develop presenile cataracts approximately four years earlier than women (2). The present study of cultured rat lenses provides strong support for this epidemiological evidence that estrogen protects against cataract.
This study goes beyond previous studies, however, in that it provides direct evidence that estrogen protects against cataract. Furthermore, it shows that estrogen does so by protecting lens cells against the cataractogenic effects of TGFβ. This protective effect is observed whether estrogen is administered in vivo or in vitro. Thus, it seems likely that estrogen confers protection against cataract by targeting lens cells directly when administered in vivo, although the possibility of additional indirect benefits is not excluded. In other cellular systems, estrogen has been shown to have a variety of effects on TGFβ. It may suppress the effects of TGFβ. For example, estrogen-induced tumorigenesis in the anterior pituitary of rats is accompanied by a loss of sensitivity to TGFβ1 in tumor cells and downregulation of the TGFβ type II receptor (33). Similarly, treating rats with estrogen in vivo reduces TGFβ binding in the uterus, possibly via downregulation of TGFβ receptors (34). On the other hand, estrogen may enhance TGFβ activity when included in vitro (35, 36) or have no effect (37).
The present study adds weight to an increasing body of evidence that hormone replacement therapy not only relieves acute menopausal symptoms, but also offers protection against various diseases (38). These include cardiovascular diseases such as myocardial infarction, atherosclerosis, and related hypertension and stroke, as well as osteoporosis and associated fractures (38–41). Ocular pressure decreases progressively in both women and men, undergoing treatment with estrogen (42). In addition, estrogen use dramatically reduces the risk of idiopathic macular hole formation, an ocular condition that is more common in untreated postmenopausal women than in men of the same age (43). Many postmenopausal women are now receiving estrogen replacement therapy, in conjunction with progesterone where appropriate, but it is not universally advocated or available. In 1994, it was reported that only 5–10% of menopausal women in the United States were receiving this treatment (38).
The opacities that form in rat lenses cultured with TGFβ are anterior subcapsular cataracts. Marked similarities between TGFβ-induced cataract and aftercataract have already been noted (8, 10). Evidence of subtle changes typical of posterior subcapsular cataract and cortical cataract is provided by the present study. We report for the first time that TGFβ can induce migration of aberrant cells along the lens capsule towards the posterior pole (as in Fig. 4 A). A similar abnormal migration of nucleated cells along the posterior capsule is thought to be the basis of human posterior subcapsular cataract formation (20). Some evidence of TGFβ-induced cataract-like change was also observed in the cortical fibers in the present study (Fig. 3 A).
TGFβ is potentially available to lens cells in situ. TGFβ and its mRNA have both been detected in the mammalian eye (44–46), and biologically active TGFβ has been found in samples of the ocular media that bathe the lens obtained from human patients suffering from cataract (47–50). Large reservoirs of inactive TGFβ are present in the ocular media (47, 49, 51, 52) which may become activated, e.g., after eye surgery (53). It is not clear what level of TGFβ stimulation lens cells receive under normal conditions in situ, and little is known about factors that regulate its activity in the eye (17). Estrogens have been detected in the ocular media in untreated female and male rabbits, but it is not clear what levels of active hormone are present (27).
The present study provides further evidence that TGFβ is involved in the aetiology of various forms of subcapsular cataract, and possibly cortical cataract. Our finding that estrogen, administered in vivo or in vitro, protects against cataract is consistent with epidemiological studies. Thus, as suggested by previous studies from this laboratory, the rat lens model appears to be a valid tool for investigating factors that influence cataractogenesis. The parallel between estrogen effects in the rat system and epidemiological evidence also raises the possibility that estrogen may provide protection against human cataract by influencing a TGFβmediated mechanism of cataractogenesis. This study represents another significant step towards elucidating the molecular basis of cataract, the foundation for developing new strategies for prevention or treatment of this debilitating disease.
Footnotes
The authors wish to thank Dr. Tim Shaw and Ms. Maria Bucci for performing the ovariectomies and administering replacement hormones, and Dr. Rebecca Mason for providing phenol red-free medium and estradiol. We also wish to thank Roland Smith for his assistance with the photography.
