Abstract
A successful T cell immune response has two major products: effector T cells which directly or indirectly remove the antigens, and memory T cells, which allow a faster and more efficient recall response when challenged by related antigens. An important issue is whether costimulatory molecules on the antigen-presenting cells are involved in determining whether T cells will differentiate into effector or memory cells after antigenic stimulation. To address this issue, we have produced mice with targeted mutations of either the heat-stable antigen (HSA), or both HSA and CD28. We show that CD28/B7 and HSA provide two alternative costimulatory pathways for induction of immunological memory to influenza virus. Furthermore, our results revealed that B7 is essential for the generation of effector T cells from either naive or memory T cells, while HSA is not necessary for the generation of effector T cells. Our results demonstrate that the induction of memory T cells and effector T cells can utilize distinct costimulatory molecules. These results have important implications on lineage relationship between effector and memory T cells.
Effector and memory T cells differ in two important aspects. First, by definition, effector T cells participate in antigen clearance without further differentiation, while memory T cells have no immediate effector function. Second, effector T cells are short-lived: anti-viral CTL effectors are detectable in a time span of 1–2 wk after infection (1), and the majority, if not all, of the effector T cells undergo programmed cell death (2–4). Memory CTL, in contrast, appear to be long-lived in the absence of intentional antigenic stimulation (5–7), although it is still debated whether a low level stimulation via the T cell receptor is required for maintenance of immunological memory (8). These differences raise an interesting possibility that the conditions required for the induction of these two functional T cell types may be different.
Induction of significant clonal expansion of naive T cells requires two types of biological signals (9–13). One is delivered by interaction of the T cell receptor with its ligand. The other, the costimulatory signal, is delivered by a variety of molecules such as the B7 family members B7-1 (14– 17) and B7-2 (18–22) which interact both with CD28 (14) and with CTLA4 at a higher affinity (23) on T cells, the heat-stable antigen (HSA)1 (24–30), CD44 (31), and intercellular adhesion molecule-1 (32, 33). Given the fact that effector cells and memory cells can be induced by the same antigen, we were interested in asking whether distinct costimulatory molecules are utilized for the induction of these two populations of cells.
Most in vitro studies suggest a major role for costimulatory molecules, particularly that of the B7 family members, B7-1 and B7-2, in the induction of effector T cells from naive T cells in vitro (34, 35). However, it is not clear whether B7 is required for the induction of effector T cells from memory T cells. Whereas the induction of effector T cells in vivo has not been systematically studied with regards to the requirement for costimulatory molecules, potent effects of CTLA4-Ig, a fusion protein with a high affinity for B7-1 and B7-2 (36), in blocking the rejection of allogeneic (37) or xenogeneic grafts (38) supports a major role of B7 in the induction of effector T cells in vivo. However, it is unknown whether induction of memory T cells requires costimulation. Several recent studies suggest that T cell priming can be achieved in the absence of a B7:CD28/CTLA4 interaction (39–41). Some of the studies were interpreted as evidence that antigen alone, if appropriately localized, would be sufficient to prime T cells (41). However, this interpretation contradicts a large collection of studies (10, 11, 42, 43) which demonstrate that engagement of T cell receptors in the absence of costimulation leads to the induction of T cell tolerance rather than immunity. An alternative possibility is that other costimulatory molecules, such as HSA, are sufficient to induce immunological memory from naive T cells.
HSA is a GPI-anchored protein (44–46), and it is expressed on multiple lineages of hemapoietic and neuronal origin (47, 48). It was implicated as a costimulatory molecule when a mAb which blocks T cell proliferation and induces T cell unresponsiveness in vitro, was shown to bind HSA by expression cloning (25). Indeed, gene-transfection experiments indicated that HSA can transfer costimulatory activity to CHO cells (25). In addition, accumulating evidence supports a role of HSA in costimulating T cells in a variety of experimental models involving several different types of antigen-presenting cells (APC). On B cells, HSA and B7 appear to act synergistically in inducing clonal expansion of T cells (24–26, 29). HSA expressed on Langerhans cells is involved in both the induction of clonal expansion and prevention of clonal anergy of Th1 clones (27). More recently, HSA was shown to be induced during macrophage phagocytosis and to be critical for in vitro priming of naive CD8 T cells (30). Furthermore, tumors transfected with HSA induce better priming of anti-tumor CTL responses (28).
To address the requirement for costimulatory molecules in the induction of effector and memory CTL, we have produced mice with a targeted mutation of the HSA gene. We have also bred HSA-deficient mice with CD28-deficient mice (39) and thereby generated mice deficient in both HSA and CD28. We have chosen influenza virus as the antigen because both effector and memory CTL responses can be easily detected in vivo (1). Our results demonstrate that induction of effector and memory CTL utilizes distinct costimulatory molecules.
Materials and Methods
Antibodies, Cell Lines, Synthetic Peptide and Experimental Animals
Anti-B7-1 mAb 3A12 (18), anti-B7-2 mAb GL-1 (20), antiCD28 mAb 37N (41), and anti-HSA mAb antibody 20C9 (25) were used in these studies. All mAbs were purified from hybridoma supernatants using a protein G column. A mixture of affinity-purified normal IgG from rat and hamsters were used as control. The thymoma cell line EL4 (H-2b), and mastocytomas cell line P815 (H-2d) were used as target cells for cytotoxicity assays. As antigen, we synthesized a peptide according to the amino acid sequence of a fragment of influenza virus A/JAP/57 nucleoprotein (NP365-380), which was originally identified by Townsend as the major antigenic epitope of this virus in H-2b mice. Wildtype (WT) C57BL6/j mice were purchased from Jackson Laboratories (Bar Harbor, ME). CD28-deficient mice (39), backcrossed to C57BL/6j for six generations, were kindly provided by Dr. Tak Mak (University of Toronto, Toronto, Canada).
Production of Mice with a Targeted Mutation of the HSA or both HSA and CD28
Mice Homozygous for a Disrupted HSA Gene.
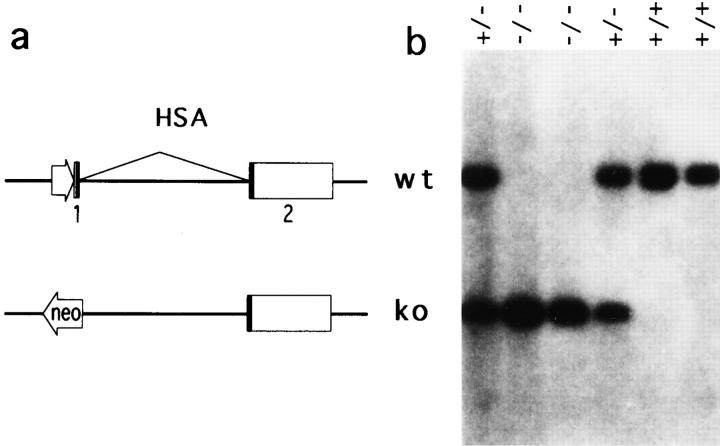
The disruption of the HSA gene in C57BL/6 ES line BL/6-III (50) was achieved by replacement of the HSA promotor and the first exon with a neomycin-resistance expression cassette. Southern blot analysis of HSA-genotypes was performed using the DNA isolated from mouse tail as described (51). The Pvu II fragment located between nucleotides −710 and −1790 relative to the transcriptional start site of the HSA gene was used as a hybridization probe.
Mice Deficient for both HSA and CD28.
