Abstract
One hypothesis seeking to explain the signaling and biological properties of T cell receptor for antigen (TCR) partial agonists and antagonists is the coreceptor density/kinetic model, which proposes that the pharmacologic behavior of a TCR ligand is largely determined by the relative rates of (a) dissociation of ligand from an engaged TCR and (b) recruitment of lck-linked coreceptors to this ligand-engaged receptor. Using several approaches to prevent or reduce the association of CD4 with occupied TCR, we demonstrate that consistent with this hypothesis, the biological and biochemical consequence of limiting this interaction is to convert typical agonists into partial agonist stimuli. Thus, adding anti-CD4 antibody to T cells recognizing a wild-type peptide–MHC class II ligand leads to disproportionate inhibition of interleukin-2 (IL-2) relative to IL-3 production, the same pattern seen using a TCR partial agonist/antagonist. In addition, T cells exposed to wild-type ligand in the presence of anti-CD4 antibodies show a pattern of TCR signaling resembling that seen using partial agonists, with predominant accumulation of the p21 tyrosine-phosphorylated form of TCR-ζ, reduced tyrosine phosphorylation of CD3ε, and no detectable phosphorylation of ZAP-70. Similar results are obtained when the wild-type ligand is presented by mutant class II MHC molecules unable to bind CD4. Likewise, antibody coligation of CD3 and CD4 results in an agonist-like phosphorylation pattern, whereas bivalent engagement of CD3 alone gives a partial agonist-like pattern. Finally, in accord with data showing that partial agonists often induce T cell anergy, CD4 blockade during antigen exposure renders cloned T cells unable to produce IL-2 upon restimulation. These results demonstrate that the biochemical and functional responses to variant TCR ligands with partial agonist properties can be largely reproduced by inhibiting recruitment of CD4 to a TCR binding a wild-type ligand, consistent with the idea that the relative rates of TCR–ligand disengagement and of association of engaged TCR with CD4 may play a key role in determining the pharmacologic properties of peptide–MHC molecule ligands. Beyond this insight into signaling through the TCR, these results have implications for models of thymocyte selection and the use of anti-coreceptor antibodies in vivo for the establishment of immunological tolerance.
Recent evidence indicates that the TCR transduces different signals depending on the precise structure of the peptide–MHC molecule ligand to which it binds (1–3). This differential signaling has been associated with selective cytokine induction (by TCR partial agonists) or inhibition of secretion (by TCR antagonists) (4–7), selective upregulation of surface molecule expression (7–9), or development of T cell anergy in the presence of adequate CD28-related costimulation (1, 8, 10). The mechanistic basis for these distinct pharmacologic properties of closely related peptide– MHC molecule ligands recognized by the same TCR is not well understood. A number of models have been proposed to explain how ligand structural variation can be translated into altered TCR signaling and T cell activation (3, 11–13). Among these, the most widely accepted hypothesis postulates that the affinity of the receptor and, most probably, the dissociation rate of the ligand from the receptor, determines what effect antigen recognition has on the T cell (3, 12–15). In its simplest form, this model argues that different biochemical events take place at distinct times after the TCR has bound ligand, and premature dissociation results in the occurrence of only some but not others of these. Increasing the amount of a ligand would then increase the amount of those signals occurring before dissociation, but would not lead to the generation of any of those events that typically require a longer time of TCR–ligand association to occur. This model provides a simple explanation for the reported data on the effects of TCR interaction with partial agonists or antagonists on early TCR-related tyrosine phosphorylation events, which show an altered pattern that varies in quantity with ligand amount, but which remain distinct from that seen using agonist at all tested concentrations of ligand (1, 2, 16). It is also in general agreement with very recent studies that have correlated the functional properties of peptide–MHC molecule ligands with their measured affinity for soluble versions of the TCR studied in the absence of coreceptor (17, 18).
The major alternative models focus on structural rather than affinity issues. One argues for an efficacy component to TCR–ligand engagement that reflects a conformational change in the TCR upon ligand binding that is necessary for all the typical downstream biochemical events seen using agonist ligands. Alternatively, emphasis is placed on a narrow requirement for alignment of the various proteins (TCR, CD4, MHC–peptide) involved in an effective signaling complex, such that misalignment, irrespective of the absolute ligand–TCR affinity, would result in aberrant signaling and altered functional response (19, 20). Only very limited data offer support for these hypotheses, such as the apparent realignment of TCR on the MHC surface when the side chains of a peptide are altered (21), and the ability of only some but not other monovalent anti-TCR antibody fragments to synergize with cross-linking antibodies in T cell activation (22).
Independently of whether kinetic or architectural models or both are correct, one still has to explain in biochemical terms how either type of change in ligand–TCR interaction gives rise to distinct early TCR-mediated signaling events. Several investigators have pointed out how variations in ligand structure leading to either diminished TCR affinity or altered receptor architecture could affect proper recruitment of the CD4 or CD8 coreceptors and associated lck molecules. Coligation of CD4 or CD8 with the TCR is well documented to augment markedly functional T cell responses to ligand (23), as well as to change the overall pattern of intracellular phosphorylation (24–27). For peptide–MHC class I ligands, cobinding of CD8 has been demonstrated to decrease the rate of ligand dissociation from the TCR (28). Recent functional studies have shown that CD8 blockade can convert a poor antagonist peptide into a good TCR antagonist (29) and that reductions in available CD4 levels can change a partial or weak agonist into an antagonist (30, 31). However, none of these studies has examined the relationship between the extent of successful TCR–coreceptor coassociation and early TCRassociated intracellular signaling events. Such studies could prove especially helpful in relating coreceptor function to the properties of variant TCR ligands, in light of the recent data on the consistent pattern of altered early TCR-associated tyrosine phosphorylation seen using partial agonists and antagonists (12, 16, 32). Here, we have directly tested the hypothesis that the particular pattern of TCR signaling seen using partial agonists and antagonists might be the result of inefficient TCR–coreceptor interactions. Results obtained from three different approaches all show that limiting recruitment of CD4 to TCR engaged by either a wild-type peptide–MHC molecule ligand that under normal circumstances shows agonist function, or by antibody, leads to biochemical and functional responses closely resembling those elicited by partial agonist ligands, including deficient IL-2 production resulting in induction of clonal anergy. These data are consistent with a key role of coreceptor recruitment to engaged TCR complexes in determining both the signal transduction and functional properties of TCR ligands. Together with previous studies, these data may help explain some apparently contradictory results involving thymocyte positive selection, as well as provide insight into the phenomenon of T cell tolerance in vivo after coadministration of nondepleting anti-CD4 antibody and antigen.
Materials and Methods
Cells.
3C6 and A.E7 are mouse CD4+ Th1 clones specific for cytochrome c fragment 81–104 (PCC[81–104]) bound to I-Ek (33); TK.G4 is a CD4+ Th1 clone specific for sperm whale myoglobin fragment 102–118 (SWmyo[102–118]) bound to I-Ad, provided by Dr. J.A. Berzofsky (National Cancer Institute, National Institutes of Health, Bethesda, MD) (34). T cell clones were maintained by cycles of antigen stimulation with irradiated spleen cells, cytokine expansion, and rest, as previously described (2). They were used for functional or biochemical experiments 10–14 d after they had last seen antigen. P13.9 L cells transfected with cDNA constructs encoding Eα and Eβ, as well as the costimulatory molecules ICAM-1 and CD80 (B7-1), were used as APCs (2, 35, 36).
Monoclonal Antibodies.
