Abstract
Although implicated in the clinical expression of human visceral leishmaniasis, a disease-exacerbating T helper cell 2 (Th2)-associated immune response involving interleukin-4 (IL-4) and/ or IL-10 is not readily detectable in experimental visceral infection. To overcome this obstacle to analyzing visceral Leishmania donovani in this relevant immunopathogenetic environment, we sought a model in which a Th2 response induces noncuring infection. Four initial approaches were tested primarily in BALB/c mice which control intracellular L. donovani via an IL-12– and interferon-γ (IFN-γ)–dependent Th1 mechanism: (a) modifying the cytokine milieu when the parasite is first encountered (treatment with exogenous IL-4 or anti–IL-12), (b) providing sustained endogenous exposure to a Th2 cytokine (infection of IL-4 transgenic mice), (c) increasing the parasite challenge inoculum, and (d) injecting heat-killed L. major promastigotes (HKLMP) to induce a cross-reactive Th2 response to live L. donovani. Only the last approach generated a functional Th2-type response that induced disease exacerbation accompanied by inhibition of tissue granuloma assembly. In HKLMP-primed BALB/c mice, prophylaxis with anti–IL-4, anti–IL-10, or exogenous IL-12 (but not IFN-γ) readily restored resistance. In primed mice with established visceral infection, treatment with either IL-12 or IFN-γ also successfully induced antileishmanial activity. The results in this model (a) suggest that rather than acting alone, IL-4 and IL-10 may act in concert to prevent acquisition of resistance to L. donovani, (b) reemphasize the capacity of IL-12 to reverse early Th2-related effects, and (c) demonstrate that Th1 cytokines (IL-12, IFN-γ) have therapeutic action in an established systemic infection despite the presence of a disease-exacerbating Th2-type response.
Emerging data derived from patients with visceral leishmaniasis, a disseminated intracellular protozoal infection primarily caused by Leishmania donovani or Leishmania chagasi, suggest that clinically apparent or progressive disease may be related to preferential expression of a downregulating Th2-associated immune response (1–11). This mechanism, which involves the action of suppressive cytokines including IL-4, IL-10, and perhaps other soluble factors (12–15), has been thoroughly characterized in a BALB/c mouse model of uncontrolled cutaneous infection caused by Leishmania major (12, 13, 16, 17) and in a distinct model of cutaneous infection induced by Leishmania mexicana (18, 19).
Although L. donovani and L. chagasi also readily parasitize and/or cause noncuring visceral infection in inbred mice (20–25), these Leishmania species do not regularly provoke an active, functional Th2 response in experimental infection as they seem to induce in human disease (1–3, 9–11). The one reported exception is in coat pigment mutant C57BL/6 ep/ep (pale ear) mice in which noncuring L. donovani infection is related to multiple host defense defects including a partially active Th2 cell response (24). However, in other models, it has been difficult to detect or assign a pathogenic role to this suppressive mechanism. In B10.D2 mice, for example, noncuring L. donovani infection has been ascribed to the failure to properly express a Th1-associated response rather than to pathologic activity of a Th2 mechanism (23). In addition, although L. donovani does induce both IL-4 and IL-10 expression in infected tissues of initially susceptible BALB/c mice, this response does not expand and is rapidly overshadowed by a protective Th1associated mechanism (26). The latter response, dependent upon IL-12 and IFN-γ (27, 28), induces macrophage activation and mediates acquisition of resistance and eventual resolution of infection (22). The course of visceral L. chagasi infection in BALB/c mice also closely mimics that of L. donovani, and mice express a susceptible but ultimately selfhealing phenotype (25). In the L. chagasi model, initially suppressed Th1 activity (IFN-γ secretion) is apparently not related to either IL-4 or IL-10 (25). Finally, rather than being less susceptible to L. donovani, IL-4 gene disrupted 129/ Sv × C57BL/6 mice, in which Th2-associated cytokine responses are blocked (29), show similar, rather than lower, parasite burdens in comparison to wild-type controls (19).
Despite the preceding experience, studying L. donovani experimentally in the presence of an active Th2 response is worthwhile in view of the apparent clinical relevance of this mechanism to human visceral infection (1–3, 7–10). Therefore, we tested a variety of approaches to develop an L. donovani-Th2 response model.
Materials and Methods
Mice and Visceral Infection.