A male CD28-deficient mouse (a mixture of 129 and C57BL/6 backgrounds) was mated to female HSA-deficient mice (strain C57BL/6). F1 offspring were mated to HSA-deficient mice (also C57BL/6) and the resulting HSA-deficient, CD28 heterozygote offspring were interbred to generate HSA/CD28 double deficient mice.
Immunization and Antibody Treatment
C57BL/6j mice (WT) and mice with targeted mutations of HSA and/or CD28 genes were injected with 1,000 HAU of influenza virus A/JAP intravenously (i.v.) or intraperitoneally (i.p.). In some experiments, these mice were injected with either a mixture of normal hamster and rat Ig or a mixture of anti-B7-1 and anti-B7-2 at the doses and intervals given in the figure legends. Spleen cells were harvested after immunization and antibody treatments. The cytotoxicity of the freshly isolated spleen cells were defined as the primary CTL response.
Evaluation of Memory CTL Responses
Three different assays were used to evaluate the activity of memory T cells.
Recall Response in 96-Well Microplate Culture without Exogenous Growth Factors.
Varying numbers of A/JAP-primed spleen cells were restimulated in vitro with irradiated A/JAP-infected syngeneic spleen cells (3 × 104/well) for 5 d at 37°C in Click's EHAA medium containing 5% FCS. At the end of culturing, the cells were washed twice with medium, the 51Cr-labeled target cells were added and CTL activity was determined by 51Cr-release.
Bulk Culture.
When CD28-deficient spleen cells are used, the recall CTL response in microplate culture is usually very weak, so the priming of CTL in experiments involving CD28-KO T cells were evaluated in bulk culture. In brief, pooled spleen cells from groups of 2–3 mice were harvested on day 7 after i.v. or i.p. injection of A/JAP (1,000 HAU) and were restimulated with A/JAPinfected (1,000 HAU/12 × 106 cells, 37°C,1 h), irradiated syngeneic spleen cells for 5 d (Responder: stimulator ratio 5:1, with responder cells at a density of 106/ml). At the end of culturing, viable cells were harvested and the cytotoxicity of these cells was determined by a 6 h 51Cr-release assay.
Limiting Dilution Analysis.
Spleen cells were harvested from either naive mice or mice that have been immunized with A/JAP virus 5–7 wk previously. Graded numbers of spleen cells (pooled from 2 mice per group, 24 wells replicates at each cell density) were stimulated with A/JAP-infected, irradiated spleen cells. Supernatants from PMA-activated EL4 cells were added at the beginning of the culture at a final concentration of 1%. After 6 d of culture, the plates were washed once with medium. 51Cr-labeled, NP366-374-pulsed EL4 cells were added at 104/well. The wells that have given equal or greater than mean plus 3× SD of medium release were scored as positive. The precursor frequency is calculated based on Poisson distribution.
Evaluation of Effector T Cells
In brief, syngeneic H-2b EL4 cells were pulsed with (EL4-NP) or without (EL4) 10 μg/ml of synthetic peptides corresponding to AA 365–380 of influenza virus A/JAP nucleoprotein and labeled with 51Cr for 1 h at 37°C. The labeled target cells were then incubated with the effector cells for 6 h, and the released 51Cr in the supernatants were determined. The specific lysis percentages were calculated by the following formula:

Spleen cells freshly isolated from the animals 6–8 d after viral infection were defined as primary effectors, and the CTL generated from primed spleen cells were defined as secondary effectors.
Results
Production of Mice Deficient for HSA and for both HSA and CD28.
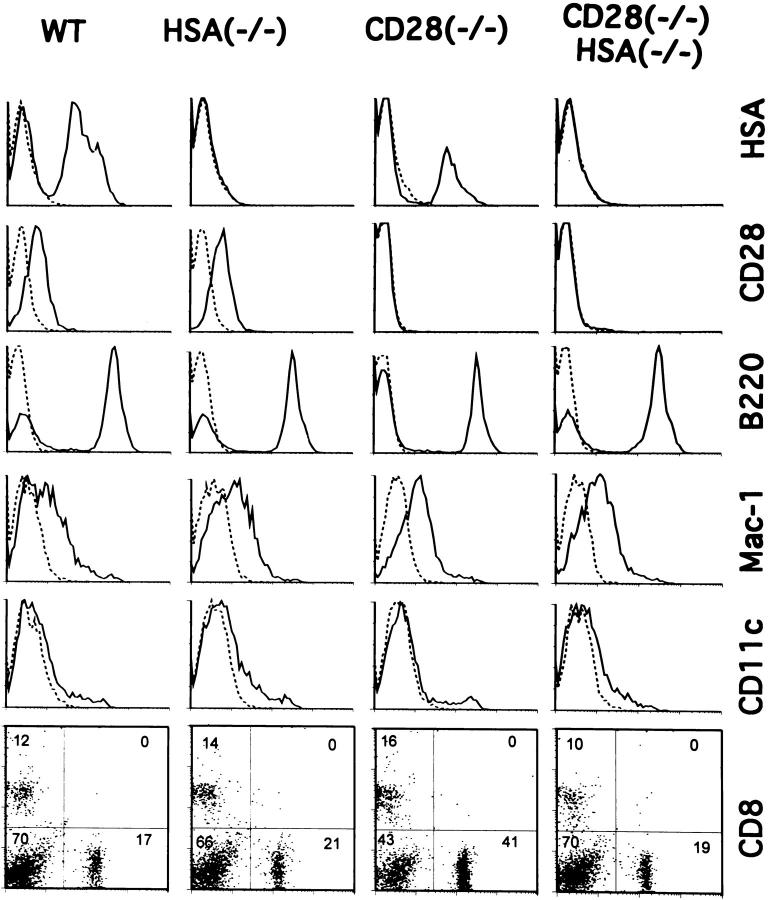
The disruption of the gene for the HSA in C57BL6ES line BL/6-III (50) was achieved by replacing the HSA promotor and the first exon with a neomycin-resistance expression cassette (Fig. 1 a). Except for a somewhat reduced litter size, the mice homozygous for the targeted mutation (HSA-KO) (Fig. 1 b) are indistinguishable from WT control in a conventional mouse facility. We have mated HSA-deficient mice with previously developed CD28deficient mice and generated mice which are deficient in both HSA and CD28. As shown in Fig. 2, normal numbers of CD4 and CD8 T cells are generated in mice deficient for either HSA and/or CD28. Furthermore, the major types of APC, such as B cells, macrophages, and dendritic cells, are produced in normal numbers in all mutant mice. Thus, these mice can be used for studying the role of costimulatory molecules in the generation effector and memory T cells.
Figure 1.
Production of mice homozygous for a disrupted HSA gene. (a) The disruption of the HSA gene in C57BL/6 ES line BL/6-III was achieved by replacement of the HSA promotor and the first exon with a neomycin-resistance expression cassette. The coding portions of both HSA exons are depicted by filled boxes. (b) Southern blot analysis of HSA genotypes using a Pvu II fragment located between nucleotides −710, and −1790 relative to the transcriptional start site of the HSA gene as a hybridization probe.
Figure 2.