The following mAbs were used in these experiments: purified RM4.5, a rat IgG against mouse CD4 (Pharmingen, San Diego, CA); GK 1.5 (37), a rat IgG against mouse CD4; 14-4-4S (38), a mouse antibody specific for class II molecules containing Eα; 4G10, a mouse IgG2b mAb to phosphotyrosine (Upstate Biotechnology, Inc., Lake Placid, NY), and 500A2 (39), a hamster IgG against mouse CD3ε (PharMingen, San Diego, CA). Blocking experiments were performed using deaggregated Abs prepared by ultracentrifugation (100,000 g) for 1 h at 4°C. The F(ab′)2 fragment, anti-CD3–fos, has been previously described (40). Anti-CD4–jun F(ab′)2 was derived from GK 1.5 (Yun Tso, J., and J. Bluestone, unpublished observations), and the bispecific F(ab′)2 anti-CD3–fos × anti-CD4–jun was prepared according to Kostelny et al. (40). Anti-ZAP-70 rabbit antiserum was the gift of Dr. L. Samelson (Cell Biology and Metabolism Branch, National Institute of Child Health and Development, NIH, Bethesda, MD).
Peptides.
The following peptides were used for these experiments: PCC(81–104) (IFAGIKKKAERADLIAYLKQATAK); PCC(88–104); and Swmyo (102–118) (KYLEFISEAIIHVLHSR). Peptides were synthesized in the Peptide Synthesis Facility of the National Institute of Allergy and Infectious Diseases (Dr. J. Coligan, NIH, Bethesda, MD), or commercially provided by Procyon Biopharma, Inc. (London, Ontario, Canada). They were purified to greater than 85% by HPLC before use.
Tyrosine Phosphorylation Analysis.
Detection of tyrosine phosphorylation of proteins in CD3ε or anti-ZAP-70 immunoprecipitates was performed as described (2). 4G10 mAb was used for immunoblotting, and 500A2 or rabbit antiserum to ZAP-70 was used to immunoprecipitate TCR subunits or ZAP-70 and associated proteins, respectively. Immunoprecipitations were performed from 1 × 107 cells per group at 10 min after ligand exposure in the form of antigen-bearing L cells or soluble antibodies, using culture conditions previously described (2). Signal intensity was quantitated using an imaging densitometer (Bio Rad, model GS 700, Hercules, CA) and the Molecular Analyst® Software (version 1.0, 1994, Bio Rad Laboratories).
Functional Assays.
Measurement of proliferation and cytokine (IL-2, IL-3) production were performed following standard procedures (2). In brief, for proliferation assays, 5 × 104 T cells were incubated with either 5 × 104 mitomycin c–treated L cells and the indicated concentration of peptide or with the indicated concentration of anti-CD3–fos, anti-CD4–jun, or anti-CD3–fos × antiCD4–jun bivalent antibody for 28 h, after which the cultures were pulsed with 1 μCi [3H]thymidine for another 20 h before harvesting and counting of incorporated label. Cytokine production was measured by ELISA of 24-h supernatants from 5 × 104 T cells stimulated with peptide and 5 × 104 APC or antibody.
Induction of T Cell Anergy.
P13.9 L cells were first loaded with magnetic beads (Dynal, Inc., Lake Success, NY) by incubating these cells (30 × 106 in 30 ml of complete medium) with 200 × 106 beads at 37°C for 48 h. P13.9 having ingested beads cells were then harvested by magnetic sorting. A.E7 T cells (1 × 106 per group in duplicate) were incubated with mitomycin c–treated bead-containing P13.9 cells as APC (1 × 106 per group in duplicate) and 10 or 100 nM PCC(81–104), in the presence of increasing concentrations of anti-CD4 mAb (GK1.5) in 24-well plates in a final volume of 2 ml at 37°C for 24 h. The T cells were harvested by two rounds of magnetic depletion of APC, followed by Ficoll centrifugation, and one wash in complete medium. Recovered antigen-free viable T cells (0.5–1.5 × 106) were then rested in 2 ml of complete medium in 24-well plates for at least 5 d, before being rechallenged with PCC(81–104) and mitomycin c–treated P13.9 cells (3 × 104 T cells plus 3 × 104 P13.9 and increasing concentrations of peptide). IL-2 production was measured by ELISA using 24-h supernatants from these restimulation cultures.
Results
Antibody Blockade of CD4 Changes Agonist-induced Cytokine Secretion and Early Phosphorylation Events to a Partial Agonist/ Antagonist Pattern.
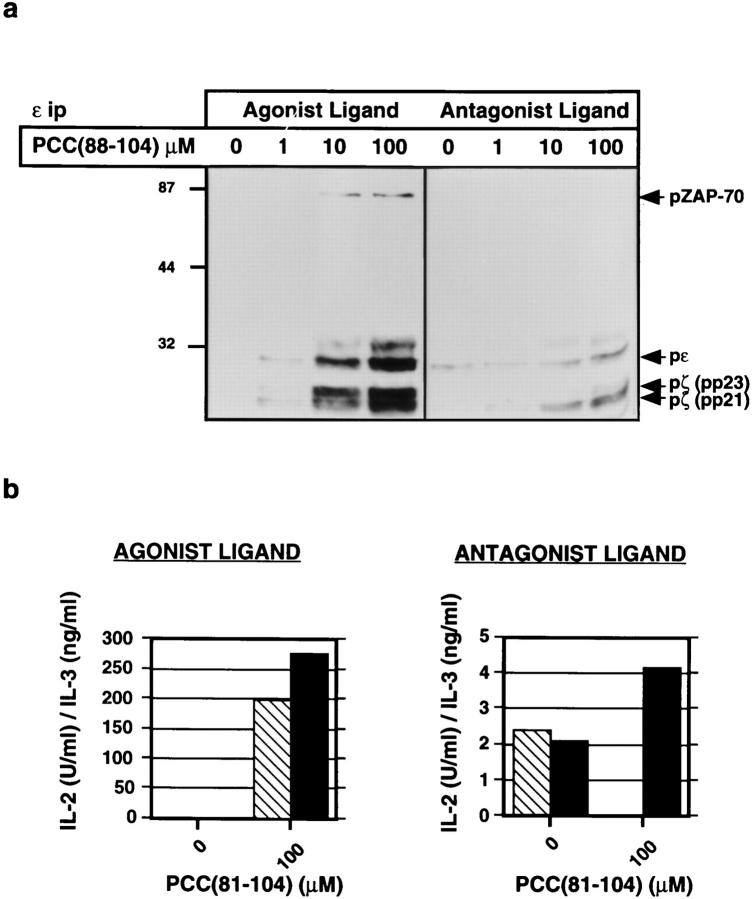
We have previously reported that 3C6 proliferates and secretes both IL-2 and IL-3 in response to a mutant I-Ek molecule agonist expressed on transfected L cell APC (7). Addition of PCC(81–104) to the culture results in the formation of an altered TCR ligand consisting of the PCC peptide and the mutant I-E molecule (7). The pattern of protein tyrosine phosphorylation in anti-CD3ε immunoprecipitates is different for 3C6 cells exposed to an agonist consisting of wild-type I-Ek and PCC(81-104) or to this peptide–mutant MHC class II combination (2). The former is characterized by the presence of similar levels of p21 and p23 tyrosine-phosphorylated forms of TCR-ζ, tyrosine-phosphorylated CD3ε, and kinase-active tyrosinephosphorylated ZAP-70. In contrast, the latter leads to the predominant presence of the p21 tyrosine phosphorylated form of TCR-ζ, little or no tyrosine phosphorylated CD3ε, and the presence of ZAP-70 that is neither detectably phosphorylated nor kinase active, as reported previously (2) and shown here (Fig. 1 a). This PCC peptide–mutant I-E molecule ligand selectively antagonizes IL-2 production, while also acting as a partial agonist capable of modestly stimulating IL-3 production (7) (Fig. 1 b).
Figure 1.
