Abstract
The lytic activity of natural killer (NK) cells is inhibited by the expression of class I major histocompatibility complex (MHC) antigens on target cells. In murine NK cells, Ly-49A mediates inhibition of cytotoxicity in response to the class I MHC antigen H-2Dd. In this report, we studied the function of mouse Ly-49A in both the rat NK cell tumor line, RNK-16, transfected with Ly-49A cDNA, and in primary NK cells. We show that ligation of Ly-49A by H-2Dd inhibits early signaling events during target cell stimulation, including polyphosphoinositide turnover and tyrosine phosphorylation. We also show that Ly-49A directly associates with the cytoplasmic tyrosine phosphatase SHP-1, and that Ly-49A function is impaired in NK cells from SHP-1 mutant viable motheaten mice and from SHP-1–deficient motheaten mice. Finally, we demonstrate that mutational substitution of the tyrosine within the proposed SHP-1 binding motif in Ly-49A completely abrogates inhibition of NK cell cytotoxicity through this receptor. These results demonstrate that Ly-49A interrupts early activating signals in NK cells, and that SHP-1 is an important mediator of Ly-49A function.
NK cells and some T cells express a variety of type II transmembrane receptors characterized by extracellular C-type lectin domains (1). In mice, these proteins include the members of the Ly-49 family (2–8), which recognize MHC class I molecules on target cells (6–10), and the NKR-P1 family, whose physiologic ligands have yet to be determined (11–13). While the NKR-P1 molecules activate NK cell cytotoxicity (14–16), at least three members of the Ly-49 family inhibit NK cell function (6–8).
Susceptible targets stimulate phosphoinositide turnover, calcium mobilization, and the induction of protein tyrosine phosphorylation in NK cells. These signals have been associated with activation of NK cell cytotoxic responses (17–20). The NKR-P1 lectin-like receptor also transduces these activating signals in NK cells (14–16). In contrast, mouse Ly49A inhibits NK cell cytotoxicity upon ligation by the target cell MHC class I molecules H-2Dd or H-2Dk (6, 9, 10). The mechanisms by which Ly-49A interrupts NK cell activation are poorly understood, but important clues can be derived from structural motifs within the Ly-49A molecule.
The Ly-49A cytoplasmic domain contains the amino acid sequence VxYxxV, which constitutes a proposed binding motif for the cytoplasmic tyrosine phosphatase, SHP-1 (21, 22). SHP-1 is an SH2 domain containing tyrosine phosphatase, expressed in hematopoietic cells that can inhibit specific cellular functions. SHP-1 negatively modulates signaling through the erythropoietin receptor (23, 24), and it inhibits activation of B cells through its association with FcγRIIB1 (25–28). In human NK cells, SHP-1 has been implicated in inhibition of cytotoxicity through its association with the killer inhibitory receptors (KIRs)1, members of the Ig family that bind to human MHC class I molecules (21, 29, 30). The presence of a proposed SHP-1 binding motif in the cytoplasmic domain of Ly-49A suggests that this murine receptor may also functionally associate with SHP-1. A tyrosine-phosphorylated synthetic tridecapeptide derived from the cytoplasmic domain of Ly-49A has recently been shown to bind to SHP-1 and to the related phosphatase SHP-2, but the functional relevance of these findings to intact Ly-49A has not yet been examined (22). In this report, we demonstrate that ligation of Ly-49A interrupts early signals for NK cell activation, inhibiting tyrosine phosphorylation and polyphosphoinositide turnover. We also demonstrate that intact Ly-49A directly associates with SHP-1. Moreover, we show that the full inhibitory effect of Ly-49A in NK cells requires intact SHP-1 function as well as the tyrosine residue within the proposed SHP-1 binding site of Ly-49A.
Materials and Methods
Cells.
RNK-16, a spontaneous NK cell leukemia from F344 rats, was the gift of C. Reynolds (National Cancer Institute, Frederick, MD) and was adapted for in vitro growth in RPMI-1640 supplemented with 10% heat-inactivated FCS, 25 μM 2-ME, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete RPMI) (31). Tumor target cell lines cultured in complete RPMI included YAC-1 (mouse lymphoma, H-2a), P388D1 (mouse macrophage, H-2d), and C1498 (mouse monocyte, H-2b) from the American Type Culture Collection (Rockville, MD). D12 (C1498 transfected with H-2Dd, C1498.Dd), a gift from W. Yokoyama, was described previously (6). B-16S, a mouse melanoma line (H-2b), was a gift from K. Kärre (Karolinska Institute, Stockholm, Sweden).
Mice.
Viable motheaten mice C57BL/6 (mev/mev) mice were obtained from the Jackson Laboratory at 6 wk of age along with littermate heterozygotes (+/mev) and age-matched wild-type C57BL/6 (+/+) mice. Motheaten C57BL/6 (me/me) mice and littermate heterozygotes (+/me) were killed at 4–5 wk of age at the Jackson Laboratory, and isolated spleen cells were obtained.
Antibodies and Flow Cytometry.
mAbs to mouse Ly-49A (A1, mouse IgG2a), NK1.1 (PK136, mouse IgG2a), gp42 (3G7, mouse IgG2a), and phosphotyrosine (APT) (4G10, mouse IgG2b) were produced from their respective hybridoma lines. Antibodies were partially purified from ascites by ammonium sulfate precipitation. Tissue culture supernatant of anti–mouse Fc receptor antibody (2.4G2, rat IgG2b) was a gift from P. Linnemeyer (University of California at San Francisco, CA). Fluorescein conjugation of protein A-purified mAb utilized standard methods (32). F(ab′)2 fragments were generated by pepsin digestion, and undigested mAb was absorbed over protein A–Sepharose columns as described (32). Purity of F(ab′)2 fragments was verified by SDS-PAGE and silver staining. For fluorescence analysis, mAbs were used at a concentration of 1 μg/106 cells. Staining of IL-2–activated NK cells was performed using directly conjugated mAb in the presence of blocking antibodies to prevent binding to Fc receptors (1 μg/106 cells mouse IgG2a myeloma antibody [Cappel, Malvern, PA] in 0.1 ml 2.4G2 supernatant). Routine analysis was performed by using a FACScan®.
Cytotoxicity Assays.
Specific lysis of NK targets was determined by using a standard 4-h 51Cr-release assay as previously described (16). In brief, target cells were harvested and labeled for 1 h at 37°C with 200 μCi of sodium 51Cr (Amersham, Arlington Heights, IL) in complete RPMI. Labeled target cells were washed and resuspended at 105 cells/ml, and 0.1 ml of this cell suspension was added to each well of 96-well plates containing 0.1 ml of effector cells at the indicated effector to target ratios. Plates were incubated at 37°C for 4 h, then centrifuged for 5 min. 100 μl of supernatant was counted in a gamma counter. All assays were performed in triplicate. For antibody inhibition studies, effector cells were preincubated for 15 min at room temperature with F(ab′)2 at a concentration of 50 μg/106 effectors or with intact antibody at a concentration of 20 μg/106 effectors before the addition of targets.
IL-2–activated NK Cells.
IL-2–activated NK cells (LAK) were prepared from fresh splenocytes as previously described (33). Spleen cells harvested from me/me and +/me mice were transported from Jackson Laboratories in complete RPMI, after red cell lysis, and received within 24 h of death. Spleen cells were then passaged through nylon wool and placed in culture with IL-2, as with fresh splenocytes (33). Ly-49A positive and negative IL-2– activated NK cells were isolated as previously described (6). In brief, day 6 IL-2–activated NK cells were panned with the anti– Ly-49A mAb, A1. The purity of the Ly-49− NK cell population was ensured by treatment with anti–Ly-49A and rabbit anti–mouse Ig (Cappel, Malvern, PA), followed by rabbit complement (Cedarlane, Westbury, NY) for 1 h at 37°C. Ly-49− and Ly-49+ cell populations were then cultured overnight in complete RPMI supplemented with 1,000 U/ml human IL-2 (National Cancer Institute, Frederick, MD). Cells were washed extensively with BSS with 3% FCS on day 7, replated and used for assays on day 9. This resulted in populations of NK cells that were >95% pure as assessed by their expression of NK1.1.
cDNA Constructs and Transfections.
