Abstract
Loss of the actin filament capping protein CapG has no apparent effect on the phenotype of mice maintained under sterile conditions; however, bone marrow-derived macrophages from CapG−/− mice exhibited distinct motility defects. We examined the ability of CapG−/− mice to clear two intracellular bacteria, Listeria monocytogenes and Salmonella enterica serovar Typhimurium. The 50% lethal dose of Listeria was 10-fold lower for CapG−/− mice than for CapG+/+ mice (6 × 103 CFU for CapG−/− mice and 6 × 104 CFU for CapG+/+ mice), while no difference was observed for Salmonella. The numbers of Listeria cells in the spleens and livers were significantly higher in CapG−/− mice than in CapG+/+ mice at days 5 to 9, while the bacterial counts were identical on day 5 for Salmonella-infected mice. Microscopic analysis revealed qualitatively similar inflammatory responses in the spleens and livers of the two types of mice. Specific immunofluorescence staining analyzed by fluorescence-activated cell sorting revealed similar numbers of macrophages and dendritic cells in infected CapG−/− and CapG+/+ spleens. However, analysis of bone marrow-derived macrophages revealed a 50% reduction in the rate of phagocytosis of Listeria in CapG−/− cells but a normal rate of phagocytosis of Salmonella. Stimulation of bone marrow-derived dendritic cells with granulocyte-macrophage colony-stimulating factor resulted in a reduction in the ruffling response of CapG−/− cells compared to the response of CapG+/+ cells, and CapG−/− bone-marrowed derived neutrophils migrated at a mean speed that was nearly 50% lower than the mean speed of CapG+/+ neutrophils. Our findings suggest that specific motility deficits in macrophages, dendritic cells, and neutrophils render CapG−/− mice more susceptible than CapG+/+ mice to Listeria infection.
Macrophages and neutrophils play a critical role in protecting the host against invading pathogens. These cells are able to crawl to the site of infection and ingest and kill invading pathogens. Chemotaxis and phagocytosis require rearrangement of the actin cytoskeleton, and a myriad of actin regulatory proteins orchestrate this rearrangement (23). One way to regulate actin filament length and concentration is to cap or block actin monomer exchange at the fast-growing or barbed (as defined by electron micrographs of heavy meromyosin-decorated filaments) ends of actin filaments. The barbed end is the site of new actin filament growth in living cells. One actin regulatory protein that can serve this function is CapG, a 38-kDa protein (21) that is particularly abundant in macrophages (1% of the total cytoplasmic protein) and neutrophils (0.5% of the total cytoplasmic protein) (5). CapG is the only member of the gelsolin-villin family that caps the barbed ends of actin filaments but does not sever them. Like other members of the gelsolin-villin family, CapG requires micromolar Ca2+ concentrations to function, and its activity is inhibited by the phosphotidylinositol-4,5-bisphosphate (30). The ability of CapG to cap the ends of filaments is completely reversible. When the concentration of Ca2+ is lowered to the nanomolar range, CapG dissociates from the barbed end (21).
A transgenic mouse strain was recently generated in which the CapG gene was disrupted, preventing mRNA transcription and blocking all CapG protein expression, as demonstrated by Northern and Western blotting (29). The CapG−/− mice appeared to be healthy when they were maintained in a pathogen-free environment and exhibited no deficiencies in peripheral white blood cells, red blood cells, or platelets. However, examination of CapG−/− bone marrow-derived macrophages, the cells that normally contain the highest CapG concentrations, revealed a marked depression of macrophage colony-stimulating factor-stimulated ruffling, a 50% reduction in phagocytic rates, and a 50% decrease in vesicle actin-based motility (29). The possibility that there was an intrinsic defect in ruffling ability was eliminated by demonstrating that exposure to Salmonella enterica serovar Typhimurium, a bacterium that induces ruffling by injecting specific proteins into the host cell cytoplasm, thus bypassing membrane receptors, induced a vigorous ruffling response in CapG−/− macrophages. Furthermore, microinjection of recombinant CapG fully restored macrophage colony-stimulating factor-induced ruffling, proving that the loss of CapG was the basis for the defect in receptor-agonist-induced ruffling (29).
Our investigations of CapG−/− bone marrow-derived macrophages suggested that CapG−/− animals should have an impaired intrinsic host defense, and we explored this possibility by using two intracellular pathogens, Listeria monocytogenes and S. enterica serovar Typhimurium. These two pathogens were chosen because their mechanisms of entry into macrophages are remarkably different. Listeria is taken up by receptor-ligand interactions (2, 15, 17), while Salmonella uptake is receptor independent and is triggered by secreted proteins (1, 9, 10, 32). We tested CapG−/− and wild-type mouse host defenses by determining the 50% lethal doses (LD50) and titers of bacteria in organs, by examining the histopathology, and by analyzing the inflammatory cells in infected splenic tissue. These whole-animal studies revealed that CapG−/− animals are more susceptible than CapG+/+ animals to Listeria infection but not to Salmonella infection. Investigation of bone marrow-derived macrophages revealed a specific defect in the ability of CapG−/− animals to phagocytose Listeria but not in the ability to phagocytose Salmonella. Studies of two other types of phagocytic cells also revealed motility defects in CapG−/− cells. Cultured CapG−/− dendritic cells exhibited depressed ruffling in response to granulocyte-macrophage colony-stimulating factor (GM-CSF), and CapG−/− bone marrow-derived neutrophils often failed to polarize and migrated at significantly lower speeds than wild-type cells in response to a chemotactic gradient. Our experiments highlight the role of CapG in receptor-mediated host defense mechanisms and emphasize the importance of barbed end capping proteins in phagocyte function.