The IL-2 response of 3C6 T cells to mutant I-Ek is selectively antagonized by the addition of PCC(81–104). (a) Tyrosine phosphorylation analysis of proteins in CD3ε immunoprecipitates from 3C6 cells stimulated with increasing concentrations of agonist (PCC[81–104]– I-Ek) or antagonist (PCC[81–104]–mutant I-E) ligands. T cells were stimulated for 10 min with APC plus PCC(81–104) and CD3ε immunoprecipitates (9 × 106 cell equivalents/lane) were electrophoresed and immunoblotted with an anti-phosphotyrosine antibody. (b) 3C6 T cells (5 × 104) were stimulated with mitomycin C–treated, wild-type I-Ek, or mutant I-E-transfected L cells and PCC(81–104) (100 μM). Production of IL-2 and IL-3 was measured in 24-h supernatants by ELISA. Crosshatched bars show IL-2 and closed bars show IL-3.
The response of 3C6 cells to antigen is CD4 dependent, as indicated by dose-dependent inhibition of proliferation or cytokine production using increasing concentrations of anti-CD4 mAb in culture (Madrenas, J., and R.N. Germain, unpublished observations). This blocking effect, or the decreased antigen sensitivity seen following loss of surface CD4 expression in T hybridomas, has generally been considered to result from lower TCR occupancy and, hence, less receptor signaling for activation. However, some published reports document changes in the pattern of overall intracellular protein phosphorylation in cell lysates when CD4 or CD8 are coligated with the TCR, and shifts between agonist and antagonist behavior of the same peptide– MHC molecule combination have been observed when peptide–MHC molecule ligands are tested under conditions of variable coreceptor expression or availability. Therefore, we examined whether anti-CD4 antibody inhibition of the 3C6 response led to a simple quantitative loss of signal at any given ligand concentration, or whether it actually altered the pattern of cytokine response to and/or proximal signaling events associated with TCR recognition of ligand.
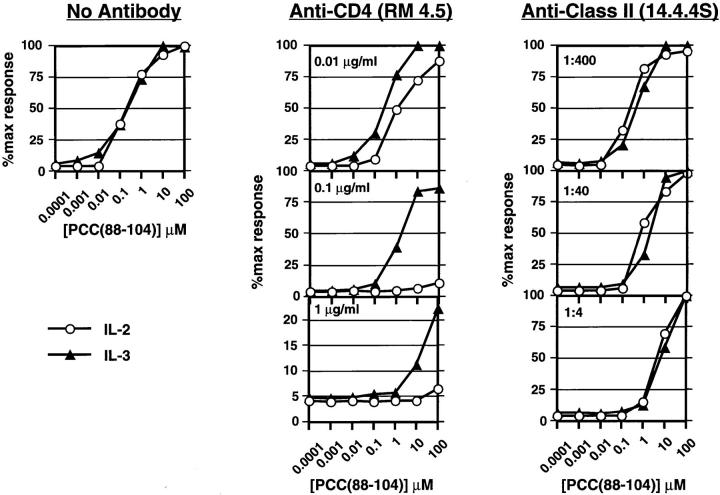
3C6 cells were exposed to PCC(88–104)–I-Ek complexes (wild-type ligand) on APC in the presence of increasing concentrations of a blocking mAb specific for CD4 (RM4.5). This was compared with stimulation of the cells in the presence of a blocking anti-class II mAb specific for I-E (14-4-4S), to limit ligand availability by a means other than peptide titration as had been done previously (2) and shown here in Fig. 2 . Addition of anti-CD4 inhibits both IL-2 and IL-3 secretion, but IL-2 release is disproportionately sensitive to the antibody. Thus, any given fractional decrease in IL-2 production requires less anti-CD4 than a similar decrease in IL-3 production, and IL-2 secretion is abolished using much less anti-CD4 than is necessary to achieve the same effect on IL-3. In contrast, anti-class II MHC antibody inhibits IL-2 and IL-3 production to a similar extent. The results obtained using anti-CD4 resemble those seen upon simultaneous exposure of 3C6 to both agonist and antagonist, under which conditions IL-2 production is preferentially inhibited (7). If PCC(81–104) peptide is used to stimulate 3C6 instead of PCC(88–104), production of IL-2 requires less TCR occupancy than production of IL-3, as indicated by the fact that 50% maximal response for IL-2 production is reached at a lower concentration of peptide than is required for 50% maximal IL-3 production (7). Strikingly, under these conditions, exposure to antiCD4 mAb leads to an inversion in the IL-2 and IL-3 dose– response relationship, with relatively greater fractional IL-3 production than IL-2 production at each point in the dose– response (data not shown). Thus, blockade of CD4 leads to a functional response similar to that seen upon engagement of the 3C6 TCR with a variant ligand, and given the distinct results obtained with anti-class II antibody (Fig. 2), this effect cannot be explained by a simple decrease in occupancy of the TCR.
Figure 2.
Effect of anti-CD4 and anti-MHC class II mAbs on IL-2 and IL-3 production by 3C6 T cells responding to the wild-type ligand PCC(88–104)–I-Ek. T cells (5 × 104 per well) were stimulated with I-Ekexpressing L cells and increasing concentrations of PCC(88–104) for 24 h in the absence or the presence of the indicated concentrations of antiCD4 or anti-class II mAb. Supernatants were then collected and IL-2 (open circles) and IL-3 (closed triangles) measured by ELISA. Results are expressed as the percent of cytokine produced considering the maximal cytokine measured in each experiment in the absence of blocking antibody as 100%.
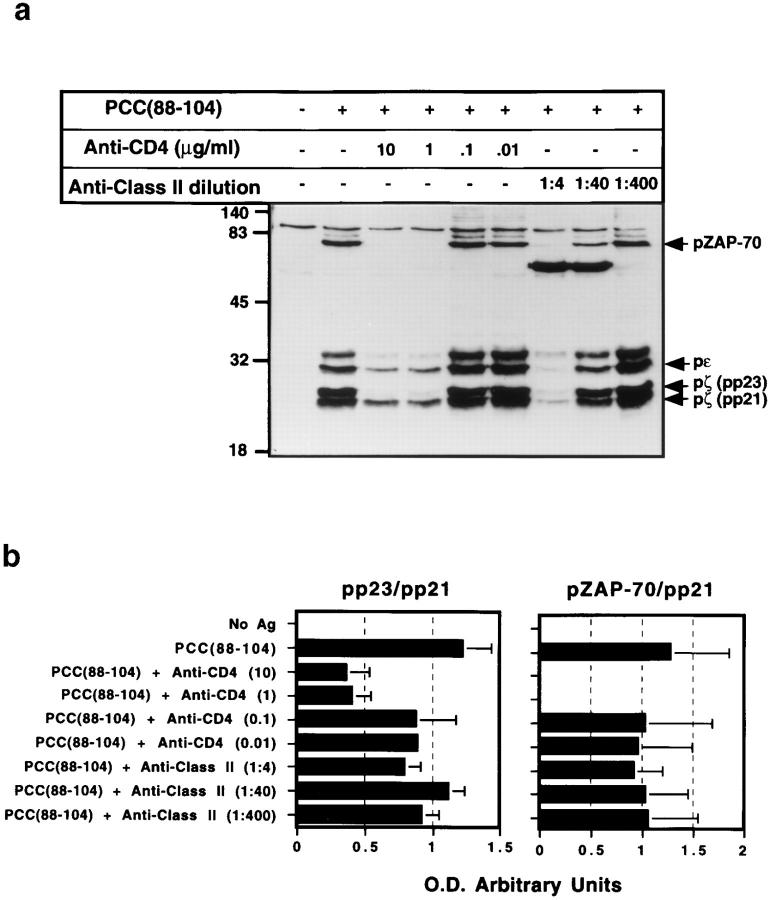
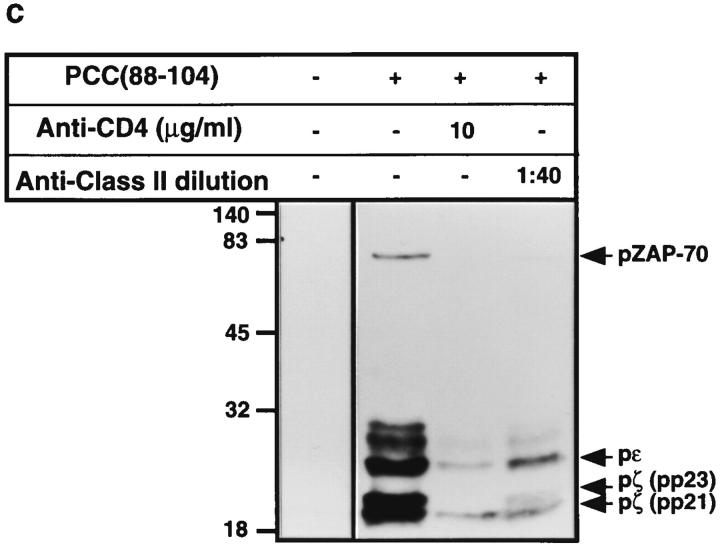