20–30 g female BALB/c and 129/ Sv/Ev mice were purchased from Charles Rivers Laboratories (Wilmington, MA) and Taconic Farms (Germantown, NY), respectively. IL-4 transgenic 129/Sv/PEP mice (30, 31) were bred at DNAX (Palo Alto, CA). Groups of mice were challenged via the tail vein with 107 L. donovani amastigotes (1 Sudan strain) obtained from infected hamster spleen homogenates (22). The course of visceral infection was measured using stained liver imprints, and microscopic counts were performed in a blinded fashion. Liver parasite burdens, expressed as Leishman–Donovan units (LDU)1, were calculated as the number of amastigotes per 500 nucleated hepatic cells × liver weight (g) (22). Formalin-fixed liver sections, stained with hematoxylin and eosin, were scored for granuloma formation as described (22).
Treatment of BALB/c Mice to Induce a Th2-associated Response.
Using the schedules described in the text and figure legends, groups of four to five BALB/c mice were treated before L. donovani challenge with (a) IL-4 complexed with anti–IL-4 (32) with or without a single injection of anti–IFN-γ, (b) anti–IL-12 (28, 33, 34), or (c) heat-killed L. major promastigotes (HKLPM; 35). 250 μg of murine recombinant IL-4 (rIL-4; lot 941-90-1; 108 U/ml, DNAX) was mixed in saline with 2.5 mg of rat anti–murine IL-4 mAb (11B.11; 10 mg/ml; DNAX) (32), and after 2 h on ice, 0.1 ml containing 10 μg of IL-4/100 μg of anti–IL-4 was injected intraperitoneally. This complexing prolongs the in vivo effects of IL-4 (32). Some IL-4/anti–IL-4–treated mice also received an intraperitoneal injection of 100 μg of anti–murine IFN-γ mAb (XMG1.2; 2.6 mg/ml; DNAX). 200 μg of normal sheep IgG or 200 μg of partially-purified sheep anti–mouse IL-12 IgG (5 mg/ ml; Genetics Institute, Cambridge, MA), provided by Dr. V. Van Cleave (Genetics Institute; 34), was also injected three times per week intraperitoneally as in earlier studies (28, 34). L. major promastigotes (WHO strain WHOM/−/173), maintained at room temperature by weekly passage in medium 199 containing 30% fetal bovine serum and antibiotics, were washed once, resuspended in saline at 1.5 × 108/ml, and then heated at 56°C for 60 min. Mice were injected subcutaneously (thigh) once per week for 4 wk with 0.1 ml containing saline alone or 1.5 × 107 HKLMP, and were challenged with L. donovani 7 d after the fourth injection.
Anticytokine and Cytokine Treatments in HKLMP-primed BALB/c Mice.
One day before L. donovani challenge (day −1) and once a week thereafter, HKLPM-primed mice were injected intraperitoneally with 1 mg of one of the following rat-derived preparations: normal IgG (26), anti–mouse IL-4 mAb (11B.11, lot 3-287880217; Biologic Response Modifiers Program, National Cancer Institute, Fredrick, MD, provided by Dr. C. Reynolds, National Cancer Institute; 26), anti–mouse IL-10 mAb (JES5-2A5, IgG1; 2.6 mg/ml; DNAX; 36), or anti–mouse β-galactosidase (GL113, IgG1; 6 mg/ml, DNAX) used as an isotype control. The latter two mAb preparations were purified from tissue culture supernatants by ion exchange chromotography and gel filtration, and each contained <3 endotoxin units/mg of protein (Limulus agglutination assay).
As in earlier studies (37, 38), treatment with murine rIL-12 (1 μg/day), murine rIFN-γ (2 × 105 U/day), or bovine serum albumin (1 μg/day) as a control was administered continuously for 7 d by subcutaneously implanted osmotic pumps (Alzet model 2001; Alza Corp., Palo Alto, CA). IL-12 (7.8 × 106 U/mg) and IFN-γ (2 × 107 U/mg) were provided by Dr. J. Sypek (Genetics Institute) and Amgen Biologicals (Thousand Oaks, CA), respectively. To test cytokine effects as prophylaxis (34, 39), pumps delivering treatment for 7 d were inserted 4 h after L. donovani challenge. To test activity in established visceral infection, 7-d pumps were implanted 3 wk after infection on day +21. In all groups of cytokine-treated mice, liver parasite burdens were measured at the end of week 4 (day +28). Percent inhibition of parasite replication at week 4 was determined as: (week 4 LDU in untreated mice − week 4 LDU in treated mice) / week 4 LDU in untreated mice × 100; percent parasite killing was determined as: (week 3 LDU in untreated mice − week 4 LDU in treated mice) / week 3 LDU in untreated mice × 100 (38).
Assay for Serum IgE.