Mice with targeted mutations of HSA and/or CD28 produce normal numbers of T cells, B cells and APC. Mouse spleen cells were analyzed by flow cytometry after staining with either anti-HSA (20C9), anti-CD28 (37N), anti-Mac-1 (TIB128), anti-dendritic cell (HB224), FITC-labeled anti-CD4, and phycoerythrin-labeled anti-CD8 mAbs. CD4, CD8, CD28, B220, and HSA typing was done with freshly isolated spleen cells in pools from three mice. Expression of CD28 on gated T cells is presented. Macrophage and dendritic cells were determined using low-density spleen cells, enriched by centrifugation over a 55% Percoll medium. The genotypes and antibodies used are marked on the panels.
B7, but not HSA, Is Required for the Induction of Effector T Cells from Naive T Cells.
C57BL6/j mice infected with influenza virus mount a primary CTL response which is detectable on day 5, which peaks at day 7 or 8, and disappears within 2 wk after infection (1). In A/JAP (H2N2)-infected H-2b mice, the major antigenic epitope is NP365-380 of A/JAP virus (52); the minimal peptide was later identified as NP366-374 (53). We therefore used NP365-380-pulsed EL4 cells to detect the primary CTL response.
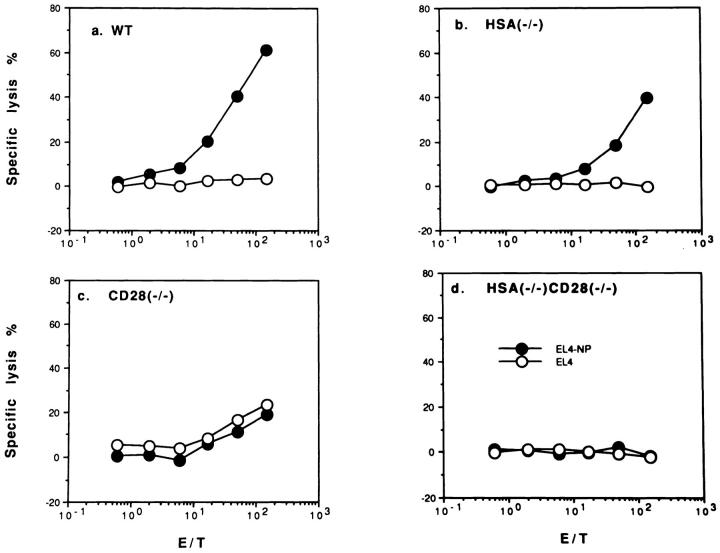
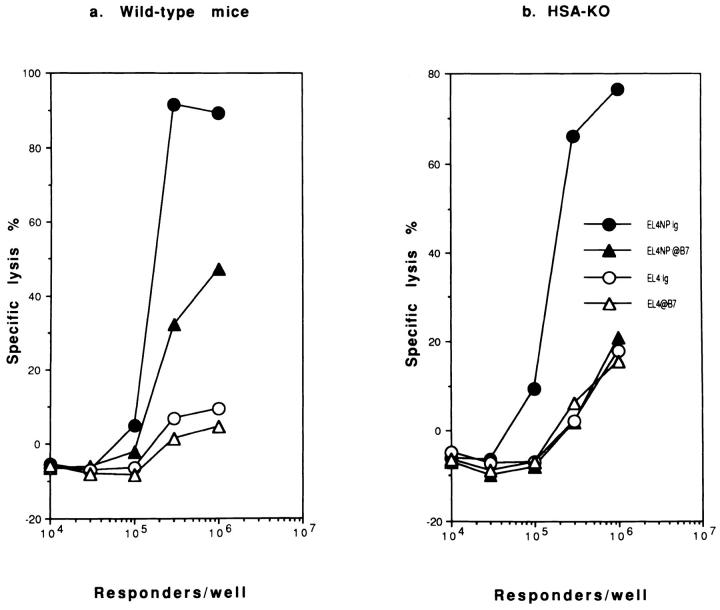
As shown in Fig. 3, WT and HSA-deficient mice mount a significant primary CTL response against NP-peptidepulsed syngeneic EL4 target cells, but not against unpulsed EL-4, (Fig. 3, a and b), or peptide-pulsed allogeneic P815 (H-2d) targets (data not shown). The kinetics of the CTL response is similar in all mice (data not shown). Although in this experiment, the primary CTL response in HSA- deficient mice is somewhat lower than WT mice, in other experiments, HSA-KO mice appear to mount a higher primary CTL response (data not shown). Thus HSA is not required for the generation of primary CTL. In contrast, no virus-specific primary CTL response is detected in mice deficient for either CD28, or both CD28 and HSA, although in some experiments, a significant nonspecific cytotoxicity was detected in CD28-deficient mice, most likely due to NK cells. This lack of CTL response was not due to a change in the kinetics, since we have been unable to detect primary responses against influenza virus between day 3 and day 14, in mice with a targeted mutation of CD28 (data not shown). Because CD28 is the major receptor for the costimulatory molecules B7-1/2, these results strongly suggest that B7 is necessary for the induction of primary CTL. This is more directly demonstrated by the results in Fig. 4, which show that a mixture of anti-B7-1/B7-2 completely eliminates the primary anti-influenza NP CTL response in both WT and HSA-deficient mice. In contrast, anti-HSA mAb 20C9 does not block primary effector CTL responses (data not shown). These results also demonstrate that anti-B7 mAbs efficiently block the function of B7 in vivo.
Figure 3.
Primary in vivo CTL response against influenza virus in WT mice (a), and mice with a targeted mutation of HSA (b), CD28 (c), or of both HSA and CD28 (d). Spleen cells were harvested from mice on day 7 after A/JAP-infection. The CTL activities were tested on either peptide (AA365-380 of the nucleoprotein from A/JAP virus)- pulsed (EL4-NP), or unpulsed EL4 cells (EL4). Representatives of three independent experiments using pooled spleen cells from 2–4 mice in each group are shown.
Figure 4.
Anti-B7 mAbs completely block the production of effector CTL from naive T cells in both WT mice (a) or HSA-KO (b) mice. WT and mutant mice were infected with 1,000 HAU/mouse of influenza virus A/JAP by intraperitoneal injection on day 0. The mice were injected with either a mixture of normal rat and hamster IgG, or anti-B7-1 + anti-B7-2 mAbs (3A12+GL1) on days −1, 0, +1, at a dose of 100 μg/ mouse/injection. Data presented are representative of three experiments using pooled spleen cells from 2–3 mice per group.
Two Costimulatory Pathways for Rapid Priming of Recall CTL Response In Vitro.