To determine whether this functional switch in response to one resembling variant ligand stimulation also reflects a corresponding change in TCR signaling, we examined TCR subunit tyrosine phosphorylation in T cells responding to wild-type ligand in the presence of CD4 blockade. At concentrations of anti-CD4 affecting cytokine production, the early TCR-dependent signaling response clearly changes from one typical of agonist to one close to that characteristic of partial agonists (preferential accumulation of the p21 tyrosine phosphorylated form of TCR-ζ, with less phosphorylated CD3ε, and no detectable phosphorylated ZAP-70) (Fig. 3 a). This shift is unlikely to reflect active signaling following antibody interaction with the CD4 molecule because we used soluble deaggregated antibodies (41), the L cell APC do not express Fc receptors, and no significant tyrosine phosphorylation of TCR subunits is observed when antigen is omitted from cultures containing anti-CD4, T cells, and APC (data not shown). The effect of anti-class II mAb is different from that of the anti-CD4 and similar to what has been reported previously for changes in antigenic peptide concentration (1, 2, 16), namely, a uniform decrease in phosphorylation of all TCR subunits or the associated ZAP-70. These results seen using anti-CD4 versus anticlass II antibodies were confirmed by quantitative densitometric analysis of the pp21, pp23, and pZAP-70 bands from three different experiments (Fig. 3 b). The ratio between pp23 and pp21 is markedly decreased with higher concentrations of mAb against CD4, whereas this ratio remains stable for samples treated with anti-class II MHC. In addition, the ratio between pZAP-70 and pp21 falls to zero in those samples with higher concentrations of anti-CD4 mAb. Nevertheless, as reported previously (2, 3), ZAP-70 is recruited to the TCR complex upon TCR engagement of ligand even if anti-CD4 antibody is present and no phosphorylated ZAP-70 is observed (Fig. 3 c).
Figure 3.
Effect of anti-CD4 antibody on antigen-induced tyrosine phosphorylation of TCR subunits. (a) 3C6 T cells (1 × 107) were stimulated with I-Ek-transfected L cells and PCC(88–104) (100 μM) for 10 min in the presence of increasing concentrations of anti-CD4 mAb (RM 4.5) or anti-class II mAb (14-4-4S). Cell were lysed with lysis buffer containing 1% Triton X-100, and TCR subunits were immunoprecipitated using a mAb against mouse CD3ε (500A2). Immunoprecipitates were immunoblotted using a mAb against phosphotyrosine (4G10). (b) Optical density of the pp21, pp23, and the pZAP-70 signals from three independent experiments was measured using an imaging densitometer, and the pp23/pp21 and pZAP-70/pp21 ratios displayed. (c) 3C6 T cells (1 × 107) were stimulated with I-Ek-transfected L cells and PCC(88–104) (100 μM) for 10 min in the presence of anti-CD4 mAb (RM 4.5) or anti-class II mAb (14-4-4S). Cell were lysed with lysis buffer containing 1% Triton X-100, and TCR subunits were immunoprecipitated using a mAb against mouse ZAP-70. Immunoprecipitates were immunoblotted using a mAb against phosphotyrosine (4G10).
An MHC Class II Ligand Unable to Bind to CD4 Induces TCR Signaling Resembling That Seen with Partial Agonists.
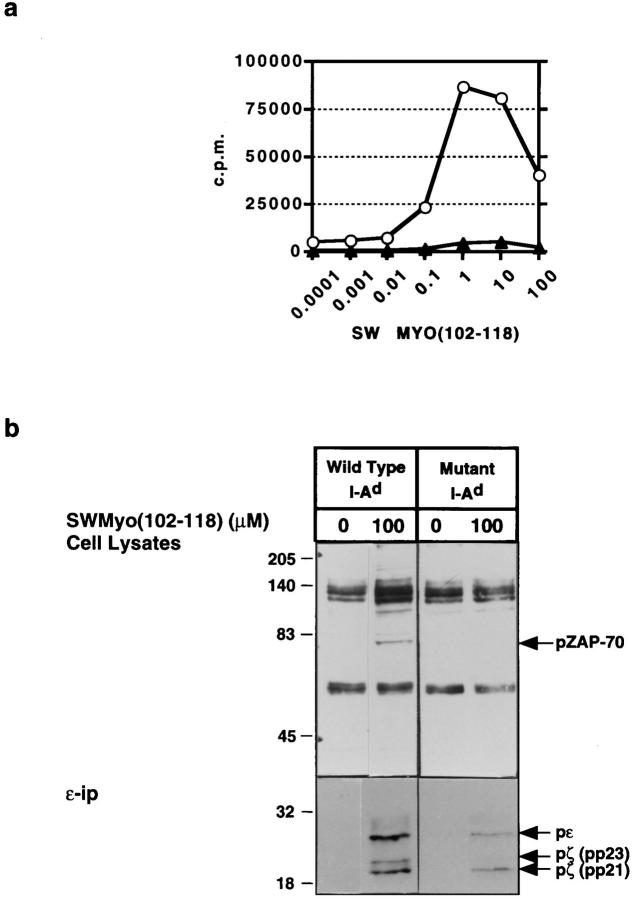
To examine whether the lack of CD4 recruitment to the TCR leads to a partial agonist pattern of response under conditions not involving antibody ligation of CD4, we examined the functional and biochemical responses of a CD4+ T cell clone to its specific peptide presented by either wild-type class II molecules or by class II molecules in which the main CD4 binding site has been mutated (36, 42, 43). TK.G4 is a Th1 clone specific for sperm whale myoglobin fragment 102–118 (SWmyo[102–118]) bound to I-Ad molecules. The proliferative response of TK.G4 to this peptide–MHC class II molecule combination is CD4 dependent as shown by a significant reduction in the magnitude of the response when CD4 cannot bind to the I-Ad molecule present on the APC (Fig. 4 a). Under these conditions involving presentation of antigen by a mutant class II molecule, a pattern of signaling similar to that typical of partial agonist stimulation is observed (Fig. 4 b). This cannot be simply attributed to lower overall signaling from the TCR because pp21 TCR-ζ reaches a higher level under these conditions than when the same clone is stimulated with 10 μM SWmyo(102–118) and wild type I-Ad, yet the latter produces a higher proliferative response and shows substantial pp23 TCR-ζ accumulation not seen with the mutant class II ligand (data not shown).
Figure 4.
TK.G4 T cell responses to cognate peptide bound to wildtype MHC class II molecules or to mutant MHC class II molecules unable to bind CD4. (a) Proliferative response of TK.G4 cells stimulated with Swmyo(102–118) + wild-type I-Ad or Swmyo(102–118) + I-Ad mutated at the primary CD4 binding site. (b) Tyrosine phosphorylation analysis of cell lysates (top) and anti-CD3ε (bottom) immunoprecipitates from TK.G4 cells stimulated under the same conditions.
