Abstract
After antigen capture, dendritic cells (DC) migrate into T cell–rich areas of secondary lymphoid organs, where they induce T cell activation, that subsequently drives B cell activation. Here, we investigate whether DC, generated in vitro, can directly modulate B cell responses, using CD40L-transfected L cells as surrogate activated T cells. DC, through the production of soluble mediators, stimulated by 3- to 6-fold the proliferation and subsequent recovery of B cells. Furthermore, after CD40 ligation, DC enhanced by 30–300-fold the secretion of IgG and IgA by sIgD− B cells (essentially memory B cells). In the presence of DC, naive sIgD+ B cells produced, in response to interleukin-2, large amounts of IgM. Thus, in addition to activating naive T cells in the extrafollicular areas of secondary lymphoid organs, DC may directly modulate B cell growth and differentiation.
At the antigen/pathogen port of entry (e.g., mucosa, epidermis), dendritic cells (DC)1, such as Langerhans cells (LC), capture the antigen and then migrate via the afferent lymphatics into the T cell–rich areas of regional lymph nodes, where they are called interdigitating dendritic cells (IDC) (for review see reference 1). There, they present processed antigen to naive T cells and generate an antigen-specific primary T cell response. Once primed by DC, T cells can promote B cell activation, both by releasing T cell–derived cytokines such as IL-2, IL-4, and IL-5, and by direct intercellular contacts (for review see reference 2). Among the signals involved in T/B cell cooperation, the interaction between CD40 (on B lymphocytes) and its ligand (CD40L) expressed on activated T cells, appears to be of critical importance (for review see reference 3). CD40, a molecule related to the TNF receptor family, is expressed on multiple cell types, including mature B cells and bone marrow– derived DC (4). Cross-linking of CD40 promotes B cell survival (5) proliferation (6) as well as B cell differentiation and immunoglobulin class switching (7, 8). The ligand for CD40, CD40L, is a TNF family member expressed on activated but not resting T cells (9). The importance of CD40/CD40L pathway in B cell immunopoiesis has been demonstrated in vivo in patients with X-linked hyper IgM syndrome (for review see reference 10).
The role of DC in humoral responses has been documented in vitro (11) and in vivo (12–15). Notably, DC incubated in vitro with antigen can induce, upon reinjection into mice, a protective humoral response (15). The critical role of DC in induction of humoral responses is viewed as a consequence of T cell priming, required for cognate interaction between T cells and B cells. However, as the primary B cell activation occurs within the extrafollicular T cell–rich areas (16), we wondered whether, in addition to priming T cells, DC were able to interact directly with B cells. Accordingly, we set up a system in which a CD40L-transfected murine cell line (CD40L L cells) was used as surrogate activated T cells, to study the effects of DC on B cell activation. Recent studies have indicated the possibility of generating large numbers of DC in vitro starting either from unseparated blood or bone marrow populations or from purified CD34+ hematopoietic progenitors (17). DC, generated by culturing human CD34+ hematopoietic progenitors with GM-CSF and TNFα have been shown earlier to induce a strong proliferation of allogeneic T cells (18, 19) and to express a functional CD40, the triggering of which induces their maturation into cells with characteristics of IDC (20). These in vitro–generated DC have been shown to contain LC as well as other DC related to dermal DC and thus were termed dendritic–Langerhans cells (D–Lc) (21).
Here, we demonstrate that D–Lc can strongly enhance both proliferation and Ig production of CD40-activated naive and memory B cells. These results suggest that DC might directly be involved in the extrafollicular plasma cell formation during induction of primary naive B cell responses or reactivation of secondary memory B cell responses.
Materials and Methods
Reagents and Cell Lines.
Cultures of CD34+ progenitors were established in RPMI-1640 (GIBCO BRL, Gaithersberg, MD) supplemented with 10% (vol/vol) FCS, 10 mM Hepes, 2 mM l-glutamine, 5 × 10−2 M 2-mercaptoethanol, and 0.08 μg/ml gentamycine (gentalline; Schering Plough, Levallois Perret, France), which will be referred to as complete RPMI. Cultures of B lymphocytes were carried out in modified Iscove's medium, supplemented with 10% inactivated FCS (MultiSer, Castell Hill, NSW), 2 mM l-glutamine (GIBCO BRL, Gaithersberg, MD) and 0.08 μg/ml gentamycine.
The murine CD40 L-transfected cell line (CD40L L cells) was produced in the laboratory as described earlier (22) and used as stimulator of B cell proliferation and differentiation. Mouse fibroblastic L cells stably transfected with the human CD32/FcγRII have been previously described (23). A chimeric fusion protein between the mouse CD8-α and the human CD40 T cell ligand was constructed in our laboratory as previously reported (22) and produced in COS cells.
rhGM-CSF (specific activity: 2 × 106 U/mg; Schering Plough Research Institute, Kenilworth, NJ) was used at a saturating concentration of 100 ng/ml (200 U/ml). rhTNF-α (specific activity, 2 × 106 U/mg; Genzyme, Boston, MA) was used at an optimal concentration of 2.5 ng/ml (50 U/ml). Purified rhIL-2 (5 × 104 U/mg) from Amgen (Thousand Oaks, CA) was used at 20 U/ml. Purified rhIL-4 and rhIL-10 (107 U/mg each; Schering Plough Research Institute, Kenilworth NJ) were used at 50 U/ml and 20 ng/ml, respectively.
Generation of D–Lc.
Umbilical cord blood samples were obtained according to institutional guidelines. Cells bearing CD34 antigen were isolated from mononuclear fractions through positive selection, using anti-CD34 mAb (10 μg/ml; Immu-133.3; Immunotech Marseille, France) and goat anti–mouse IgG-coated microbeads (Miltenyi Biotec GmBH, Bergish Gladbach, Germany). Isolation of CD34+ progenitors was achieved using Minimacs separation columns (Miltenyi Biotec) (21).
Cultures were established in the presence of GM-CSF and TNF-α in complete RPMI. CD34+ cells were seeded for expansion in 24-well culture plates (Limbro, Flow Laboratories, McLean, VA) at 2 × 104 cells/ml. Optimal conditions were maintained by splitting these cultures every 4–5 d. Cells were routinely collected at day 12 when the cultures contained between 70–90% of CD1a+ DC (18, 21). These CD1a+ cells includes LC that express Birbeck granules as well as other DC and thus will be referred to as D–Lc throughout the text (21). The CD1a− population includes granulocytes, monocytes, undifferentiated precursors and, in some instances, mature DC that have lost CD1a.
For certain experiments, after 12 d of culture in the presence of GM-CSF and TNF-α, cells generated from CD34+ progenitors were collected and labeled with FITC-conjugated OKT6 (CD1a; Ortho Diagnostic). Cells were separated according to CD1a expression into CD1a− and CD1a+ fractions using a FACStar® (Becton Dickinson, Mountain View, CA). The procedure of staining and sorting was performed in the presence of 5 mM EDTA in order to avoid cell aggregation. Reanalysis of the sorted populations showed a purity higher than 98%.
Isolation of Tonsillar B Cells.
