Abstract
The thymic medulla plays a key role in negative selection (self-tolerance induction) and contains differentiated T cells en route to the extrathymic environment. However, being relatively mature, medullary T cells are thought to be beyond the stage of tolerance induction. This paradox is resolved by the finding that medullary T cells (CD4+8− thymocytes) comprise two distinct subsets. Medullary thymocytes expressing a fully mature (HSAlo) phenotype are strongly resistant to tolerance induction, whereas cells with a semimature (HSAhi) phenotype are tolerance susceptible. These findings suggest that the differentiated T cells reaching the medulla from the cortex remain sensitive to tolerance induction for a brief period before acquiring a fully mature tolerance-resistant phenotype. The semimature subset of medullary T cells displays unique requirements for tolerance induction; depending upon the conditions used, tolerizing these cells can involve either a Fas (CD95)-dependent or a Fas-independent pathway.
Differentiation of CD4+8+ (double-positive, DP)1 thymocytes into mature single-positive (SP) CD4+8− and CD4−8+ cells involves positive and negative selection and is directed to an array of self-peptides bound to MHC molecules (1–7). Negative selection deletes T cells with high affinity for self-peptides via apoptosis, thus ensuring selftolerance, and is presumed to reflect strong signaling via TCR recognition of peptide–MHC complexes on APC. In addition to TCR ligation, negative selection appears to require second signals delivered through contact with costimulatory molecules on APC (3–5, 8–10). Although APC express a variety of costimulatory molecules, including B7-1, B7-2, ICAM-1, and HSA (11–13), which of these molecules are required for negative selection is unclear (3–5).
Unlike the cortex, the thymic medulla is packed with bone marrow (BM)–derived APC and is permeable to circulating self-antigens entering from the bloodstream (14). Thus, the medulla is a likely site for negative selection. In favor of this idea, negative selection to endogenous superantigens and to serum proteins, e.g., C5, takes place at a relatively late stage of thymocyte differentiation and is associated with the appearance of apoptotic cells in the medulla (15–19). However, a key problem with the notion that negative selection occurs in the medulla is that most of the T cells in the medulla are relatively mature (6) and thus are presumably beyond the stage of being tolerance susceptible. One explanation for this paradox is that, after positive selection in the cortex, maturing SP thymocytes remain tolerance susceptible for a brief period after reaching the medulla.
Maturation of SP cells is associated with downregulation of heat-stable antigen (HSA) (3, 20, 21) and upregulation of Qa-2 molecules (22, 23). In contrast with fully mature HSAlo Qa-2hi thymocytes, partly immature HSAhi Qa-2lo SP thymocytes are functionally incompetent in the absence of exogenous lymphokines (23–25). Whether these latter cells are tolerance susceptible is unclear, although a subset of HSAinterm CD4+8lo thymocytes is reported to undergo apoptosis in response to TCR ligation in vitro (25). In this paper, we compare purified subsets of CD4+8+, HSAhi CD4+8−, and HSAlo CD4+8− thymocytes for their relative sensitivity to TCR-mediated apoptosis in vitro and in vivo. The results suggest that immature CD4+8+ and semimature HSAhi CD4+8− thymocytes are both susceptible to negative selection. However, the conditions required for tolerizing these two subsets are distinctly different.
Materials and Methods
Mice.
Adult C57BL/6 (B6) and B6 lpr/lpr mice aged 8–12 wk were obtained from The Scripps Research Institute breeding facility.
Antibodies.
Antibodies specific for the following markers were previously described (26): CD3 (C363.29B, rat IgG), CD4 (RL172, rat IgM), CD8 (3.163.8, rat IgM), CD25 (7D4, rat IgM), HSA (J11D, rat IgM), and class II (M5/114, rat IgG). Purified mAbs from ascites specific for TCR-β (H57-597, hamster IgG) (27) and CD28 (37.51, hamster IgG) (28) were used for stimulation of cells. The following mAbs were purchased from GIBCO BRL (Gaithersburg, MD): FITC–anti-CD4 (H129.19, rat IgG), FITC–anti-CD25 (3C7, rat IgG) and Red613–anti-CD8 (53-6.7, rat IgG). FITC-conjugated mAbs specific for CD69 (H1.2F3, hamster IgG) and Qa-2 (1-1-2, mouse IgG) were purchased from PharMingen (San Diego, CA). PE-conjugated antiCD4 mAb (GK1.5, rat IgG) was purchased from Collaborative Biomedical Products (Bedford, MA).
Cell Purification.
Purification of TCRlo CD4+8+ thymocytes was performed as described previously (29). For purification of HSAhi CD4+8− cells, thymocytes were treated with mAbs specific for CD8 (3.168.8) and CD25 (7D4) plus guinea pig complement (C) for 45 min at 37°C, positively panned with anti-CD4 (RL172) mAb, then positively panned with anti-HSA (J11d) mAb; panning was performed at 4°C. HSAlo CD4+8− cells were purified by treating thymocytes with mAbs to HSA, CD8, and CD25 plus guinea pig C at 37°C followed by positive panning with anti-CD4 mAb. CD4+ LN T cells were purified by treating pooled LN cells with mAbs specific for HSA, CD8, and class II plus guinea pig C, followed by positive panning with anti-CD4 mAb.
