Abstract
The present study was designed to investigate the effect of bacterial lipopolysaccharide (LPS) on C–C chemokine receptors (CCR) expressed in human mononuclear phagocytes. LPS caused a rapid and drastic reduction of CCR2 mRNA levels, which binds MCP-1 and -3. CCR1 and CCR5 mRNAs were also reduced, though to a lesser extent, whereas CXCR2 was unaffected. The rate of nuclear transcription of CCR2 was not affected by LPS, whereas the mRNA half life was reduced from 1.5 h to 45 min. As expected, LPS-induced inhibition of CCR2 mRNA expression was associated with a reduction of both MCP-1 binding and chemotactic responsiveness. The capacity to inhibit CCR2 expression in monocytes was shared by other microbial agents and cytokines (inactivated Streptococci, Propionibacterium acnes, and to a lesser extent, IL-1 and TNF-α). In contrast, IL-2 augmented CCR2 expression and MCP-1 itself had no effect. These results suggest that, regulation of receptor expression in addition to agonist production is likely a crucial point in the regulation of the chemokine system.
The recruitment of leukocytes from the blood compartment represents one of the characteristic elements of the inflammatory process (1). Locally produced chemotactic agents are believed to play a crucial role in the activation of cells during the multistep process of leukocyte accumulation in tissues (2, 3).
In the past few years a new superfamily of chemotactic cytokines, named chemokines, has been described. The hallmark of this family are four conserved cysteine residues (4–7). According to the relative position of the first two cysteines it is possible to distinguish two main families: the C-X-C (or α) chemokines, which are primarily active on neutrophils, but show some action on T lymphocytes (4–7); and the C–C (or β) chemokines that exert their action on multiple leukocytes populations, including monocytes, basophils, eosinophils, T lymphocytes, NK and dendritic cells (4–10). Recently, a third type of protein (the C or γ chemokines) was described, which is active on T lymphocytes and NK cells. This protein is characterized by the absence of the first and third cysteines, but shows overall sequence identity with C–C chemokines (11, 12).
Chemokines, as well as classical chemotactic agonists, such as formylated peptides (of which FMLP is the prototype) and C5a, bind to and activate a family of rhodopsin-like GTP-binding protein-coupled seven-transmembrane domain receptors (13–15). Five receptors for C–C chemokines, now named CCR1 through 5, have been identified and cloned (14–17). In addition to recognizing chemokines, usually with some degree of promiscuity, C–C chemokine receptors act as coreceptors for primate lentiviruses such as HIV-1, HIV-2, and SIV (18–22). In particular CCR5 is a major determinant of the interaction of HIV-1 with mononuclear phagocytes (17–22).
MCP-1 is a prototypic C–C chemokine active on mononuclear phagocytes, basophils, T cells and NK cells (4, 8, 10). It is produced by a variety of cell types, including endothelial cells and cells of the monocyte-macrophage lineage, in response to diverse inflammatory signals, typically IL-1, TNF-α, and bacterial LPS. MCP-1 interacts with CCR2, of which two isoforms have been cloned and termed A and B (23). In monocytes and NK cells CCR2 is expressed predominantly as B isoform, with vanishingly low levels of A transcripts (23a). In addition to MCP-1, CCR2 recognizes MCP-3 (24–26). Although the regulation of chemokine production has been extensively investigated, little is known as to whether microenvironmental signals may affect the chemokine system by modulating receptor expression (27, 28). This lack of information, and previous observations demonstrating that LPS modulates the in vitro replication as well as the establishment of productive HIV-1 infection in cultured macrophages (29–31), prompted us to investigate the possible effect of LPS on the expression of C–C chemokine receptors. Here, we report that LPS rapidly downregulates CCR2 and, to a lesser extent, CCR1 and CCR5 expression in monocytes by acting on mRNA stability.
Materials and Methods
Cells.
Human monocytes were separated from peripheral blood of human healthy donors by Percoll gradient centrifugation (32). Monocytes (⩾98% pure as assessed by morphology) were resuspended at 107/ml in RPMI 1640 supplemented with 10% of fetal bovine serum, 2 mM glutamine and antibiotics. All reagents contained less than 0.125 EU/ml of endotoxin as checked by Limulus Amebocyte Lysate assay (Microbiological Associates, Walkerville, MD).
Cytokines and Reagents.
