Abstract
In anti-red blood cell autoantibody transgenic (autoAb Tg) mice almost all B cells are deleted except for B-1 cells in the peritoneal cavity and the gut. About one-half of the auto Ab Tg mice suffer from autoimmune hemolytic anemia (AIHA) in the conventional condition. Oral administration of lipopolysaccharides activates B-1 cells and induces autoimmune symptoms in the Tg mice, suggesting that the autoimmune disease in anti-RBC autoAb Tg mice is triggered by infections. To examine the association of bacterial infections with the generation of B-1 cells and the occurrence of the autoimmune disease, we analyzed anti-RBC autoAb Tg mice bred in germ-free and specific pathogen-free conditions. In germ-free conditions, few peritoneal B-1 cells were detected, while a significant number of peritoneal B-1 cells existed in specific pathogen-free conditions. In both conditions, no mice suffered from AIHA. However, when these Tg mice were transferred to the conventional condition or injected with lipopolysaccharide, peritoneal B-1 cells expanded and some of these mice suffered from AIHA. These results clearly showed that bacterial infections are responsible for both the expansion of B-1 cells and the onset of the autoimmune disease in these Tg mice.
Several lines of evidence indicate that bacterial infection may activate autoreactive lymphocytes in vivo and induce autoimmune diseases. LPS derived from gram-negative bacteria is known to activate B cells polyclonally and the administration of LPS induces the production of autoantibodies (autoAbs) even in normal mice (1). Enterotoxins derived from bacteria serve as superantigens that can bind T cell receptors, resulting in stimulation and expansion of a wide population of T cells (2). In some cases, autoreactive lymphocytes may be activated after the infection of bacteria and viruses, whose antigens have certain structural similarities with self-antigens (molecular mimicry) (3). Recently, the induction of autoimmune disease by infection was suggested in experimental allergic encephalomyelitis (EAE) transgenic (Tg) model mice, in which all T cells express myelin basic protein–specific T cell receptors. EAE did not occur in the Tg mice bred in specific pathogen-free (SPF) conditions, but some of the mice housed under conventional conditions showed the autoimmune disease (4). On the other hand, there exist several arguments against the involvement of infection in autoimmune diseases. The frequency of autoimmune gastritis in day 3 thymectomized mice remained the same under germ-free (GF) as well as under conventional conditions (5) and the frequency of autoimmune insulitis is known to increase in NOD mice bred under SPF conditions (6). Thus, it remains elusive whether or how infection is involved in autoimmunity.
B-1 cells constitute a distinct B cell population that differs in several of its functional properties from conventional B cells (7–9), and has a strong capacity for self-renewal (10). They are found predominantly in peripheral tissues such as the peritoneal and pleural cavities (11). B-1 cells preferentially use VH segments proximal to the DJ segments and produce IgM against self and bacterial antigens (7–9). They are characterized by expression of surface antigens such as IgMhigh, IgDlow, B220low, Mac-1+, and CD23− (7–9). Although B-1 cells are suggested to play an important role in autoimmune diseases (7, 8), the physiological and pathological roles of B-1 cells still remain unclear.
A key to solve these questions about B-1 cells and autoimmunity lies in creating suitable animal models to simplify genetic and pathogenic factors of autoimmunity. Previously, we established and characterized a Tg mouse line carrying the Ig genes derived from the anti-red blood cell (RBC) autoAb 4C8 (12–15). In these mice, almost all B cells were deleted in the periphery but a normal number of B-1 cells survived in the peritoneal cavity, which is sequestered from RBCs (12, 13). Under conventional breeding conditions, about one-half of the animals of this Tg line suffer from autoimmune hemolytic anemia (AIHA), even though they have the same genetic background and the same number of B-1 cells (12, 15). The production of the autoAb by peritoneal B-1 cells was shown to be responsible for AIHA in the Tg mice because apoptotic death of B-1 cells by repeated exposure to RBCs completely cured AIHA (13) and because AIHA did not occur in the anti-RBC autoAb Tg mice bearing the X-linked immunodeficiency (xid) mutation, which deletes B-1 cells (14). On the other hand, oral administration of LPS to the nonsymptomatic Tg mice caused activation of peritoneal B-1 cells and induction of AIHA (14). These lines of circumstantial evidence suggest that enteric bacteria or some infectious agents activate peritoneal B-1 cells and induce the autoimmune disease in the Tg mice (15).