In Vivo Treatment for Deletion of Immature Thymocytes.
B6 mice aged 8 wk were injected intraperitoneally with 100 μg/mouse of anti-TCR mAb (H57-597) or 1 mg/mouse of cortisone acetate (Sigma, St. Louis, MO). At 20 or 48 h after injection, the mice were sacrificed and cell surface markers of thymocytes were analyzed.
Culture Conditions.
Whole thymocytes (5 × 105) or purified cells (2–3 × 105) from B6 or B6 lpr/lpr mice were cultured in 0.2 ml of RPMI medium supplemented with 5 × 10−5 M 2-mercaptoethanol, l-glutamine and 10% FCS in 96-well tissue culture plates coated with anti-TCR (H57-597) ± anti-CD28 (37.51) mAbs or medium alone for 20 h. After harvesting, cells were stained and analyzed on a FACScan® (Becton Dickinson, San Jose, CA).
Cell Surface and TUNEL Staining.
For the in vivo studies, thymocytes were incubated with FITC-conjugated mAbs to HSA (J11d), Qa-2 (1-1-2), or CD69 (H1.2F3), followed by PE-conjugated anti-CD4 (GK1.5) and Red613-conjugated anti-CD8 (536.7) mAbs; dead cells were gated out by propidium iodide (PI) staining. For the in vitro studies, purified cells were stained with FITC-conjugated anti-CD69 or anti-CD25 (3C7) mAbs, and then TUNEL stained after cell fixation. TUNEL staining was described previously (29).
Results
Elimination of Thymocyte Subsets In Vivo.
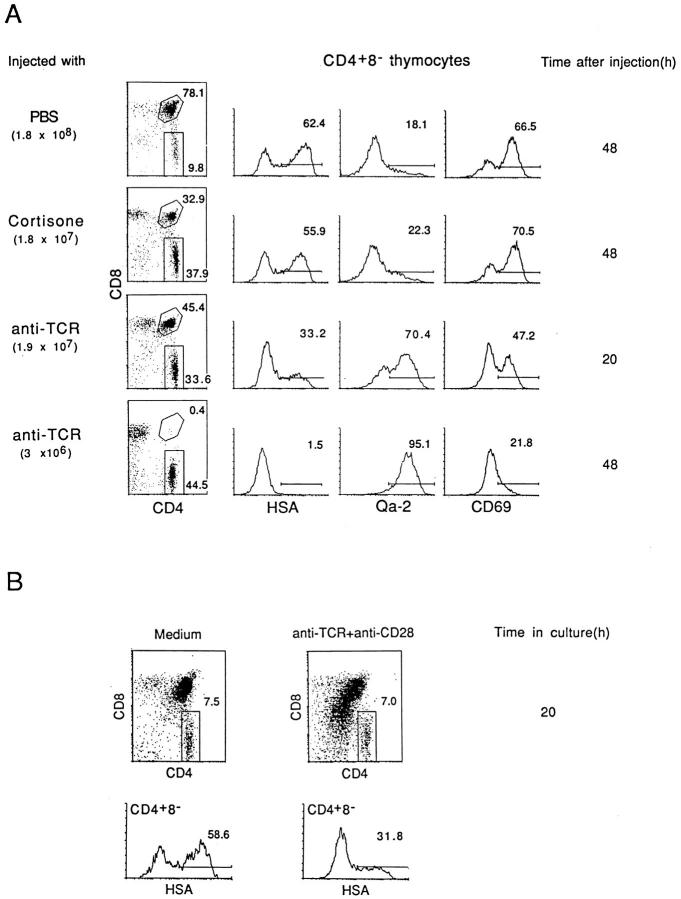
Acute negative selection of thymocytes can be induced by injecting mice with anti-TCR (or anti-CD3) mAb i.p. (30); in this model, costimulation is presumably provided by bystander APC (31, 32). As shown in Fig. 1 A, injecting mice with antiTCR mAb ablated immature CD4+8+ thymocytes by 48 h after injection and caused a reciprocal increase in SP CD4+8− and CD4−8+ cells. However, gating on CD4+8− cells at 48 h revealed that this population consisted almost entirely of fully mature HSAlo Qa-2hi CD69lo cells; the major subset of semimature HSAhi Qa-2lo CD69hi cells was virtually undetectable. These semimature T cells were prominent in normal (PBS-injected) mice and were not depleted after cortisone treatment. The data in Fig. 1 A refer to adult 8-wk-old mice. Essentially similar findings applied when anti-TCR mAb was injected into neonatal (1-wk-old) mice (data not shown). However, in this situation, the elimination of thymocytes was prominent for semimature HSAhi CD4+8− cells but not for CD4+8+ cells, suggesting that the destruction of CD4+8+ cells in adult mice (Fig. 1 A) was probably largely mediated by cytokines and/or increased steroid levels released via anti-TCR stimulation of mature postthymic T cells.
Figure 1.