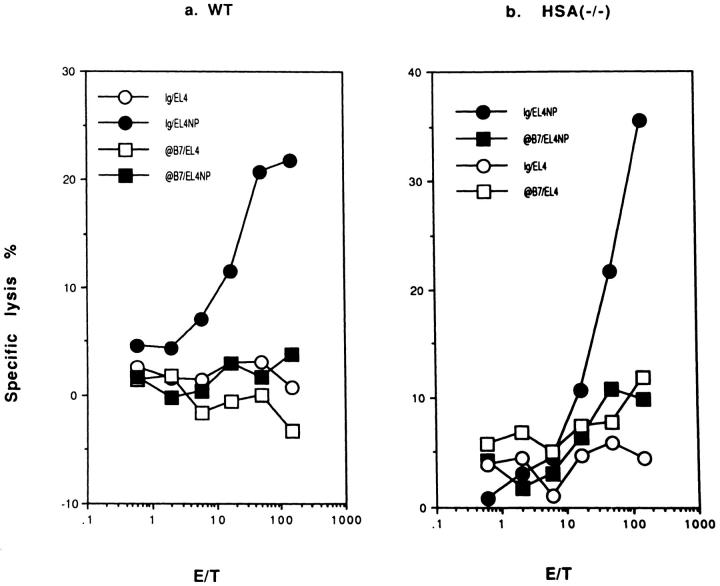
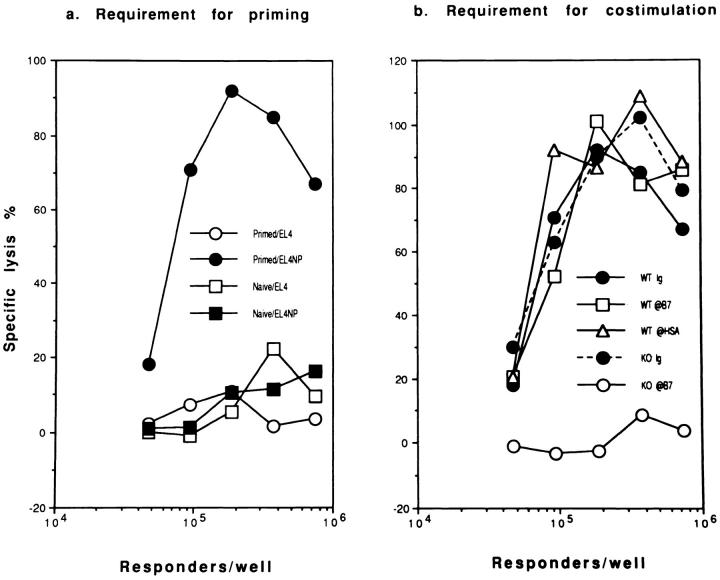
Infection of mice with influenza virus primes a recall CTL response in vitro, detectable as early as 48 h after infection (our unpublished results). To test the requirement for costimulatory molecules in the priming of the recall CTL response, we infected WT and HSA-KO mice with influenza virus either in the presence or absence of antibodies to B7 or HSA. After 8 d, spleen cells were restimulated in vitro for 5–6 d with influenza virus-infected syngeneic spleen cells in the absence of mAbs and CTL activity was measured. Fig. 5 a shows that the recall response requires in vivo priming. In the WT mice, the recall responses were not affected by treatment with a mixture of anti-B7-1 and anti-B7-2 antibodies during priming (Fig. 5 b). In contrast, a mixture of anti-B7-1/B7-2 totally abolished the priming in the HSA-KO mice (Fig. 5 b). The efficacy of the anti-B7 mAbs in vivo is confirmed because such treatment completely eliminated primary CTL response in vivo in both WT and HSA-deficient mice (Fig. 4). Thus, costimulation by either B7 or HSA is required for the in vivo priming of the recall CTL response.
Figure 5.
Requirement of costimulatory molecules for the generation of primed T cells assayed at 8 d after priming: microplate culture in the absence of exogenous cytokines. (a) Anti-viral CTL of in vitro-stimulated spleen cells from either naive or A/JAPprimed mice. (b) Requirement of B7 family members for the generation of primed T cells. WT and HSA-KO mice were infected with 1,000 HAU/mouse of influenza virus A/JAP by intraperitoneal injection on day 0. On days −1, 0, +1, these mice were injected with either a mixture of normal rat and hamster IgG, or anti-B7-1 + antiB7-2 mAbs (3A12+GL1) at a dose of 100 μg/mouse/injection. Spleen cells were harvested 8 d after viral infection and were restimulated with irradiated, A/JAP-infected syngeneic spleen cells for 6 d in vitro, and CTL activity determined. Responder cells used were from pools of two spleens. Data in b were lysis of NP peptide (AA365-380)-pulsed EL4 target (104/well) only. The lysis of unpulsed EL4 cells is not shown but was always less than 10%. Representative of three experiments, with two mice per group are shown.
Several recent studies demonstrated that T cells can be primed in mice with a targeted mutation of CD28 (CD28KO) (39), or transgenic for CTLA4-Ig (which very effectively blocks the interaction of B7-1 and B7-2 with CD28 and CTLA4) (40). To test whether costimulation by HSA accounts for this priming, we injected anti-HSA mAb into CD28KO mice. As shown in Fig. 6, the recall CTL response generated in CD28KO mice was inhibited almost completely by pre-treatment with anti-HSA during priming. In contrast, the priming to influenza virus in WT mice was not significantly affected by anti-HSA mAb. These results demonstrate that the targeted mutation of CD28 renders the CTL priming more dependent on costimulation by HSA.
Figure 6.

Either HSA or CD28-mediated costimulation is sufficient for generation of primed T cells: bulk cultures. (a and b) Anti-HSA mAb 20C9 blocks T cell memory in CD28KO (b) but not WT mice (a), as measured by recall CTL responses in vitro. CD28-deficient mice and syngeneic WT mice were treated with either normal hamster Ig or anti-HSA mAb (300 μg/mouse/injection) on day −1, day 0, and day 1. On day 0, these mice were injected intraperitoneally with influenza virus A/JAP (1,000 HAU/mouse), spleen cells were harvested on day 7; and were restimulated with A/JAP-infected (1,000 HAU/12 × 106 cells, 37°C,1 h), irradiated syngeneic spleen cells for 5 d (responder: stimulator ratio 5:1, with responder cells at a density of 106/ml). At the end of culturing, viable cells were harvested and the cytotoxicity of these cells was determined by a 6 h 51Cr-release assay. (c) Recall CTL responses in mice with different targeted mutations. The pooled spleen cells from groups of 2–3 mice were harvested on day 7 after i.v. injection of A/JAP (300 HAU), recall CTL activity determined as in a and b. Representatives of 2–3 independent experiments are shown. Note overall CTL responses in c are stronger than in a and b due to a stronger priming via i.v. injection. Dashed lines are the lysis of EL-4 cells in the absence of NP peptide.
To substantiate this conclusion, we have produced mice with targeted mutations of both the HSA and the CD28 genes (Fig. 2) and compared the recall CTL responses in mice with targeted mutations of HSA and/or CD28. Indeed, recall CTL response in mice deficient for both CD28 and HSA was ∼100-fold lower than that in the WT mice (Fig. 6 c). CD28-deficiency leads to ∼3–5-fold reduction of recall CTL responses, while HSA-deficient mice mount normal recall CTL responses (Fig. 6 c). The relative contribution of HSA- and CD28-mediated costimulation in T cell priming, however, cannot be determined by this experiment, because the CD28:B7 pathway is also required for optimal recall responses (see below). The observed reduction in recall CTL response in CD28-deficient mice could also be attributed to a role of CD28 in the generation of effector from the primed T cells.
Induction of Memory from Naive T Cells Requires Costimulation by Either B7 or HSA.
T cells responsible for long-term memory may be different from those for the early recall response measured here (54, 55). Since the above experiments used spleen cells that were primed only 8 d previously, we treated both WT and HSA-KO mice with anti-B7 mAbs for 3 wk and waited 100 d after immunization before harvesting spleen cells and assaying for memory cells in an in vitro recall culture. As shown in Fig. 7, while repeated treatment with anti-B7 mAbs reduced the recall CTL activity in the WT mice, there still were very potent recall CTL responses in such anti-B7-treated mice. In fact, the numbers of responder cells required for detectable recall responses in vitro were similar in both groups. In contrast, in HSA-KO mice, injection of a mixture of anti-B7 mAbs eliminated the generation of memory cells. These results demonstrate that costimulation by either B7 or HSA alone is sufficient for induction of memory T cells, regardless of when the memory activity is determined. The lack of longterm memory in anti-B7 treated HSA-KO mice rules out the possibility that memory cells are produced after decay of the antibodies in vivo. Moreover, the production of memory cells in anti-B7-treated WT mice depends on HSA. Thus, the induction of T cells responsible for rapid priming of recall CTL responses and long-term memory responses has a similar requirement for costimulatory molecules; either B7 or HSA can provide costimulation for induction of memory T cells.