MATERIALS AND METHODS
Animals.
The transgenic mice were generated as previously described (29). A mixed background (BALB/c × C57B/6 × 129/Sv) was required for mouse viability. To ensure a uniform genetic background, homozyote CapG−/− mice and wild-type mice (CapG+/+) were derived from heterozygote litter mates (CapG+/−). The two strains were then bred in parallel, and mice from the same generation were compared. Mice that were 7 to 12 weeks old were used in all experiments. Periodically, the progeny were randomly selected, and the tissue was subjected to Western blot analysis to confirm the mutant and wild-type phenotypes, as previously described (29). All animal experimental procedures complied with federal and institutional guidelines and policies.
Bacterial strains and growth conditions.
Experiments were performed with L. monocytogenes strain 1043S and S. enterica serovar Typhimurium strain χ3306. To maximize virulence, both strains were first inoculated into a BALB/C × C57B/6 mouse, and the spleen was harvested, homogenized, and plated onto brain heart infusion plates. A single colony from the plate was inoculated into brain heart infusion broth and grown overnight. A portion of the overnight culture was reinoculated and grown to an optical density at 600 nm of ∼0.8. The cells were then pelleted by centrifugation at 20,000 × g for 10 min and washed three times in 1× phosphate-buffered saline (PBS). For the LD50 and organ load experiments bacteria were suspended in PBS at final concentrations of 1 × 102 to 1 × 107 bacteria/ml.
LD50.
Two groups of animals were used to study the LD50: wild-type mice and CapG−/− mice. For each group, three sets of five animals were inoculated with different concentrations of either L. monocytogenes or S. enterica serovar Typhimurium. For L. monocytogenes, 200 μl of bacteria was injected through the tail vein, and for S. enterica serovar Typhimurium, 200 μl was injected subcutaneously as previously described (14). Both treatments resulted in rapid development of bacteremia. The animals were observed for 7 days. The LD50 was calculated as previously described (18).
Organ load experiments.
In the case of Listeria 6 × 103 bacteria were inoculated intravenously and in the case of Salmonella 2 × 106 bacteria were injected subcutaneously into wild-type and CapG−/−mice. Five CapG−/− mice and five wild-type mice were infected at each time point, and samples of both the liver and the spleen were obtained from each mouse. The tissues were weighed, homogenized with a Dounce homogenizer, and centrifuged at 20,000 × g for 30 min, and the resulting pellet was resuspended in 3 ml of PBS and then serially diluted and plated to determine the number of CFU. The number of CFU per gram of tissue was converted to a log10 value. Statistical analysis was performed by using a Wilcoxon nonparametric test. When a mouse died before it could be euthanized and examined, the levels of tissue infection were estimated to be the same as the highest levels in surviving mice in the same group (22).
Histology and immunofluorescence.
Mice infected with 1 LD50 of L. monocytogenes (6 × 103 CFU) or 1 LD50 of S. enterica serovar Typhimurium (2 × 106 CFU) were sacrificed on day 5, and frozen sections of the spleen and liver tissues were prepared and stained with hemotoxylin and eosin by using a standard protocol.
Frozen sections were also fluorescently stained for macrophages and Listeria or Salmonella. The antibody used for macrophages was Mac-1 (Caltag Laboratories, South San Francisco, Calif.), the antibody used for Listeria was rabbit polyclonal antisera (BD Biosciences, San Diego, Calif.), and the antibody used for Salmonella was polyclonal antisera (BD Biosciences).
Flow cytometry.
Mice were infected and sacrificed as described above for the histology studies. The spleens were excised and dissociated by needle dissection. Each cell suspension was pelleted by centrifugation at 100 × g, and then the red blood cells were lysed with 0.84% NH4Cl2. Cells were counted and diluted to obtain a concentration of 1 × 106 cells/ml. The cells were labeled with anti-CD3, anti-CD19, anti-CD11b, and anti-CD11c (all obtained from PharMingen, San Diego, Calif.), as well as anti-GR-1 (Caltag, Burlingame, Calif.), washed twice with 1× PBS, fixed with 4% formaldehyde, and analyzed by flow cytometry as previously described (13). Viable cell counts were determined by 7-aminoactinomycin D exclusion (final concentration, 2 μg/ml; Molecular Probes, Eugene, Oreg.).
Bone marrow-derived macrophages, dendritic cells, and neutrophils.
As previously described (29), bone marrow cells were obtained from the femurs of both wild-type and CapG−/− mice, allowed to adhere to glass coverslips, and exposed to L-cell supernatant for 7 to 10 days to stimulate differentiation into macrophages. To differentiate bone marrow cells from dendritic cells, cultures were exposed to GM-CSF and interleukin-4 as previously described (11, 24) and then to lipopolysaccharide (1 μg/ml) for 24 h at 37°C and to 5% CO2 to generate mature dendritic cells. The cells were then centrifuged through a 14.5% metrizamide solution, and this was followed by two washes with RPMI (16). This procedure yielded 90% mature dendritic cells from both wild-type and CapG−/− animals. Cell purity was assessed by flow cytometry by using class II, CD11c, and CD86 antibodies. To obtain mouse neutrophils, bone marrow cells were subjected to ammonium chloride lysis, followed by centrifugation through a Percoll gradient (82, 65, and 55%). Mature neutrophils were recovered at the 82%-65% interface and were demonstrated to be positive for Gr-1.
Microscope and image processing.