Human recombinant IL-1β was a gift of Dr. Boraschi (Dompè, L'Aquila, Italy); TNFα, from BASF/Knoll (Ludwighafen, Germany); IL-2, from Eurocetus (Milan, Italy); LPS (E. coli 055:B5) from Difco (Detroit, MI); Actinomycin D (ActD) from Sigma Chem. Co. (St. Louis, MO); MCP-1, from PeproTech Inc. (Rocky Hill, NC); inactivated Streptococci (OK432) from Chugai Pharmaceutical Co., Ltd. (Osaka, Japan); Candida Albicans from American Type Culture Collection (Rockville, MD); Glucan from Dr. N.R. Di Luzio (Tulane University School of Medicine, New Orleans, LA); P. Acnes from Wellcome Biotecnology Ltd. (Beckenham, UK).
Migration Assay.
Cell migration was evaluated using a chemotaxis microchamber technique (33) as previously described (32). 27 μl of chemoattractant solution or control medium (RPMI 1640 with 1% FCS) were added to the lower wells of a chemotaxis chamber (Neuroprobe, Pleasanton, CA). A polycarbonate filter (5-μm pore size; Neuroprobe) was layered onto the wells, covered with a silicon gasket and with the top plate. 50 μl of cell suspension (1.5 × 106/ml monocytes in PBMC) were seeded in the upper chamber. The chamber was incubated at 37°C in air with 5% CO2 for 90 min. At the end of the incubation, filters were removed, stained with Diff-Quik (Baxter s.p.a., Rome, Italy) and five high power oil-immersion fields were counted.
Receptor Binding Assays.
Competition for the binding of [125I] MCP-1 (specific activity 2,200 Ci/mmol; Du Pont de Nemours, Bad Homburg, Germany) to Percoll-purified human monocytes was carried out as described previously (26). Monocytes (1 × 107/ml) in binding medium (RPMI 1640 with 10 mg/ml bovine serum albumin; Sigma, Milan, Italy) were incubated with 1 nM of labeled cytokine in the presence of different concentrations of unlabeled cytokine at 4°C for 2 h. At the end of the incubation, cells were pelleted through a cushion of silicon oil by micro-centrifugation. The radioactivity present in the tip of the tubes and in the supernatants was evaluated by using a gamma counter. IC50 values for the different ligands were calculated using “ALLFIT” program as previously described (26).
Northern Blot Analysis.
Total RNA was isolated by the guanidium isothiocyanate method as previously described (34). 15 μg of total RNA from each sample were electrophoresed under denaturing conditions, blotted onto Nytran membranes (Schleicher & Schuell Inc., Keene, NH), and cross-linked by UV irradiation. cDNAs were labeled by random priming and α-[32P]dCTP. CCR2B cDNA was obtained by PCR amplification of the reported sequence (23, 35). CCR1 and CCR5 cDNAs were obtained as previously described (16). The IL-8RB (CXCR2) cDNA clone (27) was kindly donated by Dr. Ji Ming Wang (National Cancer Institute, Frederick, MD).
Nuclear Run-off Experiments.
Nuclear run-off experiments were performed essentially as described (34). Nuclei were isolated after 4 h of stimulation. Then 60 μl of 5× run-off buffer (25 mM TrisHCl, pH 8, 12.5 mM MgCl2, 750 mM KCl, and 1.25 mM each of ATP, CTP, and GTP), 2 μl of RNase inihibitors 1 U/μl (Perkin Elmer Cetus), 200 μCi of α-[32P]UTP 3,000 Ci/mmol (Amersham) were added to 220 μl of nuclei suspension and incubated at 30°C for 30 min. Elongated transcripts were then isolated using the guanidine/cesium procedure,with 50 μg of yeast tRNA added as carrier. The RNA pellet was resuspended in 180 μl of ice-cold TNE (NaCl 100 mM, 10 mM Tris-HCl, pH 8, 1 mM EDTA, pH 8) and denaturated adding 20 μl of 2N NaOH on ice for 10 min. The solution was neutralized by the addition of 200 μl of 0.48 M Hepes. RNA was precipitated by adding 880 μl of ethanol; the pellet was resuspended in 100 μl of H2O and radioactivity checked with β-counter. The RNA solution was denaturated at 65°C for 5 min and hybridized at 42°C for 48 h to 5 μg of denatured DNA immobilized on nitrocellulose filters in a few milliliters of hybridization solution (200 mM NaHPO4, pH 7.2, 1mM EDTA, pH 8, SDS 7%; deionized formamide 45%; E. coli tRNA 250 mg/ml). In a given experiment, each filter was hybridized with the same number of cpm. Filters were then washed one or two times at 37°C for 20–30 min in 40 mM NaHPO4-SDS 1% and exposed for autoradiography.