In the present study, we examined whether bacterial infections have influences on the generation and activation of B-1 cells and on the occurrence of autoimmunity in the anti-RBC autoAb Tg mice that are bred under different conditions, i.e., the GF, SPF, and conventional conditions. We report that bacterial infections most likely via the gut are necessary for the generation of B-1 cells and the occurrence of the autoimmune disease in this Tg model.
Materials and Methods
Mice.
Homozygous heavy chain and light chain Tg mice of the anti-RBC autoAb were established and maintained under conventional breeding conditions in the Center for Molecular Biology and Genetics, Kyoto University. By mating homozygous heavy and light chain Tg mice, we obtained conventionally bred antiRBC autoAb Tg mice that carry both heavy and light chain transgenes of autoAb (12). To make GF animals from anti-RBC autoAb Tg mice, two conventional pregnant mice (a heavy chain Tg mouse and a light chain Tg mouse) were aseptically hysterectomized, and young pups obtained were raised by GF foster mothers (C3H/HeMs mice) in an isolator under GF conditions. Then, a GF line of anti-RBC autoAb Tg mice was produced from F1 hybrids between two GF lines of heavy chain Tg mice and light chain Tg mice. Mice were given commercial food pellets sterilized by γ irradiation at 50 kGy (FR-1; Funabashi Farm, Inc., Chiba, Japan) and autoclaved drinking water ad libitum, and were routinely checked by testing samples of feces for the presence of bacterial and fungal contamination. No evidence of contamination could be detected before and at the time of the experiment. SPF animals of anti-RBC autoAb Tg mice, derived from the GF mice, were maintained in a barriered animal facility that has been designed to discourage the entry of undesirable microorganisms. Mice were offered commercial food pellets (F2; Funabashi Farm, Inc., Chiba, Japan) and drinking water without sterilization. Cages containing sterilized bedding were changed once per week. Mice were used at the age of 8–15 wk.
Microbacterial Examination of Mice.
To make sure enteric bacteria and fungi were absent in GF mice, feces derived from GF mice were aerobically cultured for 2 wk in thioglycollate broth (Eiken, Tokyo, Japan) at 37°C, and a small amount of the cultured fluid was inoculated on the surfaces of two brain–heart infusion agars (Nissui, Tokyo, Japan) and a Sabouraud's agar (Eiken). Each agar plate of the former was incubated for 1 wk at 37°C under aerobic or anaerobic conditions, and the latter was aerobically incubated for 1 wk at room temperature. All GF mice were negative in all these tests. To check the SPF conditions, materials were collected from blood, rhinal swab, cecum contents, duodenum contents, and anus, and were examined by ELISA, culture, or microscopic test for the following pathogenic organisms: Sendai virus, mouse hepatitis virus, Mycoplasam pulmonis, Tyzzer's organism, Corynebacterium Kutscheri, Pasteurella pneumotropica, Salmonella species, Giardia musis, Tritrichomonas species, Syphacia species, and Spironucleus muris.
Flow Cytometry.
Cells were isolated from spleen or peritoneal cavities as described before (12–14). Single cell suspension of 106 mononuclear cells was pelleted and resuspended in 10 μl of normal mouse serum for blocking the nonspecific binding of antibodies. After 10-min incubation, one of the first reagent antibodies, i.e., rat anti–mouse B220 (RA3-6B2) mAb or rat anti-Mac-1 Ab (M1/70.15.115.5HL), was added directly at the appropriate dilutions in PBS. After incubation, cells were washed twice with 1 ml of PBS containing 5% FCS and 0.05% sodium azide. The PE-conjugated anti-mouse IgM Ab (Southern Biotechnology Associates, Birmingham, AL) was added as the second reagent Ab in 10 μl of PBS at appropriate concentrations. After excluding dead cells by propidium–iodine staining and gating the lymphoid cells in forward and side scatter analysis, 104 viable lymphoid cells were analyzed on FACScan® and were plotted on quadruple logarithmic scales.