A Nikon Diaphot inverted microscope equipped with a cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan) was used to obtain digital images, which were processed by using a Metamorph computer image analysis system (Universal Imaging, Media, Pa.).
Phagocytosis assay.
Coverslips containing differentiated macrophages were removed, placed into tissue culture dishes (35 by 10 mm), and washed with PBS three times. Live bacteria were labeled with fluorescein isothiocyanate as previously described (6, 7) and added to culture dishes at a multiplicity of infection of 10. The dishes were then centrifuged at 700 × g for 5 min and incubated for 15, 30, 45, and 60 min at 37°C in the presence of 5% CO2. After these times, the coverslips were washed with PBS, fixed with 3.7% formaldehyde in PBS for 20 min at 25°C, and rinsed three times with PBS, and then ethidium bromide (final concentration, 50 μg/ml) in PBS was added to the dish. After incubation for 5 min at 25°C to allow quenching of the extracellular bacteria, the numbers of internalized bacteria were determined by microscopy (6, 7).
Intracellular speed for Listeria actin-based motility.
Coverslips containing macrophages were infected with L. monocytogenes as previously described (4). Three hours after initiation of the infection, bacteria were observed to form actin rocket tails and to move through the cytoplasm. Bacterial speed was measured by comparing the images at two times and measuring the distance traveled by each bacterium with a calibrated ×63 lens and the Metamorph track object program (Universal Imaging) as previously described (31).
Dendritic cell ruffling.
Dendritic cells were centrifuged onto glass coverslips, and GM-CSF and interleukin-4 were removed from the medium for 24 h. The cells were then exposed to GM-CSF (10 ng/ml) for 10 min to stimulate ruffling, and this was followed by fixation with 3.7% formaldehyde and staining with rhodamine phalloidin. Phalloidin staining revealed serpentine patterns that were indicative of ruffling (3), and the extent of surface ruffling for individual cells was assessed by a blinded observer as previously described (29).
Neutrophil chemotaxis.
Purified neutrophils were studied within 6 h of isolation. A 0.5-ml portion of a stock solution containing 4 × 106 cells/ml was placed in a 35-mm culture dish with a glass bottom coated with 1% bovine serum albumin (BSA). The sample was incubated for 15 to 30 min at 37°C to allow the neutrophils to adhere. A micropipette with a diameter of 0.2 μm containing the chemoattractant N-formyl-methionyl-leucyl-phenylalanine (FMLP) (Sigma) at a concentration of 10 μM was positioned at one corner of the dish by using an Eppendorf microinjection system (Eppendorf AG, Hamburg, Germany), and the solution was constantly infused, which created a chemotactic gradient as previously described (26). Phase images were captured every 10 s, and migration rates were measured by using a calibrated ×63 lens and the Metamorph track object program (Universal Imaging) (see above). The asymmetry of the shape indicating a polar morphology was assessed for adherent neutrophils following FMLP exposure, and each neutrophil was classified as polarized or nonpolarized by a blinded observer.
RESULTS
LD50.
Because the resident and peripheral macrophages are known to play a critical role in host defense against L. monocytogenes (12, 19, 20) and previous studies have demonstrated that there are defects in the actin-based motility of CapG−/− macrophages, we explored the functional consequences of the loss of CapG in the whole mouse. Groups of five mice were inoculated intravenously with different numbers of L. monocytogenes bacteria (103, 104, and 105 CFU) and observed for 7 days. The LD50, calculated as described previously (18), for CapG−/− mice (6 × 103 CFU) was 10-fold lower than the LD50 for wild-type mice (6 × 104 CFU).
We also examined the effects of Salmonella on CapG−/− and wild-type mice. Because Salmonella readily produces bacteremia following subcutaneous injection (14), we utilized this route for our analysis. We inoculated groups of five mice with 105, 106, and 107 CFU of Salmonella and observed them for 7 days, and there was no difference between the LD50 of Salmonella for CapG−/− mice and the LD50 of Salmonella for wild-type mice; both LD50s were 2 × 106 CFU.
Numbers of L. monocytogenes bacteria in the spleens and livers of CapG−/− mice.
To gain a better understanding of how the mice responded to the bacterial challenges, two target organs known to contain large populations of resident macrophages, the liver and the spleen, were analyzed by measuring the total number of bacterial CFU per gram of tissue. Quantitation was performed at specific times after the initiation of the infection. The infective dose used in these experiments was the LD50 for the knockout mice. All wild-type mice survived during the period of study; however, two of five CapG−/− mice died in the 7-day group and two of five CapG−/− mice died in the 9-day group prior to sacrifice.
Initially, the two groups of animals had similar concentrations of Listeria in their livers and spleens (Fig. 1). However, between 3 and 5 days postinfection, the bacterial counts for both the livers and the spleens of wild-type mice began to decline, while the numbers of bacteria in the CapG−/− organs remained elevated. The death of CapG−/− mice probably resulted in underestimates of the organ colony counts on days 7 and 9, because the organs of animals that succumbed to the infection would be expected to have higher bacterial titers than the organs of the surviving mice. However, cell necrosis and autolysis prevented generation of meaningful colony count data for the organs of dead mice. Despite this extenuating factor, the mean numbers of bacteria in CapG−/− organs exceeded the mean numbers of bacteria in the organs of the wild-type mice by approximately 1 log10 CFU/g on days 5 and 7 and by 2 log10 CFU/g on day 9. The differences on days 5, 7, and 9 for splenic tissue were statistically significant (P = 0.03, P = 0.05, and P = 0.05, respectively), and the difference on day 9 for liver tissue was also statistically significant (P = 0.008). In addition to quantitation of the bacteria in tissues, a spleen index (weight of the spleen divided by weight of the animal) was determined for each Listeria-infected animal. This value reflected the extent of inflammatory cell infiltration and edema in the spleen. Following infection, the spleen index increased for both wild-type and CapG−/− animals, indicating that there was increased inflammation, and the mean spleen indices were the same on days 0 to 5 for wild-type and CapG−/− animals. Because of the deaths of CapG−/− animals on days 7 and 9, meaningful comparisons of spleen weight were not possible at these times.