Results
Inhibition of CCR2 mRNA Expression by LPS.
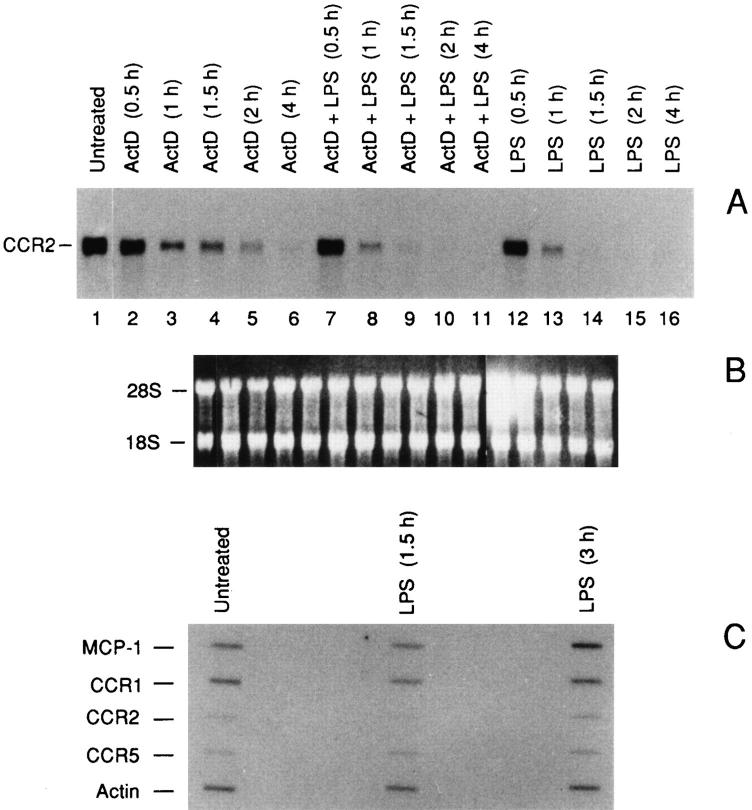
Human monocytes express high levels of CCR1, CCR2, and CCR5 transcripts (Fig. 1). CCR3 and 4 were undetectable by Northern analysis under these conditions (not shown). Using either a CCR2A or a CCR2B cDNA–specific probes, we detected a single 3.4-kb transcript, as previously reported (23, 23a). As shown in Fig. 1, LPS induced a dosedependent inhibition of the steady state level of CCR2 mRNA after 4 h, with a dramatic inhibition already at 1 ng/ml (Fig. 1 A) and virtually complete suppression at increasing concentrations, 10 and 100 ng/ml. Removal of the LPS after 4 h resulted in a gradual and complete restoration of the CCR2 mRNA level in 24 h (data not shown). Similar results were obtained by using the CCR2A cDNA– specific probe (data not shown). Likewise, but to a lesser extent, a significant LPS-dependent reduction of the CCR1 (Fig. 1 B) and CCR5 (C) mRNA levels was observed. In contrast, the mRNA level of CXCR2 was unaffected (Fig. 1 D).
Figure 1.