Heterobivalent Antibody Cross-linking of CD3 to CD4, but Not Bivalent Antibody Cross-linking of CD3 Alone, Leads to Signaling and Responses Resembling Agonist Stimulation.
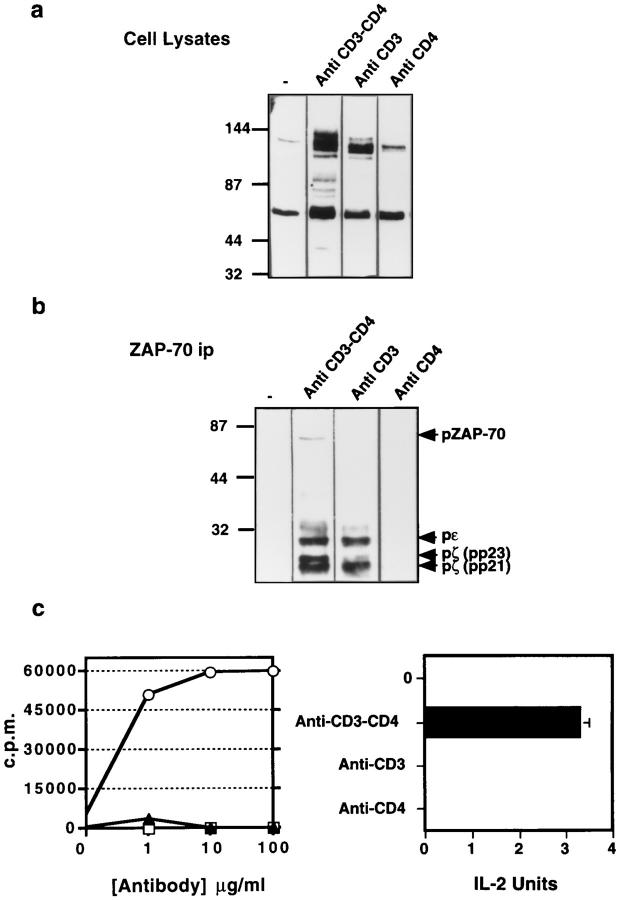
The differences in TCR-mediated signaling and response seen upon TCR engagement with peptide–MHC ligands with or without adequate CD4 coengagement are also observed when activation of T cells with heterobivalent antibodies with specificity for both CD3 and CD4 (anti-CD3–fos × anti-CD4–jun) or with bivalent antibody specific for CD3 alone (anti-CD3–fos) are compared. Immunoblotting of proteins in lysates of T cells exposed to anti-CD3–fos × anti-CD4–jun antibody shows the presence of ligand-induced tyrosine-phosphorylated species of approximately 36, 38, 42–44, 50, 60, 70, 80, 90, 120, 140, and 150 kDa. In contrast, immunoblotting of lysates of T cells exposed to antiCD3–fos antibody only shows induction of tyrosine-phosphorylated 44, 60, 90, and 120 kDa species, the latter being of variable magnitude (Fig. 5 a). When analyzed by blotting of anti-ZAP-70 immunoprecipitates, coengagement of CD3 and CD4 results in a clear induction of tyrosine-phosphorylated p21 and p23 TCR-ζ, CD3ε, and ZAP-70, similar to the pattern seen with agonist peptide–MHC class II ligands (1, 2, 16). Activation of T cells with CD3 engagement alone instead induces a pattern of TCR-associated phosphoproteins resembling that seen using partial agonists/antagonists, with pp21 TCR-ζ predominating, a very low amount of pp23 TCR-ζ, and no detectable phosphorylated ZAP-70 (Fig. 5 b). In some, but not other experiments, a limited amount of pp21 TCR-ζ was formed in response to anti-CD4–jun alone (data not shown), as reported previously (24). Functionally, substantial cell proliferation and IL-2 production is seen upon stimulation with antiCD3–fos × anti-CD4–jun, but not using anti-CD3–fos only or anti-CD4–jun only (Fig. 5 c), in agreement with previous observations that IL-2 production most closely tracks pp23 TCR-ζ accumulation and/or phosphorylation of ZAP-70 (1, 2).
Figure 5.
Biochemical and functional consequences of anti-CD3–fos, anti-CD4–jun, or anti-CD3–fos × anti-CD4–jun bivalent cross-linking. Tyrosine phosphorylation in cloned T cells after CD3 cross-linking, CD3/CD4 cocross-linking, or CD4 cross-linking. T cells (1 × 107 per sample) were stimulated with the 10 μg/ml of antibody in 100 μl of medium for 10 min. Cells were then lysed and a portion of the lysate used for immunoprecipitation with an antiserum against ZAP-70. Both cell lysates (a) and ZAP-70 immunoprecipitates (b) were electrophoresed and immunoblotted using anti-phosphotyrosine antibody. (c) Cell proliferation and IL-2 production by T cells stimulated with soluble anti-CD3– fos, anti-CD4–jun, or anti-CD3–fos × anti-CD4–jun antibodies.
Anergy Induction Accompanies the Loss of IL-2 Production and the Change in Agonist Ligand Signaling Resulting from Anti-CD4 Treatment in Culture.
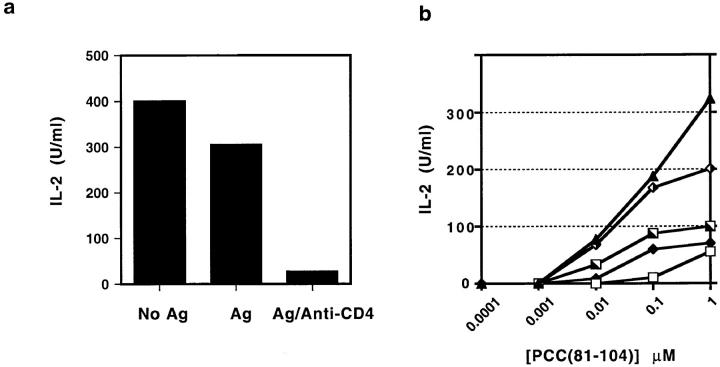
Recently, it has been shown that some partial agonists preferentially induce T cell clonal anergy rather than cytokine secretory activity even when presented by live APC able to provide costimulatory function due to expression of CD80/CD86 (1, 10, 44). The ability of these variant TCR ligands to induce anergy appeared to correlate with their ability to induce an altered pattern of TCR signaling (1). However, our own studies have indicated that the controlling factor in anergy induction is not the pattern of early phosphorylation itself, but the combination of a certain level of TCR signaling in the face of inadequate production of IL-2 due to either agonist exposure without costimulation, or partial agonist exposure even with costimulation (10). The ability of anti-CD4 to selectively inhibit IL-2 production while permitting substantial TCR signaling of the variant pattern suggested that anergy induction might accompany TCR engagement by agonist ligand on costimulatory APC in the face of antiCD4 blockade. This hypothesis would explain published findings showing that coadministration of nondepleting anti-CD4 mAb and antigen can induce a state of antigenspecific tolerance (45, 46). To investigate this possibility, A.E7 cells exposed to PCC(88–104) on I-Ek expressing, ICAM-1+, CD80+ L cells in the presence of anti-CD4 mAb were recovered after 24 h, rested for 7 d, and restimulated by agonist in the absence of anti-CD4. As shown in Fig. 6 A, the presence of anti-CD4 mAbs in the primary challenge of A.E7 T cells with antigenic peptide renders these cells less able to produce IL-2 in a subsequent rechallenge with the same antigenic peptide in the absence of the blocking antibody. The effectiveness of anergy induction by CD4 blockade correlates with the amount of anti-CD4 mAb present in the primary culture (Fig. 6 b). These results further confirm that CD4 blockade mimics the functional effects of TCR partial agonists and also provides a possible explanation for the induction of tolerance using anti-CD4 antibody in vivo.
Figure 6.