Negative selection of HSAhi CD4+8− thymocytes in vivo and in vitro. (A) Cell surface expression of HSA, Qa-2, and CD69 on CD4+8− thymocytes after injection of antiTCR mAb, cortisone acetate, or PBS; total numbers of thymocytes recovered are shown at the left. (B) Cell surface expression of HSA on CD4+8− thymocytes recovered after culturing unseparated thymocytes for 20 h on plates coated with a mixture of anti-TCR mAb (10 μg/ml) and anti-CD28 mAb (20 μg/ml) or on uncoated plates (medium alone). Surface staining on viable (PI −) cells is shown; the downregulation of CD4 and CD8 on CD4+8+ cells is typical of cells undergoing early TCR-mediated apoptosis (29); yields of total viable (PI −) cells in the cultures were 85% for cells cultured in medium alone and 52% for cells cultured with anti-TCR plus anti-CD28 mAbs.
In Vitro Studies.
Negative selection can be reproduced in vitro by culturing dissociated thymocytes with a combination of anti-TCR and anti-CD28 mAbs in cross-linked form (8); ligation of CD28 (the T cell coreceptor for B7-1 and B7-2 on APC) (11, 12) provides costimulation. As shown in Fig. 1 B, culturing unseparated thymocytes in vitro for 20 h on plates coated with a mixture of anti-TCR and anti-CD28 mAbs caused substantial, though incomplete, depletion of HSAhi CD4+8− cells. These in vitro findings correlated closely with the partial depletion of HSAhi CD4+8− cells found at 20 h after anti-TCR injection in vivo (Fig. 1 A).
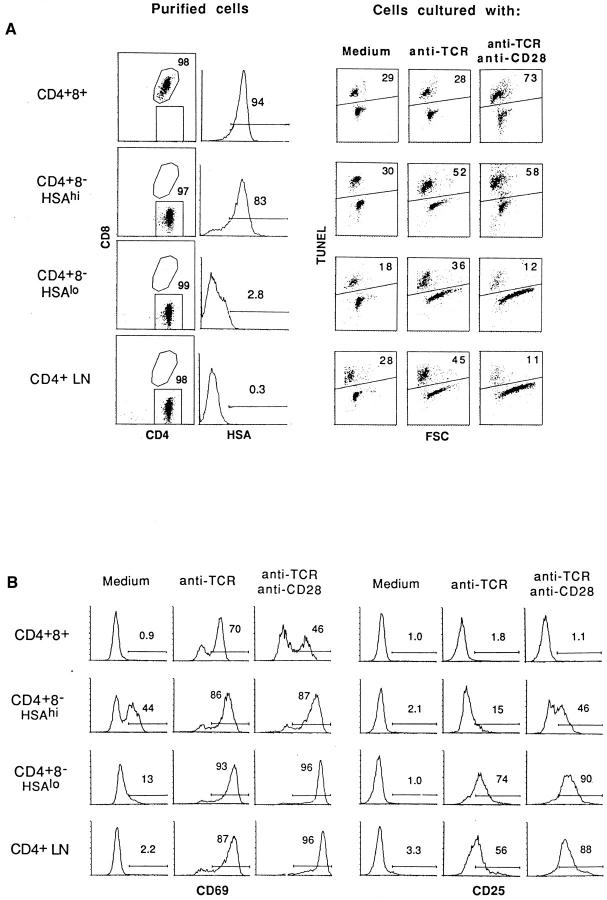
To extend these findings, purified subsets of thymocytes were tested for their sensitivity to negative selection (apoptosis) mediated by cross-linked anti-TCR plus anti-CD28 mAbs in vitro; exposure to cross-linked anti-TCR mAb alone was used as a control. Apoptosis was measured by TUNEL staining after overnight culture. Fig. 2 shows the purity of the cells tested (A, left), TUNEL staining of the cultured cells (A, right), and the expression of two activation markers, CD69 and CD25 (IL-2R), on viable (TUNEL−) cells (B); cells were cultured on plates coated with anti-CD28 (20 μg/ml) and/or anti-TCR (10 μg/ml) mAbs. Fig. 3 A shows the effects of culturing the cells with decreasing concentrations of anti-TCR mAb ± a fixed concentration of anti-CD28 mAb (20 μg/ml); background levels of apoptosis found with cells cultured in medium alone have been subtracted, and the data are shown as change in apoptosis. The data in Figs. 2 and 3 A make three points (see Fig. 2, legend).
Figure 2.
Apoptosis of thymocyte subsets and LN T cells induced by stimulation with anti-TCR mAb or anti-TCR plus anti-CD28 mAbs in vitro. Purified populations of CD4+8+, HSAhi CD4+8−, and HSAlo CD4+8− thymocytes and CD4+8− LN cells were cultured for 20 h in vitro on plates coated with anti-TCR mAb alone (H57, 10 μg/ml) or with a mixture of anti-TCR (10 μg/ml) and anti-CD28 (20 μg/ml) mAbs. (A) The purity of the subsets before culture (left) and the extent of apoptosis determined by TUNEL staining after culture (right) are shown; for TUNEL staining, the forward scatter (FSC) profile shows the relative size of the cells. (B) Shows surface expression of CD69 and CD25 (IL-2R) on viable (TUNEL −) cells after culture. To exclude the possibility that CD4 ligation during the panning procedure affected the results (50), parallel experiments were performed with cells that were purified by methods that did not involve CD4 ligation, e.g., by using unmanipulated thymocytes from β2m −/− mice as a source of purified CD4+8+ cells and CD8− IL-2R− normal thymocytes as an enriched source of CD4+8− cells. The results obtained with these cells were essentially identical to the data shown in Figs. 3 and 4.
Figure 3.