At the time mice were killed to determine parasite burdens, serum was obtained and frozen at −70°C until assayed for IgE by ELISA (40).
Statistical Analysis.
Differences between mean values were analyzed by a two-tailed Student's t test (24).
Results
Outcome of Visceral Infection in Selected Mouse Populations
Initial efforts were directed at identifying a suitable model of exacerbated visceral infection that was associated with induction of a Th2-type response. Fig. 1 summarizes preliminary experiments in which several different approaches were tested.
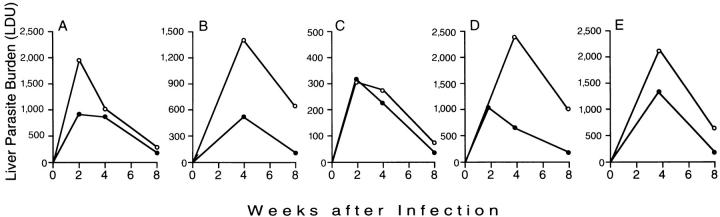
Figure 1.
Preliminary experiments to determine the course of visceral infection in five different populations of mice. (A) BALB/c mice not treated (•) or injected once with IL-4/anti–IL-4 complex one day before (day −1) L. donovani challenge (○). (B) BALB/c mice not treated (•) or injected with IL-4/anti–IL-4 starting on day −1 and twice per week for the first four weeks after challenge (○). (C) Control 129/Sv/Ev (•) and 129/Sv/PEP IL-4 transgenic mice (○). (D) BALB/c mice treated three times per week for 2 wk with normal sheep IgG (•) or sheep anti–IL-12 IgG (○) starting 2 h after challenge. (E) BALB/c mice challenged with 107 (•) or 2 × 107 (○) amastigotes. In mice challenged with 2 × 108 amastigotes, week 8 LDU were 761 ± 61 (n = 4 mice). Results in A–E indicate mean values (SE <16% in each case) from the single experiment performed with 4–5 mice per group. Note different vertical axis scale in B and C.
Effect of Single-dose Exogenous IL-4.
We focused first on IL-4, a critical regulator of the Th2 response (12, 13, 19, 41), and tested the effect of altering the host cytokine milieu at the time the parasite was initially encountered. BALB/c mice were pretreated once the day before infection (day −1) with IL-4 complexed with anti–IL-4 with or without one injection of anti–IFN-γ. 4 wk after a single dose of IL-4/anti–IL-4, the mean (± SEM) serum level of IgE (used as a marker for the effect of IL-4; 16, 42) in infected mice was 12.3 ± 1.8 μg/ml (n = 4 mice) versus 4.7 ± 0.4 μg/ml in infected controls (n = 3 mice). As shown in Fig. 1 A, whereas a single injection IL-4/anti–IL-4 exacerbated infection at week 2, this effect was short-lived in treated animals. The addition of a single injection of anti–IFN-γ on day −1 did not alter the course of hepatic infection in IL-4/anti–IL-4-treated mice (n = 5 mice, data not shown).
Effect of Multidose Exogenous IL-4.
The effect of a single injection of IL-4/anti–IL-4 at week 2, albeit brief, suggested that the continued presence of IL-4 might enhance the exacerbating action on L. donovani infection. Such an approach using IL-4 alone transiently promoted L. major infection in resistant mice in one study (42) but not in another (43). Therefore, starting on day −1 and for the first 4 wk thereafter, BALB/c mice were injected twice per week with IL-4/anti–IL-4. Visceral infection was clearly promoted during the 4-wk treatment period (Fig. 1 B). However, once IL-4 injections were discontinued, this effect waned and infection came under control by week 8.
Effect of Endogenous IL-4.
We next turned to IL-4 transgenic mice (30, 31) to test the effect of the sustained presence of endogenous IL-4. Despite a resistant 129/Sv background, these mice develop predominantly Th2-associated responses to L. major and are susceptible to cutaneous infection (31). However, in the face of active secretion of IL-4, as judged by a strikingly elevated serum IgE concentration 4 wk after infection (189 ± 35 μg/ml, n = 5 mice), IL-4 transgenic mice resisted and controlled L. donovani similar to normal 129/Sv/Ev mice (Fig. 1 C). In these latter control animals, serum IgE 4 wk after infection was 13.2 ± 2.0 μg/ml (n = 4 mice).
Effect of Anti–IL-12.