Figure 7.
Requirement for costimulatory molecules in the generation of memory T cells assayed at 100 d after priming: microplate culture in the absence of exogenous cytokines. Both WT and HSA-KO mice were injected with influenza virus A/JAP (1,000 HAU/mouse) (day 0) and treated with either a mixture of hamster and rat Ig or a mixture of anti-B7-1 plus B7-2 on days −1, 1, 3, 7, 10, 13, 21 (200 μg/ mAb/injection). At 100 d after the viral infection, pooled spleen cells (three mice per group) were stimulated in vitro, CTL activity was determined 5 d after stimulation. Similar results were obtained when memory CTL activity was measured at 30 d after infection.
We have also measured the frequency of the precursors for NP366-374-specific cytotoxic T cells by limiting dilutions. As shown in Table 1, naive WT, HSA-KO, CD28KO, and CD28/HSA-KO mice have a similar number of NP-specific CTLp in the spleen. These results support the notion that T cell development is not grossly affected by targeted-mutation of HSA and/or CD28. 5–7 wk after viral infection, the CTLp is increased by 10–50-fold in WT mice and in mice deficient for either HSA or CD28, although targeted mutation of CD28 reduced the expansion of CTLp. Most importantly, no significant increase of CTLp can be detected after mice deficient for both HSA and CD28 are infected with influenza virus. These results demonstrated that one costimulatory pathway, mediated by either HSA or CD28, is necessary and sufficient for increase of the CTLp during induction of immunological memory.
Table 1.
Numbers of the Precursors for NP366-374-specific Cytotoxic T Cells Per Million of Spleen Cells as Measured by Limiting Dilution*
| Mouse strains | Naive | Primed‡ | Fold of increase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| i.v. | i.p. | i.v. | i.p. | |||||||
| C57BL/6j | 1.47 | 71.0 | 74.1 | 48.3 | 50.4 | |||||
| HSA(−/−) | 2.44 | 45.0 | 90.9 | 18.4 | 37.3 | |||||
| CD28(−/−) | 1.25 | 30.3 | 13.5 | 24.2 | 10.8 | |||||
| CD28(−/−) HSA(−/−) | 2.80 | 6.2 | 3.92 | 2.2 | 1.4 | |||||
Graded numbers of cells (24 replicates at each density) from either naive or primed mice (2 mice per group) were stimulated in vitro with 105/well of A/JAP-infected, irradiated spleen cells from syngeneic C57BL/6j mice in the presence of T cell growth factors (PMA-activated EL4 cell supernatants used at 1% final concentration) for 6 d. The number of precursor cells was calculated by Poisson distribution.
Spleen cells were taken from mice immunized with 1,000 HAU/ mouse, either intravenously (i.v. used 5 wk after immunization) or intraperitoneally (i.p., 7 wk after immunization).
B7, but not HSA, Is Required for the Generation of Effector CTL from Memory T Cells.
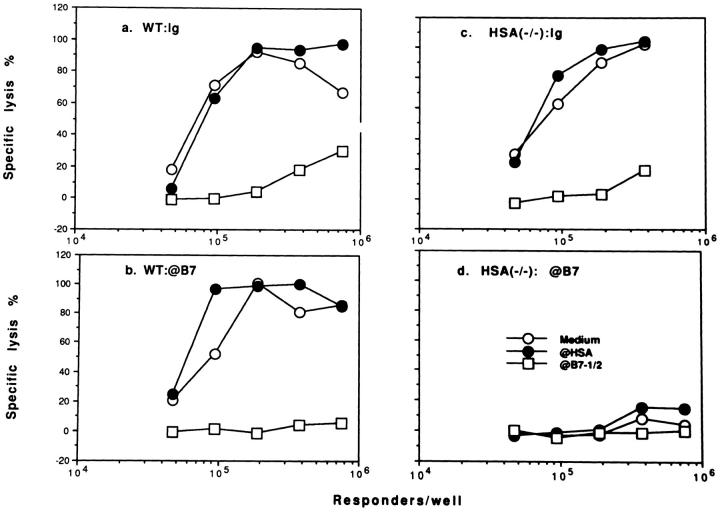
The activation of memory T cells gives rise to effector T cells. To test whether this process requires costimulatory molecules, we stimulated primed spleen cells in the presence of mAb against either B7 or HSA. As shown in Fig. 8 a, for spleen cells primed when both B7 and HSA-costimulation pathways are intact, the recall response requires costimulation by B7, but not the HSA. To test whether priming conditions affect the requirement for costimulatory molecules in eliciting effector CTL from memory T cells, spleen cells primed when B7 or HSA was blocked were restimulated in the presence of anti-HSA or anti-B7 mAbs. Again, T cells primed in the absence of costimulation by either B7 or HSA require costimulation by B7 for a recall response (Fig. 8, b and c). No recall CTL can be detected in microculture when both B7 and HSA are blocked during priming (Figs. 4 and 8 d). Thus, regardless of the priming conditions, B7 but not HSA is essential for eliciting effector from memory T cells.
Figure 8.
B7, but not HSA, plays an important role in the induction of effector T cells from memory T cells, regardless of the priming conditions: microplate culture in the absence of exogenous cytokines. Spleen cells from either WT (a and b) or HSA-deficient mice (c and d) pretreated with either normal Ig (a and c) or a mixture of anti-B7-1 and anti-B7-2 (b and d) were infected with A/JAP virus as detailed in the legend to Fig. 3. Increasing numbers of spleen cells were stimulated in vitro for 5 d with A/JAP-infected spleen cells, in the presence of either medium, or a mixture of anti-B7-1 and anti-B7-2, or anti-HSA mAb (final concentration at 5 μg/ml), and CTL activity was determined in a 6-h 51Cr-release assay. Representative of three independent experiments are shown. Similar results were obtained when memory activity is measured in bulk cultures.
Discussion
A critical issue pertinent to the basis of self-nonself discrimination in the immune system is whether induction of immunological memory requires costimulation, in addition to antigen. The two-signal theory of T cell activation (9–13) argues that stimulation of naive T cells in the absence of costimulation leads to tolerance rather than immunological memory. Classical experiments using either fixed APC (56) or transplantation of cultured allogeneic or xenogeneic endocrine grafts (57) indicate that immunological tolerance is induced when costimulatory activity is eliminated, either by depletion or by inactivation of resident APC. Recently, it has been documented that blocking the B7-CD28/ CTLA4 interaction leads to tolerance rather than immunity to xenogeneic grafts (38). However, several more recent experiments designed to test the requirement of B7:CD28/ CTLA4 interaction in the induction of memory T cells do not support an essential role of B7 family members in the induction of immunological memory. Thus, transgenic mice which constitutively express a high level of CTLA4Ig, a fusion protein consisting of the high affinity B7 receptor CTLA4 fused to the immunoglobulin Fc portion, show an even better priming than the littermate controls (40). In addition, fibroblasts transfected with viral glycoproteins induce successful priming in the absence of B7 expression (41). Because the B7:CD28 interaction is not essential, it has been proposed that induction of T cell memory does not require costimulation.