Anergy induction by stimulation with wild-type ligand in the presence of anti-CD4 antibody. (a) A.E7 T cells were incubated with I-Ek-expressing L cells without or with PCC(81–104) (100 nM) and antiCD4 mAb (1:200 dilution of supernatant) for 24 h. T cells were then isolated and rested for 7 d. At this point, T cells were restimulated with I-Ek-transfected L cells and PCC(81–104) (1 μM) for 24 h and IL-2 production was measured by ELISA. (b) Effect of variation in the concentration of anti-CD4 mAb in the primary culture on the extent of anergy induction. A. E7 cells were treated as in (a), but using various concentrations of anti-CD4 antibody (1:200, open squares; 1:2,000, closed diamonds; 1:20,000, half-filled squares; 1:200,000, half-filled diamonds; no antiCD4, closed triangles). After rest, each of these cell populations was restimulated as in (a) and IL-2 in the medium measured after 24 h by ELISA.
Discussion
The mechanistic basis of TCR partial agonism and antagonism is still unknown. Our data show that both the signaling events and functional effects characteristic of TCR engagement by variant ligands can be largely reproduced by preventing effective CD4 recruitment during TCR recognition of wild-type ligand. These observations are consistent with the hypothesis that the properties of these altered ligands may be secondary to an inability to support effective TCR–CD4 interaction during ligand recognition. This conclusion is in agreement with previous data showing differential TCR-ζ phosphorylation in CD4+ versus CD4− T cells exposed to altered peptide–MHC class II complexes (47), and also with reports indicating that blockade of coreceptor function enhances TCR antagonism (29), and converts partial agonist ligands into antagonist ligands (30, 31, 48).
CD4 and CD8 bind to monomorphic regions of MHC class II and class I molecules, respectively (49, 50). A variety of experiments indicate that during antigen presentation these proteins interact with the same MHC molecule– peptide complex bound to a clonally distributed TCR (51). This ligand-induced coassociation of the CD4 or CD8 molecule with the TCR complex is analogous to the cytokine or growth factor–induced association of distinct receptor chains (52), and both of the consequences of such heterotypic protein association as described for cytokine receptors has been observed for CD4 or CD8 and the TCR (53–58). Thus, the simultaneous interaction of the TCR and the CD4 or CD8 molecules with a single MHC molecule decreases the dissociation rate of the MHC ligand from the TCR (increases the affinity of binding) (28). In addition, the recruitment of the src kinase lck that is noncovalently associated with the cytoplasmic tail of CD4 or CD8 contributes directly to biochemical events involved in signal transduction, just as apposition of kinases associated with the cytoplasmic regions of cytokine receptors initiates such events (24, 25, 59). Because of these corecognition and cosignaling roles in antigen-specific T cell activation, CD4 and CD8 have been termed coreceptors (60, 61).
We have previously proposed that the reason these coreceptors are not an integral part of the TCR complex arises from the need of the T cell recognition system to discriminate among very structurally similar ligands (related peptides bound to identical MHC molecules) (62). The total pool of such related ligands is very large compared with the number of specific ligands whose recognition is intended to initiate cellular activation. If the TCR had a substantial MHC-related affinity for the peptide–MHC complex, as would be the case if the monomorphic recognition activity of the coreceptor were part of the TCR complex itself, then the mass action of the high total MHC molecule density on APC would constantly initiate T cell responses unless the threshold for activation was set very high. But then it would be difficult to have high sensitivity to specific ligands present at low density. By allowing the TCR to interact first on its own with potential ligands, and then permitting the coreceptor to associate with a preformed TCR–ligand complex, one could set a threshold for discrimination that is dependent on the time it takes for a coreceptor to reach such an occupied TCR on the membrane, in comparison to the dissociation rate of the TCR–ligand complex. Ligands that dissociate more rapidly than the coreceptor can bind the complex would be incapable of being stabilized or of having the coreceptor-associated lck contribute to downstream signaling events. Those that survive until the coreceptor can enter into association, stabilize the binding, and contribute lck to the signaling complex would produce an enhanced or even a different biochemical signal suitable for full T cell activation.
This model is a specific form of the kinetic proofreading model that proposes that, in an analogous way to kinetic proofreading of DNA replication and protein synthesis, there is a time lag between TCR occupancy and the onset of full signaling from the receptor (14). It is consistent with the finding that the density of coreceptor expression may determine susceptibility to TCR antagonism (19, 29–31, 48), recent evidence that the agonist versus antagonist properties of ligands are correlated with their affinities for the TCR (17, 18), and the new evidence we present here from three independent approaches that limiting recruitment of CD4 to the engaged TCR results in a qualitative change in the pattern of early tyrosine phosphorylation events from that typically seen with complete agonists to one resembling that seen with partial agonists/antagonists. Whether the TCR is stimulated using a wild-type peptide– MHC ligand and CD4 availability is reduced by anti-CD4 antibodies or mutation of the CD4 binding site of the class II molecule, or whether the corecruitment of TCR and CD4 is controlled using antibodies of various specificities, we consistently find that TCR engagement alone promotes accumulation primarily of the p21 form of phosphorylated ζ, reduced CD3ε accumulation, and receptor association of ZAP-70 without detectable phosphorylation. In contrast, effective coassociation of the TCR with CD4 leads to formation of both p21- and p23-phosphorylated ζ, strong CD3ε phosphorylation, and phosphorylation of recruited ZAP-70. These different patterns of signaling are accompanied by distinct functional responses that match those associated with partial agonist recognition and with agonist recognition, respectively. Although these data do not directly demonstrate that a limitation in coreceptor recruitment is the proximal cause of altered signaling in the case of variant peptide–MHC molecule ligands, when the available data on TCR affinity are taken together with the present results, they make a strong case that this is one likely origin of the distinct pattern of signaling that accompanies exposure of T cells to these modified ligands. Moreover, the present results emphasize the concept that the pharmacologic properties of a TCR ligand are defined not by its absolute affinity of interaction with the TCR, but by its relative affinity in the context of a variable time to recruitment of functional coreceptors whose surface density can vary from cell to cell.
A striking feature of the data derived from antibodyblocking experiments is the very rapid switch from the agonist to variant ligand signaling patterns with low amounts of blocking antibody. We have not yet specifically measured the fraction of CD4 bound by antibody under nonsaturating conditions, but clearly only a modest proportion of the CD4 molecules need to be blocked for a switch in the signaling pattern. These data agree with functional results showing that only a small change in coreceptor expression (less than a two to threefold shift) can alter the pharmacologic properties of peptide–MHC molecule ligands in vitro (29), and also can switch the pattern of thymocyte selection from positive to negative, or vice versa, in vivo (63). In the context of the kinetic model proposed above for coreceptor function, these data suggest that many T cells may operate at the limit of the system, such that even a modest decrease in coreceptor availability (a small delay in recruitment time) is sufficient to change stimulatory agonist recognition to the partial agonist pattern and an altered response. This high sensitivity to antibody blocking is also consistent with the hypothesis that CD4 does not function as a monomer, but as an oligomer (36, 64, 65), which would amplify the consequences of coreceptor blockade.