IL-12 appears likely to be the key endogenous initiator of the cell-mediated immune response which underlies experimental defense against a number of pathogens including L. donovani (28, 44). Therefore, to explore an alternative approach to establishing a Th2 response, BALB/c mice were treated with anti–IL-12 to inhibit the initiation of the Th1 mechanism, suppress the principal inducer of endogenous IFN-γ, and permit cytokines such as IL-4 and IL-10 to emerge and act unopposed (33, 34, 39). In an initial study of L. donovani–infected BALB/c mice, three injections of anti–IL-12 during the first week after challenge were sufficient to largely prevent control of visceral infection at week 4 (28). In the current experiments, we modified this protocol by (a) injecting BALB/c mice with anti–IL-12 starting 2 h after L. donovani challenge and then three times per week for 2 wk (rather than for 1 wk; 28), and then (b) observing the animals for a longer period after the last anti–IL-12 treatment. Although anti–IL-12 administration during weeks 1 and 2 exacerbated visceral infection at week 4 (Fig. 1 D), these mice nonetheless proceeded to control parasite replication and reduced liver burdens by week 8.
Effect of Increasing the Challenge Inoculum.
BALB/c mice are remarkably susceptible to L. major and develop unrelenting infection related to induction of a particularly intense Th2 response (12, 13, 45). These events have been linked in part to the size of the original challenge inoculum, because cutaneous infection is controlled when lower numbers of L. major are initially used (46). Therefore, assuming that injecting high numbers of parasites may facilitate induction or enhance the activity of an expressed but nonfunctional Th2 response (26), next we challenged BALB/c animals with 10–20-fold more amastigotes (1 and 2 × 108) than used in the standard L. donovani inoculum (107). However, after four weeks, visceral infection was controlled in a simliar fashion in all three dose groups (Fig. 1 E).
Effect of Pretreatment with HKLMP.
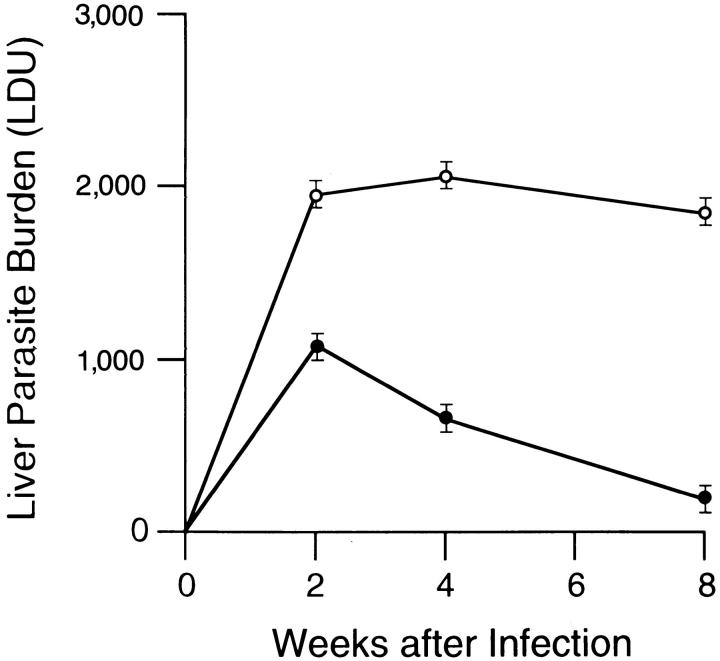
Finally, we tested whether BALB/c mice could be induced by presensitization with four once-per-week injections of HKLMP to cross-react to L. donovani with a Th2-associated response. This technique provokes a disease-exacerbating effect upon subsequent infection with live L. major (35). In a preliminary experiment, mice pretreated with four once-per-week injections of saline alone controlled and eradicated L. donovani; 4 wk after infection, mean (± SEM) liver parasite burdens were 1,144 ± 86 versus 947 ± 61 in uninjected controls, and at week 8, were 241 ± 26 versus 260 ± 31, respectively (n = 4 mice per group at each time point). In contrast, mice pretreated in a similar fashion with HKLMP failed to express acquired resistance 2 wk after L. donovani challenge, and liver burdens at week 4 were 3.2-fold higher than in control mice (Fig. 2). Visceral infection in HKLMPtreated mice then plateaued, and liver parasite burdens remained high at week 8 (8.7-fold greater than in untreated controls).
Figure 2.
Course of visceral infection in HKLMP-primed BALB/c mice. Mice were not pretreated (•) or were injected with HKLMP (○) once per week for 4 wk before being challenged with L. donovani 7 d later. Results are from four to five experiments, and indicate mean ± SEM values for 12–20 mice at each time point.