The construct for expression of Ly-49A in RNK-16 cells was prepared by subcloning the Ly-49A cDNA into the EcoRI site of the expression vector BSRαEN (A. Shaw and M. Olszowy, Washington University, St. Louis, MO). The Ly-49A/Y8F mutation was generated using sitedirected mutagenesis by PCR with the oligonucleotide 5′-ATATATGAATTCTCGAGATGAGT GAGCAGGAGGTCACTTTTTCAATGGTGAG-3′, which was cloned into the EcoRI site of BSRαEN. Constructs were confirmed by sequencing in both directions before transfection. Transfections were performed using cesium-purified plasmids or plasmids purified over two sequential Qiagen maxiprep tips according to the instructions of the manufacturer (Qiagen, Chadworth, CA). RNK-16 cells in exponential growth were transfected with 20 μg of ScaI-linearized plasmid DNA using a BTX-600 Electro Cell manipulator. Electroporation was performed using 3 × 106 cells/ml in 2-mm cuvettes in a total volume of 400 μl of complete RPMI, at 115 V, 850 μF, 129 ohms. Cuvettes were incubated on ice for 15 min after electroporation. Cells were cultured overnight, then plated in 96-well plates at a density of 104 cells/well in complete RPMI containing 1 mg/ml active G418 (Boehringer, Indianapolis, IN). G418-resistant cells grew out in 10–14 days in 5–10% of the wells. Transfected cells were maintained in 1 mg/ml active G418, but were grown in complete RPMI without G418 for at least 2 d before use in functional assays.
Measurement of Inositol–Trisphosphate.
To measure changes in water-soluble inositol trisphosphates (InsP3), cells were washed into inositol-free medium containing [3H]myoinositol (20 μCi/ml, 80–120 Ci/mmol, Amersham, Arlington Heights, IL) at a concentration of 5 × 106 cells/ml. After incubation at 37°C for 3 h, cells were washed in complete RPMI. Then 5 × 106 labeled effector cells were stimulated with 107 target cells in a total volume of 1 ml. Cells were immediately centrifuged at 50 g for 10 s and incubated at 37°C for the appropriate interval. [3H]InsP3 was extracted and resolved by ion exchange chromatography on Dowex AG-50, 1X-8 (Bio-Rad, Hercules, CA) as previously described (12).
Target Stimulation of [32P]-phosphate-labeled Effector Cells.
For 32P-metabolic labeling, effectors were incubated with 1 mCi/ml [32P]orthophosphate in phosphate-free RPMI supplemented with 10% dialyzed FCS, at 2 × 106 cells/ml for 90 min at 37°C. After labeling, cells were washed once in phosphate-free RPMI and used immediately for stimulation. In each sample, 107 labeled effector cells were stimulated with 107 unlabeled target cells in a total volume of 1 ml complete phosphate-free RPMI. Cell suspensions were immediately centrifuged for 10 s at 50 g, then incubated at 37°C for the indicated time, after which cells were rapidly centrifuged at 500 g and cell pellets were resuspended in cold HNTG lysis buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 10% glycerol, 1 mM sodium orthovanadate, and protease inhibitors) with 1% Triton X-100. Lysates were precleared with protein A–Sepharose beads (Pharmacia, Piscataway, NJ) that had been previously coated with 1 ml 2.4G2 supernatant, for 2 h at 4°C, then immunoprecipitated with 30 μl of protein A beads coated with 5 mg APT mAb (4G10) overnight at 4°C. After washing with cold HNTG buffer with 1% Triton X-100, immunoprecipitates were resolved by 8% SDS-PAGE under reducing conditions. Gels were stained, fixed, and dried, and labeled proteins were resolved by autoradiography.
Immunoprecipitations and Western Blotting.
Where indicated, transfected and wild-type RNK-16 cells (1.5 × 107 cells/ sample) were incubated for 5 min at 37°C in complete RPMI (6 × 105 cells/ml) with 0.03% H2O2 and 100 μM sodium orthovanadate (pervanadate), which pharmacologically increases protein tyrosine phosphorylation by inhibiting phosphatase activity (34). Cell pellets were lysed at 4°C in complete HNTG lysis buffer containing 1% Triton X-100. Lysates were precleared for 2 h at 4°C on Protein A–Sepharose beads that had been loaded with 10 μg control mAb (mouse IgG2a myeloma protein, Cappel, Malvern, PA). Lysates were then immunoprecipitated overnight at 4°C on 30 μl protein A–Sepharose beads loaded with anti–Ly-49A (A1) or isotypematched control mAb (anti-NK1.1). Precipitates were washed four times with complete HNTG buffer containing 0.1% Triton X-100. Precipitated samples were resolved by 8% SDS-PAGE under nonreducing conditions and transferred to PVDF membranes (Immobilon-P, Millipore, Marlborough, MA). After blocking with TBS-T (10 mM Tris, pH 8, 150 mM NaCl, 0.05% Tween-20) and 3% milk, the membranes were incubated with 0.5 μg/ml of anti–SHP-1 polyclonal rabbit antibody (UBI, Lake Placid, NY) in TBS–Tween with 3% milk for 1 h at room temperature. After extensive washing in TBS-T, blots were developed using 125I–protein A (Amersham, Arlington Heights, IL) followed by autoradiography.
Cold-target Inhibition Studies.
Cytotoxicity assays to measure cold-target inhibition were performed at an E/T ratio of 10:1. 105 cold targets were added to effectors at the same time as 104 labeled targets. Effectors were preincubated with F(ab′)2 fragments at 25 μg/106 cells for 15 min at room temperature before addition of targets when indicated. Results are expressed as percent inhibition = (1 − [percent cytotoxicity with cold target/percent cytotoxicity without cold target]) × 100.
Results
Mouse Ly-49A Inhibits Killing of H-2d Targets by RNK-16.
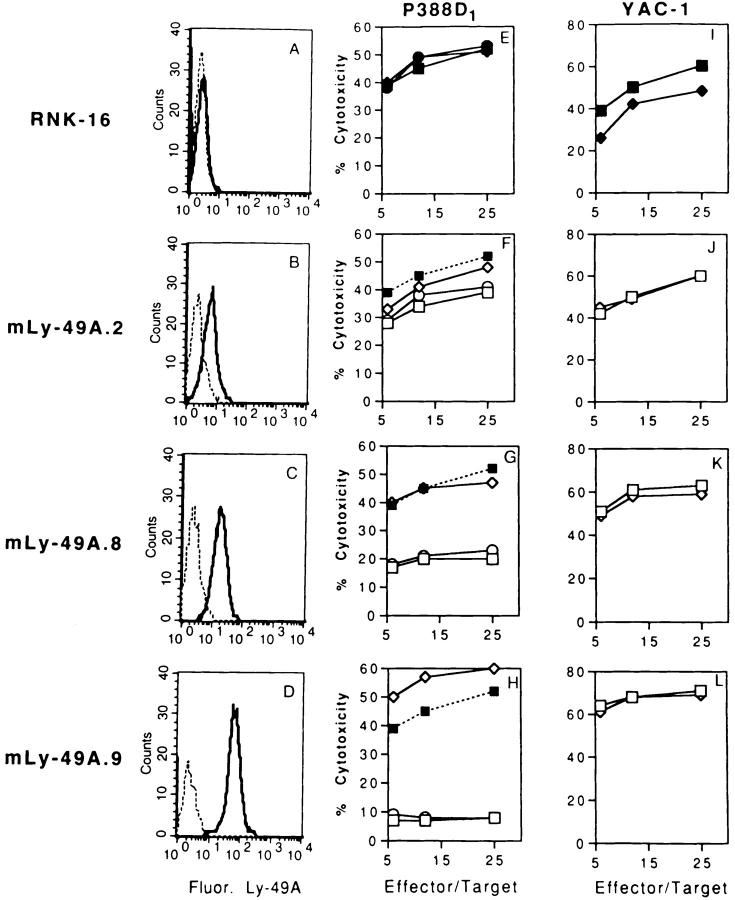
To examine the intracellular signaling pathways that mediate the inhibitory function of mouse Ly-49A, we transfected mouse Ly-49A into RNK-16, a rat cell line with phenotypic and functional characteristics of rat NK cells (31). Nine RNK-mLy-49A clones expressing Ly-49A at different levels were obtained, three of which are represented in Fig. 1. Clones 2, 8, and 9 are representative of clones with low, medium, or high expression of Ly-49A, respectively (Fig. 1, B, C, D). Wild-type RNK-16 effector cells lysed P388D1 (H-2Dd) tumor cells (Fig. 1 E), but RNK-16 cells transfected with Ly-49A (RNK-mLy-49A cells) demonstrated reduced lysis of P388D1 (F, G, H). Inhibition of lysis of P388D1 cells was proportional to the level of Ly-49A expression on the RNK-16 transfectants. RNK-mLy-49A.2 had low expression and demonstrated only minimal inhibition of lysis (Fig. 1 F), whereas RNK-mLy49A.8 and RNK-mLy-49A.9, with progressively higher levels of expression, demonstrated marked inhibition of lysis (G and H). Inhibition of lysis in Ly-49A transfectants was reversible by the addition of anti–Ly-49A F(ab′)2 fragments, while control F(ab′)2 fragments (anti-NK1.1) had no effect. Addition of intact antibody had the same effect as F(ab′)2 fragments (data not shown). YAC-1 targets have previously been shown to be susceptible to lysis by mouse Ly-49A+ NK cells (6). YAC-1 target cells were lysed equally well by wild-type RNK-16 cells and by RNK-16 cells expressing Ly-49A at various levels, and lysis of YAC-1 targets was not altered by the presence of antibodies to Ly-49A (Fig. 1, I–L). These studies demonstrate that expression of Ly-49A renders RNK-16 cells ineffective in the killing of H-2Dd targets. The data indicate that the clonal RNK-mLy-49A transfectants behave similarly to freshly isolated murine Ly49A+ NK cells, and that they are a valid model in which to study Ly-49A function.