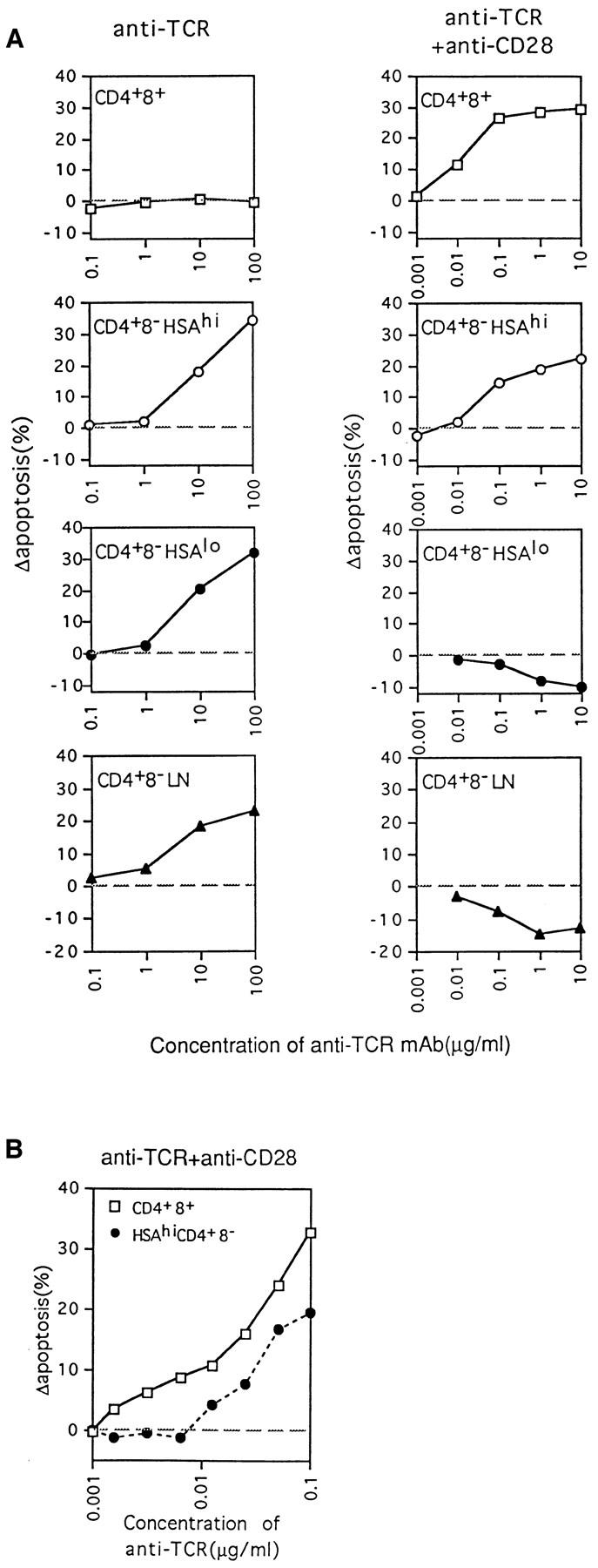
Concentration of anti-TCR mAb required to induce apoptosis of thymocyte subpopulations as measured by TUNEL staining at 20 h. (A) Purified thymocyte subsets and LN CD4+8− cells were cultured on plates coated with various concentrations of anti-TCR mAb, i.e., 100 μg/ml to 0.1 μg/ml for stimulation with anti-TCR mAb alone, and 10 μg/ml to 1 ng/ml for stimulation with anti-TCR mAb plus a fixed amount of 20 μg/ml of anti-CD28 mAb. (B) Comparison of the sensitivity of CD4+8+ and HSAhi CD4+8− thymocytes to apoptosis induced by stimulation with various concentrations of anti-TCR mAb and a fixed amount (20 μg/ml) of anti-CD28 mAb. Background levels of apoptosis found with cells cultured in medium alone have been subtracted, and the data are shown as change in apoptosis. The data represent the mean from three (A) or two (B) separate experiments.
First, confirming the findings of others (8), inducing apoptosis of immature CD4+8+ thymocytes in vitro required combined TCR–CD28 ligation; ligation of TCR alone caused strong upregulation of CD69 but not CD25 (indicative of partial activation), but failed to cause apoptosis. Negative selection of immature CD4+8+ cells thus was crucially dependent upon costimulation.
Second, diametrically opposite findings applied to fully mature thymocytes, i.e., to HSAlo CD4+8− cells, and also to mature LN CD4+8− cells. Surprisingly, these two populations of mature T cells were susceptible to apoptosis mediated by TCR ligation alone. However, combined TCR– CD28 ligation failed to cause apoptosis and, instead, led to T cell activation associated with CD69 and CD25 upregulation, an increase in cell size (increased forward scatter [FSC]), and a reduction in apoptosis relative to cells cultured alone.
Third, the semimature subset of HSAhi CD4+8− cells behaved as an intermediate population. These cells were sensitive to apoptosis mediated by TCR ligation alone, and thus resembled mature HSAlo CD4+8− cells. However, at low doses of anti-TCR mAb (Fig. 3 A), TCR-induced apoptosis of HSAhi CD4+8− cells was considerably enhanced by CD28 coligation. The properties of HSAhi CD4+8− cells thus were midway between immature CD4+8+ cells and mature HSAlo CD4+8− cells; this also applied to the extent of CD69 and CD25 upregulation after combined TCR–CD28 ligation.