Given the fact that several costimulatory molecules have been discovered, we were interested in testing whether a lack of requirement for B7 in the induction of immunological memory was due to the existence of other redundant costimulatory pathways. We have used three different assays to measure the memory CTL response. The first is a microplate restimulation culture in the absence of exogenous cytokines. Such assay allows us to estimate the number of spleen cells necessary to generate a detectable CTL response (presumably including helper and CTLp). The second assay is a bulk culture involving a larger number of cells (10 × 106 cells in 10 ml). Whereas this assay is perceived to be less quantitative than the first assay, it is more sensitive and appears to be the only assay that allows detection of optimal recall CTL response in spleen cells from CD28-deficient mice without adding exogenous cytokines. Most likely, this is due to the involvement of CD28 in eliciting CTL from memory cells. Thirdly, we have used a limiting dilution assay to measure the precursor frequency of NP366-374-specific CTL. This method is widely used to measure CTL memory, however, it should be noted that this assay only measures the persistency of expanded CTL. Utilization of three different assays allows a more comprehensive measurement of the memory CTL response.
We show here that in mice with a targeted-mutation of the HSA gene, but not in the WT mice, B7 is required for the induction of immunological memory. In contrast, in CD28deficient mice, the HSA is necessary for T cell priming. Memory T cell response is hardly detectable in mice deficient for both HSA and CD28. Thus, induction of immunological memory requires costimulation, but either B7 family members or the HSA are sufficient. It should be noted that while our studies have stressed the importance of costimulation mediated by either HSA or CD28. We cannot rule out contribution of other costimulatory molecules in the induction of memory T cells. In this regard, it is worth noting that blockade of both CD40L:CD40 and CD28/CTLA4:B7 interactions is more efficient than simply blocking either one in preventing clonal expansion of self-reactive helper T cells (58). It is likely that CD40L- induced costimulatory molecules, such as CD44 (59) and ICAM-1 (60) may participate in induction of memory T cells.
It is worth noting that targeted mutation of CD28 alone causes a three- to fivefold reduction in recall CTL response, while HSA-deficiency alone is not sufficient to affect immunological memory (Fig. 6). These results may indicate that CD28-dependent costimulatory pathway is more important than that of HSA for induction of memory CTL. However, since memory cells are quantitated by the effector T cells they produced, and since production of effector cells from memory cells requires B7 (Fig. 8), the reduction in recall CTL response may be due to lack of CD28 on the memory cells. In this regard, it is worth noting that transgenic expression of CTLA4Ig, which blocks B7:CD28/ CTLA4 interaction, does not affect induction of immunological memory when it is assayed in the absence of CTLA4Ig (40). Similarly, priming in the presence of antiB7 mAbs does not affect induction of recall response (Fig. 5). In contrast, since HSA is not involved in the induction of effector T cells from memory T cells (Fig. 8), a reduction in recall response caused by targeted mutation of HSA can be attributed to HSA involvement during priming.
An important conclusion of our study is that distinct costimulatory molecules can be utilized in the induction effector and memory T cells. Induction of effector T cells depends strictly on costimulation by B7, while induction of memory T cells requires costimulation by either B7 or the HSA. These conclusions are based on three lines of evidence. First, a mixture of anti-B7-1 and anti-B7-2 mAb completely blocks the generation of primary CTL response in WT mice, yet it does not seem to significantly affect the generation of memory CTL responses. While the magnitude of the recall response was affected somewhat in the long-term memory response, the number of memory cells was not altered. Second, a targeted mutation of CD28 eliminates the antigen-specific primary CTL responses, yet it causes only a small reduction in the number of precursor cells at 5–7 wk after priming. Third, costimulation by HSA plays no role in the induction of effector T cells from either naive or memory T cells, yet it provides an alternative costimulatory pathway for the induction of memory T cells. That HSA enhances priming CTL responses is supported by a recent study in which transfection of HSA into tumor cells lead to enhanced memory CTL response (28).
Because CD4 T cells can provide help for CD8 T cell responses in both priming and induction of effector from memory T cells, our results could be explained on the basis of a requirement for costimulation in the CD4 T cell compartment. However, previous studies from others and us have demonstrated that, in an anti-influenza CTL response, induction of memory T cells is independent of CD4 T cells (61, 62). Blocking the priming of CD4 T cells, therefore, cannot lead to a defect of CD8 T cell response as reported here. Furthermore, we demonstrate here that targeted mutation of both CD28 and HSA prevents clonal expansion of CTLp (Table 1).
A simple model can be proposed to explain the requirement for distinct but overlapping costimulatory molecules in the induction of effector and memory CTL. The strength of the total signals received by naive T cells could determine whether they will differentiate into effector or memory T cells. A stronger stimulation (integration of signals from TCR and costimulation) would lead to the production of effector cells, while a weaker stimulation would be sufficient to produce memory cells. HSA is a less potent costimulatory molecule for clonal expansion of T cells than B7 family members, so it can only be utilized for the induction of a memory T cell responses. B7, being a stronger costimulator, can be used for both memory and effector T cell responses, depending on the concentration of the molecule on the surface of APC and the strength of the TCR/antigen interaction. For example, dendritic cells, which express a high level of B7, may induce the production of effector T cells, while B cells, which express a lower level of B7, may be more likely to induce only memory T cells.
Our study may explain the interesting difference between viruses regarding the requirement for the costimulatory receptor CD28 in the induction of CTL in vivo. Shihanian et al. (39), showed that CD28 is not required for induction of CTL specific for lymphocytic choriomeningitis virus (LCMV) in vivo. Since LCMV causes a productive infection in mice, naive T cells can first be primed, and then these primed T cells can give rise to effector CTL after repeated stimulation. The influenza virus used in this study, and the vesicular stomatitis virus used by Kündig et al., do not cause productive infection, and thus the CTL response in vivo become CD28-dependent after one round of infection (63).
A successful adaptive immune response has two major T cell products: effector T cells and memory T cells. The lineage relationship between these two types of cells has not been resolved. Because effector T cells and memory T cells share certain activation conditions (64) and express several identical activation markers (65), it has been proposed that memory T cells are derived from effector T cells (55, 64, 65). A critical prediction of this hypothesis is that memory T cell responses should be eliminated when the effector T cell responses are abrogated. Results presented in this study show that memory CTL responses are largely intact when effector T cell development is completely absent. These results are not consistent with the notion that effector T cells are mandatory precursors for memory T cells. Furthermore, we showed that costimulation by HSA does not contribute to generation of effector cells, but it can and does contribute to the generation of memory cells. This qualitative difference in costimulatory molecules used for effector vs memory T cell responses strongly suggests that these two types of cells can be products of different activation pathways (see Fig. 9 for a model).