Figure 1.
Inhibition of lysis of P388D1 (H-2Dd) targets correlates with the level of expression of mouse Ly-49A on RNK-16 cells. The various levels of Ly-49A expression are shown in FACS® histograms (A–D). Cells were incubated with anti–Ly-49A (solid line) with FITC–goat anti–mouse Ab (FITC–GAM) or FITC-GAM alone (dotted line). Standard 4-h cytotoxicity assays were performed with either P388D1 cells (E–H) or YAC-1 targets (I–L). Effectors were wild-type RNK-16 cells (closed symbols) or RNK-16 transfected with Ly-49A (open symbols). Effector cells used were RNK-16 (A, E, and I) and clones of RNK-16 expressing Ly-49A: low expression, RNK-mLy-49A.2 (B, F, J); intermediate expression, RNKmLy-49A.8 (C, G, K); and high expression, RNK-mLy-49A.9 (D, H, L). Assays were carried out in the absence of antibody (squares), or in the presence of anti-Ly-49A F(ab′)2 (diamonds) and control F(ab′)2 (antiNK1.1) (circles). The dotted line in F–H is the killing curve for wild-type RNK-16 without mAb (from E for comparison).
Ly-49A Inhibits Phosphoinositide Turnover in Response to H-2Dd Target Cells.
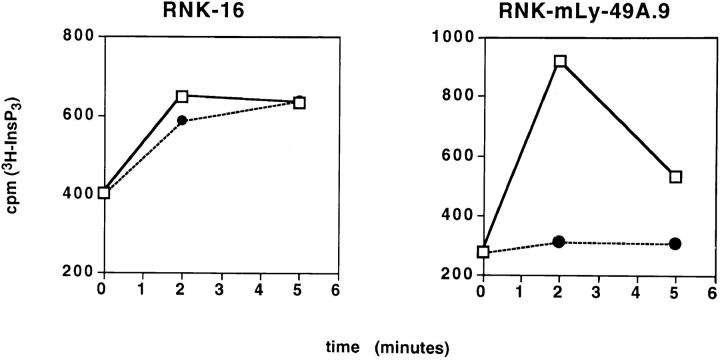
Using the RNK-mLy-49A.9 cell line, we examined the inositol phosphate response following stimulation by YAC-1 (sensitive) and P388D1 (resistant) targets. Wild-type RNK-16, but not RNK-mLy-49A.9 cells, responded to the H-2Dd target P388D1 with a rapid increase in InsP3 at 2 min, as shown in Fig. 2. YAC-1, which is susceptible to lysis by both wild-type RNK-16 and RNK-mLy-49A.9, stimulates an InsP3 response in both cell types. The lack of phosphoinositide turnover in RNKmLy-49A.9 in response to H-2Dd target cells indicates that Ly-49A interrupts proximal signaling events in RNK-16.
Figure 2.
Ly-49A inhibits phosphoinositide turnover in response to H-2Dd target cells. RNK-mLy-49A.9 cells fail to generate InsP3 upon stimulation with P388D1 targets. [3H]myoinositol-labeled RNK-16 and RNK-mLy-49A.9 effectors (5 × 106 cells) were stimulated with 107 targets in a total volume of 1 ml at 37°C. Soluble InsP3 was resolved by ion exchange chromatography. A brisk rise in InsP3 was seen in RNK-16 cells (left) in response to either YAC-1 (open squares) or P388D1 (H-2Dd) (closed circles). Phosphoinositide turnover in RNK-mLy-49A.9 (right) was stimulated by YAC-1, but not by P388D1.
Ly-49A Inhibits an Early Increase in Protein Tyrosine Phosphorylation during Target Cell Stimulation.
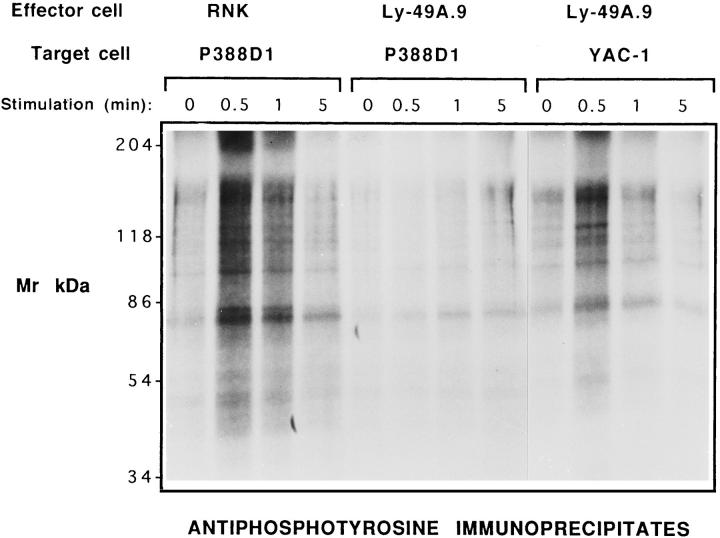
To examine the effect of Ly-49A–mediated inhibition on protein tyrosine phosphorylation, [32P]orthophosphate-labeled RNK-16 and RNK-mLy-49A.9 cells were stimulated with YAC-1 (H-2a) and P388D1 (H-2Dd) target cells. Because Ly-49A interrupts InsP3 turnover, an early signaling event, we examined the effect of target stimulation at 30 s, 1 min, and 5 min time points. Lysates from cells stimulated for these time intervals were immunoprecipitated with APT (4G10) and resolved by SDS-PAGE (Fig. 3). RNK-16 cells stimulated with P388D1 (H-2Dd) showed a rapid increase in protein tyrosine phosphorylation at 30 s to 1 min, which diminished toward basal levels at 5 min (Fig. 3, left). In contrast, RNK-mLy-49A.9 cells stimulated with P388D1 failed to show an increase in protein tyrosine phosphorylation at 30 s to 1 min, but showed a minimal increase at 5 min (Fig. 3, center). Nonetheless, RNK-mLy-49A.9 cells demonstrated rapid protein tyrosine phosphorylation in response to YAC-1 after 30 s, demonstrating that this signaling pathway was intact (Fig. 3, right). Wild-type RNK-16 showed a similar response to YAC-1 target cells (data not shown). Thus, susceptible, but not resistant, targets induce very brisk increases in protein tyrosine phosphorylation, and mouse Ly-49A specifically interrupts rapid tyrosine phosphorylation in response to P388D1 (H-2Dd) targets.
Figure 3.
Ly-49A inhibits an early rise in tyrosine phosphorylation induced by target cell stimulation. Tyrosine phosphorylation of proteins in RNK-16 cells stimulated with P388D1 cells is shown on the left side of the figure. RNK-mLy-49A.9 cells stimulated with P388D1 cells are in the center, and RNK-mLy-49A.9 cells stimulated with YAC-1 cells are on the right. Target cell stimulation time points were 0, 0.5, 1.0, and 5.0 min. RNK-16 and RNKmLy-49A.9 effector cells were metabolically labeled with 32Porthophosphate. After washing, 107 labeled effector cells and 107 unlabeled target cells were stimulated in a total volume of 1 ml complete phosphate-free RPMI with a brief 50 g contact spin, followed by incubation at 37°C for the indicated time. Cells were then immediately lysed in cold HNTG buffer with 1% Triton X-100. Clarifed precleared cell lysates were immunoprecipitated with APT (4G10), washed, and resolved by reducing 8% SDS-PAGE. Gels were dried and developed by autoradiography.
SHP-1 Directly Associates with Mouse Ly-49A.