This work was supported by grants from the National Health & Medical Research Council of Australia, the National Eye Institute, Department of Health, Education and Welfare, USA (R01 EYO3177), and a University of Sydney Faculty of Medicine Postgraduate Scholarship to A.M. Hales.
References
- 1.Harding, J.J. 1991. Cataract: Biochemistry, Epidemiology and Pharmacology. Chapman and Hall, London. 83–124.
- 2.Schwab IR, Armstrong MA, Frienman GD, Wong IG, Carpantieri AC, Dawson CR. Caratact extraction. Risk factors in a health maintenance organization population under 60 years of age. Arch Ophthalmol. 1988;106:1062–1065. doi: 10.1001/archopht.1988.01060140218027. [DOI] [PubMed] [Google Scholar]
- 3.Klein BEK, Klein R, Linton KLP. Prevalence of age-related lens opacities in a population. The Beaver Dam eye study. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JR, Deane JS, Hall AB, Rosenthal AR. Oestrogen and lens opacities in the Melton eye study. Invest Ophthalmol Visual Sci. 1996;37:S585. [Google Scholar]
- 5.Klein BEK. Lens opacities in women in Beaver Dam, Wisconsin: is there evidence of an effect of sex hormones? . Trans Am Ophthalmol Soc. 1993;91:517–544. [PMC free article] [PubMed] [Google Scholar]
- 6.Harding JJ. Estrogens and cataract. Arch Ophthalmol. 1994;112:1511. doi: 10.1001/archopht.1994.01090240017008. [DOI] [PubMed] [Google Scholar]
- 7.Klein BEK, Klein R, Ritter LL. Is there evidence of an estrogen effect on age-related lens opacities? . Arch Ophthalmol. 1994;112:85–91. doi: 10.1001/archopht.1994.01090130095025. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor-β. Invest Ophthalmol Visual Sci. 1994;35:388–401. [PubMed] [Google Scholar]
- 9.Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-β1 induces lens cells to accumulate α-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res. 1994;13:885–890. doi: 10.3109/02713689409015091. [DOI] [PubMed] [Google Scholar]
- 10.Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-β. Invest Ophthalmol Visual Sci. 1995;36:1709–1713. [PubMed] [Google Scholar]
- 11.Worgul, B.V. 1982. Lens. In Ocular Anatomy, Embryology and Teratology. F.A. Jakobiec, editor. Harper and Row, Philadelphia. 355–389.
- 12.Border WA, Noble NA. Targeting TGF-β for treatment of disease. Nat Med. 1995;1:1000–1001. doi: 10.1038/nm1095-1000. [DOI] [PubMed] [Google Scholar]
- 13.Wahl S. Transforming growth factor β: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy CR, Rogers AW. Effect of ovarian hormones on cell membranes in the rat uterus. III. The surface carbohydrates at the apex of the luminal epithelium. Cell Biophys. 1981;3:305–320. doi: 10.1007/BF02785116. [DOI] [PubMed] [Google Scholar]
- 15.Hales AM, Chamberlain CG, McAvoy JW. Induction of subcapsular cataract in cultured weanling and adult rat lenses by TGFβ2. Invest Ophthalmol Visual Sci. 1996;37:S983. [Google Scholar]
- 16.McAvoy JW, Chamberlain CG, Liu J, Hales AM. TGFβ induces cataract-like changes in lens epithelial explants. Invest Ophthalmol Visual Sci. 1994;35:1103. [PubMed] [Google Scholar]
- 17.Schulz MW, Chamberlain CG, McAvoy JW. Inhibition of TGFβ-induced cataractous changes in lens explants by ocular media and α2-macroglobulin. Invest Ophthalmol Visual Sci. 1996;37:1509–1519. [PubMed] [Google Scholar]
- 18.McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development (Camb) 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- 19.Font RL, Brownstein SA. A light and electron microscopic study of anterior subcapsular cataract. Am J Ophthalmol. 1974;78:972–984. doi: 10.1016/0002-9394(74)90811-3. [DOI] [PubMed] [Google Scholar]