FIG. 1.
Numbers of Listeria CFU per gram of tissue from infected wild-type and CapG−/− mouse spleens (A) and livers (B). At time zero the animals were inoculated intravenously with 3.7 log10 CFU of Listeria. The error bars indicate the standard errors of the means. The numbers of spleens and livers cultured were identical for each time point and varied from three to five (CapG−/− mice on days 1 and 3, five organs; CapG−/− mice on day 5, four organs; CapG−/− mice on days 7 and 9, three organs; wild-type mice on days 1, 5, 7, and 9, five organs; wild-type mice on day 3, three organs). Asterisks indicate times at which the differences in bacterial counts were statistically significant (on day 5, P = 0.03 for spleens; on day 7, P = 0.05 for spleens; and on day 9, P = 0.05 for spleens and P = 0.008 for livers). Symbols: •, CapG−/− mice; ○, wild-type mice.
Because no significant difference in LD50 was observed for the S. enterica serovar Typhimurium-infected animals, mice were sacrificed at a single time, 5 days postinfection. The bacterial loads in both the spleen and the liver were determined. As shown in Table 1, the average numbers of bacteria per gram of spleen or liver for the CapG−/− and wild-type animals were not significantly different (P = 0.5).
TABLE 1.
Numbers of bacterial colonies per gram of tissue in Listeria- and Salmonella-infected mice on day 5
| Organism | Log10 colonies/g in:
|
|||
|---|---|---|---|---|
| Spleens of CapG+/+ mice | Spleens of CapG−/− mice | Livers of CapG+/+ mice | Livers of CapG−/− mice | |
| L. monocytogenes | 5.24 ± 0.15a | 6.53 ± 0.66 | 4.93 ± 0.43 | 5.68 ± 0.32 |
| S. enterica serovar Typhimurium | 7.78 ± 0.86 | 8.03 ± 0.25 | 7.02 ± 0.59 | 7.86 ± 1.06 |
The difference between CapG+/+ and CapG−/− mice was statistically significant (P = 0.02).
Histopathology and flow cytometry.
The wild-type and knockout animals were inoculated with 6.0 × 103 L. monocytogenes cells or 2 × 106 S. enterica serovar Typhimurium cells and then were sacrificed on day 5. The livers and spleens were extracted, and frozen sections were stained with hematoxylin and eosin. In Listeria-infected animals no qualitative differences were apparent in the inflammatory responses generated by the wild-type and knockout animals. Infected CapG−/− spleens exhibited levels of inflammation roughly similar to those exhibited by CapG+/+ spleens (Fig. 2). Infected CapG−/− livers also exhibited degrees of vascular congestion and inflammation similar to those exhibited by wild-type livers and had numbers of necrotic foci similar to the numbers in wild-type livers (Fig. 3). We also found that Salmonella infection produced similar inflammatory responses in CapG−/− and CapG+/+ livers and spleens (data not shown).
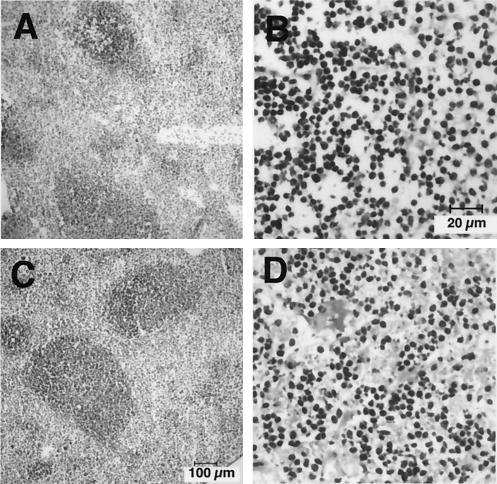
FIG. 2.
Hematoxylin- and eosin-stained frozen sections of splenic tissue from Listeria-infected wild-type mice at a low magnification (A) and a high magnification (B) and from Listeria-infected CapG−/− mice at a low magnification (C) and a high magnification (D). Animals had been infected for 5 days at the time that the organs were sampled (see Materials and Methods).
FIG. 3.
Hematoxylin- and eosin-stained frozen sections of liver tissue from Listeria-infected wild-type mice at a low magnification (A) and a high magnification (B) and from Listeria-infected CapG−/− mice at a low magnification (C) and a high magnification (D). Animals had been infected for 5 days at the time that the organs were sampled.
Because CapG is most abundant in macrophages and the loss of this protein could affect macrophage migration and proliferation in the reticuloendothelial system, we used Mac-1 antibody and immunofluorescence microscopy to estimate the numbers of macrophages in the inflammatory foci of Listeria-infected spleens and livers from CapG−/− and wild-type animals. We detected no differences in the numbers of Mac-1-positive cells in the two groups, confirming our findings obtained with hematoxylin and eosin staining (Fig. 4). Similar immunofluorescence studies of Salmonella-infected organs also failed to demonstrate that there were significant differences in the numbers of Mac-1-positive cells in the livers and spleens of CapG−/− and wild-type animals (data not shown).