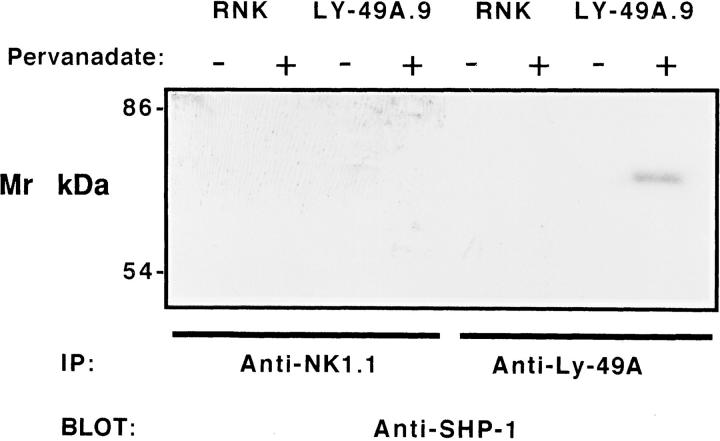
In other lymphoid cells, SHP-1 has been shown to interrupt early tyrosine phosphorylation events. The mouse Ly-49A cytoplasmic domain includes the proposed SHP-1 binding motif VxYxxV (21, 22). These structural features of Ly-49A, and the results obtained in Fig. 3, suggested that Ly-49A– dependent inhibition of NK cell function might be mediated by SHP-1. Therefore, we first investigated the binding of SHP-1 to Ly-49A in RNK-16 cells by performing immunoprecipitation experiments. RNK-16 and RNK-mLy49A.9 cells were stimulated with pervanadate, a phosphatase inhibitor that pharmacologically increases protein tyrosine phosphorylation (34). Lysates from stimulated and unstimulated cells were precipitated with anti–Ly-49A, and precipitates were examined for the presence of Ly-49A–associated SHP-1 by Western blot analysis. As shown in Fig. 4, SHP-1 was present only in anti–Ly-49A immunoprecipitates from pervanadate-stimulated RNK-mLy-49A.9 cells. SHP-1 was not detected in anti–Ly-49A immunoprecipitates from unstimulated RNK-mLy-49A.9 cells or in isotype-matched control mAb immunoprecipitates from unstimulated or stimulated RNK-mLy-49A.9 cells. SHP-1 was not detected in anti–Ly-49A or control mAb immunoprecipitates from RNK-16 cells, regardless of stimulation.
Figure 4.
The tyrosine phosphatase SHP-1 associates with mouse Ly-49A in pervanadatestimulated RNK-16 cells. 1.5 × 107 unstimulated or pervanadatestimulated RNK-16 cells or RNK-mLy-49A.9 cells were incubated in complete RPMI for 5 min at 37°C, washed, and lysed in cold HNTG lysis buffer containing 1% Triton X-100. Clarified precleared lysates were immunoprecipitated with anti–Ly-49A (A1, lanes 5–8) or isotypematched control mAb (NK1.1, PK136, lanes 1–4), washed, and resolved by 8% SDS-PAGE under nonreducing conditions. Proteins were transferred to PVDF membranes, immunoblotted with anti-SHP-1 antiserum, and developed with 125I–protein A followed by autoradiography.
Ly-49A Function Is Impaired in NK Cells from Viable Motheaten and Motheaten Mice.
To examine the role of SHP-1 in mouse Ly-49A–mediated inhibition, we isolated Ly49A+ and Ly-49A− IL-2–activated NK cells from SHP-1 mutant mice. These included viable motheaten (mev/mev) mice, which have an incomplete defect in SHP-1, and the completely SHP-1–deficient motheaten (me/me) mice. We first examined NK cells from SHP-1 mutant viable motheaten (mev/mev) mice because they survive to 8–9 wk of age, when NK cell development is complete. These mice contain a point mutation in the SHP-1 gene that destroys a donor splice site. This mutation results in aberrant splicing, creating either an in-frame insertion or deletion in the catalytic domain of the SHP-1 phosphatase (35–37). Although SHP-1 activity is reduced, some residual SHP-1 function remains and mev/mev mice have an attenuated motheaten phenotype.
We isolated Ly-49A+ and Ly-49A− NK cells from homozygous mev/mev, heterozygous +/mev, and wild-type C57BL/6 +/+ mice. Using these effectors, we tested Ly49A function in cytotoxicity assays against C1498 (H-2b) cells and D12 (C1498.Dd) cells as targets. Ly-49A+ cells from all mice expressed Ly-49A at similar levels and >95% of all cells were positive for NK1.1 and negative for CD3 by FACS® (data not shown).
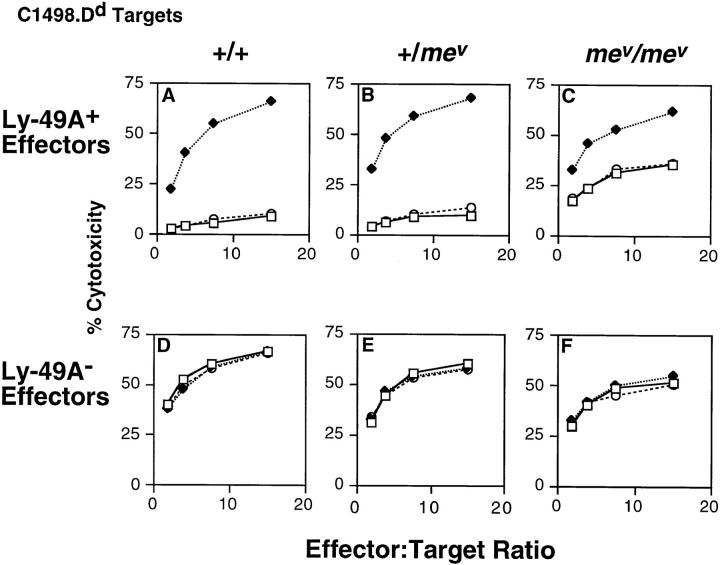
In each of four experiments, with homozygous mev/mev mice the function of Ly-49A was impaired in that C1498.Dd targets were lysed by Ly-49A+ effectors, albeit less effectively than by Ly-49A− effectors. However, Ly-49A remained partially effective in cells from mev/mev mice in that addition of anti–Ly-49A still increased lysis of the C1498.Dd targets. Fig. 5 shows a representative experiment in which Ly-49A+ cells from wild-type C57BL/6 +/+ or from heterozygous +/mev mice were unable to lyse C1498.Dd targets (A and B), but Ly-49A+ cells from homozygous mice were able to lyse these targets (C). Ly-49A− cells from all mice were able to lyse C1498.Dd equally well (Fig. 5, D–F). Addition of anti–Ly-49A mAb reversed the Ly-49A–mediated inhibition to levels similar to those of Ly-49A− cells from all mice, whereas isotype-matched control anti-gp42 mAb had no effect. Ly-49A+ and Ly-49A− cells from all mice were able to lyse the H-2b target C1498 and addition of mAb anti–Ly-49A or anti-gp42 had no effect (data not shown). These findings indicate that the function of Ly49A is partially impaired in IL-2–activated NK cells isolated from homozygous mev/mev mice.
Figure 5.
Ly-49A function is impaired in mev/mev LAK cells. 9-d Ly-49A+ and Ly-49A− LAK cells were tested in 4-h cytotoxicity assays against D12 (C1498.Dd) targets against Ly-49A+ effector cells (A–C) or Ly-49A− effector cells (D–F) from +/+ (A and D), +/mev (B and E), or mev/mev mice (C and F). Assays were done in the absence of antibody (open squares), or in the presence of anti–Ly-49A (A1, closed diamonds), or isotype-matched control antibody (anti-gp42, 3G7, open circles).
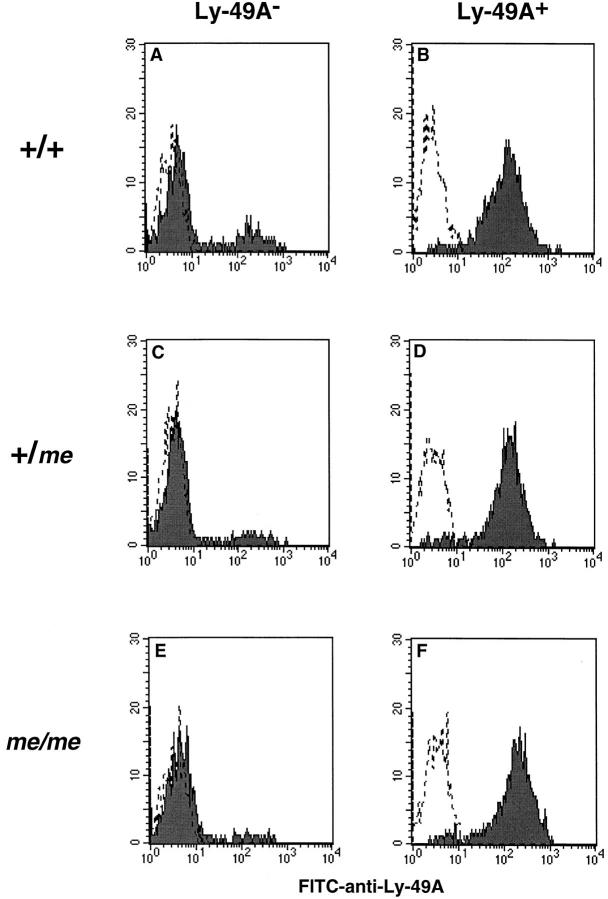
The partial function of Ly-49A in mev/mev mice could reflect the residual activity of SHP-1 in these mice. To examine the effect of the complete absence of SHP-1, we next examined Ly-49A function in IL-2–activated NK cells isolated from me/me mice. Using spleen cells harvested from me/me mice (sacrificed just before their natural demise), +/me littermate heterozygote controls, and wild-type +/+ mice, we isolated Ly-49A+ and Ly-49A− cells. As shown in Fig. 6 (B, D, F), Ly-49A expression was equivalent on cells isolated from all mice. Ly-49A− cells (Fig. 6, C and E) contained <5% Ly-49A+ cells, whereas the +/+ Ly-49A− population (A) contained ∼10% Ly-49A+ cells. >95% of all cells were positive for NK1.1 and negative for CD3 by FACS® (data not shown).
