Abstract
Jak3 mediates growth signals through cytokine receptors such as interleukin-2 (IL-2), IL-4, and IL-7, and its deficiency results in autosomal recessive SCID in mice and humans. In spite of the severely reduced number of lymphocytes in Jak3-deficient mice, the differentiation profile of thymocytes was normal and mature T cells accumulated in the periphery with age. However, we found that self-reactive T cells were not deleted in the thymus and the peripheral tissues in Jak3-deficient mice. All peripheral T cells were in the activation state and thus were unable to be activated further, as demonstrated by the failure of eliciting Ca2+ response upon T cell receptor (TCR) stimulation. From the analysis of TCR-transgenic Jak3-deficient mice, only self-reactive T cells appeared to be in the activated state and anergic. These findings demonstrate a crucial function of Jak3 in the negative selection of autoreactive T cells and the maintenance of functional peripheral T cells.
Jak3 is associated constitutively with the common γ chain (γc), a subunit of the cytokine receptors of IL-2, IL-4, IL-7, IL-9, and IL-15, and has been shown in vitro to be essential for their signal transduction (1–8). In the case of IL-2 signaling, Jak3 is activated as one of the earliest events upon IL-2 binding to IL-2 receptor (2, 8–9), and then activates Stat5 to translocate into the nucleus for mediating growth signals. In addition, the constitutive activation of the Jak3–Stat5 signaling pathway causes IL-2-independent growth for HTLV-1-transformed T cells (10). These in vitro analyses have demonstrated that the signal through Jak3 is essential for and regulates cell growth of lymphocytes.
SCID is a disease based on several different genetic abnormalities, one of them being X-linked SCID (XSCID) caused by functional mutations of the γc (11). It has recently been shown that Jak3 deficiency caused autosomal recessive SCID (AR-SCID) in humans, with symptoms similar to XSCID (12–13). Recently, we and others established Jak3-deficient mice (Jak3 −/−) as an animal model of human AR-SCID (14–16). Jak3 −/− mice revealed severe developmental inhibition of B cells, NK cells, and γδ T cells in the skin and small intestine. These results demonstrated the important roles of Jak3 in the development of lymphocytes and NK cells in vivo. Although thymocytes and peripheral T cells were drastically reduced in young Jak3-deficient mice, T cells gradually accumulated in the peripheral organs with age in the absence of functional growth signals mediated by Jak3. We analyzed the repertoire and function of these thymocytes and peripheral T cells in Jak3 −/− mice and found defects of negative selection of self-reactive T cells in the thymus and the periphery, demonstrating a novel function of Jak3 in mediating negative selection and in maintaining functional T cells.
Materials and Methods
Mice.
C57BL/6 mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). DO11.10 TCR-transgenic mice (DO-Tg) were provided by Dr. D. Loh (Nippon Roche Research Center, Kamakura, Japan) (17). Jak3-deficient mice were previously described (15).
Analysis of Cell Proliferation and IL-2 Secretion.
For proliferation assay, splenocytes (2 × 105) from 8-wk-old mice were stimulated with Con A (2.5 μg/ml), PMA (5 ng/ml) plus A23187 (100 ng/ ml), and anti-CD3ε mAb (145-2C11) cross-linking for 48 h, pulsed with 37 kBq [3H]thymidine (Amersham Corp., Arlington Heights, IL) for the last 8 h of culture, and harvested. [3H]thymidine uptake was measured with a MicroBeta™ liquid scintillation counter (Pharmacia, Uppsala, Sweden). For IL-2 production, proliferation of the IL-2-dependent cell line CTLL-2 was measured. CTLL-2 cells (6 × 103) were cultured with supernatants from the proliferation assay after stimulation for 48 h. Cells were pulsed with [3H]thymidine and harvested as described above.
Measurement of Intracellular Ca2+ Response.
Intracellular Ca2+ mobilization was measured using Epics Elite ER-3 (Coulter Corp., Hialeah, FL) with a Cell Quest analyzing program. 2 × 107 cells were incubated with 4 μM Indo-1 (Molecular Probes, Inc., Eugene, OR) for 30 min at 37°C for loading. For TCR stimulation, cells were incubated with anti-CD3ε mAb (1452C11) for 30 min on ice and washed. After measuring the basal level, 10 μg goat anti–hamster Ig was added for cross-linking. For the response by Ca2+ ionophore, 1 μg A23187 (Sigma) was added after measurement of the basal level.