Responses in HKLMP-treated BALB/c Mice
Induction of an Active Th2-associated Cytokine Response.
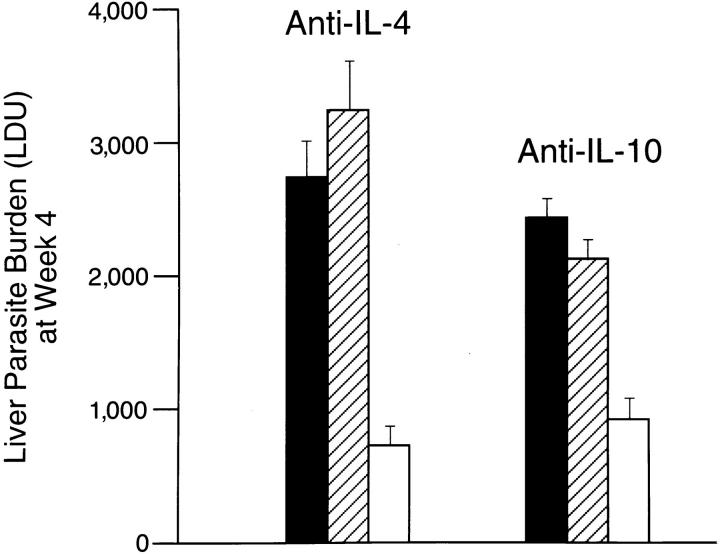
In normal unmanipulated BALB/c mice, L. donovani infection induces both IL-4 and IL-10 mRNA expression in the liver and serum IgE levels increase as well (26). Nevertheless, we concluded that this Th2 response is not functional and/or is rapidly overshadowed by a Th1 mechanism in normal mice since treatment with anti–IL-4 (26) or anti– IL-10 (unpublished) does not affect the kinetics of visceral infection and resistance is acquired (22, 26). In contrast, as judged by overt increases in serum IgE levels both before and after L. donovani challenge (Table 1), and more importantly, by the effects of injecting anti–IL-4 and anti–IL-10, HKLMP-primed mice showed clear-cut evidence of an active, disease-promoting Th2-associated cytokine response. As shown in Fig. 3, treatment with either anti–IL-4 or anti–IL-10 permitted HKLMP-sensitized mice to exert essentially normal control over intracellular visceral infection at week 4. Thus, whereas both IL-4 and IL-10 were required, neither by itself appeared sufficient to mediate the suppressive, antihost defense effect of the provoked Th2 response. We concluded from these results that IL-4 and IL-10 likely acted in concert in this model.
Table 1.
Serum IgE Levels before and after L. donovani Infection*
| Pretreatment of BALB/c mice | Week | Serum IgE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | |||||||
| μg/ml | ||||||||||
| Experiment 1 | ||||||||||
| None (control) | 0.6 ± 0.1 | 1.8 ± 0.3 | 4.0 ± 0.9 | 5.3 ± 1.2 | ||||||
| HKLMP | 6.2 ± 4.1 | 4.7 ± 0.4 | 15.3 ± 1.8 | 26.2 ± 3.7 | ||||||
| Experiment 2 | ||||||||||
| None (control) | 1.7 ± 0.4 | 3.9 ± 0.9 | 5.8 ± 0.8 | 18.8 ± 2.7 | ||||||
| HKLMP | 17.9 ± 2.9 | 13.9 ± 1.8 | 41.5 ± 8.9 | 97.8 ± 14.1 | ||||||
Mice received no pretreatment or once-per-week injections of HKLMP for 4 wk before L. donovani challenge. Week 0 indicates samples obtained just before L. donovani infection. Results are mean ± SEM values for three to four mice per group.
Figure 3.
Effect of anti–IL-4 or anti–IL-10 treatment on visceral infection of HKLPM-primed mice. BALB/c mice were pretreated once per week for 4 wk with HKLMP and then challenged with L. donovani. The day before challenge and once per week thereafter for 4 wk, mice received no further treatment (filled bar), injections of control antibody (hatched bar), or anti-cytokine mAb (open bar). Results are from two experiments, and indicate mean ± SEM LDU values for six to seven mice per group.
Effect on the Tissue Immune Response.