Figure 6.
Ly-49A expression on Ly-49A+ and Ly-49A− LAK cells isolated from +/+, +/me, me/me mice. 6-d LAK cells were separated into Ly-49A+ and Ly-49A− populations by panning with anti–Ly-49A Ab. Ly-49A− cells were additionally treated with rabbit anti–mouse Ab and complement depletion. FACS® analysis was performed on day 9 LAK cells using FITC–anti-Ly-49A (A1). Staining was performed in the presence of unlabeled blocking antibodies (IgG2a mouse myeloma protein, 1 μg/106 cells in 0.1 ml 2.4G2 supernatant). Ly-49A expression is shown in FACS® histograms (A–F). Dotted lines represent cells incubated with saline; solid lines represent FITC–anti-Ly-49A staining.
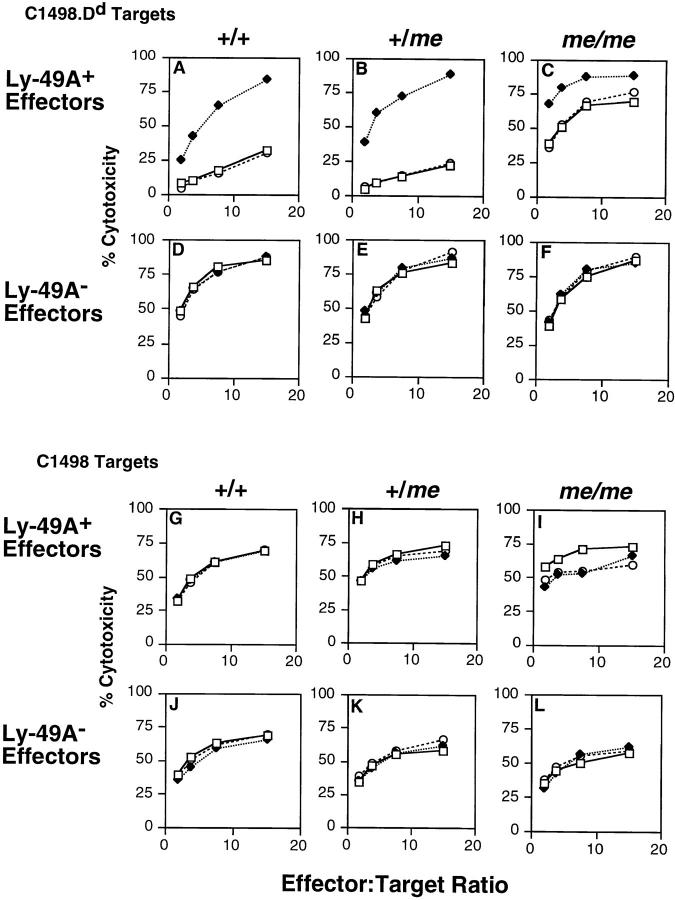
As shown in Fig. 7, Ly-49A function was significantly impaired in NK cells isolated from me/me mice, but, as in mev/mev mice, it was not completely absent. Ly-49A+ cells from wild-type +/+ or from heterozygous +/me were unable to lyse C1498.Dd targets (A and B), but Ly-49A+ cells from me/me mice were able to lyse these targets (C), albeit less effectively than Ly-49A− cells. Ly-49A− cells from all mice were able to lyse C1498.Dd targets equally well (Fig. 7, D–F). Addition of anti–Ly-49A mAb reversed Ly-49A–mediated inhibition of Ly-49A+ cells from all mice (Fig. 7, A–C), whereas control mAb had no effect. Ly-49A+ and Ly-49A− cells from all mice were able to lyse the H-2b target C1498 (Fig. 7, G–L), and addition of anti–Ly-49A or control mAb had no effect. These findings indicate that Ly-49A is functionally impaired in NK cells isolated from homozygous me/me mice. However, even in the complete absence of SHP-1, Ly-49A had some remaining inhibitory activity.
Figure 7.
Ly-49A function is impaired in me/me LAK cells. 9-d Ly-49A+ and Ly-49A− LAK cells were tested in 4-h cytotoxicity assays against D12 (C1498.Dd) and C1498 (H-2b) targets. D12 targets (A–F) and C1498 targets (G–L) were tested against Ly-49A+ effector cells (A–C and G–I) or Ly-49A− effector cells (D–F and J–L) from +/+ (A, D, G, J), +/me (B, E, H, K), or me/me mice (C, F, I, L). Assays were done in the absence of antibody (open squares), or in the presence of anti–Ly-49A (A1, closed diamonds), or isotype-matched control antibody (anti-gp42, 3G7, open circles).
The Position 8 Tyrosine Is Required for Mouse Ly-49A Function.
To examine further the role of SHP-1 in the function of Ly-49A, we mutated the tyrosine residue within the proposed SHP-1 binding motif in the Ly-49A cytoplasmic domain. RNK-16 cells transfected with this tyrosine mutant, (RNK-mLy-49A/Y8F) stained with anti– Ly-49A mAb at levels similar to those seen in RNK-mLy49A.9 (Fig. 8, D, F, H). Despite this level of Ly-49A expression, RNK-mLy-49A/Y8F clones 1 and 4, derived from separate transfections, were not inhibited in their capacity to lyse P388D1 (H-2Dd) cells (Fig. 8, E and G). Consistent with our earlier results, RNK-mLy-49A.9 cells could not lyse P388D1 (Fig. 8 C), whereas wild-type RNK-16 lysed P388D1 efficiently (A). Notably, neither anti–Ly-49A nor control mAb (anti–NK1.1) had any effect on lysis of P388D1 by RNK-mLy-49A/Y8F (Fig. 8, E and G), but anti–Ly-49A reversed the inhibition of P388D1 lysis by RNK-mLy-49A.9 (C). RNK-mLy-49A/Y8F clones 1 and 4 effectively lysed YAC-1 targets (data not shown). These data show that the tyrosine within the proposed immunoreceptor tyrosine-based inhibitory motif (ITIM) is required for the inhibitory effect of Ly-49A on RNK-16 cytotoxicity. These experiments complement the studies of motheaten mice, demonstrating a functional role for SHP-1 in the inhibitory activity of Ly-49A.
Figure 8.