In terms of their sensitivity to TCR–CD28 ligation, HSAhi CD4+8− cells differed from immature CD4+8+ cells in two respects. First, titrating the dose of anti-TCR mAb (with a fixed amount of anti-CD28 mAb) revealed that HSAhi CD4+8− cells were appreciably less sensitive to TCR–CD28-mediated apoptosis than CD4+8+ cells (Fig. 3 B). Thus, the dose of anti-TCR mAb required to induce apoptosis was about fivefold higher for CD4+8− cells than for CD4+8+ cells; this difference was surprising because the TCR density on HSAhi CD4+8− cells was 5- to 10-fold higher than on CD4+8+ cells (data not shown). Second, as discussed below, these two populations differed in their sensitivity to Fas (CD95)-mediated apoptosis.
Role of Fas.
It is well accepted that activation-induced cell death of mature postthymic T cells is largely Fas-mediated and reflects contact with Fas ligand (FasL) (33–38). However, to date there is no clear evidence that Fas plays a role in negative selection in the thymus (39, 40). In agreement with this view, TCR–CD28-mediated apoptosis of immature CD4+8+ thymocytes could not be blocked with a soluble Fas fusion protein (Fas–Ig) (41) and was unimpaired when CD4+8+ thymocytes were prepared from Fas-deficient B6 lpr/lpr mice (Fig. 4 A). Interestingly, however, TCR– CD28-mediated apoptosis of semimature HSAhi CD4+8− cells was abolished by Fas–Ig and was almost undetectable with cells from B6 lpr/lpr mice. These findings also applied to ligation with TCR alone (Fig. 4 A).
Figure 4.
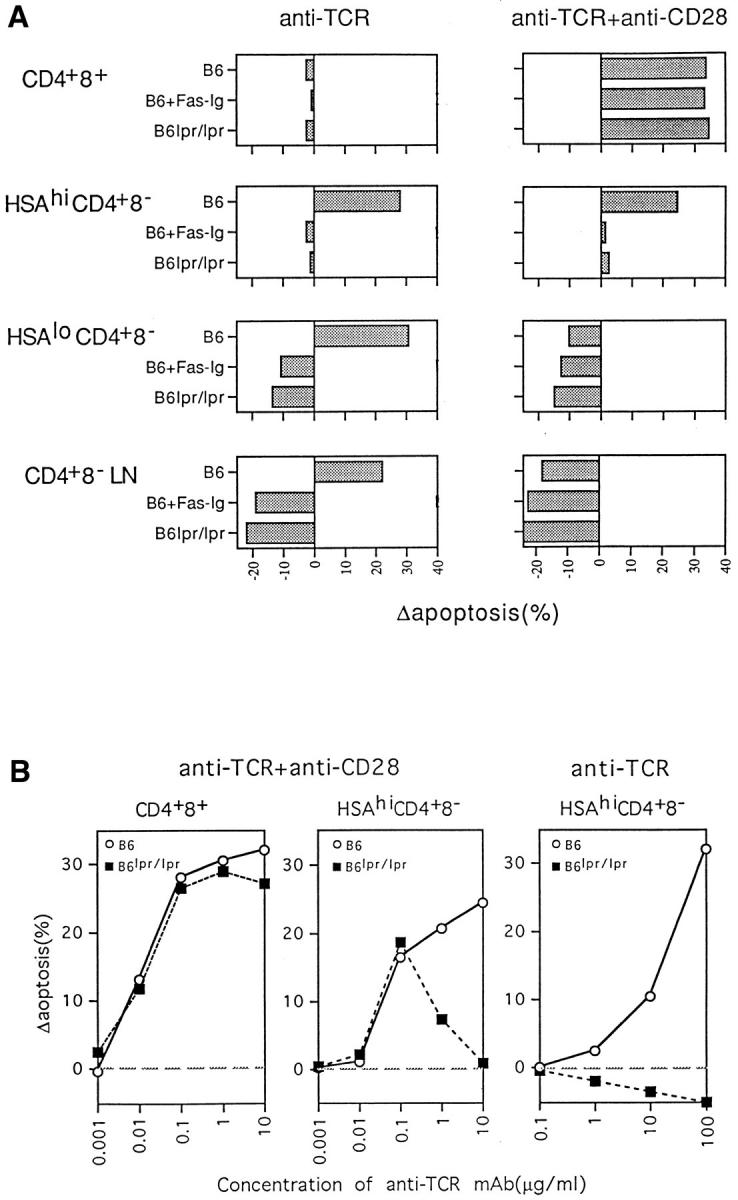
Effect of Fas (CD95) on negative selection of thymocyte subpopulations. (A) Purified cells from B6 or B6 lpr/lpr mice were cultured for 20 h on plates coated with either anti-TCR mAb alone (20 μg/ml) or with anti-TCR (10 μg/ml) plus anti-CD28 (20 μg/ml) mAb. Where indicated, Fas–Ig fusion protein (provided by Dr. D. Lynch; 10 μg/ml) was added to the cultures. (B) Relative sensitivity of normal B6 and B6 lpr/lpr CD4+8+ and HSAhi CD4+8− cells to TCR–CD28-mediated apoptosis in vitro. Cells were cultured for 20 h on plates coated with various concentrations of anti-TCR mAb ± a fixed concentration of anti-CD28 mAb as described in Fig. 3. Apoptosis was measured by TUNEL staining. The data represent the mean of two separate experiments.