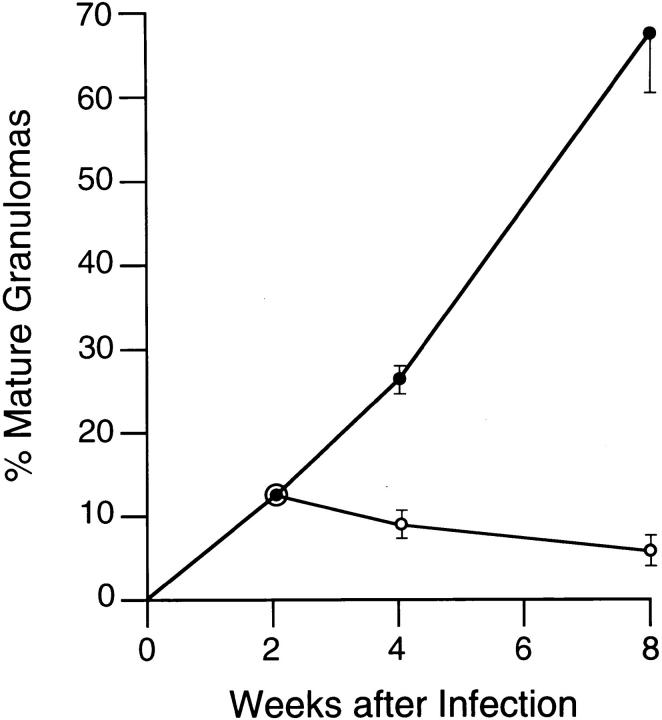
Since successful resistance to L. donovani in BALB/c mice is expressed in the tissues by granuloma formation (22, 47), we also examined liver sections from HKLMP-pretreated animals. In normal BALB/c mice, granuloma assembly in the liver proceeds in an orderly fashion; each infected focus, consisting initially of single, infected resident macrophages (Kupffer cells), gives rise to a core of fused parasitized Kupffer cells which comes to be surrounded by a mononuclear cell mantle comprised of influxing T cells and monocytes (22, 27, 47). This histologic reaction is detectable by week 2 after challenge and is fully developed by or after week 4 (22, 47). The early tissue reaction to L. donovani at week 2 was similar in HKLMP-treated and control BALB/c animals: (a) no cellular reaction was present at 32 ± 6% versus 40 ± 4% of infected foci, respectively; (b) developing granulomas were present at 56 ± 8% versus 48 ± 7% of sites; and (c) 12 ± 1% versus 12 ± 2% of foci were scored as mature granulomas (two experiments, n = 4 mice). However, after week 2, responses diverged. While normal mice converted the bulk of infected foci to mature granulomas by week 8, HKLMP-primed mice did not, and in these animals the tissue reaction failed to properly progress to yield formed granulomas at the majority of infected sites (Fig. 4). In normal BALB/c mice, single parasitized Kupffer cells with no surrounding mononuclear cell reaction were seldom encountered 4 (3 ± 1%) or 8 wk (1 ± 1%) after infection (two experiments, n = 4 mice). Thus, observing little or no histologic reaction at these latter two time points at 23 ± 4% and 29 ± 5% of parasitized foci in HKLMP-primed mice also demonstrated the extent of inhibition of mononuclear cell recruitment (Fig. 5). Together, these data emphasized the overall failure of granuloma assembly in this Th2 response environment.
Figure 4.
Inhibition of granuloma assembly in HKLMP-primed BALB/c mice. Liver sections from untreated control (•) and HKLPM-primed mice (○) were examined for infected foci. All foci in 50 consecutive ×63 microscopic fields were scored as follows for granuloma formation: none, single or fused parasitized Kupffer cells with no mononuclear cell infiltrate; developing granuloma, core of fused infected Kupffer cells with some cell infiltrate; or mature granuloma, fused Kupffer cell core surrounded by a well-developed mononuclear cell mantle (22, 27). Results are from two experiments, and indicate mean ± SEM values for four mice (see Fig. 5).