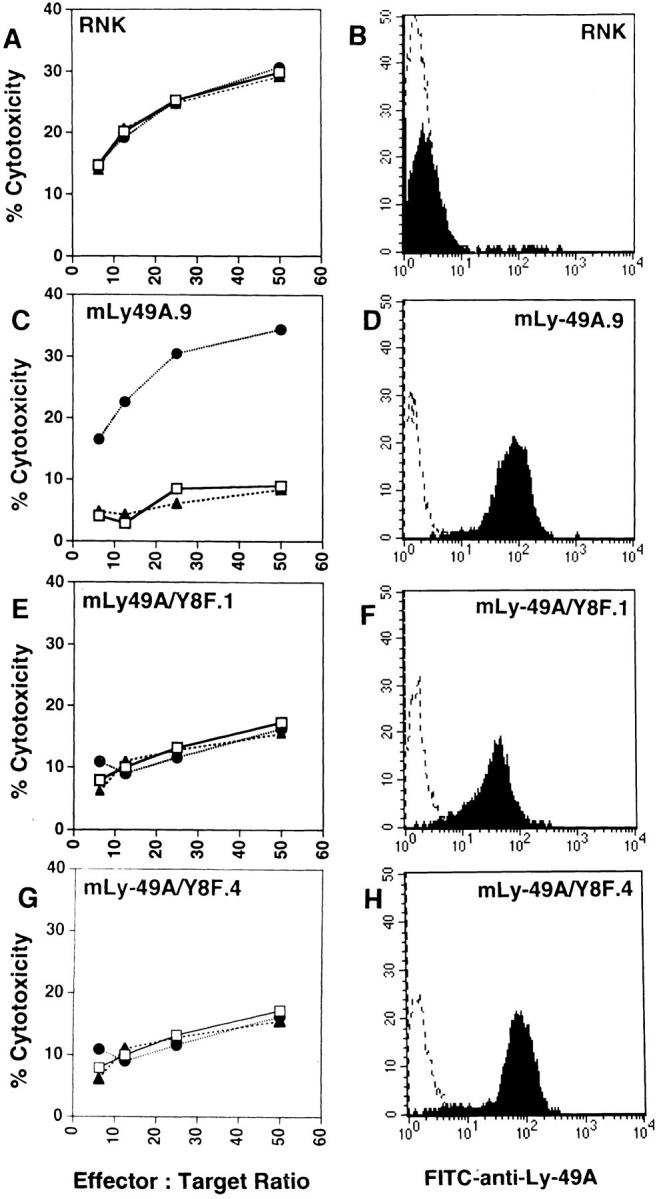
Lysis of P388D1 (H-2Dd) cells is not altered in RNK-mLy49A/Y8F transfectants. Ly-49A expression on RNK transfectants was assessed by staining cells with either saline (dotted line) or FITC–anti-Ly-49A (solid line). FACS® histograms show Ly-49A expression in wild-type RNK-16 (B), RNK-mLy-49A.9 (D), RNK-mLy-49A/Y8F.1 (F) and RNK-mLy-49A/Y8F.4 (H). Standard 4-h cytotoxicity assays were performed using P388D1 (H-2Dd) as targets. Effector cells were wild-type RNK-16 (A), RNK-mLy-49A.9 (C), RNK-mLy-49A/Y8F.1 (E), or RNK-mLy-49A/Y8F.4 (G). Effectors were preincubated with either media alone (open squares), anti-Ly-49A (closed circles), or isotype-matched control antibody (anti-NK1.1, PK136, closed triangles), before addition of targets.
Addition of H-2Dd (Resistant) Targets Does Not Affect the Killing of Non-H-2Dd (Susceptible) Targets by RNK-mLy49A.9.
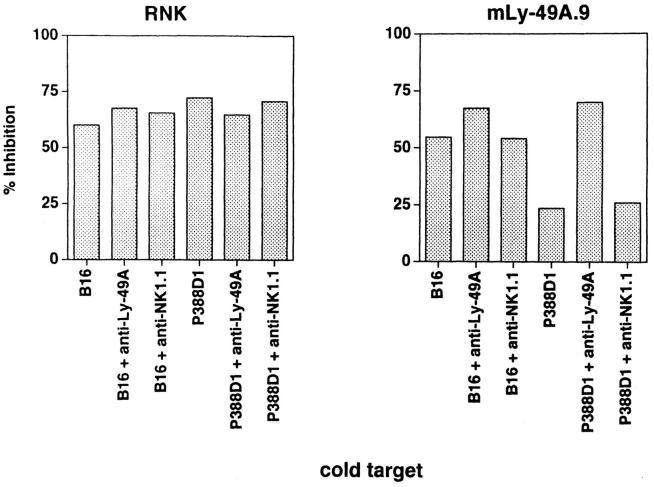
Initial reports demonstrated that ligation of H-2Dd targets by the Ly-49A receptor resulted in inhibition of natural killing, of antibody-dependent cellular cytoxicity (ADCC), and of lectin-induced cytotoxicity by NK cells (6, 38). These studies, performed on bulk populations of Ly-49A+ NK cells, suggested that Ly-49A might transduce signals that globally inhibit NK cell function. Using our RNK-Ly-49A transfectants, we were able to examine mouse Ly-49A function in a clonal cell population. To examine the effect of inhibitory H-2Dd targets on the lysis of labeled bystander non–H-2Dd targets, we performed cold-target competition experiments with wild-type RNK-16 or RNK-mLy-49A.9 as effectors, and 51Cr-labeled B-16S (H-2b) melanoma target cells. The effect of unlabeled H-2Dd or non–H-2Dd targets on lysis of B-16S was determined for each effector (Fig. 9). As expected, unlabeled B-16S (H-2b) were effective cold-target competitors for the lysis of 51Cr-labeled B-16S by both RNK-16 and by RNK-mLy-49A.9. Unlabeled P388D1 (H-2Dd) targets, which are sensitive to lysis by RNK-16, effectively inhibited B-16S lysis by RNK-16. In contrast, P388D1, which was not killed by RNK-mLy49A.9, was relatively ineffective as a cold-target inhibitor of B-16S lysis by RNK-mLy-49A.9. In the presence of F(ab′)2 anti–Ly-49A, P388D1 became sensitive to lysis by RNKmLy-49A.9. This allowed cold P388D1 cells to compete effectively with labeled B-16S for the lytic machinery of RNK-mLy-49A.9. Control F(ab′)2 anti-NK1.1 fragments had no effect on cold-target competition.
Figure 9.
Cold-target competition of unlabeled P388D1 and B-16S in the lysis of labeled B-16S targets. 4-h cytotoxicity assays were performed with RNK-16 (left) or RNK-mLy-49A.9 cells (right) as effectors. B-16S (H-2b) target cells were labeled with 51Cr and tested at an effector to labeled target ratio of 10:1. Cold targets were either unlabeled B-16S cells (H-2b) or P388D1 cells (H-2Dd). Cold targets were added in 10-fold excess to labeled targets. 105 cold targets and 104 labeled targets were added at the same time to 105 effectors in a total volume of 0.2 ml. Effectors were preincubated with no antibody, F(ab′)2 anti–Ly-49A or F(ab′)2 antiNK1.1. Results are expressed as percent inhibition = (1 − [percent cytotoxicity with cold target/percent cytotoxicity without cold target]) × 100.
Thus, the expression of H-2Dd on bystander targets does not globally inhibit the Ly-49A+ effector cell response towards a non–H-2Dd cell. Rather, because H-2Dd cells are not susceptible to NK cell lysis by Ly-49A+ effectors, they compete less well than susceptible (B-16S) cold targets for the lysis of labeled B-16S. These data indicate that there is no bystander inhibition through Ly-49A. They suggest that inhibitory effects mediated through this receptor are spatially oriented toward ligand-bearing H-2Dd targets on the NK cell membrane, and this does not affect killing of susceptible targets recognized by the same NK cell. Alternatively, the duration of Ly-49A–mediated inhibition could be brief, and temporally limited to periods of NK cell contact with H-2Dd targets. Temporal restriction would allow the subsequent lysis of susceptible H-2b targets by the same NK cell.
Discussion
In an attempt to elucidate the pathways through which Ly-49 molecules inhibit natural killing, we examined mouse Ly-49A, which prevents NK cell lysis of targets expressing H-2Dd or H-2Dk (6). To study Ly-49A function in a uniform clonal NK cell population, we transfected the rat NK cell line RNK-16 with the mouse Ly-49A cDNA. Ly-49A was functional in these RNK-16 transfectants, specifically inhibiting lysis of the H-2Dd target P388D1.
Because activation of cytotoxicity is associated with phosphoinositide turnover and an increase in protein tyrosine phosphorylation in NK cells, we examined the effect of Ly-49A ligation on these early signaling events during target-induced NK cell activation. Rapid rises in InsP3 were stimulated in wild-type RNK-16 cells in response to P388D1 (H-2Dd) targets, but the expression of Ly-49A on RNK-16 cells prevented this response. Ligation of Ly-49A also inhibited target cell–induced protein tyrosine phosphorylation in response to P388D1 targets. In contrast, YAC-1 targets stimulated a prompt increase in protein tyrosine phosphorylation in wild-type RNK-16 cells and in RNK-mLy-49A.9 cells.
The ability of mouse Ly-49A to inhibit the generation of InsP3 is similar to the effect previously shown in human NK cells, where inhibition of inositol phosphate turnover parallels the inhibition of NK cell cytotoxicity by class I molecules (39). Although no crosshybridizing human Ly-49 homologues have yet been identified, human NK cells express a different family of KIRs (40). The structure of KIRs, which are type I immunoglobulin-like receptors, is not related to that of Ly-49A. However, like Ly-49A, the KIRs inhibit NK cell cytotoxicity upon ligation by specific MHC class I antigens on target cells (41–46).
Initial reports of human NK cells indicated that protein tyrosine phosphorylation was not inhibited by the expression of MHC class I on targets (29, 39). One study examined target-induced phosphorylation in NK cells stimulated for 1, 5, and 30 min, and another study examined NK cells stimulated for 5 min (29, 39). In these human studies, there were no appreciable differences in levels of phosphorylation when NK cells were stimulated with susceptible or resistant targets. In our RNK-mLy-49A.9 transfectant, we also could detect no significant differences in protein tyrosine phosphorylation at 5 min (Fig. 3), or at 15 and 30 min (data not shown) after target cell stimulation with either sensitive or resistant targets. However, we were able to demonstrate clearly that rapid (30–60 s) protein tyrosine phosphorylation is markedly reduced when Ly-49A mediates inhibition of cytotoxicity. The inhibitory effects of Ly49A on phosphoinositide turnover and on tyrosine phosphorylation indicate that Ly-49A interrupts signaling events early in target cell–induced activation of NK cells.