Because negative selection in vivo is not thought to be Fas dependent, the dominant role of Fas in TCR–CD28mediated apoptosis of HSAhi CD4+8− cells in vitro was surprising. However, titration experiments showed that the Fas dependency of these cells to TCR–CD28-mediated apoptosis only applied with high doses of anti-TCR mAb, e.g., 10–20 μg/ml (Fig. 4, A and B, middle). At this concentration, TCR–CD28-mediated apoptosis of HSAhi CD4+8− cells was prominent with normal thymocytes but undetectable with B6 lpr/lpr thymocytes. Paradoxically, however, apoptosis of HSAhi CD4+8− lpr/lpr thymocytes became clearly apparent when the dose of anti-TCR mAb was reduced to a low level, i.e., to 0.1 μg/ml (Fig. 4 B, middle). In fact, at this low dose, apoptosis of lpr/lpr cells was as high as with normal cells. TCR–CD28-mediated apoptosis of HSAhi CD4+8− cells thus was Fas dependent at a high dose of anti-TCR but Fas independent at a low dose. This phenomenon was not seen with CD4+8+ cells (Fig. 4 B, left) and did not apply when lpr/lpr HSAhi CD4+8− cells were exposed to anti-TCR mAb in the absence of anti-CD28 mAb (Fig. 4 B, right); here, apoptosis of lpr/lpr cells was undetectable over a wide dose range of anti-TCR mAb. The conditions required to induce Fas-independent apoptosis of HSAhi CD4+8− cells thus were precise in two respects: (a) the dose of anti-TCR mAb had to be low, and (b) costimulation via CD28 was essential.
In contrast with semimature HSAhi CD4+8− cells, apoptosis of fully mature HSAlo CD4+8− thymocytes and CD4+8− LN cells in vitro appeared to be completely Fas dependent. Thus, apoptosis of these populations after ligation with TCR alone was totally blocked by Fas–Ig and was not seen with Fas-deficient lpr/lpr cells (Fig. 4 A, left). In fact, with Fas inactivation/blockade, TCR ligation alone considerably enhanced cell survival relative to cells cultured alone.
Discussion
This paper demonstrates that under defined conditions immature (CD4+8+), semimature (HSAhi CD4+8−) and fully mature (HSAlo CD4+8−) thymocytes are all susceptible to rapid induction of apoptosis after TCR ligation in vitro. However, each population of thymocytes displayed different requirements for apoptosis induction.
Contrary to expectation, fully mature naive HSAlo CD4+8− thymocytes and CD4+8− LN cells were susceptible to apoptosis mediated by TCR ligation alone. This form of apoptosis was Fas dependent and was not seen after combined TCR–CD28 ligation, implying that costimulation blocked apoptosis. Likewise, TCR-mediated apoptosis of mature T cells was not detectable after addition of small numbers of spleen APC as a source of bystander costimulation (data not shown). Since APC are found in all lymphoid organs, including the thymus, the chances of mature T cells being subjected to TCR ligation in vivo in the absence of costimulation would seem unlikely. For this reason, we suggest that the rapid onset of Fas-mediated apoptosis described here for naive T cells after TCR ligation in vitro may not operate under in vivo conditions.
It is important to emphasize that the rapid (<20 h) onset of TCR-mediated apoptosis of mature naive T cells was not associated with entry into cell cycle (although entry into G1 has not been excluded) (data not shown). This finding differs from activation-induced cell death (AICD), in which apoptosis of mature T cells generally follows a prior proliferative response (42). Nevertheless, it is striking that, as for naive T cells, AICD is Fas dependent (36–38, 43); in addition, some groups (44, 45) but not others (43) find that costimulation prevents AICD. Hence, AICD and the rapid death of naive T cells described here may be closely related. It should be stressed that demonstrating TCR-mediated apoptosis of naive T cells required (a) a high concentration of anti-TCR mAb in cross-linked form, (b) a virtual absence of APC, and (c) a highly sensitive technique (TUNEL) for detecting apoptosis. These strict requirements may explain why early TCR-mediated apoptosis of naive T cells is not seen routinely by other groups.
For mature and semimature T cells, susceptibility to Fasdependent apoptosis following TCR ligation in the absence of costimulation correlated with rapid upregulation of FasL (our unpublished data). Thus, apoptosis presumably reflected Fas–FasL interaction. Interestingly, no upregulation of FasL was seen when purified CD4+8+ cells were exposed to either TCR or TCR–CD28 ligation. Hence, the failure to detect a role for Fas in apoptosis of CD4+8+ cells (Fig. 4) could simply reflect that, despite their high density of Fas, CD4+8+ cells are unable to upregulate FasL. It should be mentioned that exposing CD4+8+ cells to crosslinked anti-Fas mAb causes rapid apoptosis (our unpublished data), indicating that the Fas death pathway is intact in CD4+8+ cells.