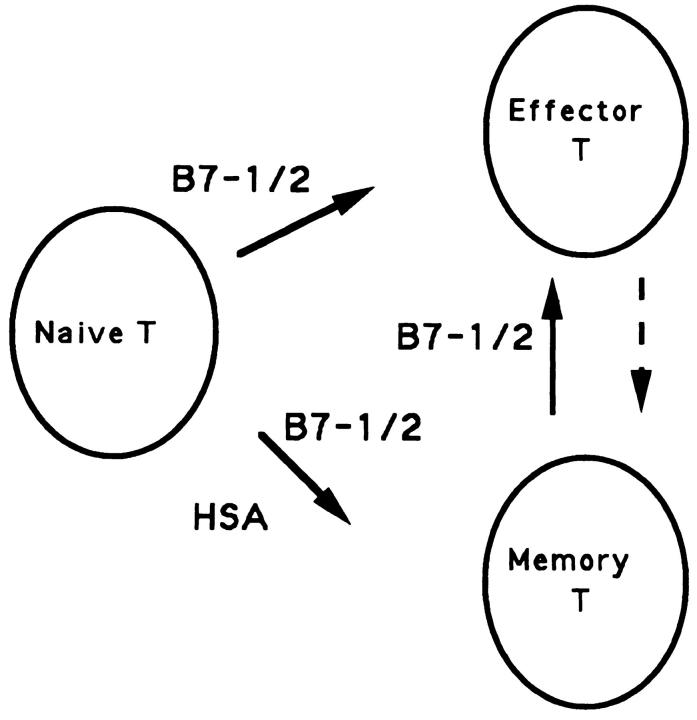
Figure 9.
A model for the involvement of costimulatory signals in the generation of effector and memory T cells. Two major products, effector and memory T cells, are produced from naive T cells after viral infection. The production of memory T cells requires costimulation by either HSA or B7, while the production of effector T cells utilizes B7 but not HSA. By definition, memory T cells give rise to effector T cells after further stimulation by antigen. However, effector T cells are unlikely to be mandatory precursors for memory T cells and distinct costimulatory molecules could be used at different phases of the immune response.
A critical test for the model is whether memory cells can be produced when effector T cells are ablated. A major obstacle to such a test is that memory and effector T cells are traditionally measured at different times after antigenic stimulation. Effector CTL disappears within two weeks after influenza viral infection, yet memory cells are generally measured long after that. This is necessary not because of the kinetics of production of memory, since elegant studies demonstrated that T cell memory is produced as early as three days after antigen challenge (66); rather, it is because of the need to differentiate memory from effector cells. Since blocking B7 or CD28 efficiently eliminates production of effector T cells, we can use the same spleen cells to test whether priming can take place when effector CTL are not generated. Such an analysis strongly supports the notion that the primed T cells are not derived from effector T cells. Experiments are under way to test whether the activity measured in this early recall assay represents true immunological memory.
In conclusion, we have produced mice with a targeted mutation of HSA, and mice deficient for both HSA and CD28. We have demonstrated that the induction of memory T cells requires costimulation by either B7 or HSA, while induction of effector T cells depends on B7 but not HSA. Our study firmly establishes that induction of immunological memory requires costimulation, and that either B7 or HSA is sufficient for this step. It also raises an interesting possibility that memory T cells can be induced without going through an effector phase.
Acknowledgments
We thank Drs. Tak Mak and K. Pfeffer for providing CD28KO mice, Dr. Brigit Ledermann for supplying the C57BL/6 ES line BL/6-III, Dr. Charles A. Janeway for helpful discussions, Drs. Jon Yewdell and Jack Bennik for influenza virus, and Drs. Charlie Janeway, Victor Nussenzweig, Dan Littman, Jeanette Thorbecke, and Stan Vukmanovic for critical reading of the manuscript. This study is supported by National Institutes of Health grant AI32981.
Footnotes
1 Abbreviations used in this paper: CTLp, the precursor for cytolytic lymphocytes; HSA, The heat-stable antigen; WT, wild-type mice; KO, mice with a homozygous targeted-mutation; LCMV, lymphocytic choriomeningitis virus; NP, nucleoprotein of influenza virus A/JAP.
References
- 1.Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus-persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 3.Razvi ES, Welsh RM. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993;67:5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripp RA, Lahti JM, Doherty PC. Laser light suicide of proliferating virus-specific CD8 T cells in an in vivoresponse. J Immunol. 1995;155:3719–3721. [PubMed] [Google Scholar]
- 5.Müllbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature (Lond) 1994;369:648–652. doi: 10.1038/369648a0. [DOI] [PubMed] [Google Scholar]
- 7.Hou S, Hyland L, Ryan M, Portner A, Doherty PC. Virus-specific CD8 T-cell memory determined by clonal burst size. Nature (Lond) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 8.Matzinger P. Immunology. Memories are made of this? . Nature (Lond) 1994;369:605–606. doi: 10.1038/369605a0. [DOI] [PubMed] [Google Scholar]
- 9.Brestcher P, Cohn M. A theory of self-nonself discrimination. Science (Wash DC) 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 10.Lafferty KJ, Cunningham AJ. A new analysis of alloreactivity. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 11.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion vs functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 12.JanewayJr. C.A. Immunogenicity: signal 1, 2, 3... and 0. Immunol Today. 1989;10:283–288. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- 13.JanewayJr. C.A. Approaching the asymptote: revolution and evolution in the immune system. Cold Spring Harbor Symp Quant Biol. 1990;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Linsley PS, Clark EA, Ledbetter JA. The T cell antigen, CD28, mediates adhesion with B cells by interacting with the activation antigen B7. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin-2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman GJ, Freeman AS, Segil JM, Lee G, Whitman JF, Nadler L M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143:2714–2722. [PubMed] [Google Scholar]
- 17.Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou LJ, White M, Figeroth JD, Gribben JG, Nadler LM. Structure, expression and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991;174:625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Guo Y, Liu Y. A major co-stimulatory molecule, CTLA4 ligand A, is distinct from B7. J Exp Med. 1993;178:1789–1793. doi: 10.1084/jem.178.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azuma M, Ito D, Yagita H, Okumura K, Philips JH, Lanier LL, Somora C. B70 antigen is a second ligand for CTLA4 and CD28. Nature (Lond) 1993;366:76–78. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 20.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley PS, Hodes RJ. Identification of an alternative CTLA4 ligand co-stimulatory for T cell activation. Science (Wash DC) 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 21.Freeman GJ, Borriello F, Hodes RJ, Reiser H, Gribben JG, Ng JW, Kim J, Goldberg JW, Hathcock K, Laszlo G, et al. Murine B7-2, an alternative CTLA4 counter receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman GL, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard GS, Nadler LM. Cloning of B7-2: a CTLA4 counter-receptor that costimulates human T cell proliferation. Science (Wash DC) 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 23.Linsley PS, Brady W, Grosmaire L, Damle NK, Ledbetter JA. CTLA4 is a second receptor for B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Jones B, Brady W, Janeway CA, Jr, Linsley PS. Costimulation for CD4 T cell growth: Co-operation of B7 and the heat-stable antigen. Eur J Immunol. 1992;22:2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Jones B, Sullivan K, Aruffo A, Linsley PS, Janeway CA., Jr The heat-stable antigen is a co-stimulatory molecule for CD4 T cells. J Exp Med. 1992;175:437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Janeway CA., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci USA. 1992;89:3845–3849. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enk A, Katz S. Heat-stable antigen is an important costimulatory molecule on epidermal Langerhans' cells. J Immunol. 1994;152:3264–3270. [PubMed] [Google Scholar]
- 28.Wang Y-C, Zhu L, McHugh R, Sell KW, Selvaraj P. Expression of heat-stable antigen on tumor cells provides co-stimulation for tumor-specific T cell proliferation and cytotoxicity in mice. Eur J Immunol. 1995;25:1163–1167. doi: 10.1002/eji.1830250505. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy MK, Mohler KM, Shanebeck KD, Baum PR, Picha KS, Otten-Evans CA, Janeway CA, Jr, Grabstein KH. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur J Immunol. 1994;24:116–123. doi: 10.1002/eji.1830240118. [DOI] [PubMed] [Google Scholar]