Flow Cytometric Analysis.
Splenocytes and lymph node T cells were incubated with fluorescence- or biotin-conjugated antibodies and analyzed with a FACScan® (Becton Dickinson, Mountain View, CA) using Cell Quest software. 5 × 105 cells were analyzed for each sample. For multicolor analysis, cells were first incubated with anti-FcRγ receptor mAb 2.4G2 to prevent nonspecific staining. The following antibodies were used: CD4 (RM4-4), CD8α (53-6.7), CD69 (H1.2F3), CD44 (1M7), CD25 (7D4), and MEL14. These Abs, as well as all anti-Vβ mAbs (Vβ2–Vβ14), were purchased from PharMingen (San Diego, CA). Anti-clonotypic mAb against DO11.10 TCR, KJ1-26, was provided by Dr. P. Marrack (Denver, CO).
Results
Failure of Deletion of Self-reactive T Cells in the Thymus and Periphery of Jak3-deficient Mice.
When the Vβ repertoire of splenic T cells from Jak3-deficient mice with C57BL/6 background was analyzed, there was no difference in the frequency of Vβ usage between Jak3 −/− mice and wildtype littermates (data not shown). To analyze the repertoire of T cells reactive to endogenous MMTV products presented on I-E molecule, we backcrossed with BALB/c and analyzed the Vβ usage. As shown in Fig. 1 A, the frequency of mature T cells expressing Vβ5 and Vβ11, but not others, was significantly increased in Jak3 −/− mice as compared with heterozygous mice. These Vβ-expressing T cells are known to be deleted in BALB/c mice, whereas C57BL/6 mice fail to delete them due to the lack of MHC class II I-E molecules. Although extensive analysis could not be performed on thymocytes due to their limited number in Jak3 −/− mice, similar increases of CD4 single-positive thymocytes expressing Vβ5 and Vβ11 were observed (Fig. 1 B). It is noteworthy that, while the percentage of Vβ11+ T cells in Jak3 −/− mice with H-2d background was almost restored to the level of C57BL/6 mice, the frequency of Vβ5 was only partly recovered. These data demonstrate that Jak3-deficient mice failed to delete self-reactive T cells. Furthermore, the observation that the percentage of Vβ10+ T cells was reduced in Jak3 −/− mice suggests additional defects.
Figure 1.
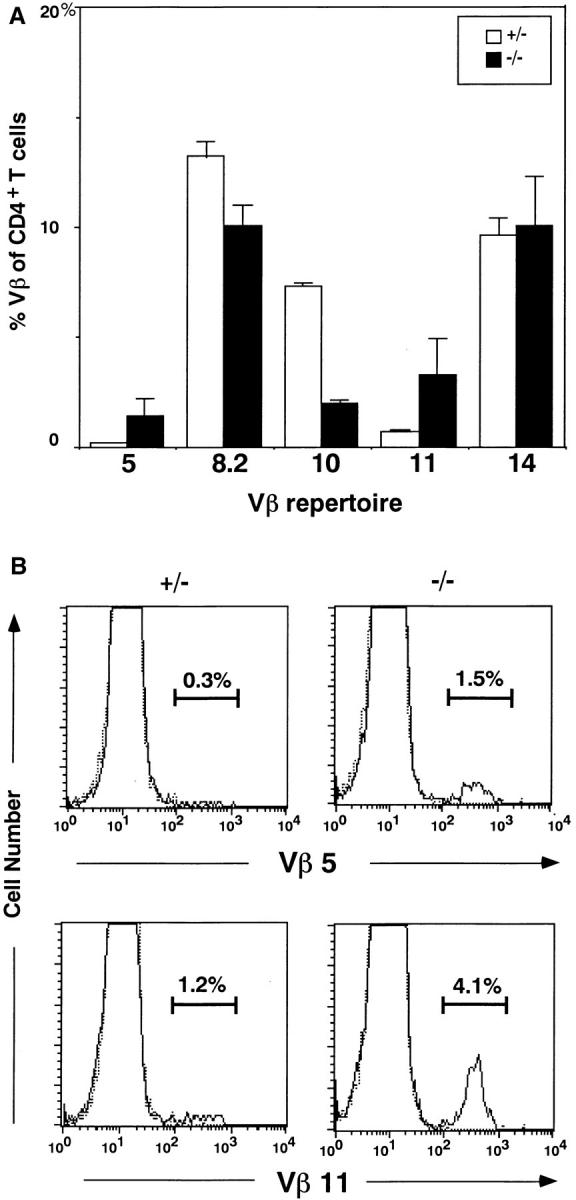
Vβ repertoire of T cells in the thymus and periphery of Jak3deficient mice. (A) Vβ repertoire of CD4+ peripheral T cells from Jak3homozygous (−/−) and heterozygous (+/−) mice. The percentages of T cells expressing representative Vβs among CD4+ T cells were shown as the average ± SD of three experiments. (B) Expression of Vβ5 and Vβ11 on CD4+ CD8− thymocytes from Jak3-homozygous (−/−) and heterozygous (+/−) mice. Staining profiles with anti-Vβ5 and anti-Vβ11 mAbs (solid line) and control mAb (dotted line) on CD4+CD8− thymocytes. The percentages of Vβ5- and Vβ11-expressing T cells among CD4+CD8− thymocytes were indicated in each panel. For both (A) and (B), thymocytes or B cell–depleted splenocytes were incubated first with each anti-Vβ mAb and then with a mixture of biotin-coupled goat anti– mouse Ig and rat Ig Abs. After blocking with mouse and rat Ig, the cells were stained with FITC–anti-CD4 and PE–anti-CD8 and streptavidin– Quantum Red.
Peripheral T Cells in Jak3-deficient Mice Are Preactivated.
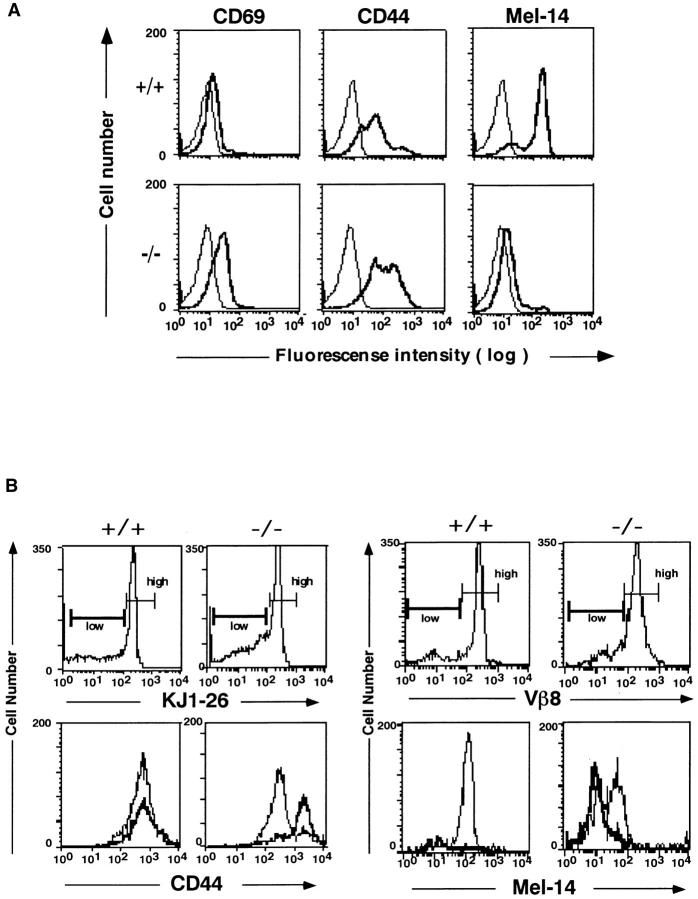
In addition to the existence of autoreactive T cells in thymus, spleen, and lymph node, all peripheral T cells from Jak3 −/− mice were activated as determined by surface expression of several markers. These T cells expressed high levels of CD44 and CD69 (18–20) and a low level of Mel14, representing the phenotype of activated T cells (Fig. 2 A).