FIG. 4.
Immunofluorescence micrographs of frozen spleen sections from wild-type (A) and CapG−/− (B) mice infected with Listeria. Splenic tissue was stained with Mac-1 antibody (fluorescein, green) and polyclonal anti-Listeria antibody (rhodamine, red). Bar = 100 μm.
To more accurately quantify the inflammatory cells populating the uninfected and infected livers and spleens, we utilized flow cytometry analysis. A total of 1 × 105 cells were counted per sample. The results obtained for representative samples are shown in Fig. 5. No significant differences in the numbers and types of cells found in the spleens or livers of CapG−/− animals and wild-type uninfected, Salmonella-infected, or Listeria-infected animals were detected (P > 0.1 for all comparisons).
FIG. 5.
FACS analysis of splenic cells derived from wild-type and CapG−/− mice that were not infected (A) or were infected with Listeria (B) or Salmonella (C). Staining was performed as described in Materials and Methods. Monoclonal antibodies were used to detect T lymphocytes (anti-CD3), macrophages (anti-CD11b), dendritic cells (anti-CD11c), and polymorphonuclear leukocytes (anti-GR-1). The vertical axis indicates the percentage of each cell type. The error bars indicate the standard errors of the means (n = 3). Open striped bars, wild-type mice; solid striped bars, CapG−/− mice.
Macrophage phagocytosis assay.
It has recently been demonstrated that CapG−/− macrophages ingest immunoglobulin G-labeled zymosan particles at one-half the rate that wild-type macrophages ingest such particles (29). To further explore host-pathogen interactions with CapG−/− macrophages, we examined phagocytosis of L. monocytogenes and S. enterica serovar Typhimurium. As observed with opsonized zymosan particles, CapG−/− macrophages ingested L. monocytogenes at one-half the rate that CapG+/+ macrophages ingested such particles (Fig. 6A). However, the phagocytic rates for Salmonella were identical for CapG−/− and wild-type macrophages (Fig. 6B).
FIG. 6.
Comparison of the rates of phagocytosis of L. monocytogenes (A) and S. enterica serovar Typhimurium (B) by wild-type and CapG−/− bone marrow-derived macrophages. Each value is the mean number of bacteria per cell, as determined by counting the intracellular bacteria in 100 macrophages at each time point. Symbols: •, CapG−/− mice; ○, wild-type mice.
L. monocytogenes intracellular speed.
Once Listeria is ingested, this bacterium escapes from the phagolysosome and induces actin filament rocket tails that propel the bacterium through the cytoplasm (4). Actin-based motility allows the bacterium to spread from cell to cell and cause disease. We have previously shown that vesicle actin-based motility is impaired in CapG−/− macrophages; therefore, it was of interest to examine Listeria actin-based motility in the same cells. Using phase-contrast microscopy, we measured the speed of intracellular bacteria in CapG−/− and wild-type macrophages. Unlike vesicle motility, we found no difference in the Listeria actin-based velocities in CapG−/− and CapG+/+ macrophages, which were 0.152 ± 0.049 and 0.164 ± 0.060 μm/s, respectively (means ± standard errors for 50 intracellular motile bacteria).
Dendritic cell ruffling.
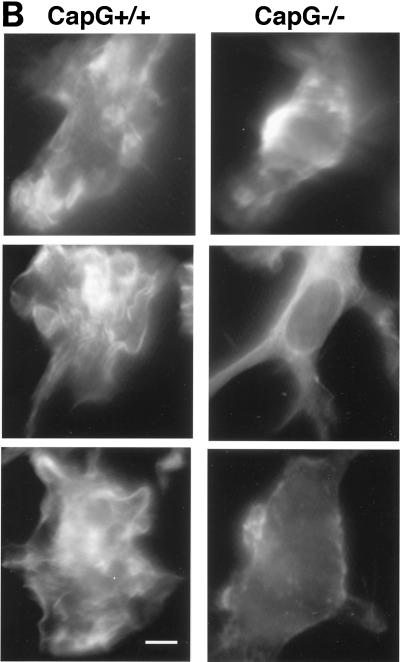
Dendritic cells play a critical role in the processing of antigens and in generating specific cell-mediated immune responses. Dendritic cells are derived from monocytes, as well as lymphoid or myeloid precursors, and reside primarily in the lymph nodes and spleen. Western blotting of wild-type dendritic cells revealed that the cytoplasmic concentrations of CapG were similar to the concentrations in macrophages (Fig. 7A). CapG has been shown to play a critical role in receptor-stimulated ruffling, as well as phagocytosis, in macrophages. Dendritic cells utilize ruffling to sample the extracellular environment, and this motile process is likely to play an important role in antigen uptake. Using the ruffling assay previously described for macrophages (29), we compared the ruffling responses of wild-type and CapG−/− dendritic cells. The unstimulated and GM-CSF-stimulated ruffling responses of the CapG−/− cells were significantly reduced compared to the responses of wild-type cells (P < 0.0001) (Fig. 7B and C).
FIG. 7.