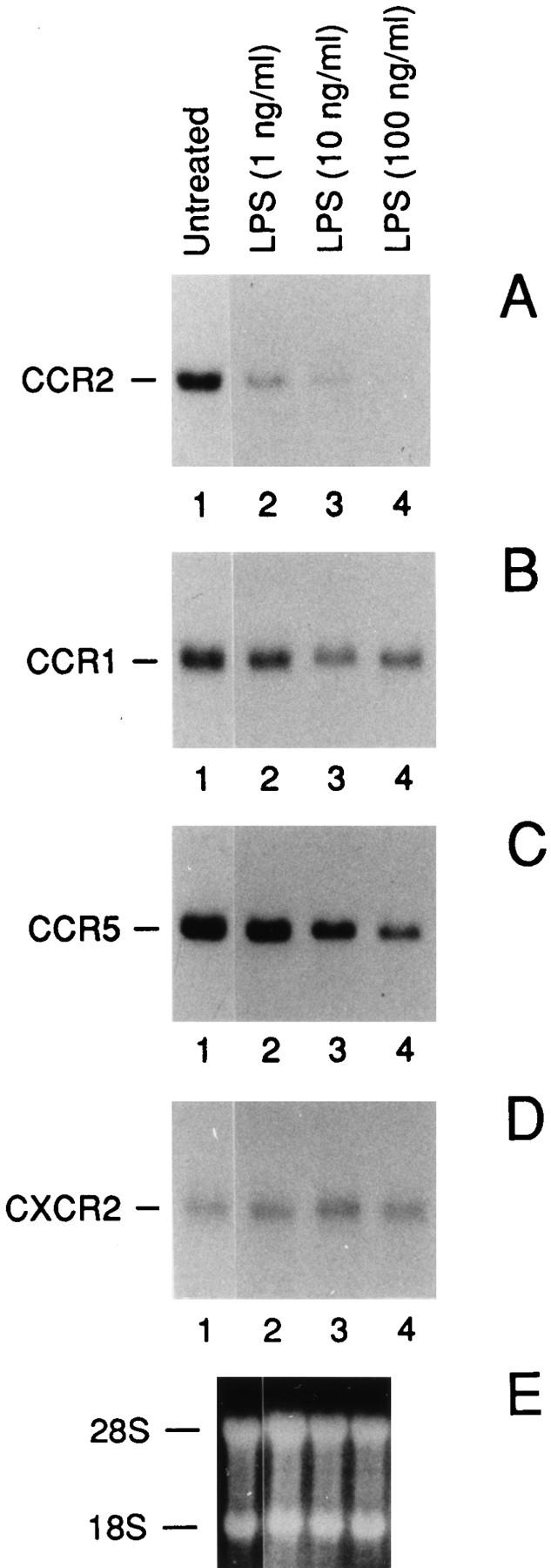
Effect of LPS on the CCR2 (A), CCR1 (B), CCR5 (C) and CXCR2 (D) mRNA expression: dose-response analysis. E shows the ethidium bromide stained ribosomal RNA. A is representative of four different donors. B and C are representative of two different donors. Total RNA was purified from fresh human monocytes incubated for 4 h as indicated.
LPS Destabilizes CCR2 mRNA.
Having established that low concentrations of LPS cause a rapid and drastic reduction of CCR2, subsequent work was focused mainly on this receptor. To investigate the mechanism of LPS action, we estimated its effects on both mRNA stability and gene transcription (Fig. 2). ActD (1 μg/ml) was added to fresh human monocytes in the presence or absence of 100 ng/ml of LPS and total RNA was extracted at different times as indicated. In the presence of ActD the estimated half-life of the transcript was about 1.5 h (Fig. 2, lanes 2–6), while cotreatment with ActD and LPS (lanes 7–11) considerably shortened this time (∼45 min). To evaluate the effects of LPS on gene transcription, we used nuclear run-off analysis (Fig. 2 C). In agreement with published results (34), LPS was able to induce MCP-1 gene transcription. In contrast, LPS did not induce appreciable variations on the transcription rate of the CCR2, CCR1, and CCR5 genes. Taken together, these data indicate that the inhibitory action of LPS on CCR2 gene expression is posttranscriptional and suggest similarities of the mechanisms controlling gene expression of the three β chemokine receptors studied here.
Figure 2.
Destabilization of CCR2 mRNA by LPS. (A) effect of LPS (100 ng/ml) on the CCR2B mRNA transcript stability. Total RNA was purified from fresh human monocytes incubated for 4 h as indicated. B shows the ethidium bromide stained ribosomal RNA. (C) Nuclear runoff analysis of the MCP-1, CCR1, CCR2, and CCR5 genes. Fresh human monocytes were incubated with 100 ng/ml of LPS for different periods as indicated.
LPS Downregulates MCP-1 Binding and Chemotaxis.