The capacity of costimulation to prevent TCR-mediated apoptosis was unique to mature T cells and did not apply to CD4+8+ or HSAhi CD4+8− thymocytes. For these populations, the presence of costimulation was either essential for TCR-mediated apoptosis (for CD4+8+ cells) or considerably enhanced apoptosis (for HSAhi CD4+8− cells). How costimulation alters the susceptibility of thymocyte subsets to TCR-mediated apoptosis is unclear. For fully mature HSAlo CD4+8− thymocytes and LN cells, preliminary data has shown that combined TCR–CD28 ligation causes strong upregulation of Bcl-xL (our unpublished data); this finding is not seen with TCR ligation alone. Thus, for mature T cells, the capacity of costimulation to prevent TCR-mediated apoptosis could be attributed to rapid upregulation of antiapoptotic molecules such as Bcl-xL (44). Interestingly, in the case of semimature HSAhi CD4+8− thymocytes, we have found that upregulation of Bcl-xL following TCR– CD28 ligation is minimal. Hence, the inability of costimulation to prevent apoptosis of semimature T cells may reflect a failure of these cells to upregulate Bcl-xL.
Because a requirement for costimulation is an important feature of negative selection in the thymus, the susceptibility of thymocytes to TCR–CD28-mediated apoptosis in vitro would seem to be a valid model for defining which subsets of thymocytes are subject to negative selection in vivo. With this assumption, the present data suggest that negative selection is not restricted to immature cortical CD4+8+ cells but also includes the semimature population of HSAhi CD4+8− cells found in the medulla. In support of this view, each of these populations was eliminated after injection of anti-TCR mAb in vivo; under these conditions, costimulation was presumably presented in bystander form by thymic APC. It is notable that anti-TCR injection failed to destroy HSAlo CD4+8− thymocytes. This finding is in agreement with the current dogma that fully mature T cells are resistant to negative selection.
The finding that negative selection includes the semimature subset of HSAhi CD4+8− cells found in the medulla provides direct support for the view that the medulla is an important site of negative selection. Thus, one can envisage that purging the T cell repertoire of reactivity to circulating self-antigens may occur at a relatively late stage of T cell differentiation and be delayed until semimature CD4+8− cells reach the APC-enriched environment of the medulla and cortico–medullary junction. After being screened for self-reactivity in these sites, the surviving T cells then differentiate into fully mature T cells and are exported to the periphery. It is of interest that, under in vitro conditions, HSAhi CD4+8− cells were significantly (5-fold) less sensitive to TCR–CD28-mediated apoptosis than immature CD4+8+ cells. While having only minimal effects on the efficiency of negative selection, this mild reduction in tolerance susceptibility could be vital for enabling SP cells to be refractory to weak (antagonist or partial agonist) selfpeptides on APC, i.e., to the ubiquitous self-peptides that initially signaled positive selection in the cortex at the DP stage (7, 46). This notion would explain why the reactivity of early thymocytes for the weak peptides controlling positive selection is apparently lost when the cells begin to mature and move from the cortex to the medulla (47).
The signaling pathways involved in negative selection of medullary HSAhi CD4+8− cells remain to be clarified. Because the density and TCR–MHC affinity of each selfantigen expressed on medullary APC is likely to vary considerably, efficient negative selection of HSAhi CD4+8− cells could depend upon multiple signaling pathways, the involvement of each pathway being determined by the relative strength and density of the self-antigen concerned. In this respect, it is notable that a Fas-independent pathway controlled TCR–CD28-mediated apoptosis of HSAhi CD4+8− cells in vitro, but only at a low dose of anti-TCR mAb. At higher doses, the Fas-independent pathway failed, and negative selection required a secondary mechanism, i.e., a Fasmediated pathway. This finding raises the possibility that negative selection in Fas-deficient lpr/lpr mice would not occur if the concentration of antigen were raised to a high level. In fact, recent studies with soluble superantigens have shown that this is indeed the case (our unpublished data).
The participation of multiple signaling pathways in negative selection could explain the difficulty of defining which particular costimulatory molecules are required for this process. Thus, despite the in vitro results reported here, costimulation via CD28–B7 interaction apparently is not mandatory for negative selection in vivo (9, 48, 49). However, it does not necessarily follow that this pathway is unimportant. Bearing in mind the large variety of costimulatory molecules on APC, many or all of these molecules could provide effective costimulation for negative selection under in vivo conditions. If so, the consequences of deleting only one of these molecules, e.g., B7 (or CD28) or Fas, would then be relatively minor. The participation of multiple signaling pathways and various costimulatory molecules in negative selection could represent a fail-safe device to ensure that self-tolerance induction is as efficient as possible.
Irrespective of which particular signaling pathways are involved in negative selection, the main finding in this paper is that the tolerance susceptibility of thymocytes is not restricted to immature cortical CD4+8+ cells, but includes the semimature subset of HSAhi CD4+8− cells found in the medulla. Thus, the data support the view that negative selection to circulating self-antigens occurs at a relatively late stage of thymocyte differentiation, i.e., after the differentiation of DP cells to SP cells and after migration of early SP cells into the medulla.
Footnotes
We wish to express our thanks to Dr. David Lynch and Immunex for providing Fas–Ig and to Ms. Barbara Marchand for typing the manuscript.