Results and Discussion
Breeding Conditions Determine the Occurrence of AIHA in Anti-RBC AutoAb Tg Mice.
We previously reported that only one-half of anti-RBC autoAb Tg mice suffered from AIHA when housed under conventional breeding conditions (12). AIHA was induced in nonsymptomatic Tg mice by the administration of LPS (14), suggesting that some environmental factors such as infections are involved in the occurrence of AIHA. To demonstrate the involvement of infection in the onset of autoimmunity, we first examined the occurrence of AIHA in Tg mice bred in the GF and SPF conditions. GF Tg mice are not supposed to have any bacteria or pathogens in vivo at all, and SPF Tg mice are not supposed to be infected by at least 11 tested microorganisms described in Materials and Methods. AIHA did not occur in either SPF or GF anti-RBC autoAb Tg mice, while 46% of Tg mice suffered from AIHA in the conventional condition (Table 1). These results directly demonstrated that some pathogenic microorganisms were involved in the occurrence of AIHA in the anti-RBC autoAb Tg mice.
Table 1.
Occurrence of AIHA in Anti-RBC AutoAb Tg Mice in Different Breeding Environments
| ConV | SPF | GF | ConV* (LPS injected) | GF/ConV‡ | SPF/ConV‡ | GF* (LPS injected) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of autoimmune | ||||||||||||||
| hemolytic anemia % | 46 | 0 | 0 | 100 | 33 | 33 | 25 | |||||||
| Number of mice with | ||||||||||||||
| autoimmune hemolytic anemia | 29 | 0 | 0 | 13 | 2 | 4 | 2 | |||||||
| Number of tested mice | 63 | 26 | 30 | 13 | 6 | 12 | 8 |
We considered Tg mice with hematocrit values below 40% as AIHA mice and examined the occurrence of AIHA in 6- to 8-wk-old anti-RBC autoAb Tg mice in different breeding conditions: GF, SPF, and conventional conditions (ConV).
Anti-RBC autoAb Tg mice bred in the conventional or GF condition were examined 7 d after oral administration of 100 μg of LPS.
GF Tg mice or SPF Tg mice were transferred to the conventional condition, bred there for 4–6 wk, and then examined.
To identify the pathogenic microorganisms that induce AIHA in Tg mice, we checked microorganisms in serum, rhinal swab, cecum, and duodenal contents of conventionally bred Tg mice. 5 Tg mice bred under the conventional condition were randomly chosen and examined for the infection of at least 11 microorganisms (Table 2). In these Tg mice, Sendai virus, mouse hepatitis virus, Tyzzer's organism, and Syphacia obvelata were detected. However, this result does not imply that any of these 4 microorganisms detected directly induced AIHA in Tg mice, because it remains possible that other untested pathogens are involved in the occurrence of AIHA.
Table 2.
Examination of Microorganisms in Conventionally Bred Anti-RBC AutoAb Tg Mice
| Organism | Method | Material | Results | |||
|---|---|---|---|---|---|---|
| Sendai virus | ELISA | Serum | + | |||
| Mouse hepatitis | ||||||
| virus | ELISA | Serum | + | |||
| Mycoplasma | ||||||
| pulmonis | ELISA | Serum | − | |||
| Tyzzer's organism | ELISA | Serum | + | |||
| Corynebacterium | ||||||
| kutscheri | Culture | Rhinal swab | − | |||
| Pasteurella | ||||||
| pneumotropica | Culture | Rhinal swab | − | |||
| Salmonella species | Culture | Cecum contents | − | |||
| Giardia muris | Microscopic test | Cecum contents | − | |||
| Trictrihomonas | ||||||
| species | Microscopic test | Cecum contents | − | |||
| Syphacia species | Microscopic test | Anus | + | |||
| Spironucleus muris | Microscopic test | Duodenum contents | − |
Generation and Activation of B-1 Cells in Anti-RBC AutoAb Tg Mice.