In terms of thymocyte selection, this concept of a critical role for coreceptor density in the signaling properties of self-ligands can help explain the results obtained in organ culture analysis of the peptide requirements for positive selection. Data obtained from fetal thymic organ cultures using β2m−/− donors expressing an OVA-specific transgenic TCR clearly show that no concentration of a potent agonist can induce net positive selection, and that if an antagonist or a partial agonist for the mature T cell bearing this same TCR is offered to the developing thymocytes, the cells that emerge as CD8+ mature cells cannot respond to this same ligand (66, 67). However, in another transgenic model an agonist for the mature T cell source of the TCR was shown to be capable of promoting differentiation to the CD8+ state (68, 69). Nonetheless, these cells also do not show responses to the same peptide used to promote their differentiation, even though this is an agonist ligand for the donor T cell (70). In the OVA case, the lack of response correlates with a small but clearcut reduction in mean CD8 expression by the effectively selected cells, and this has been shown to be associated with a loss of partial agonist function for the selecting ligand (29). One might predict that with the other system, a similar effect is occurring such that among the thymocyte precursors with a wider range of coreceptor expression than on the TCR donor clone, those cells with the correct CD8 density to signal primarily in the antagonist/partial agonist mode rather than agonist mode are the ones that avoid negative selection and mature, emerging as unresponsive to the same ligand that has agonist properties for the donor clone. Thus, rather than operating in a strict avidity mode, with just the intensity of signaling controlling thymocyte selection, these other data could be reconciled with the OVA results to argue that successful selection demands a match of ligand–TCR affinity and thymocyte coreceptor expression level that results in the altered signaling pattern characteristic of variant ligands. Interactions outside this range that allow very effective coreceptor recruitment and support agonist pattern signaling would instead lead to negative selection.
Several investigators have shown that antigen administration in the presence of nondepleting anti-coreceptor antibodies induces the development of an antigen-specific state of tolerance that does not appear to result from deletion of the antigen-specific T cells (49, 71–75). Here, we provide a possible explanation for these results by showing that the blocking of coreceptors is distinct in its effects from decreasing the density of ligand, with the former producing a change in signaling that can have a selective effect on IL-2 secretion despite continued intracellular biochemical changes due to receptor ligation. This combination of partial TCR signaling without adequate IL-2 production has been shown to induce anergy in established The 1 clones when using altered ligands (10); here, we demonstrate that wild-type ligands for the TCR offered in the presence of blocking anti-CD4 antibody also induce an anergic state in these clones. If such a phenomenon occurs with primary T lymphocytes in vivo, they would be likely candidates for the suppressors cells seen in antibody plus antigen-treated animals by Waldmann and associates (74, 76), as they would fail to generate effector function but could either compete for growth factors with potential effectors in their environment, or secrete a subset of cytokines that deviate the response away from that being measured.
In summary, we have shown that interference with CD4 recruitment to an engaged TCR complex leads to a change in early TCR-mediated signaling, with conversion from an agonist to a partial agonist-like pattern. As a consequence, the response to what was initially an agonist ligand when CD4 is fully available becomes a partial agonist response, with a change in the pattern of cytokine production and/or the induction of T cell anergy. These findings support the concept that the relative affinity of a ligand for the TCR and the density of coreceptor together define the functional properties of TCR ligands in both the thymus and periphery, and provide additional insight into the possible molecular basis of variant ligand function.
Footnotes
This work was partially supported by the Medical Research Council of Canada, the Kidney Foundation of Canada, and PO1 AI29531. J. Smith was supported by HL 07605.
References
- 1.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of ZAP-70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Madrenas J, Wange RL, Wang JL, Isakov NA, Samelson LE, Germain RN. ζ phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science (Wash DC) 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 3.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature (Lond) 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 4.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science (Wash DC) 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 5.De Magistris M, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog– major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 6.Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity. 1995;2:373–380. doi: 10.1016/1074-7613(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 7.Racioppi L, Ronchese F, Matis LA, Germain RN. Peptide–major histocompatibility complex class II complexes with mixed agonist/antagonist properties provide evidence for ligand-related differences in T cell receptor–dependent intracellular signaling. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature (Lond) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 9.Ruppert J, Alexander J, Snoke K, Coggeshall M, Herbert E, McKenzie D, Grey HM, Sette A. Effect of T-cell receptor antagonism on interaction between T cells and antigen-presenting cells and on T-cell signaling events. Proc Natl Acad Sci USA. 1993;90:2671–2675. doi: 10.1073/pnas.90.7.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madrenas, J., R.H. Schwartz, and R.N. Germain. 1996. IL-2 and not TCR signaling patterns control anergy induction by agonists or partial agonists. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 11.Evavold BD, Sloan-Lancaster J, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 12.Madrenas J, Germain RN. Variant TCR ligands: new insights into the molecular basis of antigen-dependent signal transduction and T cell activation. Semin Immunol. 1996;8:83–101. doi: 10.1006/smim.1996.0011. [DOI] [PubMed] [Google Scholar]
- 13.Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 14.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConnell HM, Wada HG, Arimilli S, Fok KS, Nag B. Stimulation of T cells by antigen-presenting cells is kinetically controlled by antigenic peptide binding to major histocompatibility complex class II molecules. Proc Natl Acad Sci USA. 1995;92:2750–2754. doi: 10.1073/pnas.92.7.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis e Sousa C, Levine EH, Germain RN. Partial signaling by CD8+T cells in response to antagonist ligands. J Exp Med. 1996;184:149–157. doi: 10.1084/jem.184.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T-cell-receptor affinity and thymocyte positive selection. Nature (Lond) 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 18.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:51–59. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoon ST, Dianzani U, Bottomly K, Janeway CA., Jr Both high and low avidity antibodies to the T cell receptor can have agonist or antagonist activity. Immunity. 1994;1:563–569. doi: 10.1016/1074-7613(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 20.Janeway CA., Jr Ligands for the T-cell receptor: hard times for avidity models. Immunol Today. 1995;16:223–225. doi: 10.1016/0167-5699(95)80163-4. [DOI] [PubMed] [Google Scholar]
- 21.Ehrich EW, Devaux B, Rock EP, Jorgensen JL, Davis MM, Chien Y-h. T cell receptor interaction with peptide/major histocompatibility complex (MHC) and superantigen/MHC ligands is dominated by antigen. J Exp Med. 1993;178:713–722. doi: 10.1084/jem.178.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janeway CA., Jr T cell receptor signaling: high fives or hand clasps? . Curr Biol. 1992;2:591–593. doi: 10.1016/0960-9822(92)90163-5. [DOI] [PubMed] [Google Scholar]
- 23.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 24.Veillette A, Bookman MA, Horak EM, Samelson LE, Bolen JB. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck . Nature (Lond) 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 25.Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck . Nature (Lond) 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 26.Dianzani U, Shaw A, al-Ramadi BK, Kubo RT, Janeway CJ., Jr Physical association of CD4 with the T cell receptor. J Immunol. 1992;148:678–688. [PubMed] [Google Scholar]