MCP-1 is a high-affinity ligand for the CCR2 receptor (23). Therefore we measured the [125I]MCP-1 binding in untreated and LPS-treated fresh human monocytes. As shown in Fig. 3 A, a significant time-dependent inhibition of the [125I]MCP-1 binding was observed after LPS treatment, which resulted in 60% of reduction after 5 h. Likewise, LPS treatment induced a strong decrease in the number of monocytes migrating in response to MCP-1 (Fig. 3 B). Thus, in human monocytes the LPS-mediated inhibition of CCR2 expression results in a marked reduction of both binding and migratory response to MCP-1, with the latter being much more sensitive to the action of LPS. A stronger inhibition of chemotaxis compared to receptor binding was previously described also for IL-8 (27).
Figure 3.
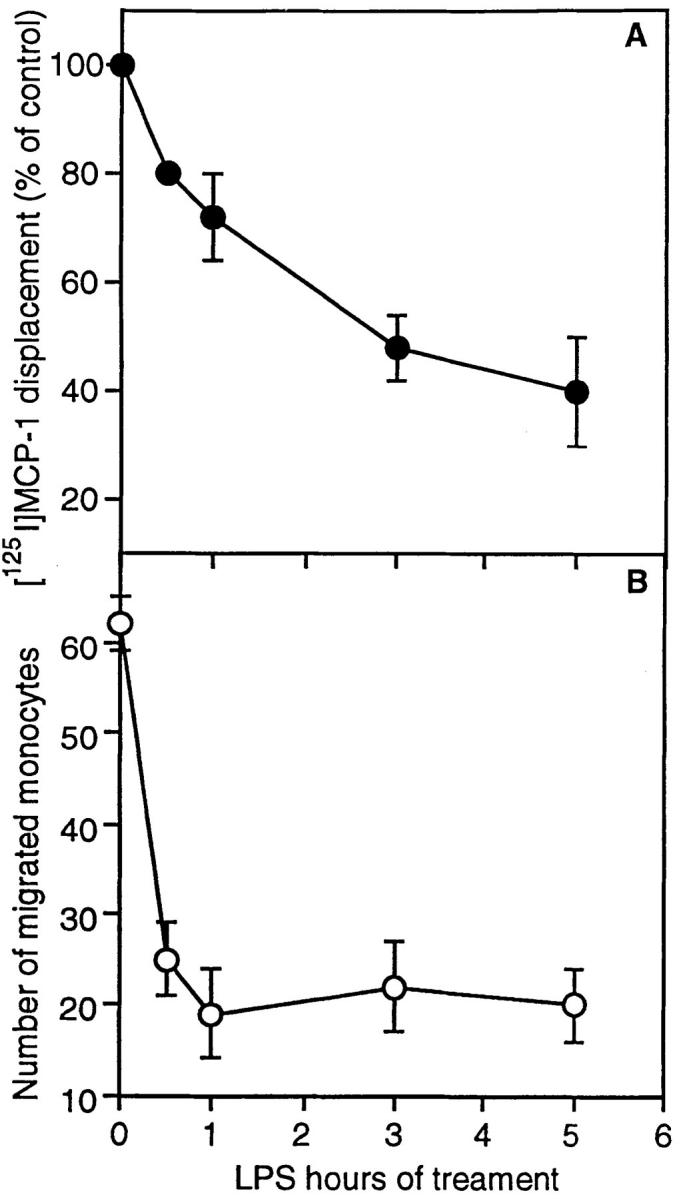
Inhibition by LPS of MCP-1 binding and chemotaxis. (A) Binding. fresh human monocytes were incubated for 1–5 h in the presence or absence of 100 ng/ml of LPS before [125I]MCP-1 binding assay. Results are expressed as percent of bound on total counts. (B) Chemotaxis. Fresh human monocytes were stimulated with 100 ng/ml of LPS and chemotactic response to MCP-1 (100 ng/ml) was measured after different periods as indicated.
Effect of Other Microbial Agents and Cytokines on CCR2 Expression.
Having established that LPS rapidly inhibits CCR2 expression, we wanted to investigate whether other microbial agents or components and cytokines affected this chemokine receptor. We tested agents (e.g., inactivated Streptococci, Propionibacterium acnes, Candida albicans, glucan) known to affect various functions of monocytes, including chemokine production (36, 37). As shown in Fig. 4 A, CCR2 gene expression was completely suppressed either by treatment with inactivated Streptococci or P. acnes. Furthermore, the CCR2 mRNA level was also partially affected by Glucan, but not by C. albicans. Among cytokines (Fig. 4 C), we found that IL-2 stimulates CCR2 expression thus extending to monocytes recent results with T lymphocytes and NK cells (23, 28, 35). In contrast, TNF-α and IL-1β reduced CCR2 expression and MCP-1 itself had no effect (not shown).