This work was supported by grants CA38355, CA25803, AI21487, and AI32068 from the United States Public Health Service. Dr. Hidehiro Kishimoto is the recipient of a fellowship from the Cancer Research Institute. This is publication number 9928-IMM from The Scripps Research Institute.
1 Abbreviations used in this paper: AICD, activation-induced cell death; BM, bone marrow; C, constant; DP, double positive; FasL, Fas ligand; HSA, heatstable antigen; PI, propidium iodide; SP, single positive; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP-biotin nick end labeling.
References
- 1.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 3.Fowlkes BJ, Schweighoffer E. Positive selection of T cells. Curr Opin Immunol. 1995;7:188–195. doi: 10.1016/0952-7915(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 4.Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 5.Sprent J, Webb SR. Intrathymic and extrathymic clonal deletion of T cells. Curr Opin Immunol. 1995;7:196–205. doi: 10.1016/0952-7915(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 6.Shortman, K., and R. Scollay. 1985. Cortical and medullary thymocytes. In Recognition and Regulation in Cell-Mediated Immunity. J.D. Watson and J. Marbrook, editors. Marcell Dekker, New York. 31–60.
- 7.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 8.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+thymocytes by T cell receptor–induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiba Y, Mazda O, Davis MM, Muramatsu S, Katsura Y. Requirement of a second signal from antigen presenting cells in the clonal deletion of immature T cells. Int Immunol. 1994;6:1475–1483. doi: 10.1093/intimm/6.10.1475. [DOI] [PubMed] [Google Scholar]
- 10.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+thymocytes. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 11.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 12.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 13.Janeway CA, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 14.Raviola E, Karnovsky MJ. Evidence for a blood–thymus barrier using electron-opaque tracers. J Exp Med. 1972;136:466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengartner H, Odermatt B, Schneider R, Schreyer M, Wälle G, MacDonald HR, Zinkernagel RM. Deletion of self-reactive T cells before entry into the thymus medulla. Nature (Lond) 1988;336:388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- 16.Hugo P, Boyd RL, Waanders GA, Petrie HT, Scollay R. Timing of deletion of autoreactive Vβ6+ cells and down-modulation of either CD4 or CD8 on phenotypically distinct CD4+CD8+subsets of thymocytes expressing intermediate or high levels of T cell receptor. Int Immunol. 1990;3:265–272. doi: 10.1093/intimm/3.3.265. [DOI] [PubMed] [Google Scholar]
- 17.Guidos CJ, Danska JS, Fathman CG, Weissman IL. T cell receptor–mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990;172:835–845. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature (Lond) 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 20.Bruce J, Symington FW, McKearn TJ, Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981;127:2496–2501. [PubMed] [Google Scholar]
- 21.Crispe IN, Bevan MJ. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987;138:2013–2018. [PubMed] [Google Scholar]
- 22.Vernachio J, Li M, Donnenberg AD, Soloski MJ. Qa-2 expression in the adult murine thymus: a unique marker for a mature thymic subset. J Immunol. 1989;142:48–56. [PubMed] [Google Scholar]
- 23.Ramsdell F, Jenkins M, Dinh Q, Fowlkes BJ. The majority of CD4+8−thymocytes are functionally immature. J Immunol. 1991;147:1779–1785. [PubMed] [Google Scholar]
- 24.Nikolic-Zugic J, Bevan MJ. Functional and phenotypic delineation of two subsets of CD4 single positive cells in the thymus. Int Immunol. 1990;2:135–141. doi: 10.1093/intimm/2.2.135. [DOI] [PubMed] [Google Scholar]
- 25.Dyall R, Nikolic-Zugic J. The majority of postselection CD4+single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosaka H, Surh CD, Sprent J. Stimulation of mature unprimed CD8+T cells by semiprofessional antigenpresenting cells in vivo. J Exp Med. 1992;176:1291–1302. doi: 10.1084/jem.176.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–2742. [Google Scholar]
- 28.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 29.Kishimoto H, Surh CD, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Y, Bissonnette RP, Parfrey N, Szalay M, Kubo RT, Green DR. In vivo administration of antibodies to the CD3–T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991;146:3340–3346. [PubMed] [Google Scholar]
- 31.Mueller DL, Jenkins MK, Schwartz RH. Clonal expression versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Janeway CA., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci USA. 1992;89:3845–3849. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crispe IN. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994;1:347–349. doi: 10.1016/1074-7613(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 34.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 35.Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, Green DR. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 36.Dhein J H, Walczak, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/ (Fas/CD95) Nature (Lond) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 37.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/ Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 38.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature (Lond) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 39.Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 40.Theofilopoulos AN. The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognition. Immunol Today. 1995;16:90–98. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 41.Ramsdell F, Seaman MS, Miller RE, Tough TW, Alderson MR, Lynch DH. gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol. 1994;24:928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- 42.Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;6:476–487. doi: 10.1016/0952-7915(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 43.van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 44.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 45.Radvanyi LG, Shi Y, Vaziri H, Sharman A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 46.Rothenberg E. Developmental biology of lymphocytes. The Immunologist. 1995;3:172–175. [Google Scholar]
- 47.Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak S-Y, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PS. Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science (Wash DC) 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 49.Walunas TL, Sperling AI, Khattri R, Thompson CB, Bluestone JA. CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 50.Newell MK, Haughn LJ, Maroun CR, Julius MH. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature (Lond) 1990;347:286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]