Mononuclear cells from tonsils were isolated by a standard Ficoll–HyPaque (dose = 1,077 g/ml) gradient method. Tonsillar B cells were first enriched in the E− fraction and submitted to anti-CD2, anti-CD4, anti-CD8, antiCD14, anti-CD16 mAb negative selection with magnetic beads coated with anti-mouse IgG (Dynabeads; Dynal, Oslo, Norway). In the isolated population, >99% expressed CD19 and CD20 and <1% expressed CD2 or CD14 antigens. Isolation of sIgD+ and sIgD− B cells subpopulations was performed using a preparative magnetic cell sorter (MACS; Becton Dickinson) (24). IgD was expressed on >99% of the sIgD+ B cell subpopulation and <1% of sIgD− B cell subpopulation, as assessed by fluorescence analysis using a FACScan® (Becton Dickinson). For certain experiments, sIgD− B cells were further separated according to CD38 and CD39 expression into CD38+CD39− germinal center (GC) cells and CD38−CD39+ memory B cells using anti-CD38 and anti-CD39 mAbs and bead depletion as described earlier (25).
In some experiments, total B cells were separated according to their size using a discontinuous gradient of Percoll (Pharmacia, Uppsala, Sweden) as described earlier (22). B cells recovered at the interface of 35–55% layers were referred to as low density B cells, and cells recovered at the interface of 65–55% Percoll solutions were referred to as high density or resting B cells. In some cases, resting B cells were further labeled with FITC-conjugated anti-CD20, PEconjugated anti-CD38 (Becton Dickinson) and biotinylated antiIgD (Sigma), followed by incubation with Streptavidin–Tricolor (Kallestad, Austin, TX) and sorted into CD20+IgD+CD38− naive B cells and CD20+IgD−CD38− memory B cells (25). Reanalysis of the sorted populations showed a purity higher than 99%.
Isolation of Human Monocytes.
Total PBMC were isolated from healthy donors by Ficoll–HyPaque centrifugation; monocytes were isolated by elutrial centrifugation as described elsewhere (26). The preparation was >90% pure as controlled by flow cytometry with FITC-conjugated anti CD14 (Leu-M3; Becton Dickinson).
Coculture of B Cells and D–Lc.
2.5 × 103 irradiated CD40L L cells (7,500 rads) were seeded together with 104 B lymphocytes (either total B cells or B cells subpopulations) in the presence or absence of in vitro generated D–Lc (104) in 96-well culture plate (Nunc, Roskilde, Denmark). B cell proliferation was monitored by tritiated thymidine ([3H]TdR) incorporation after 6 d of coculture, except for kinetic experiments. Cells were incubated for the last 16 h with 1 μCi of [3H]TdR. Tests were carried out in triplicate, and results were expressed as cpm ± SD. For determination of Ig production, supernatants were recovered after 15 d and used for indirect ELISA (27). For Giemsa stainings and immunostainings, 105 B cells were cultured together with 105 irradiated D–Lc over 2.5 × 104 irradiated CD40L L cells in a final volume of 1 ml in 24-well culture plates. After 6 d of culture, cells were gently harvested and used for staining experiments.
In other experiments, B cells and D–Lc were cultured in separate compartments using transwells (Costar, Wilmington, MA). 105 D–Lc cultured in the presence or absence of CD40 triggering (2.5 × 104 CD40L L cells or CD32 L cells used as control) in the lower compartment (in a total volume of 0.8 ml) were assayed for their ability to stimulate growth and differentiation of 1.5 × 104 B cells activated by 3.75 × 103 CD40L L cells or control CD32 L cells in the top of the transwells (in a total volume of 0.2 ml). DNA synthesis of B cells was performed by transferring, at day 6, the cells present in the top of the transwells into flat-bottomed 96-well plates and pulsing them with [3H]TdR for the last 16 h of the culture period.
Control phenotype of the cultures was routinely performed using FITC-labeled anti-CD3 and anti-CD19 (Immunotech) and FITC-labeled IgG1 (Kallestad).
Giemsa and Immunostainings.
Cells from cocultures of B cells and D–Lc over CD40L L cells were cytocentrifuged for 5 min at 500 rpm on microscope slides. Some slides were used for MayGrünwald-Giemsa staining and the others were fixed either in cold acetone or 4% paraformaldehyde during 10 min for immunocytology. Single stainings were performed using the alkaline phosphatase–anti-alkaline phosphatase system (APAAP technique) with mouse anti–HLA-DR (IgG1 from Immunotech) or anti-CD80 (IgG1 from Becton Dickinson) revealed by the Fast Red substrate (DAKO). Double stainings were performed using the biotin–avidin–peroxidase system (mouse IgG2a anti–HLA-DR from Becton Dickinson) and the APAAP technique (mouse IgG1 anti-CD20 from Becton Dickinson) as described elsewhere (30). Peroxidase activity was developed by 3-amino-9-ethylcarbazole, which gives a red color, and alkaline phosphatase activity was developed by Fast Blue substrate, which gives a blue color.
Results
D–Lc Enhance the Growth of CD40-activated B Cells.
In early experiments, using allogeneic cord blood DC and resting tonsillar T cells and B cells, DC were found to induce the activation of T cells, subsequently resulting in the differentiation of B cells as measured by Ig production (Caux, C., B. Vanbervliet, and J. Banchereau, unpublished results). Accordingly, we wondered whether D–Lc generated in vitro by culturing cord blood CD34+ cells for 12 d in the presence of GM-CSF and TNF-α (later on referred to as D–Lc) (18, 21) could directly modulate B cell responses. Inasmuch as CD40L on activated T cells appears to signal both B cells and DC, CD40L-transfected L cells (CD40L L cells) were used as surrogate activated T cells.
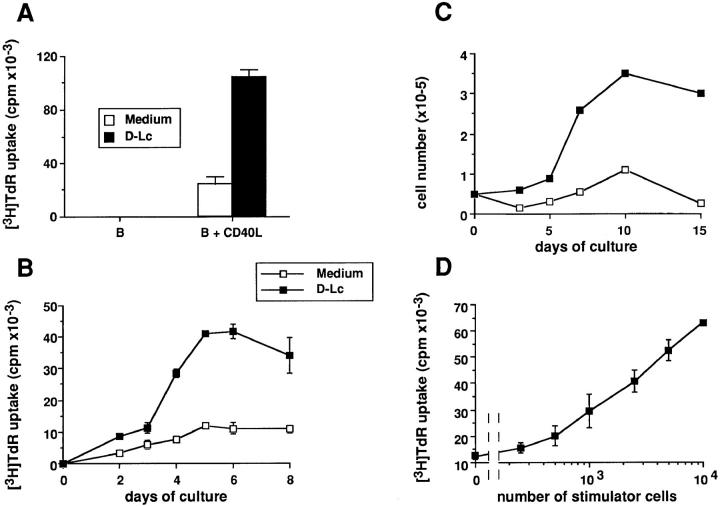
The D–Lc preparation used in this study contains 70– 90% CD1a+ as well as CD1a− cells including granulocytes and monocytes (see Materials and Methods). The role of the latter cells will be discussed later on (Fig. 4). As shown in Fig. 1 A, D–Lc strongly enhanced CD40-induced B cell proliferation as measured by [3H]TdR uptake after 6 d of coculture (three to eight-fold enhancement of [3H]TdR uptake). Note that in the absence of CD40L L cells, D–Lc can not induce B cell proliferation (Fig. 1 A, left). Kinetics analysis shows that addition of 104 D–Lc to CD40 triggering resulted in a twofold increase of [3H]TdR incorporation at day 2, which reached fourfold at day 4, when a plateau is reached (Fig. 1 B). This enhancement of B cell DNA synthesis by D–Lc is associated to an important increase in number of viable B cells (Fig. 1 C) that reaches a plateau at day 10, a time when D–Lc increased B cell number by fivefold. As few as 500 D–Lc are sufficient to induce a two- to threefold increase of CD40-induced B cell proliferation (Fig. 1 D), a maximal effect being obtained with 104 D–Lc (ratio 1:1). Thus, D–Lc enhance the proliferation of CD40-activated B cells in absence of any exogenous cytokine.