Figure 2.
Expression of activation markers on splenic T cells from Jak3-deficient mice (A) and DO-Tg·Jak3 −/− mice (B). (A) Staining profiles of CD69, CD44, and Mel-14 on splenic T cells from Jak3-deficient mice (−/−) and wild-type littermates (+/+). Cells were stained for either CD69, CD44, or Mel-14 in addition to CD4 and CD8, and the profiles were shown for CD4+ T cells. The profiles for CD8+ T cells were almost the same as for CD4+ T cells. Thick and thin lines indicated staining with each Ab and control staining, respectively. (B) Expression of CD44 (left) and Mel-14 (right) on CD4+ splenic T cells from DO-Tg (+/+) and DOTg·Jak3 −/− (−/−) mice. (Top left) Staining profiles with the clonotypic mAb KJ1-26; cells were divided into two groups, KJ1-26 high and low. (Bottom left) Thin and thick lines indicate CD44 staining for KJ1-26 high and low cells, respectively. (Top right) Staining profiles with antiVβ8 mAb; cells were divided into two groups, Vβ8 high and low. (Bottom right) Thin and thick lines indicate Mel-14 staining for Vβ8 high and low cells, respectively.
To analyze further the origin of the preactivated T cells in the periphery of Jak3 −/− mice, we crossed Jak3 −/− mice with OVA-specific DO-Tg mice whose TCR (DO-TCR) was detected by staining with anti-clonotypic mAb KJ1-26 for the TCRαβ dimer and anti-Vβ8 mAb F23.1 for the TCRβ chain. ∼20% of the T cells from DO-Tg mice expressed endogenous TCR, but the rest of the cells were stained with KJ1-26 and F23.1 (17). Whereas KJ1-26 positive and negative populations from DO-Tg mice did not show a significant difference in CD44 expression, the DOTCR-expressing (KJ1-26high) T cells from DO-Tg·Jak3 −/− mice were CD44low (Fig. 2 B). In contrast, most of the T cells expressing endogenous TCR (KJ1-26low) were CD44high, which is the same phenotype as Jak3 −/− splenic T cells (Fig. 2 B). This was also shown by Mel-14 expression. Whereas DO-TCR expressing T cells (Vβ8high) were composed of both Mel-14high and Mel-14low populations, T cells expressing endogenous TCR (Vβ8low) were all Mel-14low (Fig. 2 B). These data demonstrate that T cells with endogenous TCR but not DO-TCR-expressing T cells were reactivated in DO-Tg·Jak3 −/− mice, suggesting that splenic T cells in Jak3 −/− mice may be autoreactive and have been activated with self-antigens.
Defects of TCR Signaling in Peripheral T Cells from Jak3- deficient Mice.
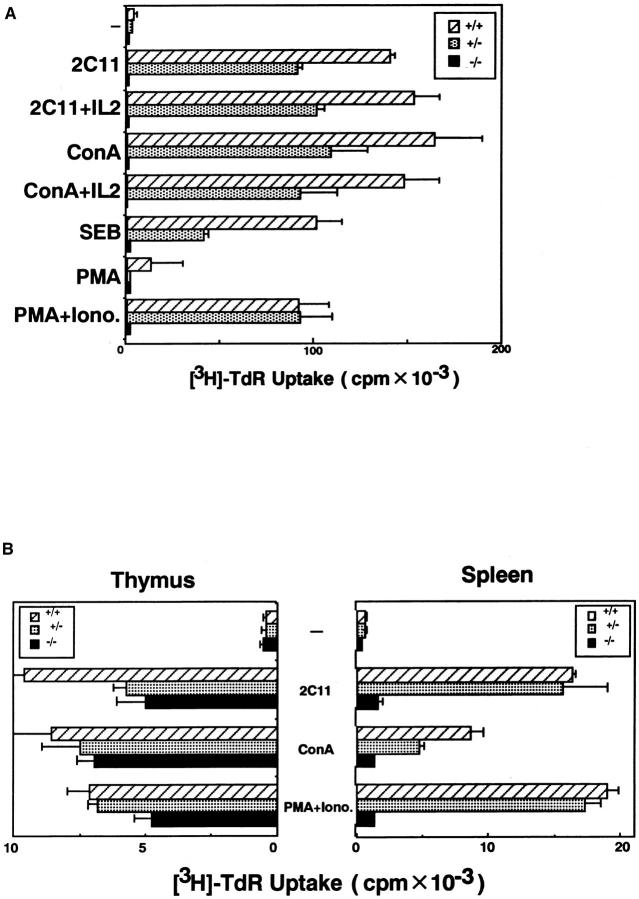