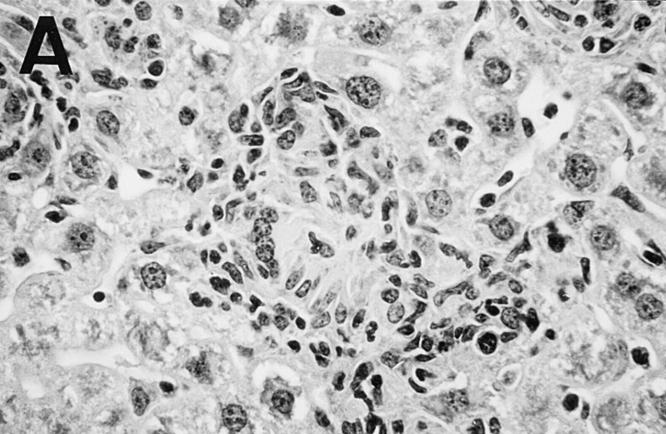
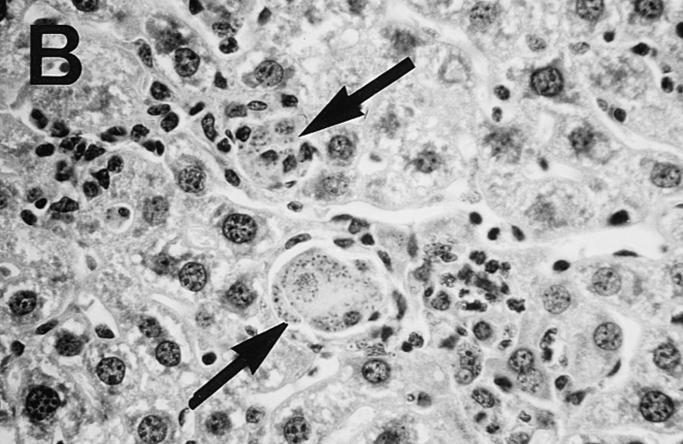
Figure 5.
Photomicrograph of liver sections from untreated control (A) and HKLMP-primed BALB/c mice (B) 4 wk after L. donovani infection. (A) shows a large, well-developed mature granuloma at an infected focus in normal mice. The granuloma contains few amastigotes. In B, HKLMP-primed mice show fused, heavily parasitized Kupffer cells (arrows), but little or no surrounding mononuclear cell infiltrate. ×200.
Effect of Prophylactic and Therapeutic Administration of Th1associated Cytokines IL-12 and IFN-γ.
We concluded the experiments in HKLMP-sensitized mice by asking (a) whether the induced Th2 response was reversible by early (prophylactic) treatment with IL-12 or IFN-γ, two Th1-associated cytokines with well-recognized antileishmanial effects (34, 37–39), and (b) could exogenous IL-12 or IFN-γ also induce activity once the Th2 response and visceral infection were well established. Consistent with earlier results derived from L. major–infected BALB/c mice (34, 39), early treatment with IL-12 (days +1 to +7) entirely reversed the inability of HKLMP mice to control visceral infection measured at week 4 (Table 2). However, in the same experiments, prophylactic treatment with IFN-γ had considerably less effect. Nevertheless, when given as therapy in HKLMPsensitized mice with firmly established infection, both IL-12 and IFN-γ induced antileishmanial activity (Table 2) at a level comparable to that achieved in parallel studies carried out in normal BALB/c mice (37, 48). While the effect of cytokine treatment in Table 2 is expressed as percentage inhibition of parasite replication, both IFN-γ and, to a considerably greater extent, IL-12, also induced leishmanicidal effects. In cytokine-treated HKLMP-primed mice, liver parasite burdens were reduced by 26% (IFN-γ) and 55% (IL-12) at the end of 7 d of cytokine administration (week 3 versus 4 LDU).
Table 2.
Effect of IL-12 and IFN-γ Treatment in HKLMP-primed BALB/c Mice*
| Treatment of HKLMP- primed mice | Liver parasite burden | % Inhibition of Replication‡ | ||||
|---|---|---|---|---|---|---|
| Day +21 | +28 | |||||
| LDU | ||||||
| Day +1 to +7 | ||||||
| None | (see below) | 2,561 ± 170 | − | |||
| Saline | NT§ | 2,682 ± 101 | 0 | |||
| IL-12 | NT | 546 ± 93+ | 79 | |||
| IFN-γ | NT | 1,893 ± 107 | 26 | |||
| Day +21 to +28 | ||||||
| None | 1,761 ± 132 | 2,561 ± 170 | − | |||
| Saline | − | 2,351 ± 97 | 8 | |||
| IL-12 | − | 767 ± 63++ | 70 | |||
| IFN-γ | − | 1,267 ± 139+ | 51 | |||
BALB/c mice were pretreated for 4 wk with HKLMP, challenged with L. donovani, and received either no treatment or were subsequently treated for 7 d with pump-administered saline, IL-12 (1 μg/d), or IFN-γ (2 × 105 U/d). 7-d pumps were implanted either 4 h after challenge or not until 3 wk later (day +21). All treated mice were killed on day +28. Results are from two (IL-12) to three experiments (IFN-γ), and indicate mean ± SEM values for 6–10 mice per group.
Compared to day +28 LDU in untreated control mice.
NT, not tested +P <.05 versus untreated controls on day +28. ++P <.05 versus untreated controls on both +21 and +28.