- 20.Eshagian J. Human posterior subcapsular cataracts. Trans Ophthalmol Soc UK. 1982;102:364–368. [PubMed] [Google Scholar]
- 21.Novotny GEK, Pau H. Myofibroblast-like cells in human anterior subcapsular cataract. Virchows Arch A Pathol Anat Histopathol. 1984;404:393–401. doi: 10.1007/BF00695223. [DOI] [PubMed] [Google Scholar]
- 22.Green WR, McDonnell PJ. Opacification of the posterior capsule. Trans Ophthalmol Soc UK. 1985;104:727–739. [PubMed] [Google Scholar]
- 23.Frezzotti R, Caporossi A, Mastrangelo D, Hadjistilianou T, Tosi P, Cintorino M, Minacci C. Pathogenesis of posterior capsular opacification. II. Histopathological and in vitro culture findings. J Cataract Refractive Surg. 1990;16:353–360. doi: 10.1016/s0886-3350(13)80708-0. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt-Graff A, Pau H, Spahr R, Piper HM, Skalli O, Gabbiani G. Appearance of alpha-smooth muscle actin in human eye lens cells of anterior capsular cataract and in cultured bovine lens-forming cells. Differentiation. 1990;43:115–122. doi: 10.1111/j.1432-0436.1990.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 25.Hatae T, Ishibashi T, Yoshitomo F, Shibata Y. Immunocytochemistry of type I–IV collagen in human anterior subcapsular cataracts. Graefe's Arch Clin Exp Ophthalmol. 1993;231:586–590. doi: 10.1007/BF00936523. [DOI] [PubMed] [Google Scholar]
- 26.Namiki M, Tagami Y, Morino I, Kano M, Sugiura T. Findings from slit lamp and histological examination of the anterior capsule in patients with severe anterior capsular shrinkage and opacities after implantation of intraocular lenses. J Jpn Ophthalmol Soc. 1993;97:716–720. [PubMed] [Google Scholar]
- 27.Starka L, Hampl R, Bicikova M, Obenberger J. Identification and radioimmunologic estimation of sexual steroid hormones in aqueous humor and vitreous of rabbit eye. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1976;199:261–266. doi: 10.1007/BF00417296. [DOI] [PubMed] [Google Scholar]
- 28.Ljungkvist I. Attachment reaction of rat uterine luminal epithelium. Acta Soc Med Ups. 1971;76:91–109. [PubMed] [Google Scholar]
- 29.Chan CC, Davis VL, Schoen T, Li Q, Korach KS, Chader GJ. The role of estrogen on cataract formation: a study of transgenic mice with mutation of estrogen receptor. Invest Ophthalmol Visual Sci. 1996;37:S988. [Google Scholar]
- 30.Nahoul K, Roger M. Age-related decline of plasma bioavailable testosterone in adult men. J Steroid Biochem. 1990;35:293–299. doi: 10.1016/0022-4731(90)90287-3. [DOI] [PubMed] [Google Scholar]
- 31.Livingston PM, Guest CS, Stanislavsky Y, Lee S, Walker C, McKean C, Taylor HR. A populationbased estimate of cataract prevalence: The Melbourne visual impairment project experience. Dev Ophthalmol. 1994;26:1–6. doi: 10.1159/000423753. [DOI] [PubMed] [Google Scholar]
- 32.Cernea P, Ignat F, Danciulescu D. Cataracta pathologica din insuficienta ovariana. Rev Chir Oncol Radiol ORL Oftalmol Stomatol Ser Oftalmol. 1989;33:187–192. [PubMed] [Google Scholar]
- 33.Pastorcic M, De A, Boyadjieva N, Vale W, Sarkar DK. Reduction in the expression and action of transforming growth factor beta 1 on lactotropes during estrogen-induced tumorigenesis in the anterior pituitary. Cancer Res. 1995;55:4892–4898. [PubMed] [Google Scholar]
- 34.Takahashi T, Eitzman B, Bossert NL, Walmer D, Sparrow K, Flanders KC, McLachlan J, Nelson K. Transforming growth factors β1, β2, and β3 messenger RNA and protein expression in mouse uterus and vagina during estrogen-induced growth: a comparison to other estrogen regulated genes. Cell Growth Differ. 1994;5:919–935. [PubMed] [Google Scholar]