- 27.Caron L, Abraham N, Pawson T, Veillette A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck . Mol Cell Biol. 1992;12:2720–2729. doi: 10.1128/mcb.12.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor–ligand interactions on living cytotoxic T lymphocytes. Nature (Lond) 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 29.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature (Lond) 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannie MD, Rosser JM, White GA. Autologous rat myelin basic protein is a partial agonist that is converted into a full antagonist upon blockade of CD4. Evidence for the integration of efficacious and nonefficacious signals during T cell antigen recognition. J Immunol. 1995;154:2642–2654. [PubMed] [Google Scholar]
- 31.Vidal K, Hsu BL, Williams CB, Allen PM. Endogenous altered peptide ligands can affect peripheral T cell responses. J Exp Med. 1996;183:1311–1321. doi: 10.1084/jem.183.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Matis LA, Longo DL, Hedrick SM, Hannum C, Margoliash E, Schwartz RH. Clonal analysis of the major histocompatibility complex restriction and the fine specificity of antigen recognition in the T cell proliferative response to cytochrome C. J Immunol. 1983;130:1527–1535. [PubMed] [Google Scholar]
- 34.Berkower I, Kawamura H, Matis LA, Berzofsky JA. T cell clones to two major T cell epitopes of myoglobin: effect of I-A/I-E restriction on epitope dominance. J Immunol. 1985;135:2628–2634. [PubMed] [Google Scholar]
- 35.Ronchese F, Schwartz RH, Germain RN. Functionally distinct subsites on a class II major histocompatibility complex molecule. Nature (Lond) 1987;329:254–256. doi: 10.1038/329254a0. [DOI] [PubMed] [Google Scholar]
- 36.König R, Shen X, Germain RN. Involvement of both major histocompatibility complex class II α and β chains in CD4 function indicates a role for ordered oligomerization in T cell activation. J Exp Med. 1995;182:779–787. doi: 10.1084/jem.182.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 38.Ozato K, Mayer N, Sachs DH. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980;124:533–540. [PubMed] [Google Scholar]
- 39.Havran WL, Poenie M, Kimura J, Tsien R, Weiss A, Allison JP. Expression and function of the CD3antigen receptor on murine CD4+8+ thymocytes. Nature (Lond) 1987;330:170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- 40.Kostelny SA, Cole MS, Tso JY. Formation of a bispecific antibody by the use of leucine zippers. J Immunol. 1992;148:1547–1553. [PubMed] [Google Scholar]
- 41.Janeway CA., Jr The T cell receptor as a multicomponent signaling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 42.König R, Huang LY, Germain RN. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature (Lond) 1992;356:796–798. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 43.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannwarth W, Guardiola J, Sinigaglia F. Identification of a CD4 binding site on the β2 domain of HLA-DR molecules. Nature (Lond) 1992;356:799–801. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 44.Tsitoura DC, Holter W, Cerwenka A, Gelder CM, Lamb JR. Induction of anergy in human Th0 cells by stimulation with altered T cell antigen receptor ligands. J Immunol. 1996;156:2801–2808. [PubMed] [Google Scholar]
- 45.Alters SE, Song HK, Fathman CG. Evidence that clonal anergy is induced in thymic migrant cells after anti-CD4-mediated transplantation tolerance. Transplantation. 1993;56:633–638. doi: 10.1097/00007890-199309000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Waldmann H, Cobbold S. The use of monoclonal antibodies to achieve immunological tolerance. Immunol Today. 1993;14:247–251. doi: 10.1016/0167-5699(93)90040-R. [DOI] [PubMed] [Google Scholar]
- 47.Vignali DA, Strominger JL. Amino acid residues that flank core peptide epitopes and the extracellular domains of CD4 modulate differential signaling through the T cell receptor. J Exp Med. 1994;179:1945–1956. doi: 10.1084/jem.179.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabinowitz JD, Beeson C, Wülfing C, Tate K, Allen PM, Davis MM, McConnell HM. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5:125–135. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 49.Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature (Lond) 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 50.Norment AM, Salter RD, Parham P, Engelhard VH, Littman DR. Cell–cell adhesion mediated by CD8 and MHC class I molecules. Nature (Lond) 1988;336:79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- 51.Saizawa K, Rojo J, Janeway CA., Jr Evidence for a physical association of CD4 and the CD3:α:β T-cell receptor. Nature (Lond) 1987;328:260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- 52.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 53.Tite JP, Sloan A, Janeway CA., Jr The role of L3T4 in T cell activation: L3T4 may be both an Ia-binding protein and a receptor that transduces a negative signal. J Mol Cell Immunol. 1986;2:179–190. [PubMed] [Google Scholar]
- 54.Saizawa K, Haque S, Jones B, Rojo J, Tite JP, Kaye J, Janeway CA., Jr The L3T4 molecule is part of the helper T-cell antigen/Ia recognition complex. Ann Inst Pasteur Immunol. 1987;138:138–143. doi: 10.1016/s0769-2625(87)80105-8. [DOI] [PubMed] [Google Scholar]
- 55.McCluskey J, Singer A, Germain RN, Margulies DH. The role of CD4/L3T4 in T-lymphocyte function. Ann Inst Pasteur Immunol. 1987;138:150–157. doi: 10.1016/s0769-2625(87)80108-3. [DOI] [PubMed] [Google Scholar]
- 56.Janeway CA, Jr, Haque S, Smith LA, Saizawa K. The role of the murine L3T4 molecule in T cell activation: differential effects of anti-L3T4 on activation by monoclonal anti-receptor antibodies. J Mol Cell Immunol. 1987;3:121–131. [PubMed] [Google Scholar]
- 57.Anderson P, Blue ML, Schlossman SF. Comodulation of CD3 and CD4. Evidence for a specific association between CD4 and approximately 5% of the CD3:T cell receptor complexes on helper T lymphocytes. J Immunol. 1988;140:1732–1737. [PubMed] [Google Scholar]
- 58.Eichmann K, Jonsson JI, Falk I, Emmrich F. Effective activation of resting mouse T lymphocytes by crosslinking submitogenic concentrations of the T cell antigen receptor with either Lyt-2 or L3T4. Eur J Immunol. 1987;17:643–650. doi: 10.1002/eji.1830170510. [DOI] [PubMed] [Google Scholar]
- 59.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck . Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 60.Janeway CA., Jr T-cell development. Accessories or coreceptors? . Nature (Lond) 1988;335:208–210. doi: 10.1038/335208a0. [DOI] [PubMed] [Google Scholar]
- 61.Janeway CA., Jr The co-receptor function of CD4. Semin Immunol. 1991;3:153–160. [PubMed] [Google Scholar]
- 62.König R, Fleury S, Germain RN. The structural basis of CD4–MHC class II interactions: coreceptor contributions to T cell receptor antigen recognition and oligomerization-dependent signal transduction. Curr Topics Microbiol Immunol. 1996;205:19–46. doi: 10.1007/978-3-642-79798-9_2. [DOI] [PubMed] [Google Scholar]
- 63.Robey EA, Ramsdell F, Kioussis D, Sha W, Loh D, Axel R, Fowlkes BJ. The level of CD8 expression can determine the outcome of thymic selection. Cell. 1992;69:1089–1096. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- 64.Sakihama T, Smolyar A, Reinherz EL. Oligomerization of CD4 is required for stable binding to class II major histocompatibility complex proteins but not for interaction with human immunodeficiency virus gp120. Proc Natl Acad Sci USA. 1995;92:6444–6448. doi: 10.1073/pnas.92.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakihama T, Smolyar A, Reinherz EL. Molecular recognition of antigen involves lattice formation between CD4, MHC class II and TCR molecules. Immunol Today. 1995;16:581–587. doi: 10.1016/0167-5699(95)80081-6. [DOI] [PubMed] [Google Scholar]
- 66.Hogquist KA, Jameson SC, Bevan MJ. The ligand for positive selection of T lymphocytes in the thymus. Curr Opin Immunol. 1994;6:273–278. doi: 10.1016/0952-7915(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 67.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 68.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 69.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science (Wash DC) 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 70.Rothenberg E. Developmental biology of lymphocytes. The Immunologist. 1995;3:172–175. [Google Scholar]
- 71.Alters SE, Shizuru JA, Ackerman J, Grossman D, Seydel KB, Fathman CG. Anti-CD4 mediates clonal anergy during transplantation tolerance induction. J Exp Med. 1991;173:491–494. doi: 10.1084/jem.173.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pearson TC, Madsen JC, Larsen CP, Morris PJ, Wood KJ. Induction of transplantation tolerance in adults using donor antigen and anti-CD4 monoclonal antibody. Transplantation. 1992;54:475–483. doi: 10.1097/00007890-199209000-00018. [DOI] [PubMed] [Google Scholar]
- 73.Shizuru JA, Alters SE, Fathman CG. AntiCD4 monoclonal antibodies in therapy: creation of nonclassical tolerance in the adult. Immunol Rev. 1992;129:105–130. doi: 10.1111/j.1600-065x.1992.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 74.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science (Wash DC) 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 75.Darby CR, Bushell A, Morris PJ, Wood KJ. Nondepleting anti-CD4 antibodies in transplantation. Evidence that modulation is far less effective than prolonged CD4 blockade. Transplantation. 1994;57:1419–1426. [PubMed] [Google Scholar]
- 76.Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994;24:2383–2392. doi: 10.1002/eji.1830241019. [DOI] [PubMed] [Google Scholar]