Figure 4.
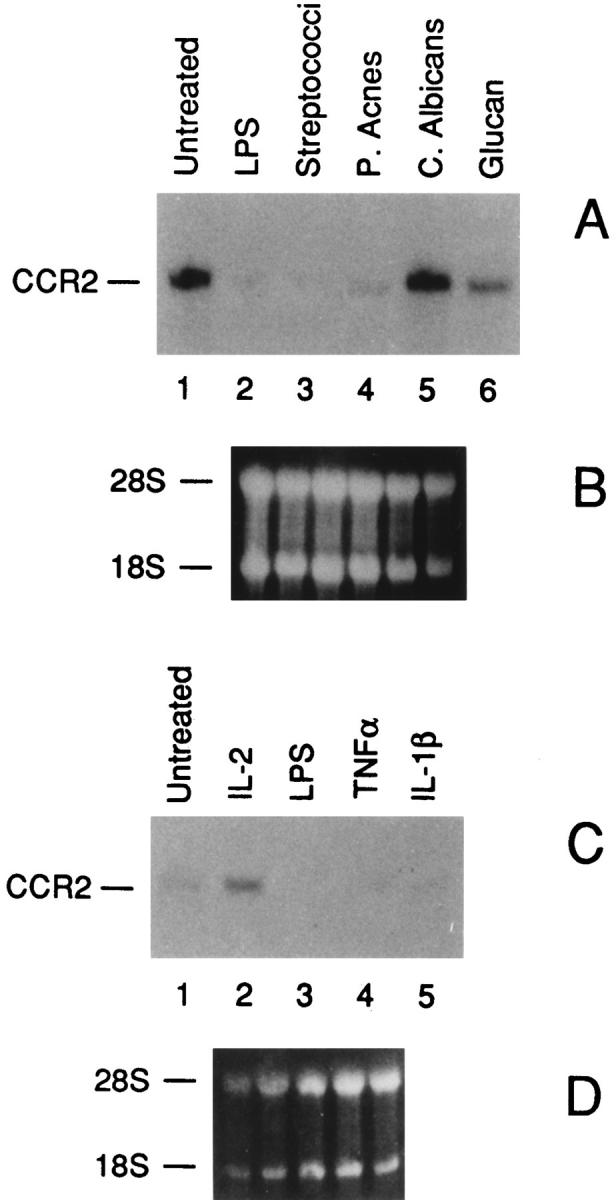
Effects of microbial products (A) and cytokines (C) on CCR2 mRNA expression. B and D show the ethidium bromide stained ribosomal RNA of A and C, respectively. Total RNA was purified from fresh human monocytes incubated for 4 h as indicated. (A) lane 1, untreated; lane 2, LPS 100 ng/ml; lane 3, inactivated Streptococci OK432 (0.015 KE/ml); lane 4, P. acnes (10 μg/ml); lane 5, C. albicans (100 μg/ ml); lane 6, Glucan (100 μg/ml). (C) lane 1, untreated; lane 2, IL-2 (1,000 U/ml); lane 3, LPS (100 ng/ml); lane 4, TNF-α (500 U/ml); lane 5, IL-1-β (20 ng/ml).
Discussion
The results presented here show that LPS causes a drastic and rapid downregulation of the expression of CCR2, a receptor for MCP-1 and -3. The ED50 of LPS was ∼1 ng/ml and half-maximal effect was reached with an optimal dose in ∼45 min. Inhibition of MCP-1 receptor expression was functionally relevant since LPS-treated monocytes showed a reduced capacity to bind and to respond to MCP-1 chemotactically. Two isoforms of CCR2 (A and B) have been cloned (23). Monocytes and activated NK cells express predominantly a 3.5-kb mRNA encoding the B form, with low levels of A transcripts (23, 35). LPS reduced expression of both isoforms (Fig. 1 and data not shown). Moreover LPS also inhibited expression of CCR1 and CCR5, though to a lesser variable extent than CCR2. The action of LPS on C–C chemokine receptors was specific in that CXCR2 was unaffected. It was previously reported that LPS does not affect the FMLP receptor in neutrophils (7, 27).