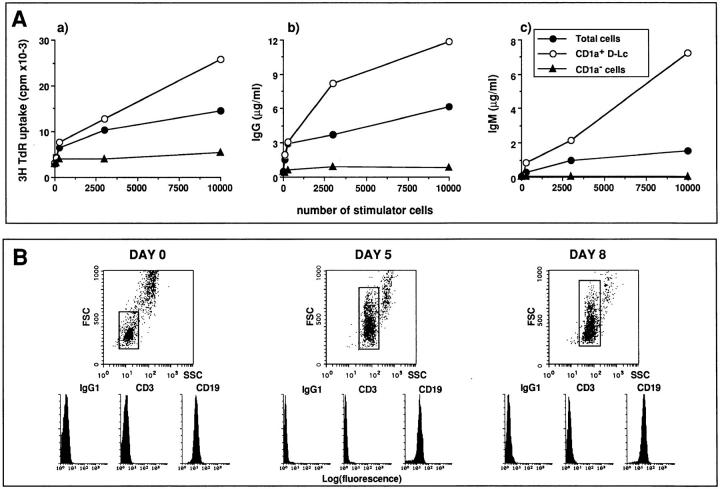
Figure 4.
The effects of D–Lc on CD40-activated B cell are restricted to CD1a+ dendritic cells and are not due to potentially contaminating T cells. (A) In vitro generated D–Lc were FACS® sorted into CD1a− cells and CD1a+ DC and used as stimulators of 104 CD40-activated B cells. Increasing numbers of either total D–Lc or FACS® sorted populations were added to the culture to study (a) the cytokine-independent proliferation of B cells, (b) the cytokineindependent IgG production, and (c) the IL-2-dependent IgM production. Results are expressed as mean of triplicate cultures (SD ⩽ 10%). (results from 1 experiment representative of 3). No significant B cell proliferation and differentiation was observed in absence of CD40L L cells. (B) The phenotype of cultures consisting of total B cells, D–Lc, CD40L L cells, and IL-2 was routinely followed during the culture period. Fluorescence histograms gated on small cells have been obtained with FITC–anti-CD3 and anti-CD19 after exclusion of dead cells using propidium iodide. High FSC/SSC cells correspond to the D–Lc population.
Figure 1.
In vitro generated D–Lc enhance DNA synthesis and viable cell number of B lymphocytes activated through their CD40. (A) 104 highly purified B cells were cultured over 2.5 × 103 irradiated CD40L L cells (right) or in medium alone (left) in the presence or absence of 104 irradiated D–Lc, as detailed in Materials and Methods. Thymidine uptake, determined after 6 d, is expressed as mean ± SD of triplicate cultures. (B) Kinetic of DNA synthesis of 104 CD40-activated B cells cultured in the presence or absence of 104 D–Lc was monitored between day 0 and day 8. (C) For numeration experiments, 5 × 104 highly purified B cells were cultured over 2.5 × 104 irradiated CD40L L cells in the presence or absence of 5 × 104 D–Lc in 24-well plates. Viable cell recovery was determined at many timepoints using Trypan blue dye exclusion between day 0 and day 15. (D) Increasing D–Lc numbers were added to 104 CD40activated B cells, and thymidine uptake was determined after 6 d of coculture (results from experiments representative of 15).
D–Lc Induce CD40-activated Memory B Cells to Secrete Igs.
To investigate the effect of D–Lc on the differentiation of CD40-activated B cells into Ig-secreting cells, tonsillar B cells were cultured over CD40L L cells with or without D–Lc. In the absence of D–Lc, 104 B cells produced only marginal amounts of Igs (<0.2 μg/ml; Fig. 2 A). Addition of 104 D–Lc to CD40-activated B cells results in the production of microgram amounts of IgG (range, 4–70 μg/ml; mean increase, 125; n = 12) and IgA (range, 0.8–6 μg/ml; mean increase, 33; n = 12). In contrast, IgM secretion was increased only 5- to 15-fold (range, 0.5–2 μg/ml; mean increase, 10; n = 12). As few as 330 D–Lc induced an eightfold increase in total Ig secretion, maximal effect being obtained with 104 D–Lc per well (40-fold increase) (Fig. 2 B).
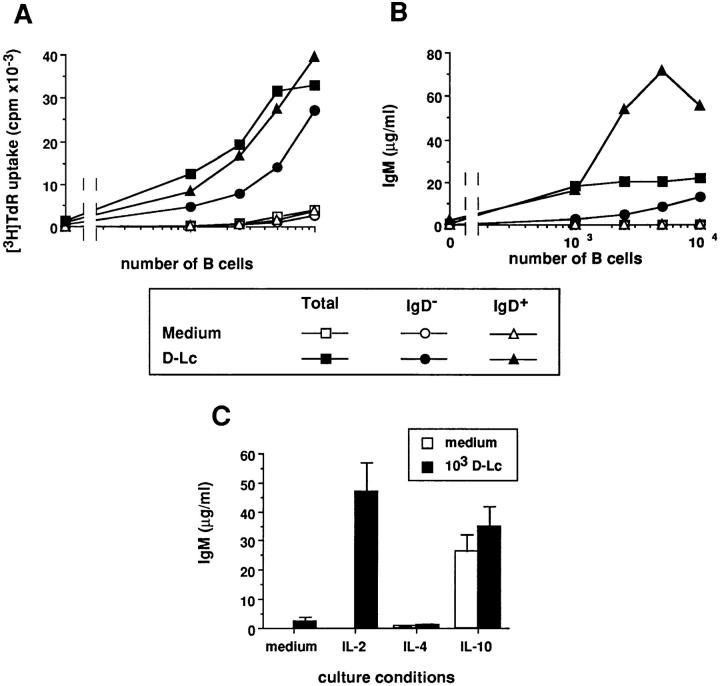
Figure 2.