Since splenic T cells in Jak3 −/− mice are in the activated state, we asked whether these T cells might be functionally unresponsive to further stimulation. Indeed, splenic T cells from Jak3 −/− mice failed to proliferate upon stimulation with either Con A or anti-CD3ε mAb crosslinking, regardless of the presence of exogenous IL-2. Furthermore, these T cells did not proliferate even after stimulation with PMA and Ca2+ ionophore (Fig. 3 A). In contrast with the splenic T cells, thymocytes from Jak3 −/− mice responded to both anti-CD3ε cross-linking and stimulation with PMA plus Ca2+ ionophore (15). As shown in Fig. 3 B, thymocytes from Jak3−/− mice secreted a considerable amount of IL-2 compared with normal thymocytes, while splenic T cells from Jak3−/− mice produced very little. Cell surface staining revealed no difference in TCR expression between Jak3 −/− and wild-type mice (15). These data demonstrated that splenic T cells in Jak3 −/− mice possess defects in the signal transduction pathway leading to IL-2 production upon TCR stimulation, in addition to growth signal defects.
Figure 3.
Functional analysis of thymocytes and splenic T cells from Jak3-deficient mice. (A) Proliferation of splenic T cells upon mitogenic stimulation. Splenocytes (2 × 105) from Jak3 homozygous (−/−), heterozygous (+/−) mutant mice, and wild-type littermates (+/+) were stimulated with anti-CD3ε mAb (145-2C11, 10 μg/ml), Con A (2.5 μg/ml), IL-2 (40 U/ ml), Staphyloccocal enterotoxin B (10 μg/ml), and the combination of PMA (5 ng/ml) and A23187 (100 ng/ml). Cells were cultured for 48 h and pulsed with [3H]thymidine for 8 h. (B) IL-2 production of thymocytes and splenic T cells upon mitogenic stimulation. Thymocytes and splenic T cells from Jak3homozygous (−/−), and heterozygous (+/−) mutant mice, and wild-type littermates (+/+) were stimulated with 145-2C11, Con A, and PMA plus A23187 as described in (A). All results were presented as mean ± SD from triplicate cultures.
To investigate the defects in TCR activation, we analyzed intracellular Ca2+ mobilization as an indicator of the early signal transduction pathway upon TCR stimulation. As shown in Fig. 4 A, thymocytes from Jak3 −/− mice elicited almost comparable Ca2+ response to that of thymocytes from wild-type mice upon stimulation with both anti-CD3ε mAb cross-linking and Ca2+ ionophore. In contrast, splenic T cells from Jak3 −/− mice failed to elicit Ca2+ response upon TCR cross-linking in spite of the fact that these cells showed Ca2+ flux upon stimulation with Ca2+ ionophore (Fig. 4 B). These data clearly demonstrate that splenic T cells from Jak3 −/− mice have defects in early Ca2+ signaling upon activation through the TCR complex.
Figure 4.