- 30.De Bruijn MLH, Peterson PA, Jackson MR. Induction of heat-stable antigen expression by phagocytosis is involved in in vitroactivation of unprimed cytotoxic T lymphocytes by macrophages. J Immunol. 1996;156:2686–2692. [PubMed] [Google Scholar]
- 31.Guo Y, Wu Y, Shinde S, Liu Y. Identification of a CD40 ligand-induced costimulatory molecule as CD44H. J Exp Med. 1996;184:955–961. doi: 10.1084/jem.184.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubey C, Croft M, Swain S. Costimulatory requirement of naive CD4 T cells: ICAM-1 or B7-1 can costimulate naive CD4 T cell activation, but both are required for optimal responses. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- 33.Isobe M, Yagita H, Okumura K, Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science (Wash DC) 1992;255:1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Hellstrom KE, Mizuno M, Chen L. In vitropriming of tumor-reactive cytolytic T lymphocytes by combining interleukin-10 with B7-CD28 interaction. J Immunol. 1995;155:3897–3903. [PubMed] [Google Scholar]
- 35.Gajewski TF, Renauld JC, Van Pel A ,, and T. Boon. Costimulation with B7-1, IL-6, and IL-12 is sufficient for primary generation of murine anti-tumor cytolytic lymphocytes in vitro . J Immunol. 1995;154:5637–5648. [PubMed] [Google Scholar]
- 36.Linsley PS, Green JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) binds with similar avidity but distinct kinetics to CD28 and CTLA4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 37.Turka A, Linsley PS, Lin H, Brady W, Leiden JM, Wei R-Q, Gibson M-L, Zheng X-G, Myrdal S, Gordon D, et al. T cell activation by CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–11106. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenschow DJ, Zeng J, Thistlewaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islets grafts induced by CTLA4Ig. Science (Wash DC) 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 39.Shahinian A, Pfeffer K, Lee K, Kundig KP, Kishihara TM, Wakham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science (Wash DC) 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 40.Ronchese F, Hausmann B, Hubele S, Lane P. Mice transgenic for a soluble form of murine CTLA-4 show enhanced expansion of antigen-specific CD4+T cells and defective antibody production in vivo. J Exp Med. 1994;179:809–817. doi: 10.1084/jem.179.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kündig TM, Bachmann MF, DiPaolo C, Simard JL, Battegay M, Lother H, Gessner A, Kühlcke K, Ohashi P, Hengartner H, Zinkernagel RM. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science (Wash DC) 1995;268:1343–1346. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Linsley PS. T cell costimulation. Curr Opin Immunol. 1992;4:265–270. doi: 10.1016/0952-7915(92)90075-p. [DOI] [PubMed] [Google Scholar]
- 43.Liu, Y. 1994. The costimulatory pathway for T cell responses. R.G. Landes Company, Austin. 122 pp.
- 44.Springer T, Galfre G, Secher S, Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978;8:539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- 45.Kay R, Takei F, Humphries R. Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J Immunol. 1991;146:1952–1959. [PubMed] [Google Scholar]
- 46.Wenger RH, Ayane M, Bose R, Köhler G, Niesen PJ. The genes for a mouse hematopoietic differentiation marker called the heat-stable antigen. Eur J Immunol. 1991;21:1039–1046. doi: 10.1002/eji.1830210427. [DOI] [PubMed] [Google Scholar]
- 47.Alterman LA, Crispe N, Kinnon C. Characterization of the murine heat-stable antigen: an hematolymphoid differentiation antigen defined by the J11d, M1/69 and B2A2 antibodies. Eur J Immunol. 1990;20:1597–1602. doi: 10.1002/eji.1830200728. [DOI] [PubMed] [Google Scholar]
- 48.Rougon G, Alterman LA, Dennis K, Guo X-J, Kinnon C. The murine heat-stable antigen: a differentiation antigen expressed in both the hematolymphoid and neural cell lineages. Eur J Immunol. 1991;21:1397–1402. doi: 10.1002/eji.1830210611. [DOI] [PubMed] [Google Scholar]
- 49.Harding F, McAuther J, Gross JA, Allison JP. CD28-mediated signalling costimulates murine T cells and prevents induction of anergy in T cells. Nature (Lond) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 50.Ledermann B, Bürki K. Establishment of germline competent C57BL/bj embryonic stem-cell lines. Exp Cell Res. 1991;197:254–258. doi: 10.1016/0014-4827(91)90430-3. [DOI] [PubMed] [Google Scholar]
- 51.Wenger RH, Kopf M, Nitschke L, Lamers MC, Köhler G, Nielsen PJ. B-cell maturation in chimeric mice deficient for the heat-stable antigen (HSA/mouse CD24) Trans Res. 1995;4:173–183. doi: 10.1007/BF01968782. [DOI] [PubMed] [Google Scholar]
- 52.Townsend ARM, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael A. The epitopes of influenza nucleoprotein recognized by CTL can be defined by short peptide. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 53.Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee HG. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature (Lond) 1990;348:195–197. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 54.Askonas BA, Müllbacher A, Ashman RB. Cytotoxic memory cells in virus infection and specificity of helper cells. Immunology. 1982;45:79–84. [PMC free article] [PubMed] [Google Scholar]
- 55.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins MK, Schwartz RH. Antigen-presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lafferty KJ, Prowse SJ, Simeonovich CJ. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Ann Rev Immunol. 1983;1:143–174. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 58.Griggs ND, Agersborg SA, Noelle RJ, Ledbetter JA, Linsley PS, Tung SK. The relative contribution of the CD28 and gp39 costimulatory pathway in the clonal expansion and pathogenic acquisition of self-reactive T cells. J Exp Med. 1996;183:801–810. doi: 10.1084/jem.183.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Y, Wu Y, Shinde S, Liu Y. Identification of CD44H as a costimulatory molecule rapidly induced by CD40 ligand. J Exp Med. 1996;184:955–961. doi: 10.1084/jem.184.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinde S, Wu Y, Guo Y, Xu J, Grewal IS, Flavell RA, Liu Y. CD40L is important for induction of, but not response to costimulatory activity: ICAM-1 as the second costimulatory molecule rapidly up-regulated by CD40L. J Immunol. 1996;157:2764–2768. [PubMed] [Google Scholar]
- 61.Liu Y, Müllbacher A. The generation and activation of memory T cell responses to influenza A virus in vivo do not require CD4+ T cells. Cell Biol Immunol. 1989;67:413–420. doi: 10.1038/icb.1989.58. [DOI] [PubMed] [Google Scholar]
- 62.Allen W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice infected with influenza: consequences of depleting CD4 T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 63.Kündig M, Shahinian A, Kawai K, Mittrücker H-W, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 64.Swain S. Generation and in vivopersistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 65.Cerottini J-C, MacDonald H-R. The cellular basis of T cell memory. Ann Rev Immunol. 1989;7:77–89. doi: 10.1146/annurev.iy.07.040189.000453. [DOI] [PubMed] [Google Scholar]
- 66.Black SJ, Inchley CJ. Characteristics of immunological memory in mice. I. Separate early generation of cells mediating IgM and IgG memory to sheep erythrocytes. J Exp Med. 1974;140:333–348. doi: 10.1084/jem.140.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]