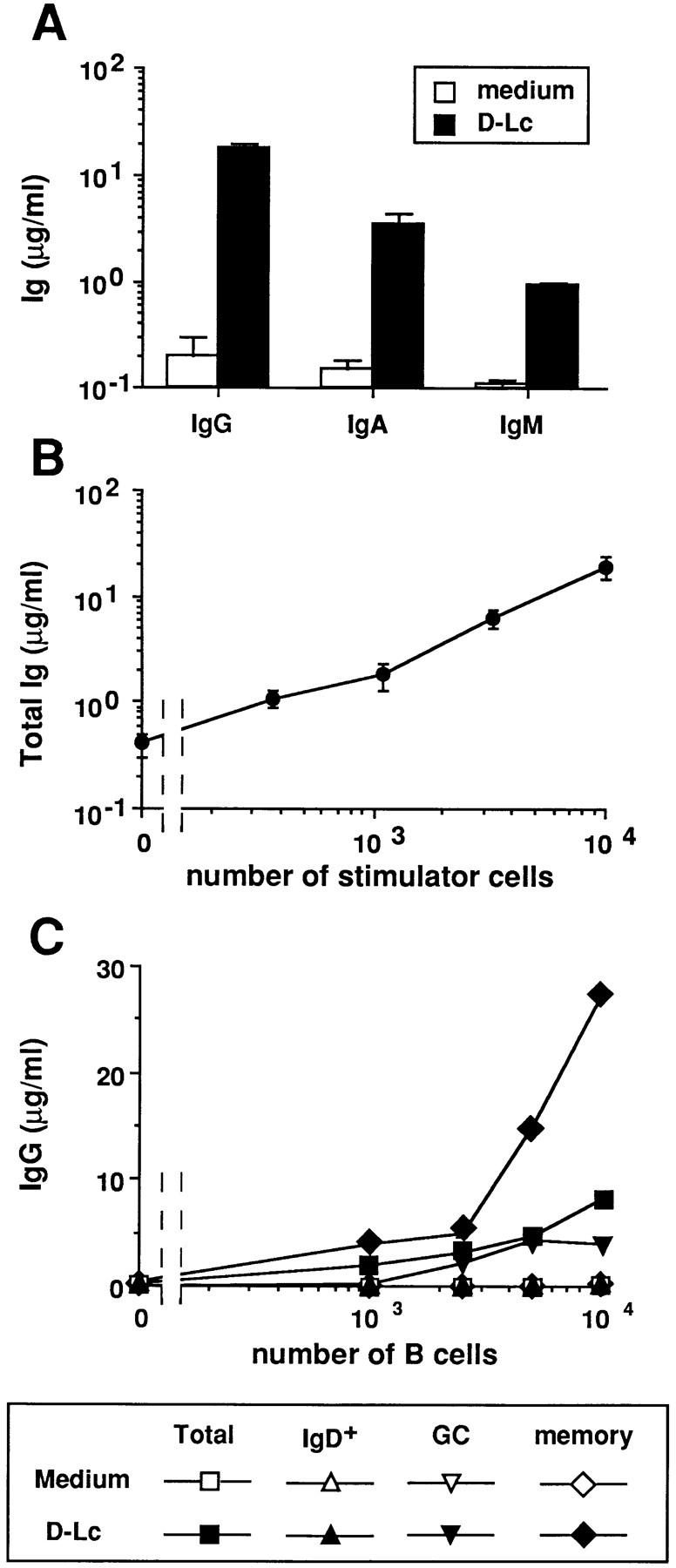
D–Lc strongly enhance Ig production by CD40-activated memory B cells. B cells were cultured over CD40L L cells in the presence or absence of D–Lc and their supernatants were harvested after 15 d and assayed for presence of (A) IgG, IgA, and IgM. (B) Increasing numbers of D–Lc were added to 104 CD40-activated B cells and total Ig production was measured after 15 d of coculture. Igs levels are expressed as mean ± SD of triplicate cultures (results from 1 experiment representative of 10). (C) B cells were purified into IgD+ B cells and IgD− B cells using the MACS system (purification detailed in Materials and Methods). IgD− B cells were further separated into CD38+CD39− germinal center B cells (GC) and CD38−CD39+ memory B cells using mAbs and bead depletion as detailed in Materials and Methods. Increasing numbers of either total B cells, MACS purified sIgD+ B cells, GC cells, or memory B cells were cultured over 2.5 × 103 irradiated CD40L L cells in medium alone or in the presence of 104 irradiated D–Lc. (SD ⩽ 10%). (results from 1 of 3 experiments). No significant B cell proliferation and differentiation was observed in absence of CD40L L cells.
Tonsillar B cells consist of naive B cells, expressing surface IgM and IgD as well as GC B cells and memory B cells that have mostly undergone isotype switch, thus resulting in a loss of IgD expression (28, 29). Therefore, we analyzed whether either purified sIgD+ B cells (naive B cells) or sIgD− B cells (GC and memory B cells) were preferential targets for D–Lc effect on IgG and IgA secretions. As shown in Fig. 2 C, CD40-activated naive sIgD+ B cells did not produce IgG whether or not D–Lc are added. Separation of sIgD− B cells into CD38+CD39− GC cells and CD38−CD39+ memory B cells (using mAbs and bead depletion) (25), allowed identification of the responding cell population. As shown in Fig. 2 C, memory B cells represent the main source of IgG as, when cultured in the presence of D–Lc, 103 memory B cells produced 5 μg/ml IgG and 104 27 μg/ml, while GC cells produced <0.5 μg/ml and 4 μg/ml, respectively, under such culture conditions.
To exclude that these observations could be the result of expansion of in vivo–activated B cells, resting and activated B cells were separated according to their density by Percoll gradients. As shown in Table 1 (experiment 1), in the absence of exogenous cytokine, D–Lc enhanced IgG production of both resting (high density) and activated (low density) B cells. Finally, CD20+ small resting B cells FACS® sorted into IgD−CD38− cells (resting memory B cells) produced high levels of IgG, when stimulated with D–Lc (90fold enhancement) in the absence of cytokine (Table 1, experiment 2). Similar results were obtained for IgA (data not shown).
Table 1.
D–Lc Enhance Growth and Ig Secretion of Resting B Lymphocytes Activated Through CD40
| [3H]TdR uptake (cpm × 10−3) | IgG (μg/ml) | IgM (μg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | D–Lc | Medium | D–Lc | IL-2 | IL-2 + D–Lc | |||||||||
| Exp. 1 | Total B cells | 7.3 ± 0.02 | 52.4 ± 0.1 | 0.1 ± 0.05 | 23.9 ± 0.9 | 0.1 ± 0.02 | 26.8 ± 2.6 | |||||||
| Low density | 5.6 ± 0.06 | 39.0 ± 2.5 | 0.2 ± 0.05 | 10.9 ± 1.2 | 0.1 ± 0.02 | 16.6 ± 1.5 | ||||||||
| High density | 10.0 ± 1.6 | 56.2 ± 1.7 | 0.7 ± 0.01 | 27.5 ± 1.0 | 0.2 ± 0.06 | 41.9 ± 5.1 | ||||||||
| Exp. 2 | Total B cells | 3.5 ± 0.6 | 17.3 ± 0.2 | 0.5 ± 0.01 | 6.5 ± 0.9 | 0.1 ± 0.02 | 6.0 ± 2.0 | |||||||
| High density IgD+CD38+ | 3.1 ± 0.6 | 15.4 ± 1.7 | 0.1 ± 0.01 | 0.5 ± 0.2 | 0.3 ± 0.1 | 21.5 ± 5.8 | ||||||||
| High density IgD−CD38− | 3.5 ± 0.2 | 18.9 ± 4.7 | 0.1 ± 0.05 | 9.0 ± 1.2 | 0.1 ± 0.05 | 0.6 ± 0.2 | ||||||||
D–Lc stimulate differentiation of resting naive and memory B cells activated through their CD40. Highly purified B cells were separated according to their size, using Percoll gradients, into low density B cells (activated B cells) and high density B cells (resting B cells). In experiment 1, 104 B cells were cultured over DC40L L cells in the presence or absence of D–Lc. For expeirment 2, CD20+ resting B cells (high density) were further separated into IgD+CD38− naive B cells and IgD−CD38− memory B cells, using three-color FACS® sorting (as described in Materials and Methods). These cells were dispensed at 5 × 103/well together with 2.5 × 103 CD40L L cells with or without 104 D–Lc. DNA synthesis was assayed after 6 d of coculture, and levels of IgG (in the absence of cytokine) and IgM (in the presence of IL-2) were determined at 15 d (1 experiment representative of 3). No significant B cell proliferation and differentiation was observed in absence of CD40L L cells.