Comparison of bone marrow dendritic cells from CapG−/− and CapG+/+ mice. (A) Western blots of bone marrow-derived macrophages and dendritic cells. Lanes 1 and 2 contained extracts from wild-type macrophages and CapG−/− macrophages, respectively, and the results demonstrated that CapG was not present in CapG−/− cells. Twenty micrograms (total amount) of cytoplasmic protein was loaded into each lane. Lane 3 contained 20 ng of purified CapG protein, and lanes 4 and 5 contained cytoplasmic extracts from macrophages (lane 4) and dendritic cells (lane 5) from CapG+/+ mice. Densitometry of the two peaks revealed comparable areas under the curves (2,059 and 1,973 integration units for macrophages and dendritic cells, respectively). Thirty micrograms of cytoplasmic protein was loaded into lanes 4 and 5. (B) Rhodamine phalloidin-stained CapG+/+ and CapG−/− dendritic cells before stimulation with GM-CSF (top two images) and following stimulation for 15 min with GM-CSF (bottom four images). Note the serpentine actin filament staining pattern of CapG+/+ cells following stimulation, indicating a marked ruffling response. The staining pattern was less pronounced in unstimulated cells of both genotypes and in stimulated CapG−/− dendritic cells, indicating that there was a reduced ruffling response. Bar = 5 μm. (C) Graphic comparison of the ruffling responses of CapG+/+ and CapG−/− dendritic cells before and after GM-CSF stimulation. Ruffling was quantified as described in Materials and Methods. Both the resting and stimulated ruffling responses of CapG−/− cells were significantly reduced compared to those of CapG+/+ dendritic cells (P < 0.0001).
The motility of dendritic cells varies with the level of maturity. Therefore, to reduce the possible confounding problem of variation in maturation between wild-type and CapG−/− dendritic cells, we utilized fully differentiated dendritic cells for our experiments and by fluorescence-activated cell sorting (FACS) analysis demonstrated that CapG−/− and wild-type cells had achieved similar levels of maturation (see Materials and Methods).
Neutrophil chemotaxis.
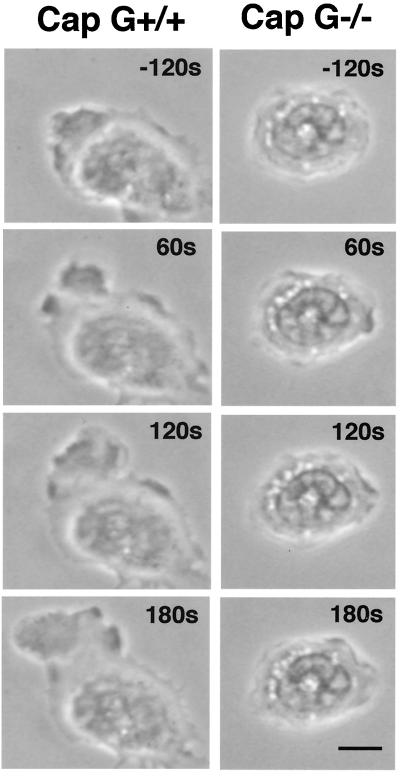
As shown in Fig. 8, CapG−/− bone marrow-derived neutrophils became minimally polarized in the presence or absence of a chemotactic gradient, while the majority of wild-type neutrophils were polarized, forming a discrete leading edge (lamellipod) and tail (uropod). In the presence of a chemotactic gradient only 25% of CapG−/− neutrophils (10 of 40 neutrophils) had asymmetric shapes consistent with a polarized morphology, compared to 90% of the wild-type neutrophils (55 of 61 neutrophils) (P = 0.0016). Also, the mean rate of migration of CapG−/− neutrophils on BSA-coated glass was approximately 50% lower than that of wild-type neutrophils; the migration speed of CapG−/− neutrophils was 1.68 ± 0.12 μm/min (mean ± standard error of the mean; n = 84), compared to a wild-type speed of 3.24 ± 0.12 μm/min (n = 135). The difference was highly significant (P < 0.0001).
FIG. 8.
Time lapse phase-contrast micrographs of representative bone marrow-derived CapG+/+ and CapG−/− neutrophils adhering to BSA-coated glass before and after exposure to an FMLP chemotactic gradient. The times are indicated in the right upper corners. Time zero was the time at which the FMLP gradient was introduced at the left upper corner. Note the lack of polarization of the CapG−/− neutrophil and the absence of significant movement compared to the movement of the wild-type neutrophil. Bar = 5 μm.
DISCUSSION
Our whole-animal investigations demonstrated that CapG−/− mice are more susceptible to Listeria infection than wild-type mice. The LD50 for CapG−/− animals was 10-fold lower than the LD50 for CapG+/+ mice, while no difference in the susceptibilities to Salmonella was observed. The finding that there was a normal response to Salmonella infection suggests that the loss of CapG does not lead to a generalized depression of host defenses. Examination of the numbers of bacteria in the liver and spleen provided insight into the kinetics of bacterial growth in the animals. During the early stages of infection, days 1 to 3, no differences in the numbers of Listeria colonies were observed. However, by day 5, at the time when cell-mediated and humoral immune responses might be expected to begin, higher organ loads of Listeria were found in CapG−/− livers and spleens than in wild-type livers and spleens. The differences were significant in the spleens at 5 and 7 days and in both organs after 9 days. At 9 days the numbers of Listeria colonies per gram of tissue were 100 times higher in CapG−/− tissues than in wild-type tissues.
The higher numbers of Listeria cells in the organs of CapG−/− animals were in contrast to the numbers of cells observed after Salmonella infection, after which no differences in the numbers of bacteria in CapG−/− and CapG+/+ livers and spleens were observed. It is unlikely that these differences in organ counts and LD50 for Listeria and Salmonella were due to differences in the mode of administration. As demonstrated by our counts of bacteria in organs on day 5, both intravenous administration of Listeria and subcutaneous injection of Salmonella led to high levels of bacteria in the spleens and livers and would be expected to present comparable challenges to the animals' immune systems (Table 1).