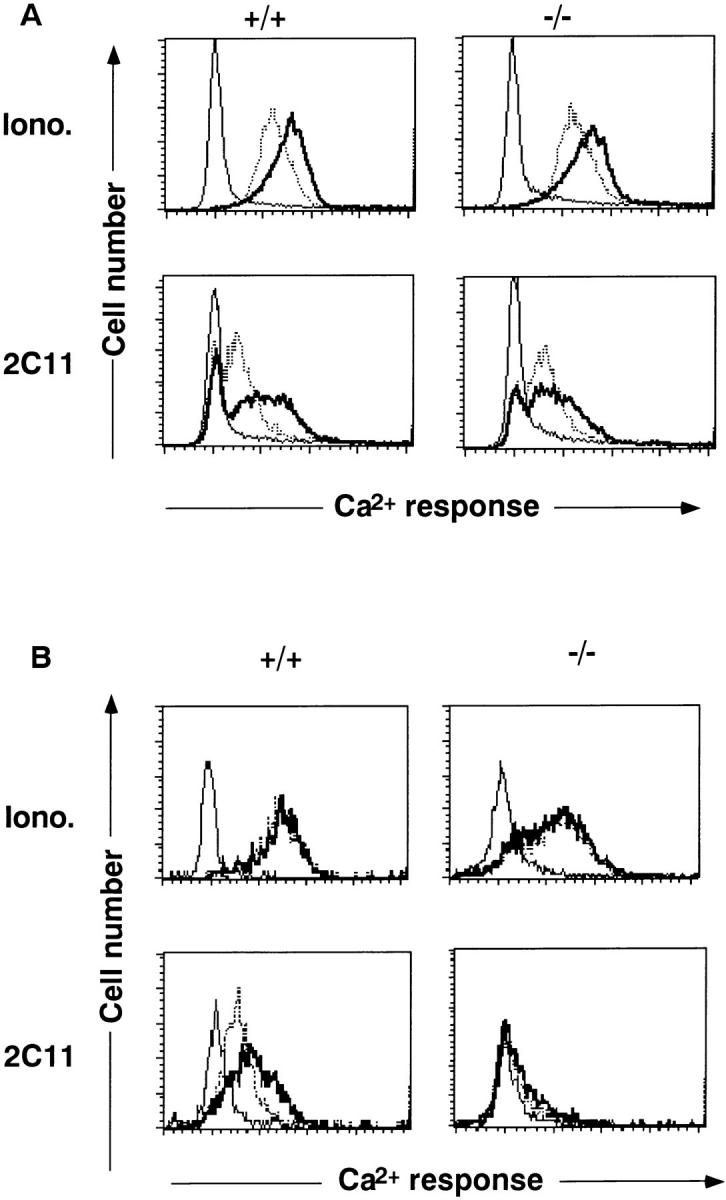
Intracellular Ca2+ mobilization of thymocytes (A) and splenic T cells (B) from Jak3-deficient mice (−/−) and wild-type littermates (+/+). Thymocytes and splenocytes were stained with anti-CD4 and anti-CD8 mAbs, loaded with Indo-1, stimulated with cross-linking with 2C11 or A23187 (Iono.), and then Ca2+ responses were measured. Data for CD4+ cells were represented. In both A and B, the peak (thick line) and sustained line (dotted line) Ca2+ responses were shown.
Discussion
Mutations in γc and Jak3 in the patients of XSCID (11) and AR-SCID (12–13), respectively, caused growth defects in T cells because γc and Jak3 are associated (2, 8) and are both required for growth signal in T cells (9). The failure of cell growth has been thought to be due to defective cytokine receptor signaling. In the present study, we have demonstrated that, in addition to the growth defects, AR-SCID model mice have defects in negative selection of self-reactive T cells. Thus, Jak3-deficient mice possess forbidden autoreactive T cells in the thymus and periphery. The reason these mice do not develop autoimmune diseases may be because these autoreactive T cells are anergic to further stimulation. Although Jak3 deficiency resulted in a dramatic decrease in the number of precursor cells in the thymus, once they were seeded in the thymus, thymocyte differentiation appeared to take place normally (15). However, we found that Jak3 deficiency resulted in a failure to eliminate self-reactive thymocytes, consequently leading to the accumulation of autoreactive but anergic peripheral T cells.
How Jak3 is involved in negative selection is unknown at present. One possibility is that Jak3-mediated growth signal is crucial for the subsequent deletion of self-reactive thymocytes in addition to signals through TCR. The other intriguing possibility is that Jak3 is directly involved in T cell activation. Our observation that PMA plus Ca2+ ionophore did not stimulate IL-2 production in splenic T cells from Jak3 −/− mice, as well as the previous finding that Jak3 is crucial for preventing the induction of anergy in T cells (21), are consistent with this idea.