Taken together, these data indicate that, D–Lc induce CD40-activated memory B cells to secrete considerable amounts of IgG and IgA.
CD40-activated Naive B Cells Cultured in the Presence of D–Lc Secrete IgM in Response to IL-2.
In view of the weak stimulation of IgM production, we wondered whether sIgD+ B cells were sensitive to the stimulatory effect of D–Lc. As shown in Fig. 3 A and Table 1, D–Lc equally enhanced the CD40-induced proliferation of all B cell subsets (sIgD+, sIgD−, resting, and in vivo-activated B cells) in absence of any exogenous cytokines. As D–Lc enhanced growth of naive B cells, we wondered whether addition of exogenous cytokines could enhance the IgM production induced by D–Lc. Among the tested cytokines (IL-2, IL-3, IL-4, IL-6, IL-10, IL-12, IFN-γ and their combinations), only IL-2 yielded significant results. D–Lc did not significantly affect the low levels of IgM obtained in response to IL-4 and the high levels obtained in response to IL-10 (Fig. 3 C). However, in IL-2 supplemented cultures, addition of D–Lc induced CD40-activated B cells to secrete high levels of IgM, which reached 50 μg/ml (mean increase, 161-fold; n = 16). Separation of B cells into sIgD+ and sIgD− B cells showed that production of IgM was mainly a property of the naive sIgD+ B cell population (Fig. 3 B). Note, however, that sIgD− B cells can be induced to produce significant levels of IgM provided 10-fold more B cells (104) are added to cultures. In addition, results presented in Table 1 show that D–Lc can induce small resting naive B cells (high density sIgD+CD38− cells) to produce high amounts of IgM in the presence of CD40 triggering and IL-2.
Figure 3.
In the presence of IL-2, D–Lc strongly enhance IgM production by naive sIgD+ B cells. B cells were purified into IgD+ B cells and IgD− B cells using the MACS system (purification detailed in Materials and Methods). Increasing numbers of either total B cells, MACS-purified IgD+ B cells or IgD− B cells were cultured under CD40 activation in medium alone or in the presence of irradiated D–Lc. (A) Cytokine-independent proliferation of B cells was measured after 6 d of coculture. (B) IL-2–dependent IgM production was measured after 15 d. Results are expressed as mean of triplicate cultures (SD ⩽ 10%). (results from 1 experiment representative of 6). (C) 104 highly purified B cells were cultured over 2.5 × 103 irradiated CD40L L cells in the presence or absence of 103 D–Lc, either in medium alone or with IL-2 (20 U/ml), IL-4 (50 U/ml), or IL-10 (20 ng/ml). Superposable results were obtained with IgD+ B cells. Ig levels were determined after 15 d of culture. Results are expressed as mean ± SD of triplicate cultures (1 experiment representative of 10). No significant B cell proliferation and differentiation was observed in absence of CD40L L cells.
Thus, D–Lc induce CD40-activated naive B cells to secrete high amounts of IgM in response to IL-2.
The Effects of D–Lc on CD40-activated B Cells Are Borne by the CD1a+ Population and Are Independent of the Outgrowth of Potentially Contaminating T Cells.
Because a population of D–Lc contains 70–90% CD1a+ cells as well as other cell types, including granulocytes and monocytes, D–Lc were FACS® sorted into CD1a+ and CD1a− cells. 104 CD1a+ cells were found to induce a 5-fold increase of B cell proliferation, whereas CD1a− cells were ineffective (Fig. 4 Aa). In addition, as few as 250 CD1a+ cells induced a 10-fold increase in IgG production, in absence of exogenous cytokines (Fig. 4 Ab), and in IgM production, in the presence of IL-2 (Fig. 4 Ac). In contrast, even at high cell density (104 cells), CD1a− cells remained ineffective in stimulating either IgG or IgM production, demonstrating that the described stimulatory effects of in vitro–generated D–Lc on B cells are restricted to CD1a+ D–Lc.
Although highly purified B cells (>99% CD20+ cells), isolated using either bead depletion or cell sorting (Table 1), were used in all experiments, the presence of IL-2 and D-Lc, known for their strong capacity to stimulate alloreactive T cells, renders it necessary to exclude a possible contribution of potentially contaminating T cells. For that purpose, phenotype of cultured cells was performed routinely. As an example, Fig. 4 B shows the phenotype of cells proliferating in cultures performed with total B cells, D–Lc, CD40L L cells, and IL-2 (the condition that would most likely allow the outgrowth of contaminating T cells). No significant outgrowth of CD3+ cells can be detected at any timepoint tested, and >98% of the small cells are CD19+ B cells. Similar results were observed in cultures set up with sIgD+ B cells and in cultures set up in the absence of IL-2 (data not shown). D–Lc (high FSC/SSC) were detected during all the culture period (50% at day 0 to <10% at day 8). These results support the conclusion that CD1a+ D–Lc can directly enhance the growth and differentiation of CD40-activated B cells.
D–Lc Are More Efficient than Monocytes in Enhancing CD40-induced B Cell Differentiation.
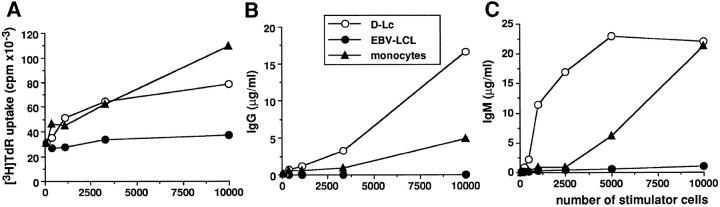
We compared the effects of various antigen-presenting cells including D–Lc, elutriated monocytes (>90% CD14), and lymphoblastoid cell lines (EBV–LCL) on the proliferation and differentiation of CD40-activated B cells. As shown in Fig. 5 A, monocytes were as potent as D–Lc in enhancing CD40induced B cell proliferation, whereas EBV cell lines were totally inefficient (only one of the three tested is shown in Fig. 5). Significant IgG production, in absence of cytokine, was observed with 3.5 × 102–103 D–Lc, whereas more than 5 × 103 monocytes were required to observe the same effects. At higher cell density (104 cells), monocytes induced levels of IgG in the order of 5 μg/ml compared with 17 μg/ml for D–Lc (Fig. 5 B). Concerning the IL-2–dependent IgM production, a significant effect was observed with less than 450 D–Lc, whereas more than 2.5 × 103 monocytes were required to reach similar levels of IgM secretion. Thus, whereas D–Lc and monocytes shared an equal ability to enhance B cell proliferation, D–Lc are more efficient that monocytes in inducing B cells to secrete IgG and IgM.