Little information is available on regulation of expression of chemokine receptors. In neutrophils, LPS and TNF-α were reported to inhibit the expression of IL-8 receptors, while G-CSF increased it (27). IL-2 was shown to induce CCR2 in T lymphocytes and NK cells (23a, 28, 35), an observation confirmed here for monocytes. Interestingly, CCR2 induction in T cells was a slow process, requiring four days of exposure to the cytokine (28). The results reported here show a dramatic, rapid and differential downregulation of chemokine receptors by LPS in monocytes.
LPS did not inhibit the rate of nuclear transcription of CCR2, but did reduce the mRNA half life from 1.5 h to 45 min. This result is in agreement with the observation that the 3′-untranslated region of the mRNAs of several rhodopsin family members, contain AU-rich elements, which correlate with highly regulated short-lived mRNAs (38, 39). Chemokine receptors play a central role in HIV-1 infection (40, 41). In particular CCR5, CCR1, and CCR3 have been involved as coreceptors for macrophage-tropic HIV-1 strains (18–22). The finding that LPS and cytokines reduce expression of these coreceptors may to some extent explain the bidirectional effects of these agents on HIV infection (e.g., 29–31). More in general, they point to the feasibility of targeting C–C chemokine receptor expression, as an alternative to occupancy, to inhibit infection.
LPS and bacterial agents used in the present study, as well as IL-1 and TNF, are potent inducers of various chemokines in monocytes including MCP-1 and -3 (10). The results reported here show that these agents have divergent actions on C–C chemokine production and receptor expression in monocytes. Regulation of CCR chemokine receptor expression, in addition to agonist production, is likely a crucial point for regulation of the chemokine system. We speculate that the divergent effect of certain proinflammatory signals on agonist versus receptor expression may serve to retain mononuclear phagocytes at sites of inflammation, to prevent their reverse transmigration (42), and, possibly, to limit excessive recruitment.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC) and by special project AIDS from Istituto Superiore di Sanita, Italy.
Footnotes
Note added in proof: During handling of this manuscript, we became aware of a study (Verani, A., G. Scarlatti, M. Comar, E. Tresoldi, S. Polo, M. Giacca, P. Lusso, A.G. Siccardi, and D. Vercelli. C–C chemokines released by LPS-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J. Exp. Med. In press) showing that LPS-induced chemokines may inhibit HIV infection of macrophages. Thus, inhibition of CCR2 expression and induction of chemokine production may both contribute to the observations with LPS reported in references 30 and 31.
References
- 1.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signal for lymphocyte recirculation and leukocyte emigration: the mutistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:99–179. [PubMed] [Google Scholar]
- 5.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 6.Schall, T.J. 1994. The Chemokines. In The Cytokine Handbook. A. Thomson, editor. Academic Press, London. 419–460.
- 7.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 8.Sozzani S, Locati M, Zhou D, Rieppi M, Luini W, Lamorte G, Bianchi G, Polentarutti N, Allavena P, Mantovani A. Receptors, signal transduction and spectrum of action of monocyte chemotactic protein-1 and related chemokines. J Leukoc Biol. 1995;57:788–794. doi: 10.1002/jlb.57.5.788. [DOI] [PubMed] [Google Scholar]
- 9.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 10.Mantovani, A., S. Sozzani, P. Proost, and J. Van Damme. 1996. The monocyte chemoattractant protein family. In Chemoattractant Ligands and Their Receptors. R. Horuk, editor. CRC Press, Inc., Boca Raton, FL. 169–192.