Signaling defects in splenic T cells from Jak3 −/− mice were observed in association with reactivated status, namely the high expression of activation markers such as CD44 and CD69 as well as the downregulation of Mel-14. Splenic T cells were reactivated in Jak3-deficient mice and were all refractory to further activation. From the analysis of DO-Tg·Jak3 −/− mice, we showed that the appearance of reactivated and refractory T cells depended on the specificity of TCR. Because thymocytes from Jak3-deficient mice do not exhibit the activated phenotype and proliferate, secrete IL-2 and exhibit Ca2+ flux upon TCR stimulation, preactivation of splenic T cells probably takes place during immigration after leaving the thymus or within the periphery. Considering that only T cells with endogenous TCRs exhibited the activated phenotype, it is likely that these T cells were activated with self-peptides in the periphery, while OVA-specific T cells could not be activated in the absence of OVA peptide. The fact that defects in negative selection were influenced by TCR specificity is consistent with our observation that some Vβ+ T cells were completely restored from deletion, while some others were only partly recovered by Jak3 deficiency (Fig. 1). Such autoreactive T cells are in an anergic state after activation. Alternatively, provided that all T cells had been activated during immigration from thymus to the periphery and then returned to the resting state, T cells may fail to return in the absence of Jak3, although some of them can still return to the resting state depending on their TCR specificity. In either case, Jak3 plays a pivotal role in maintaining the normal phenotype and function of peripheral T cells.
Further analysis will be required to elucidate the molecular basis of defects of thymic negative selection and signaling in peripheral lymphocytes from Jak3-deficient mice.
Acknowledgments
We thank Dr. D. Loh for providing DO11.10 Tg mice, Dr. P. Marrack for mAb, Drs. S. Miyatake and T. Shirasawa for helpful discussions, Ms. M. Sakuma for technical assistance, and Ms. H. Yamaguchi for preparing the manuscript.
Footnotes
This work was supported by grants to T. Saito from the Ministry for Education, Science, and Culture and from the Agency for Science and Technology, Japan and partly by a grant from Ciba-Geigy Foundation (Japan) for the Promotion of Science.
References
- 1.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of β and γ chains of IL-2 receptor by the novel cytokine IL-15. EMBO (Eur Mol Biol Organ) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in the response to interleukin-2. Nature (Lond) 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura M, McVicar DW, Johnston JJ, Blake TB, Chen YQ, LaL BK, Lloyd AR, Kelvin DJ, Staples JE, Ortaldo JR, O'Shea JJ. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA. 1994;91:6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science (Wash DC) 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 5.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S, Sugamura K. Functional participation of the IL-2 receptor γ chain in IL-7 complex. Science (Wash DC) 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science (Wash DC) 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Cloning of the γ chain of the human IL-2 receptor. Science (Wash DC) 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 8.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signaling by interleukin-2 and -4 in lymphoid and myeloid cells. Nature (Lond) 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu ZJ, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activator of Jak1 and Jak3 by selective association with IL-2 receptor subunit. Science (Wash DC) 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 10.Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G, Leonard WJ. Constitutively activated Jak–STAT pathway in the T cells transformed with HTLV-1. Science (Wash DC) 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 12.Macci P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O'Shea JJ, et al. Mutation of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature (Lond) 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 13.Russel SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, Migone T-S, Noguchi M, Markert ML, Buckley RH, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Science (Wash DC) 1996;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 14.Nosaka T, van Deursen JMA, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking Jak3. Science (Wash DC) 1996;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase–deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 16.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science (Wash DC) 1995;270:794–796. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlothymus in vivo. Science (Wash DC) 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 18.Budd RC, Cerottini JC, Horvath C, Bron C, Pedreazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 19.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+CD8+thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita I, Nagata T, Tada T, Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor–mediated positive selection. Int Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- 21.Boussiotis VA, Barber DL, Nakarai T, Freeman GJ, Gribben JG, Bernstein GM, D'Andrea AD, Ritz J, Nadler LM. Prevention of T cell anergy by signaling through the γc chain of IL-2 receptor. Science (Wash DC) 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]