Figure 5.
D–Lc are more efficient than monocytes in enhancing CD40-induced B cell differentiation. D–Lc, one EBV cell line, and elutriated monocytes were compared, after irradiation, in their ability to enhance (A) cytokine-independent CD40-induced B cell proliferation, (B) cytokine-independent IgG production, and (C) IL-2–dependent IgM production. Increasing numbers of stimulator cells were added to 104 highly purified B cells cultured over 2.5 × 103 irradiated CD40L L cells. Results are expressed as mean of triplicate cultures (SD ⩽10%). (results from 1 experiment representative of 3). No significant B cell proliferation and differentiation was observed in absence of CD40L L cells.
The Effect of D–Lc Can Be Obtained in the Presence of Soluble CD40L.
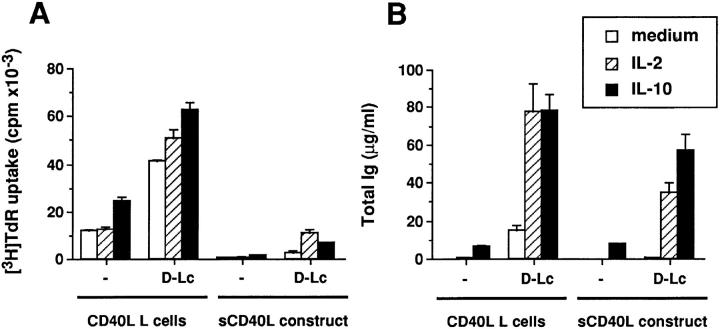
To analyze a possible contribution of the fibroblastic cell line (CD40L L cells), CD40 activation was carried out with a soluble form of CD40L using a fusion protein between the mouse CD8-α and the human CD40L. The soluble protein was a less efficient activator than the CD40L L cells (Fig. 6), particularly for proliferation of B cells, probably reflecting differences in the mode of CD40 engagement. Yet, addition of D–Lc resulted in enhanced B cell proliferation (Fig. 6 A) and differentiation (Fig. 6 B). The effect was particularly remarkable when cytokines, such as IL-2 or IL-10, were added to the cultures. Thus, the presence of the fibroblastic line is not mandatory for the B cell to undergo growth and differentiation in response to surrogate activated T cells (in the form of soluble CD40L and recombinant cytokines) and D–Lc.
Figure 6.
D–Lc enhance both proliferation and differentiation of B cells activated with a soluble form of CD40L. A soluble form of CD40L (sCD40L) was expressed as a dimeric fusion protein between the mouse CD8α and the human CD40L. sCD40L was used as a supernatant of COS cells at a final concentration of 25% (right) to stimulate (A) B cell proliferation (104 cells per well) and (B) B cell differentiation (104 cells per well). IL-2 and IL-10 were added at concentration indicated in Materials and Methods. Results obtained with CD40L L cells are indicated for comparison (left). (results from 1 experiment representative of 3). No significant B cell proliferation and differentiation was observed in absence of CD40 engagement.
The Effect of D–Lc on B Cell Activation Is Mediated by Soluble Factor(s): Partial Dependence on CD40 Ligation on D–Lc.
As previously described, B lymphocytes proliferate in clusters over CD40L L cells. Addition of D–Lc enhance the size and number of these clusters, which include L cells and D–Lc (data not shown). Giemsa staining on 6-d coculture cytospins shows close contacts between CD40-activated B cells and D–Lc (Fig. 7 A). Immunostainings on such cytospins showed large HLA-DR2+ CD80high cells with dendritic morphology surrounded by HLA-DR2+ CD80low B cells (Fig. 7, B and C). Immunostainings using anti-CD3 mAb failed to detect any positive cells (data not shown). Double stainings definitely identifies CD40-activated B cells (CD20+/HLA-DRlow) in close contacts with D–Lc (CD20−/ HLA-DRhigh), suggesting that these interactions may contribute to the D–Lc-dependent stimulation of B cell growth and differentiation described in this study.
Figure 7.
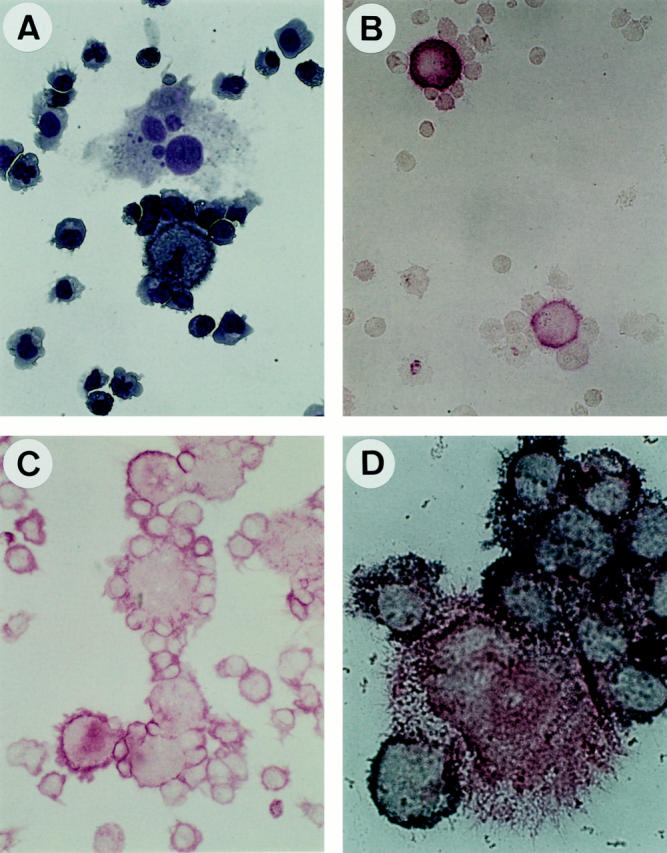
CD40-activated B cells are in close contact with D–Lc in coculture. 105 highly purified B cells were cultured in 24-well plates over 2.5 × 104 irradiated CD40L L cells and 5 × 104 D–Lc. After 8 d of coculture, cells were gently harvested, cytocentrifuged, and used for (A) MGG staining, (B) anti-HLA-DR staining, (C) anti-CD80 (B7-1) staining, and (D) double anti-HLA-DR (red) and anti-CD20 (blue) staining (as detailed in Materials and Methods). Magnification, ×400 (A, B, C); ×1000 (D).