A possible mechanism for the inhibitory activity of Ly49A was suggested by the observation that several other inhibitory receptors bind to SHP-1, a cytoplasmic tyrosine phosphatase (47–50). In other hematopoetic cells, SHP-1 has been implicated in the downregulation of signals for cellular activation. Thus, SHP-1 inhibits activation through the erythropoietin receptor, and erythropoietin receptors mutated at the SHP-1 binding site exhibit prolonged activation in response to ligand (23, 24). In B cells, activation through the immunoglobulin receptor is inhibited by coligation with FcγRIIB1 and this effect correlates with SHP-1 binding to FcγRIIB1 (25–28). SHP-1 binding has also been correlated with inhibition of human NK cell function (21, 29, 30). The KIRs associate with SHP-1 in human NK cells (21, 29, 30), and expression of a dominant negative mutant SHP-1 molecule in a human NK clone inhibits KIR function (21). Thus, in NK cells and in other immune cells, SHP-1 has been functionally implicated in the inhibition of cellular activation signals.
Because of this inhibitory effect, the binding domains for SHP-1 on immune receptors have been termed ITIMs. As originally proposed, the ITIM motif was described as S/TxxYxxL (25), but the KIR motifs are V/IxYxxL, and phosphopeptides containing this motif have been shown to bind to SHP-1 (21, 22, 29). Similar motifs, constrained by the sequence VxYxxV/L are found in Ly-49A, in the inhibitory receptors Ly-49C and Ly-49G2, and in the functionally uncharacterized murine molecules Ly-49B, E, F, G1, and G3. Interestingly, mouse Ly- 49D, which has been implicated in NK cell activation (rather than inhibition), lacks an ITIM motif as does the functionally uncharacterized molecule Ly-49H (2, 5, 51). Previous studies by Olcese et al. (22) showed that a synthetic, tyrosine-phosphorylated, 13amino acid peptide derived from the mouse Ly-49A cytoplasmic domain binds SHP-1 in vitro. In the present experiments, we have demonstrated that intact mouse Ly-49A also binds to SHP-1 in pharmacologically stimulated RNK16 transfectants.
In mouse NK cells, we examined the functional importance of the Ly-49A/SHP-1 association. To clarify the role of SHP-1 in NK cell inhibition through Ly-49A, we examined NK cells from viable motheaten (mev/mev) and motheaten (me/me) mice. Consistent with previous reports, the lysis of YAC-1 targets by mev/mev or me/me NK cells was somewhat diminished compared with lysis by wild-type, heterozygous (+/mev or +/me) NK cells (data not shown) (52). However, lysis of C1498 target cells by mev/mev or me/me NK cells was essentially normal. When compared with C1498 (H-2b), lysis of D12 (C1498.Dd) was almost completely inhibited by Ly-49A in NK cells from wildtype or from heterozygous (+/mev or +/me) mice. By contrast, Ly-49A-mediated inhibition of C1498.Dd lysis in both mev/mev and me/me NK cells was significantly impaired. We initially thought that incomplete impairment of Ly-49A function in mev/mev mice might be due to the incomplete loss of SHP-1 activity in these mice. However, Ly-49A also retained partial activity in NK cells from me/me mice, which are completely deficient in SHP-1. This implies that other cytoplasmic mediators must also be capable of facilitating inhibition by Ly-49A. One candidate mediator is the structurally related tyrosine phosphatase SHP-2, which, like SHP-1, has been shown to bind to a tyrosinephosphorylated peptide containing the ITIM of Ly-49A (22). SHP-2 has recently been shown to associate with the CTLA-4 receptor on T cells, suggesting a role for SHP-2 in the down regulation of T cell activation (53). In support of a role for SHP-2, we have recently been able to demonstrate an association between intact Ly-49A and SHP-2 in anti–Ly-49A immunoprecipitates from pervanadate-stimulated RNK-mLy-49A cells (data not shown).
Site-directed mutational analysis of the Ly-49A molecule confirmed the functional significance of the Ly-49A/SHP-1 interaction. Disruption of the putative ITIM motif in Ly49A by mutating the tyrosine to phenylalanine at residue 8 (Y8F), completely eliminated Ly-49A–mediated inhibition of cytotoxicity against H-2Dd target cells by RNK-16 transfectants.
Our studies demonstrate that Ly-49A interrupts proximal signaling events during natural killing, and they provide evidence that this inhibitory function is largely mediated through the SHP-1 phosphatase. The mechanisms by which SHP-1 inhibits cytotoxicity have not been fully elucidated. Our cold-target inhibition studies suggest that the Ly-49A–mediated inhibitory effect is localized within the NK cell, because targets expressing H-2Dd are protected from lysis but fail to inhibit NK cell cytotoxicity against non–H-2Dd targets. Thus, Ly-49A does not transduce global inhibitory signals to NK cells. Rather, it appears that Ly-49A may locally interrupt activating signals transduced by other receptors during natural killing.
In summary, we have demonstrated that the ligation of Ly-49A by its MHC class I ligand, H-2Dd, interrupts early signaling events stimulated during target-induced activation of NK cells, including phosphoinositide turnover and protein tyrosine phosphorylation. We have also shown the direct association of the cytoplasmic tyrosine phosphatase SHP-1 with Ly-49A. Our studies indicate a requirement for SHP-1 for the optimal function of Ly-49A, as we found that Ly49A function is markedly impaired in SHP-1 mutant mev/mev and me/me mice. The finding of partial Ly-49A function despite the complete absence of SHP-1 in me/me mice indicates the possible involvement of other cytoplasmic mediators, such as SHP-2. In addition, we have shown that the tyrosine within the proposed SHP-1 binding motif in Ly-49A is strictly required for the inhibitory effects of this receptor on cytotoxicity. Finally, we have demonstrated that inhibition through Ly-49A is target specific. Although a number of candidate activating receptors on NK cells have been described, including the lectin-like NKR-P1 molecule (54), the specific receptors that bind tumors and activate NK cell cytotoxicity have not yet been identified. Our studies indicate that the inhibitory Ly-49A receptor interrupts target-induced activation signals through its recruitment of inhibitory mediators to the effector/target interface in a spatially or temporally restricted manner.
Acknowledgments
We thank F.M. Karlhofer and W. Yokoyama for providing important reagents that enabled these studies, M. Olszowy and A. Shaw for the BSRα vector and invaluable help with transfection of RNK-16, S. Christianson for preparing me/me splenocytes, D.A. Lacy for assistance with the viable motheaten studies, E. Gum for sequencing, and T.P. Quinn for helpful discussions.
This work was supported by the Veterans Administration and National Institutes of Health grant RO1 CA69299 (W.E. Seaman). J.C. Ryan is the recipient of National Institutes of Health grant R29 CA60944 and is supported by the International Human Frontiers in Science program. M.C. Nakamura is supported by National Institutes of Health grant K11 AR01927, the Rosalind Russell Arthritis Foundation, and Multipurpose Arthritis Center grant P60AR20684. L.D. Shultz is the recipient of National Institutes of Health grant RO1 CA20408.
Footnotes
1 Abbreviations used in this paper: APT, phosphotyrosine; InsP3, inositol triphosphates; ITIM, immunoreceptor tyrosine-based inhibitory motif; KIRs, killer inhibitory receptors; LAK, IL-2-activated NK cells.