What mechanism or mechanisms underlie the differences in the abilities of the CapG−/− and wild-type livers and spleens to clear Listeria? One possibility is that CapG−/− organs do not contain the same numbers of inflammatory cells that wild-type organs contain. To explore this possibility, we first compared the values for the spleen index, a gross indicator of splenic inflammation, and found that these values were similar for wild-type and CapG−/− animals. Next, we examined the histopathology of infected livers and spleens. We observed no significant differences in the types and extents of the inflammatory responses in these organs. Immunofluorescence microscopy also failed to reveal any significant differences in the numbers of macrophages in the infected spleens or livers of CapG−/− and wild-type animals. Finally, our FACS analysis quantitatively supported these qualitative impressions. Similar numbers of macrophages, dendritic cells, neutrophils, and T cells were observed in the CapG−/− and wild-type spleens before and after infection with Listeria or Salmonella.
These findings eliminate the possibility that there is a quantitative defect and raise the possibility that there is a functional defect in one or more cells important for host defense. Macrophages play a critical role in protecting a host against infection, and both Listeria and Salmonella interact with macrophages. Salmonella is taken up by macrophages by a trigger-type mechanism (1, 9, 10, 32). Initial contact by Salmonella induces dramatic cytoskeleton rearrangements in macrophages, causing these cells to form giant membrane ruffles (1, 9). These large plasma membrane projections engulf the bacteria by a process similar to macropinocytosis. Salmonella contains a type III secretion system that allows the translocation of effector proteins into the cytoplasm of mammalian cells. It has been shown that injection of the Salmonella proteins SipB and SipC induces membrane ruffles and that ruffling and macropinocytosis are not impaired by tyrosine kinase inhibitors (10, 32). Listeria enters macrophages by a zipper-type mechanism. Listeria expresses surface proteins called internalins that bind to specific host cell membrane receptors (2). Receptor occupancy activates tyrosine phosphorylation and stimulates cytoskeletal rearrangements to form pseudopods that surround the bacterium and bring it into the cytoplasm by phagocytosis (15). Thus, Salmonella is internalized by a mechanism that bypasses receptors, while Listeria requires receptor-mediated signal transduction to enter cells.
It has been shown previously that loss of CapG disrupts receptor-mediated ruffling but not Salmonella Sip-mediated ruffling (29). This finding suggested that phagocytosis of Listeria, but not macropinocytosis of Salmonella, could be impaired in CapG−/− macrophages. Measurement of the rates of macrophage internalization of Listeria and Salmonella confirmed these predictions. The rate of phagocytosis of Listeria was reduced by 50% in CapG−/− macrophages, while the rate of internalization of Salmonella was unimpaired. We suggest that this functional macrophage defect may at least partially account for the inability of CapG−/− spleens and livers to control Listeria growth. Under normal conditions the generation of a cell-mediated TH-1 response would be expected to activate macrophages to kill Listeria. However, bacterial killing requires that macrophages are capable of ingesting Listeria. The reduction in the rate of phagocytosis, therefore, could explain the increased survival of Listeria in CapG−/− mice.
Additional defects in immune function could also contribute to the inability of CapG−/− mice to control Listeria infection. Dendritic cells play a critical role in processing antigens and presenting antigens to activate T cells. We report here that dendritic cells contain high concentrations of CapG and that CapG−/− dendritic cells have a reduced ability to ruffle in response to GM-CSF. It is of interest that dendritic cells lacking gelsolin have a normal ruffling response (27), and the difference in actin-based motility between CapG−/− dendritic cells and dendritic cells lacking gelsolin further supports the previous conclusion that CapG serves a unique and different in vivo function than gelsolin, a member of the same family of proteins (29). This defect in CapG−/− dendritic cells could lead to defective processing of antigens and defective activation of T cells. Future studies are planned to further explore dendritic and T-cell function in CapG−/− mice. Finally, we observed a significant defect in the ability of CapG−/− neutrophils to polarize and crawl toward a chemotactic gradient. This defect may be important in the early stages of Listeria infection, when neutrophils are known to play a major role in the killing of this pathogen in the liver (28). In our FACS analysis of cells in the CapG−/− spleens we did not find any significant reductions in neutrophil numbers; however, the percentage of neutrophils in the spleen is small compared to the percentages of T cells and macrophages, making differences in this cell population difficult to detect.
Genetic defects in host cell immunity are a major concern for pediatric immunologists. Previous work has identified defects in immunoglobulin production, superoxide production, vesicle trafficking, and phagocyte adherence (8, 25). Patients with such defects have served as natural experiments, and the findings obtained have led to major advances in our understanding of the immune system. To date, a defect in CapG expression has not been identified in humans; however, our experiments with mice indicate that CapG has a central role in phagocyte function and demonstrate that there is a distinct phenotype that renders the host more susceptible to intracellular Listeria infection but not to Salmonella infection. Our experiments should encourage clinicians to consider the possibility that there is a CapG deficiency in human patients with unexplained listeriosis.
Acknowledgments
We thank William Zeile for helpful suggestions.
This study was funded by National Institutes of Health grants RO1 AI-23262 and RO1 AI-34276.