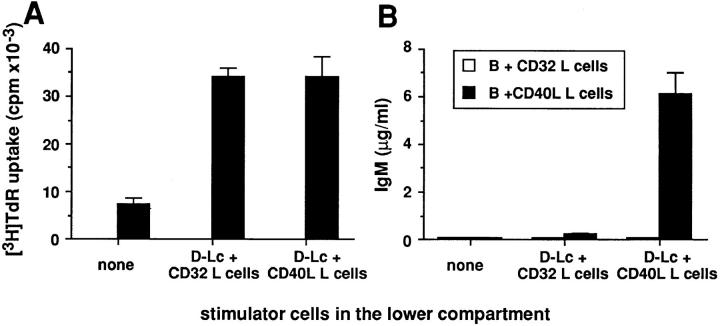
Experiments were designed to determine whether the functions of D–Lc on B cells were mediated through soluble factors and/or cell–cell interactions and whether D–Lc had to be activated through their CD40 antigen. Neither D–Lc supernatants nor fixed D–Lc significantly stimulated B cell growth and differentiation. As an alternative, D–Lc were separated from B cells by a permeable membrane using Transwells®. As shown in Fig. 8 A, D–Lc cultured in the lower compartment, with or without CD40 triggering, can enhance CD40-dependent B cell proliferation as much as if they were cocultured in direct contact. In the presence of IL-2, CD40-activated D–Lc, but not unactivated D-Lc, can support IgM production by B cells activated through CD40 (Fig. 8 B). However, IgM levels obtained were lower than those observed in direct contact coculture (6.1 ± 0.8 μg/ml versus 15.5 ± 2.5 μg/ml), suggesting that cell– cell contacts may also contribute. Note that D–Lc do not deliver any signal when B cells are not activated (Fig. 8; B plus CD32 L cells).
Figure 8.
The secretion of IgM by B cells is dependent on the production of soluble factor(s) by D–Lc after CD40 engagement. 1.5 × 104 sIgD+ B cells were cultured in the presence of 3.75 × 103 CD40L L cells or CD32 control L cells in the top of the transwell. 105 D–Lc were cultured in the lower compartment with 2.5 × 104 CD40L L cells or CD32 L cells used as controls. (A) DNA synthesis of B cells of the upper compartment, in absence of cytokine, was determined after 6 d of coculture. (B) IgM production in the presence of IL-2 was determined by ELISA after 15 d. Cultures were carried out in triplicates (one experiment representative of three).
Thus, the effect of D–Lc on B cell proliferation appears to be mediated by soluble factor(s), the production of which is independent of CD40 engagement on the D–Lc. In contrast, the effect on B cell differentiation, also mediated through soluble mediator(s), requires CD40 ligation of the D–Lc.
Discussion
The present study demonstrates that DC can directly provide signals to CD40-activated B cells, leading to enhanced proliferation as well as differentiation. To our knowledge, this study represents the first study demonstrating a direct effect of DC on normal B lymphocytes. Such information represents an important complement to studies performed earlier with mouse B lymphocytes, pointing out the critical role of DC during the course of the humoral response, including the following: (a) the demonstration that clusters of helper T lymphocytes, histocompatible B cells, and DC represented the site in which antibody-producing cells develop during the response to thymus-dependent antigens in vitro (11); (b) the observation that DC-oriented switch towards IgA of normal mouse B lymphocytes or pre B cell lines, stimulated with activated T cells or LPS (30, 31). However, potential direct effects of DC on B cells (in addition to effects on activated T cells) have never been studied. To address this question, CD40L L cells were used as surrogate activated T cells and DC were derived from cord blood CD34+ hematopoietic progenitors cultured for 12 d in the presence of GM-CSF plus TNF-α (D–Lc) (18, 21). CD1a+ D–Lc strongly enhance, in absence of any exogenous cytokines, CD40-dependent B cell proliferation (2–6-fold increase of viable cells) as well as differentiation (10–125fold increase of Ig secretion, depending on the isotype). This striking direct effect of CD1a+ D–Lc on Ig secretion was restricted to memory B cells. Furthermore, in the presence of IL-2, which by itself has only limited effects on in vitro CD40-dependent B cell activation (8, 32, 33) D–Lc stimulate resting naive IgD+ B cells to secrete large amounts of IgM.
The direct modulation of B cell activation by D–Lc, under CD40 triggering, reported here, suggests an important role of the latter cells during secondary immune responses (reactivation of memory B cells) and in the initiation of primary humoral responses (activation of naive B cells) and should actually be discussed in the context of a series of pertinent in vitro and in vivo observations: (a) CD40 engagement of cultured DC (20) yields cells with features of IDC (including low CD1a as well as high CD25, CD80, and CD86), which are found in the extrafollicular areas of secondary lymphoid organs (34–36); (b) antigen-induced T cell– dependent B cell activation and differentiation into plasma cells occurs within extrafollicular areas during both primary (naive B cells) and secondary (memory B cells) humoral responses (16, 37, 38); (c) after antigenic challenge, CD40Lexpressing T cells are mainly found within periarteriolar lymphatic sheaths (39), thus indicating that CD40-dependent activation of DC may occur within the extrafollicular areas of secondary lymphoid organs; (d) the IL-2 requirement for naive B cells to produce IgM is consistent with immunohistochemical studies showing, after immunization, the colocalization of CD40L+ IL-2 producing CD4+ T cells and specific antibody-secreting B cells in the extrafollicular areas of secondary lymphoid organs (39–41). In keeping with this, in vitro studies have shown IL-2 to be an important factor of primary humoral response (42–44). In particular, recently activated CD4+ T cells generate effector T cells that secrete mainly IL-2 and induce B cells to secrete essentially IgM (44).
The mechanisms by which D–Lc regulate B cell responses is not yet totally elucidated. However, the fact that D–Lc stimulate the proliferation of IgD+ naive B cells without altering their Ig secretion indicate that D–Lc affect the growth and the differentiation of B cells through different mechanisms and suggest the contribution of several molecular entities. Furthermore, Transwells® experiments show that D–Lc enhance growth of CD40-activated B cells through the production of soluble factor(s) independently of CD40 triggering. In contrast, the effect on IgM secretion in the presence of IL-2 is partly dependent on the release by D–Lc of soluble mediator(s) after CD40 engagement. Among known soluble mediators, IL-10, which has been shown to act in synergy with IL-2 to induce strong B cell differentiation (45), was excluded using MAbs (data not shown). The mechanism of induction of IL-2 responsiveness is not yet clear. As D–Lc expressed CD25 after CD40 engagement (20), IL-2 could signal D–Lc, which in turn induce B cells to produce IgM. Alternatively, such an effect might be due to the ability of D–Lc to upregulate CD25 expression on CD40-activated naive B cells (data not shown).