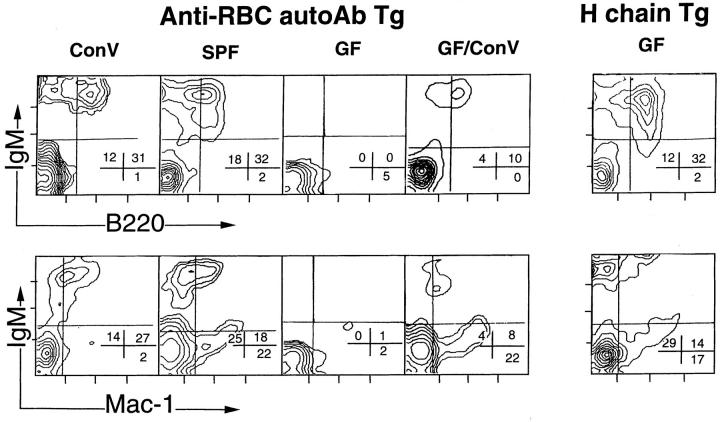
In our previous studies, we showed that conventional B cells are almost completely deleted in this Tg model and that B-1 cells, which survive in the peritoneal cavity, are responsible for the autoimmune disease (12–15). To examine the effects of environmental conditions on generation of B-1 cells, we collected lymphocytes from peritoneal cavities, spleens, and bone marrows of Tg mice, and analyzed them by flow cytometry after staining with fluorescence-congugated mAbs. In spleens and bone marrows, B cells, including B-1 cells and conventional B cells, were hardly detected in Tg mice bred in the SPF and GF conditions as well as in the conventional condition (data not shown). By contrast, the numbers of B-1 cells in the peritoneal cavity of Tg mice are different depending on environmental conditions. The peritoneal cavity of GF Tg mice contained no or few B cells, whereas SPF Tg mice had a lesser but significant number of B-1 cells (B220+, IgM+, Mac-1+) as compared with Tg mice bred in the conventional condition (Fig. 1). Moreover, when GF Tg mice were transferred to the conventional condition, a small but significant number of B-1 cells appeared in the peritonal cavity (Fig. 1).
Figure 1.
The number of peritoneal B-1 cells in anti-RBC autoAb Tg mice depends on breeding conditions. Peritoneal cells of 8- to 16-wk-old anti-RBC autoAb Tg mice bred in different environments were examined by flow cytometric analysis with anti-IgM and anti-B220 or antiMac-1 Abs. GF/ConV: anti-RBC autoAb Tg mice bred in the GF condition were transferred to the conventional condition (ConV ) and kept there for 4–6 wk before examination. Percentages of cell numbers in each quadrant are indicated.
To demonstrate more clearly the relationship between the occurrence of AIHA and infection, we transferred GF and SPF Tg mice into the conventional condition. Interestingly, one-third of the transferred Tg mice suffered from AIHA when transferred to the conventional facility (Table 1). Oral administration of LPS also induced AIHA in GF Tg mice. These results imply that autoAb-producing cells or their progenitors exist in GF and SPF Tg mice, even though their number is very small. A negligible number of peritoneal B-1 cells in GF Tg mice can expand in the peritoneal cavity by stimulation with enteric bacteria. Because a small number of peritoneal B-1 cells in SPF Tg mice do not cause AIHA, some additional infections of pathogenic microorganisms would be required to activate B-1 cells to induce the autoimmune disease. In other words, in this Tg model, the expansion and activation of peritoneal B-1 cells may be induced by two steps: enteric bacteria increases the number of peritoneal B-1 cells and pathogenic infection induces peritoneal B-1 cells to produce autoAbs.
B-1 Cells, Infection, and Autoimmunity.