Editor: F. C. Fang
REFERENCES
- 1.Alpuche-Aranda, C. M., E. L. Racoosin, J. A. Swanson, and S. I. Miller. 1994. Salmonellae stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp Med. 179:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossart, P., and M. Lecuit. 1998. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 17:3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox, D., P. Chang, Q. Zhang, P. G. Reddy, G. M. Bokoch, and S. Greenberg. 1997. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186:1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabiri, G. A., J. M. Sanger, D. A. Portnoy, and F. S. Southwick. 1990. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc. Natl. Acad. Sci. USA 87:6068-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabiri, G. A., C. L. Young, J. Rosenbloom, and F. S. Southwick. 1992. Molecular cloning of human macrophage capping protein cDNA. A unique member of the gelsolin/villin family expressed primarily in macrophages. J. Biol. Chem. 267:16545-16552. [PubMed] [Google Scholar]
- 6.Drevets, D. A., and P. A. Campbell. 1991. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods 142:31-38. [DOI] [PubMed] [Google Scholar]
- 7.Drevets, D. A., and A. M. Elliott. 1995. Fluorescence labeling of bacteria for studies of intracellular pathogenesis. J. Immunol. Methods 187:69-79. [DOI] [PubMed] [Google Scholar]
- 8.Fleisher, T. A., and J. J. Bleesing. 2000. Immune function. Pediatr. Clin. N. Am. 47:1197-1209. [DOI] [PubMed] [Google Scholar]
- 9.Francis, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 10.Hayward, R. D., and V. Koronakis. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2002. Colony-stimulating factor 1-dependent cells protect against systemic infection with Listeria monocytogenes but facilitate neuroinvasion. Infect. Immun. 70:4682-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litherland, S. A., X. T. Xie, A. D. Hutson, C. Wasserfall, D. S. Whittaker, J. X. She, A. Hofig, M. A. Dennis, K. Fuller, R. Cook, D. Schatz, L. L. Moldawer, and M. J. Clare-Salzler. 1999. Aberrant prostaglandin synthase 2 expression defines an antigen-presenting cell defect for insulin-dependent diabetes mellitus. J. Clin. Investig. 104:515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui, H., C. M. Bacot, W. A. Garlington, T. J. Doyle, S. Roberts, and P. A. Gulig. 2001. Virulence plasmid-borne spvB and spvC genes can replace the 90-kilobase plasmid in conferring virulence to Salmonella enterica serovar Typhimurium in subcutaneously inoculated mice. J. Bacteriol. 183:4652-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 16.Naumov, Y. N., K. S. Bahjat, R. Gausling, R. Abraham, M. A. Exley, Y. Koezuka, S. B. Balk, J. L. Strominger, M. Clare-Salzer, and S. B. Wilson. 2001. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc. Natl. Acad. Sci. USA 98:13838-13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parida, S. K., E. Domann, M. Rohde, S. Muller, A. Darji, T. Hain, J. Wehland, and T. Chakraborty. 1998. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol. Microbiol. 28:81-93. [DOI] [PubMed] [Google Scholar]
- 18.Reed, R. L., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 19.Rosen, H., S. Gordon, and R. J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 170:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samsom, J. N., A. Annema, P. H. Groeneveld, N. van Rooijen, J. A. Langermans, and R. van Furth. 1997. Elimination of resident macrophages from the livers and spleens of immune mice impairs acquired resistance against a secondary Listeria monocytogenes infection. Infect. Immun. 65:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southwick, F. S., and M. J. DiNubile. 1986. Rabbit alveolar macrophages contain a Ca2+-sensitive, 41,000-dalton protein which reversibly blocks the “barbed” ends of actin filaments but does not sever them. J. Biol. Chem. 261:14191-14195. [PubMed] [Google Scholar]
- 22.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stossel, T. P. 1993. On the crawling of animal cells. Science 260:1086-1094. [DOI] [PubMed] [Google Scholar]
- 24.Talmor, M., A. Mirza, S. Turley, I. Mellman, L. A. Hoffman, and R. M. Steinman. 1998. Generation of large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur. J. Immunol. 28:811-817. [DOI] [PubMed] [Google Scholar]
- 25.Uzel, G., and S. M. Holland. 2002. White blood cell defects: molecular discoveries and clinical management. Curr. Allergy Asthma Rep. 2:385-391. [DOI] [PubMed] [Google Scholar]
- 26.Weiner, O. D., G. Servant, M. D. Welch, T. J. Mitchison, J. W. Sedat, and H. R. Bourne. 1999. Spatial control of actin polymerization during neutrophil chemotaxis. Nat. Cell Biol. 1:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West, M. A., A. N. Antoniou, A. R. Prescott, T. Azuma, D. J. Kwiatkowski, and C. Watts. 1999. Membrane ruffling, macropinocytosis and antigen presentation in the absence of gelsolin in murine dendritic cells. Eur. J. Immunol. 29:3450-3455. [DOI] [PubMed] [Google Scholar]
- 28.Wing, E. J., and S. H. Gregory. 2002. Listeria monocytogenes: clinical and experimental update. J. Infect. Dis. 185(Suppl. 1):S18-S24. [DOI] [PubMed] [Google Scholar]
- 29.Witke, W., W. Li, D. J. Kwiatkowski, and F. S. Southwick. 2001. Comparisons of CapG and gelsolin-null macrophages: demonstration of a unique role for CapG in receptor-mediated ruffling, phagocytosis, and vesicle rocketing. J. Cell Biol. 154:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu, F. X., P. A. Johnston, T. C. Sudhof, and H. L. Yin. 1990. gCap39, a calcium ion- and polyphosphoinositide-regulated actin capping protein. Science 250:1413-1415. [DOI] [PubMed] [Google Scholar]
- 31.Zeile, W. L., D. L. Purich, and F. S. Southwick. 1996. Recognition of two classes of oligoproline sequences in profilin-mediated acceleration of actin-based Shigella motility. J. Cell Biol. 133:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, D., M. S. Mooseker, and J. E. Galan. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]