References
- 1.Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: The NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- 2.Smith HRC, Karlhofer FM, Yokoyama WM. Ly-49 multigene family expressed by IL-2-activated NK cells. J Immunol. 1994;153:1068–1079. [PubMed] [Google Scholar]
- 3.Yokoyama WM, Jacobs LB, Kanagawa O, Shevach EM, Cohen DI. A murine T lymphocyte antigen belongs to a supergene family of type II integral membrane proteins. J Immunol. 1989;143:1379–1386. [PubMed] [Google Scholar]
- 4.Wong S, Freeman JD, Kelleher C, Mager D, Takei F. Ly-49 multigene family: new members of a supergene family of type II membrane proteins with lectin-like domains. J Immunol. 1991;147:1417–1423. [PubMed] [Google Scholar]
- 5.Brennan J, Mager D, Jefferies W, Takei F. Expression of different members of the Ly-49 gene family defines distinct natural killer cell subsets and cell adhesion properties. J Exp Med. 1994;180:2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+IL-2-activated natural killer cells. Nature (Lond) 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 7.Stoneman ER, Bennett M, An J, Chesnut KA, Wakeland EK, Scheerer JB, Siliciano MJ, Kumar V, Matthew PA. Cloning and characterization of 5E6 (Ly49C), a receptor expressed on a subset of murine natural killer cells. J Exp Med. 1995;182:305–313. doi: 10.1084/jem.182.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason LH, Ortaldo JR, Young HA, Kumar V, Bennett M, Anderson SK. Cloning and functional characteristics of murine LGL-1: A member of the Ly-49 gene family (Ly-49G2) J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels BF, Karlhofer FM, Seaman WE, Yokoyama WM. A natural killer cell receptor specific for a major histocompatibility complex class I molecule. J Exp Med. 1994;180:687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane KP. Ly-49 mediates EL-4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J Exp Med. 1994;179:1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorda R, Trucco M. Mouse NKR-P1. A family of genes selectively co-expressed in adherent lymphokineactivated killer cells. J Immunol. 1991;147:1701–1708. [PubMed] [Google Scholar]
- 12.Ryan JC, Turck J, Niemi EC, Yokoyama WM, Seaman WE. Molecular cloning of the NK1.1 antigen, a member of the NKR-P1 family of natural killer cell activation molecules. J Immunol. 1992;149:1631–1635. [PubMed] [Google Scholar]
- 13.Yokoyama WM, Ryan JC, Hunter JJ, Smith HRC, Stark M, Seaman WE. cDNA cloning of mouse NKR-P1 and genetic linkage with Ly-49: identification of a natural killer cell gene complex on mouse chromosome 6. J Immunol. 1991;147:3229–3236. [PubMed] [Google Scholar]
- 14.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen: IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 15.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan JC, Niemi EC, Goldfien RD, Hiserodt JC, Seaman WE. NKR-P1, an activating molecule on rat natural killer cells, stimulates phosphoinositide turnover and a rise in intracellular calcium. J Immunol. 1991;147:3244–3250. [PubMed] [Google Scholar]
- 17.Seaman WE, Eriksson E, Dobrow R, Imboden JB. Inositol trisphosphate is generated by a rat natural killer cell tumor in response to target cells or to crosslinked monoclonal antibody OX-34: Possible role for the OX-34 determinant during activation by target cells. Proc Natl Acad Sci USA. 1987;84:4239–4243. doi: 10.1073/pnas.84.12.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassatella MA, Anegón I, Cuturi MC, Griskey P, Trinchieri G, Perussia B. FcγR(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+in Fc gamma R (CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989;1169:549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einspahr KJ, Abraham RT, Binstadt BA, Uehara Y, Leibson PJ. Tyrosine phosphorylation provides an early and requisite signal for the activation of natural killer cell function. Proc Natl Acad Sci USA. 1991;88:6279–6283. doi: 10.1073/pnas.88.14.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Shea JJ, McVicar DW, Kuhns DB, Ortaldo JR. A role for protein tyrosine kinase activity in natural cytotoxicity as well as antibody-dependent cellular cytotoxicity. Effects of herbimycin A. J Immunol. 1992;148:2497–2502. [PubMed] [Google Scholar]
- 21.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet J-P, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olcese L, Lang P, Vely F, Cambiaggi A, Marguet D, Blery M, Hippen KL, Biassoni R, Moretta A, Moretta L, Cambier JC, Vivier E. Human and mouse killer cell inhibitory receptors recruit PTP-1C and PTP-1D protein tyrosine phosphatases. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 23.Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–38. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 24.Yi T, Zhang J, Miura O, Ihle JN. Hematopoietic cell phosphatase associates with erythropoietin (Epo) receptor after Epo-induced receptor tyrosine phosphorylation: identification of potential binding sites. Blood. 1995;85:87–95. [PubMed] [Google Scholar]
- 25.Thomas ML. Of ITAMS and ITIMS: turning on and off the B cell antigen receptor. J Exp Med. 1995;181:1953–1956. doi: 10.1084/jem.181.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzwig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature (Lond) 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 27.D'Ambrosio D, Hippen KL, Minskoff SA, Mellman I, Pani G, Siminovitch KA, Cambier JC. Recruitment and activation of PTP-1C in negative regulation of antigen receptor signaling by FcγRIIB1. Science (Wash DC) 1995;268:293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- 28.Pani, G., M. Kozlowski, J.C. Cambier, G.B. Mills, and K.A. Siminovitch. Identification of the tyrosine phosphatase PTP1-C as a B cell antigen receptor–associated protein involved in the regulation of B cell signaling. J. Exp. Med. 181: 2077–2084. [DOI] [PMC free article] [PubMed]
- 29.Campbell KS, Dessing M, López-Botet M, Cella M, Colonna M. Tyrosine phosphorylation of a human killer inhibitory receptor recruits protein tyrosine phosphatase 1C. J Exp Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry A, Lanier LL, Weiss A. Phosphotyrosines in the KIR motif of NKB1 are required for negative signaling and for association with PTP-1C. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds CW, Bere EW, Jr, Ward JM. Natural killer activity in the rat. III. Characterization of transplantable large granular lymphocyte (LGL) leukemias in the F344 rat. J Immunol. 1984;132:534–540. [PubMed] [Google Scholar]
- 32.Coligan, J.E., A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. 1992. In Current Protocols in Immunology. John Wiley and Sons, New York.
- 33.Vujanovic NL, Herberman RB, Maghazachi AA, Hiserodt JC. Lymphokine-activated killer cells in rats. III. A simple method for the purification of large granular lymphocytes and their rapid expansion and conversion into lymphokine-activated killer cells. J Exp Med. 1988;167:15–29. doi: 10.1084/jem.167.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Shea JJ, McVicar DW, Bailey TL, Burns C, Smith MJ. Activation of human peripheral blood T lymphocytes by pharmacologic induction of protein-tyrosine phosphorylation. Proc Natl Acad Sci USA. 1992;89:10306–10310. doi: 10.1073/pnas.89.21.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph)gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 36.Kozlowski M, Mlinaric-Rascan I, Feng GS, Shen R, Pawson A, Siminovitch KA. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsui HW, Siminovitch KA, de Souza L, Tsui FWL. Motheaten and viable motheaten mice have mutations in the hematopoietic cell phosphatase gene. Nature Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 38.Karlhofer FM, Ribaudo RK, Yokoyama WM. The interaction of Ly-49 with H-2Ddglobally inactivates NK cell cytolytic activity. Trans Assoc Am Phys. 1992;105:72–85. [PubMed] [Google Scholar]
- 39.Kaufman DS, Schoon RA, Robertson MJ, Liebson PJ. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc Natl Acad Sci USA. 1995;92:6484–6488. doi: 10.1073/pnas.92.14.6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 41.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. p58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I–protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. NKB1: a natural killer cell receptor involved in the recognition of polymorphic HLA-B molecules. J Exp Med. 1994;180:537–543. doi: 10.1084/jem.180.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagtmann N, Rajagopalan S, Winter CC, Peruzzi M, Long EO. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity. 1995;3:801–809. doi: 10.1016/1074-7613(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 44.Colonna M, Samaridis J. Cloning of Ig-superfamily members associated with HLA-C and HLA-B recognition by human NK cells. Science (Wash DC) 1995;268:405–408. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 45.D'Andrea A, Chang C, Franz-Bacon K, McClananhan T, Phillips JH, Lanier LL. Molecular cloning of NKB1, a natural killer cell receptor for HLA-B allotypes. J Immunol. 1995;155:2306–2310. [PubMed] [Google Scholar]
- 46.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen S-H, Bastien L, Posner BI, Chretien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature (Lond) 1991;352:736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- 48.Yi T, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hemopoietic cells and localization to human chromosome 12p12–p13. Mol Cell Biol. 1992;12:2386–2405. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plutzsky J, Neel BG, Rosenberg RD. Isolation of a src homology 2 containing tyrosine phosphatase. Proc Natl Acad Sci USA. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthew RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatase: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid- , serine- and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason LH, Anderson SK, Smith HRC, Yokoyama WM, Ortaldo JR. Recognition and potential triggering of Ly-49D+NK cells by monoclonal antibody 12A8. Natural Immunity. 1995;14:95. [Google Scholar]
- 52.Koo GC, Manyak CL, Dasch J, Ellingsworth L, Shultz LD. Suppressive effects of monocytic cells and transforming growth factor-beta on natural killer cell differentiation in autoimmune viable motheaten mutant mice. J Immunol. 1991;147:1194–1200. [PubMed] [Google Scholar]
- 53.Marengère LEM, Waterhouse P, Duncan GS, Mittrücker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science (Wash DC) 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 54.Ryan JC, Niemi EC, Nakamura MC, Seaman WE. NKR-P1A is a target specific receptor that activates natural killer cell cytotoxicity. J Exp Med. 1995;181:1911–1915. doi: 10.1084/jem.181.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]