The anti-RBC Tg line clearly provided clear evidence for the involvement of B-1 cells and intestinal bacterial infection in autoimmunity. It remains to be seen whether this conclusion can be more generalized in other types of autoimmune diseases, although similar observations were reported for the EAE Tg model (4). It is also important to indicate that the generation of peritoneal B-1 cells is also dependent on bacterial infection in this Tg line. However, this finding contradicts a previous report that the number of peritoneal B-1 cells in GF BALB/c mice was the same as that of conventionally bred BALB/c mice (16). In fact, the H chain Tg mouse bred in the GF condition contained the same level of B-1 cells as anti-RBC Tg bred in the conventional condition (Fig. 1). There are two distinct properties of GF anti-RBC Tg mice as compared with GF BALB/c mice: autoAb-producing B-1 cells and clonal deletion of conventional B cells. The above discrepancy is likely due to the absence of conventional B cells in anti-RBC Tg, although it is totally unknown whether the presence of conventional B cells and their interaction with other immunocytes like B and T cells provide growth stimulation factors of self-reactive B-1 cells under the GF condition. It is intriguing that enteric bacteria can stimulate and activate the peritoneal B-1 cells. We previously showed that there is cell traffic between B-1 cells in the peritoneal cavity and the lamina propria of the gut (14). Bacterial polyclonal stimulants may directly stimulate B-1 cells in the lamina propria. Alternatively, bacterial infection activates other immune cells like T cells and dendritic cells, resulting in secretion of lymphokines such as IL-5 and IL-10 (17).
Acknowledgments
We thank Ms. K. Fukui and N. Nakahashi for their assistance in preparation of the manuscript. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan, and Japan Society for the Promotion of Science.
References
- 1.Izui S, Lambert PH, Fournie GJ, Trurler H, Miesher PA. Features of systemic lupus erythematosus in mice injected with bacterial lipopolysaccharides. J Exp Med. 1977;145:1115–1130. doi: 10.1084/jem.145.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 3.Oldstone MBA. Molecular mimicry and autoimmune diseases. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- 4.Govermann J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein–specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 5.Murakami K, Maruyama H, Hosono M, Shinagawa K, Yamada J, Kuribayashi K, Masuda T. Germ-free condition and the susceptibility of Balb/c mice to post-thymectomy autoimmune gastritis. Autoimmunity. 1992;12:69–70. doi: 10.3109/08916939209146132. [DOI] [PubMed] [Google Scholar]
- 6.Leter, E.H. 1990. The environmental factors in modulating insulin dependent diabetes. In The Role of Micro-organisms on Non-infectious Disease. 16th Argenteuil Symposium, Belgium. Springer Verlag, Brussels.
- 7.Herzenberg LA, Stall AM, Lalor PA, Sidman C, Moore WA, Parks DR, Herzenberg LA. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 8.Hardy RR, Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Hayakawa K. Generation of Ly-1 B cells from developmentally distinct precursors. Ann NY Acad Sci. 1992;651:99–111. doi: 10.1111/j.1749-6632.1992.tb24600.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K, Hardy RR, Stall AM, Herzenberg LA, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- 11.Marcos MAR, Huetz F, Pereira P, Andreu J-L, Martinez AC, Coutino A. Further evidence for coelomic associated B lymphocytes. Eur J Immunol. 1989;19:2031–2035. doi: 10.1002/eji.1830191110. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto M, Murakami M, Shimizu A, Ozaki S, Tsubata T, Kumagai S-i, Honjo T. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992;175:71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature (Lond) 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 14.Murakami M, Tsubata T, Shinkura R, Nisitani S, Okamoto M, Yoshioka H, Usui T, Miyawaki S, Honjo T. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, Honjo T. Involvement of B-1 cells in mucosal immunity and autoimmunity. Immunol Today. 1995;16:534–538. doi: 10.1016/0167-5699(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 16.Forster I, Muller W, Schittek B, Rajewsky K. Generation of long-lived B cells in germ free mice. Eur J Immunol. 1991;21:1779–1782. doi: 10.1002/eji.1830210732. [DOI] [PubMed] [Google Scholar]
- 17.Nisitani S, Tsubata T, Murakami M, Honjo T. Administration of interleukin-5 or -10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte. Eur J Immunol. 1995;25:3047–3052. doi: 10.1002/eji.1830251110. [DOI] [PubMed] [Google Scholar]