- 11.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, Zlotnik A. Lymphotactin: A cytokine that represents a new class of chemokine. Science (Wash DC) 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi G, Sozzani S, Zlotnik A, Mantovani A, Allavena P. Migratory response of human NK cells to lymphotactin. Eur J Immunol. 1996;26:3238–3242. doi: 10.1002/eji.1830261260. [DOI] [PubMed] [Google Scholar]
- 13.Horuk R. The interleukin-8-receptor family—from chemokines to malaria. Immunol Today. 1994;15:169–174. doi: 10.1016/0167-5699(94)90314-X. [DOI] [PubMed] [Google Scholar]
- 14.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 15.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 16.Power CA, Proudfoot AEI, Magnenat E, Bacon KB, Wells TNC. Molecular cloning and characterisation of a neutrophil chemotactic protein from porcine platelets. Eur J Biochem. 1994;221:713–719. doi: 10.1111/j.1432-1033.1994.tb18784.x. [DOI] [PubMed] [Google Scholar]
- 17.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 18.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature(Lond) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway GP, Martin SR, Huang YX, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4(+) cells is mediated by the chemokine receptor CC-CKR-5. Nature(Lond) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu LJ, Mackay CR, Larosa G, Newman W, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 21.Doranz BJ, Rucker J, Yi YJ, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 22.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKRS: a RANTES, MIP-1 alpha, MIP-1 beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science (Wash DC) 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 23.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein-1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Polentarutti, N., P. Allavena, G. Bianchi, G. Giardina, A. Basile, S. Sozzani, A. Montovani, and M. Introna. 1997. IL-2 regulated expression of the monocyte chemotactic protein-1 receptor (CCR2) in human NK cells: characterization of a predominant 3.4 Kb transcript containing CCR2B and CCR2A sequences. J. Immunol. In press. [PubMed]
- 24.Franci C, Wong LM, Van Damme J, Proost P, Charo IF. Monocyte chemoattractant protein-3, but not monocyte chemoattractant protein-2, is a functional ligand of the human monocyte chemoattractant protein-1 receptor. J Immunol. 1995;154:6511–6517. [PubMed] [Google Scholar]
- 25.Combadiere C, Ahuja SK, Van Damme J, Tiffany HL, Gao JL, Murphy PM. Monocyte chemoattractant protein-3 is a functional ligand for CC chemokine receptors 1 and 2B. J Biol Chem. 1995;270:29671–29675. doi: 10.1074/jbc.270.50.29671. [DOI] [PubMed] [Google Scholar]
- 26.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, Van Damme J, Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3—similarities and differences with MCP-1. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- 27.Lloyd AR, Biragyn A, Johnston JA, Taub DD, Xu LL, Michiel D, Sprenger H, Oppenheim JJ, Kelvin DJ. Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin 8 receptors on polymorphonuclear leukocytes. J Biol Chem. 1995;270:28188–28192. doi: 10.1074/jbc.270.47.28188. [DOI] [PubMed] [Google Scholar]
- 28.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomerantz RJ, Feinberg MB, Trono D, Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990;172:253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein MS, Tong-Starksen SE, Locksley RM. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Invest. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sozzani S, Luini W, Molino M, Jílek P, Bottazzi B, Cerletti C, Matsushima K, Mantovani A. The signal transduction pathway involved in the migration induced by a monocyte chemotactic cytokine. J Immunol. 1991;147:2215–2221. [PubMed] [Google Scholar]
- 33.Falk W, Goodwin RH, Jr, Leonard EJ. A 48well microchemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–245. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 34.Sica A, Wang JM, Colotta F, Dejana E, Mantovani A, Oppenheim JJ, Larsen CG, Zachariae CO, Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990;144:3034–3038. [PubMed] [Google Scholar]
- 35.Allavena P, Bianchi G, Giardina P, Polentarutti N, Zhou D, Introna M, Sozzani S, Mantovani A. Migratory response of human NK cells to monocyte-chemotactic proteins. Methods. 1996;10:145–149. doi: 10.1006/meth.1996.0088. [DOI] [PubMed] [Google Scholar]
- 36.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 37.Barlin E, Leser H-L, Resch K, Gemsa D. In vivo activation of macrophages by Corynebacterium parcum, pyran copolymer and glucan. Int Arch Allergy Appl Immunol. 1981;66:180–182. [Google Scholar]
- 38.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 39.Collins S, Caron MG, Lefkowitz RJ. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu Rev Physiol. 1991;53:497–508. doi: 10.1146/annurev.ph.53.030191.002433. [DOI] [PubMed] [Google Scholar]
- 40.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature (Lond) 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 41.Bates P. Chemokine receptors and HIV-1: an attractive pair? . Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 42.Randolph GJ, Furie MB. Mononuclear phagocytes egress from an in vitro model of the vascular wall by migrating across endothelium in the basal to apical direction: role of intracellular adhesion molecule 1 and the CD11/ CD18 integrins. J Exp Med. 1996;183:451–462. doi: 10.1084/jem.183.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]