Together with the above considerations, our results emphasize the importance of a three-party cellular interaction, where DC allow the encounter and interaction of antigenspecific T cells with antigen-specific B cells during primary humoral responses. First, DC, homing into the T cell–rich areas, present processed antigen to naive T cells, through an MHC–TCR interaction, resulting in upregulation of CD40L on the T cell. In return, the activated T cell stimulates the DC through CD40, resulting in CD80 and CD86 upregulation and cytokine production (20). Then, the triggering of CD28 on the T cell, in the presence of DC-derived cofactors induces a full T cell activation followed by IL-2 production. Second, these reciprocal DC–T cell interactions are likely to occur concomitantly with cognate T–B cell interactions. Antigen-specific B cells, through CD40 engagement and T cell–derived IL-2 differentiate into IgMsecreting plasma cells, after close contact with DC previously activated through CD40. There is presently no evidence that DC present unprocessed antigen to B lymphocytes directly, though it is tempting to speculate that they may use surface Fc receptors to carry the antigens in the form of immune complexes or use lectins such as DEC 205 (46) or the mannose receptor (47) to present unprocessed antigen to specific B cells.
As a conclusion, the present results demonstrate that DC can directly modulate CD40-dependent B cell activation, suggesting that the critical role of DC in initiation of T-dependent humoral response might be related to direct modulation of B cell responses in the extrafollicular areas of lymphoid organs.
Acknowledgments
We are grateful to M.C. Rissoan and P. Chomarat for elutriation of monocytes; I. Durand for FACS® sorting; N. Courbière and M. Vatan for editorial assistance; doctors from clinics and hospitals in Lyon who provided us with umbilical cord blood samples. We thank N. Burdin, L. Galibert, G. Grouard, and F. Rousset for helpful discussion and careful reading of the manuscript.
Footnotes
J. Fayette and B. Dubois were supported by Ecole Normale Supérieure de Lyon.
1 Abbreviations used in this paper: DC, dendritic cells; D–Lc, dendritic– Langerhans cells; GC, germinal center; IDC, interdigitating dendritic cells.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature (Lond) 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 3.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand–receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–433. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 5.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan ICM. Mechanisms of antigendriven selection in germinal centers. Nature (Lond) 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, de Paoli P, Vallé A, Garcia E, Rousset F. Long term human B cell lines dependent on interleukin 4 and antibody to CD40. Science (Wash DC) 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 7.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousset F, Garcia E, Banchereau J. Cytokineinduced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature (Lond) 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 10.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyperIgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Witmer MD, Steinman RM. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells, during primary antibody responses in vitro. J Exp Med. 1984;160:858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba K, Granelli-Piperno A, Steinman RM. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and man. Proc Natl Acad Sci USA. 1983;80:6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sornasse T, Flamand V, de Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Oberdan L, Moser M. Antigen-pulse dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24:605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 16.Gray D. Recruitment of virgin B cells into an immune response is restricted to activation outside lymphoid follicles. Immunology. 1988;65:73–79. [PMC free article] [PubMed] [Google Scholar]
- 17.Caux, C., and J. Banchereau. 1996. In vitro regulation of dendritic cell development and function. In Blood Cell Biochemistry. T. Whetton and J. Gordon, editors. Plenum Press, London. 263–301.
- 18.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature (Lond) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 19.Caux C, Massacrier C, Dezutter-Dambuyant C, Vanbervliet B, Jacquet C, Schmitt D, Banchereau J. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+T cells and process soluble antigen. J Immunol. 1995;155:5427–5435. [PubMed] [Google Scholar]
- 20.Caux C, Massacrier C, Vanbervliet B, Dubois B, van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GMCSF+TNFα. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltz GA, Trounstine ML, Moore KW. Cloned and expressed human Fc receptor for IgG mediates anti-CD3 dependent lymphoproliferation. J Immunol. 1988;141:1891–1896. [PubMed] [Google Scholar]
- 24.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YJ, Barthélémy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:238–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 26.Fidgor CG, Bont WS, Touw I, Roos D, Roosnek EE, de Vries JE. Isolation of functionally different human monocytes by counterflow centrifugation elutriation. Blood. 1982;60:46–53. [PubMed] [Google Scholar]
- 27.Defrance T, Vanbervliet B, Pène J, Banchereau J. Human recombinant IL-4 induces activated B lymphocytes to produce IgG and IgM. J Immunol. 1988;141:2000–2005. [PubMed] [Google Scholar]
- 28.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YJ, Banchereau J. The paths and molecular controls of peripheral B-cell development. The Immunologist. 1996;4:55–66. [Google Scholar]
- 30.Spalding DM, Griffin JA. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986;44:507–515. doi: 10.1016/0092-8674(86)90472-1. [DOI] [PubMed] [Google Scholar]
- 31.Schrader CE, Geroge A, Kerlin RL, Cebra JJ. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Int Immunol. 1990;2:563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- 32.Nonoyama S, Hollenbaugh D, Aruffo A, Ledbetter JA, Ochs HD. B cell activation via CD40 is required for specific antibody production by antigen-stimulated human B cells. J Exp Med. 1993;178:1097–1102. doi: 10.1084/jem.178.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard D, Gaillard C, Hermann P, Banchereau J. Role of CD40 antigen and interleukin-2 in T cell dependent human B lymphocyte growth. Eur J Immunol. 1994;24:330–335. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- 34.Fossum S. Lymph-borne dendritic leucocytes do not recirculate, but enter the lymph node paracortex to become interdigitating cells. Scand J Immunol. 1988;27:97–105. doi: 10.1111/j.1365-3083.1988.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 35.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 36.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens: a novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T cell–dependent and T cell– independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 38.Kelsoe G, Zheng B. Sites of B-cell activation in vivo. Curr Opinion Immunol. 1993;5:418–422. doi: 10.1016/0952-7915(93)90062-w. [DOI] [PubMed] [Google Scholar]
- 39.Van den Eertwegh AJM, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Bocrsma WJA, Claassen E. In vivo CD40–gp39 interactions are essential for thymus dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoefakker S, Van't Erve EHM, Deen C, Van den Eertwegh A, Boersma WJA, Notten WRS, Claassen E. Immunohistochemical detection of co-localizing cytokine and antibody producing cells in the extrafollicular area of human palatine tonsils. Clin Exp Immunol. 1993;93:223–228. doi: 10.1111/j.1365-2249.1993.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bogen SA, Fogelman I, Abbas AK. Analysis of IL-2, IL-4, and IFN-γ-producing cells in situ during immune responses to protein antigens. J Immunol. 1993;150:4197–4205. [PubMed] [Google Scholar]
- 42.Grabstein KH, Maliszewski CR, Shanebeck K, Sato TA, Sprigg MK, Fanslow WC, Armitage RJ. The regulation of T cell–dependent antibody formation in vitro by CD40 ligand and IL-2. J Immunol. 1993;150:3141–3147. [PubMed] [Google Scholar]
- 43.Forman MS, Puré E. T-independent and T-dependent B lymphoblasts: helper T cells prime for interleukin 2-induced growth and secretion of immunoglobulins that utilize downstream heavy chains. J Exp Med. 1991;173:687–697. doi: 10.1084/jem.173.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–4282. [PubMed] [Google Scholar]
- 45.Fluckiger AC, Garrone P, Durand I, Galizzi JP, Banchereau J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J Exp Med. 1993;178:1473–1481. doi: 10.1084/jem.178.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature (Lond) 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 47.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: down-regulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]