- 35.Dorrington JH, Bendell JJ, Khan SA. Interactions between FSH, estradiol-17 beta and transforming growth factor-beta regulate growth and differentiation in the rat gonad. J Steroid Biochem Mol Biol. 1993;44:441–447. doi: 10.1016/0960-0760(93)90248-u. [DOI] [PubMed] [Google Scholar]
- 36.Herman ME, Katzenellenbogen BS. Alterations in transforming growth factor-alpha and -beta production and cell responsiveness during the progression of MCF-7 human breast cancer cells to estrogen-autonomous growth. Cancer Res. 1994;54:5867–5874. [PubMed] [Google Scholar]
- 37.Ni N, Yager JD. Comitogenic effects of estrogens on DNA synthesis induced by various growth factors in cultured female rat hepatocytes. Hepatology. 1994;19:183–192. [PubMed] [Google Scholar]
- 38.Griffing GT, Allen SH. Estrogen replacement therapy at menopause. Postgrad Med. 1994;96:131–140. doi: 10.1080/00325481.1994.11945912. [DOI] [PubMed] [Google Scholar]
- 39.Compston JE. HRT and osteoporosis. Br Med Bull. 1992;48:309–344. doi: 10.1093/oxfordjournals.bmb.a072549. [DOI] [PubMed] [Google Scholar]
- 40.Meade TW, Berra A. Hormone replacement therapy and cardiovascular disease. Br Med Bull. 1992;48:276–308. doi: 10.1093/oxfordjournals.bmb.a072548. [DOI] [PubMed] [Google Scholar]
- 41.Wren BG. The effect of oestrogen on the female cardiovascular system. Med J Aust. 1992;157:204–208. doi: 10.5694/j.1326-5377.1992.tb137091.x. [DOI] [PubMed] [Google Scholar]
- 42.Treister G, Mannor S. Intraocular pressure and outflow facility. Arch Ophthalmol. 1970;83:311–318. doi: 10.1001/archopht.1970.00990030313008. [DOI] [PubMed] [Google Scholar]
- 43.The Eye Disease Case-Control Study Group. Risk factors for idiopathic macular holes. Am J Ophthalmol. 1994;118:754–761. [PubMed] [Google Scholar]
- 44.Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF-beta1, beta 2 and beta 3 suggest different developmental functions in vivo. Development (Camb) 1991;111:131–144. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- 45.Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGFβ1, TGFβ2, and TGFβ3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD. Immunolocalization of TGF-beta1, TGF-beta2 and TGF-beta3 in the anterior segment of the human eye. Invest Ophthalmol Visual Sci. 1993;34:23–30. [PubMed] [Google Scholar]
- 47.Granstein RD, Stasezewski R, Knisely TL, Zeira E, Nazareno R, Latina M, Albert DM. Aqueous humor contains transforming growth factor-β and a small (<3500 daltons) inhibitor of thymocyte proliferation. J Immunol. 1990;144:3021–3027. [PubMed] [Google Scholar]
- 48.de Boer JH, Limpens J, Orengo-Nania S, de Jong PTVM, La E, Heij, Kijlstra A. Low mature TGF-β2 levels in aqueous humor during uveitis. Invest Ophthalmol Visual Sci. 1994;35:3702–3710. [PubMed] [Google Scholar]
- 49.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-β in human aqueous humor. Curr Eye Res. 1990;9:963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 50.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factorbeta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Visual Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 51.Connor TB, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M, et al. Correlation of fibrosis and transforming growth factor-β type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathi RC, Li J, Chan WFA, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-β2. Exp Eye Res. 1994;59:723–728. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 53.Tripathi RC, Borisuth NSC, Tripathi BJ. Growth factors in the aqueous humor and their therapeutic implications in glaucoma and anterior segment disorders of the human eye. Drug Dev Res. 1991;22:1–23. [Google Scholar]