Discussion
These results in HKLMP-primed BALB/c mice (a) describe a model for studying visceral L. donovani in a predominant Th2-associated response environment, (b) not unexpectedly demonstrate that this response inhibits the capacity to acquire effective antileishmanial resistance measured by tissue parasite burden and granuloma assembly, and (c) suggest that the disease-exacerbating mechanism in this model requires the participation of both IL-4 and IL-10. Among other actions, IL-4 is thought to be primarily responsible for expanding the Th2 response (12, 13, 31, 41); both IL-4 and -10 are capable of suppressing the secretion and macrophage-activating effects of Th1-associated cytokines including IFN-γ (4, 12, 13, 31, 41–43, 49, 50).
While some of our observations, including the efficacy of prophylactic IL-12 (34, 39), mirror lessons already learned from models of infection caused by L. major and L. mexicana (12, 13, 16–19, 31, 33, 41), two findings differ. First, although endogenous IL-4 clearly played a resistance-inhibiting role in HKLMP-primed BALB/c mice, 129/Sv IL-4 transgenic mice showed no difficulty in controlling visceral L. donovani. We suspect these results indicate that sustained IL-4 secretion by itself may not be sufficient to overcome the innate resistance (20) imparted by the 129/Sv background of the transgenic animals. However, we did not directly measure IL-4 production, nor investigate other cytokine or cellular responses in infected transgenic mice; thus, potentially suboptimal IL-4 production (30), insufficient cofactor (IL-10) secretion, or the remote possibility of a simultaneously enhanced Th1 response also remain as other potential explanations for our observations.
Second, both IL-12– and IFN-γ–induced visceral antileishmanial activity in the face of an established, disease-exacerbating Th2-associated cytokine response. This new finding contrasts directly with treatment data derived from models of established L. major cutaneous infection in BALB/c mice in which neither exogenous IL-12 nor IFN-γ achieved antileishmanial activity (34, 39, 51). There are a number of possible explanations for these differing observations including the pathogens themselves, the site where infection is introduced, the requirement in the L. donovani model for prechallenge manipulation (sensitization), the heterogeneity and/or intensity of a naturally-occurring versus a provoked, cross-reacting type of Th2 response, and the timing and doses of cytokine treatment used. Nevertheless, experience in other models has also raised the possibility of overriding at least some components of an established Th2 response using exogenous IL-12 (52–59). In addition, treatment with IFN-γ alone can also induce measureable antileishmanial activity in patients with visceral leishmaniasis who also express a Th2 response (9, 60).
Finally, it is worth noting that in HKLPM-primed mice, exogenous IL-12 was considerably more active than IFN-γ when used either prophylactically or as treatment. Since this study did not include analysis of the mechanisms underlying the efficacy of IL-12 or IFN-γ in HKLMP-primed mice, we have not identified which prohost defense pathway was enhanced or which suppressive pathway was inhibited. However, because exogenous IL-12 readily induces IFN-γ (37), the enhanced effect of IL-12 in established infection (Table 2) suggests that in addition to macrophage activation stimulated by endogenous IFN-γ (38), multiple antileishmanial actions may mediate the efficacy of IL-12 in HKLMPtreated mice. These effects might include the capacity of IL-12 to expand other Th1-related mechanisms, suppress the deactivating effects of IL-4 and -10, and perhaps enhance the activity of natural killer cells (44).
Footnotes
This research was supported by National Institutes of Health research grant AI 16963. The DNAX Research Institute is supported by the Schering-Plough Corporation.
1 Abbreviations used in this paper: HKLPM, heat-killed L. major promastigotes; LDU, Leishman–Donovan units; r, recombinant.
References
- 1.Karp C, Wynn T, Satti M, Kordofani A, Hashim F, Neva F, Nutman T, Sacks D. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghalib H, Piuvezam M, Skeily Y, Siddig M, Hashim F, Russo D, Reed S. Interleukin-10 production correlates with pathology in human Leishmania donovaniinfections. J Clin Invest. 1993;92:324–329. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holaday B, de Lima M, Pompeu, Jeronimo S, Texeira M, de Queiroz A, Sousa, Vasconcelos A, Pearson RD, Abrams JS, Locksley RM. Potential role for interleukin 10 in the immunosuppression associated with kalaazar. J Clin Invest. 1993;92:2626–2632. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho E, Bacellar O, Regis T, Coffman R, Reed S. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–5956. [PubMed] [Google Scholar]
- 5.Raziuddin S, Abdalla R, El-Awad E, Al-Janadi M. Immunoregulatory cytokine production in visceral and cutaneous leishmaniasis. J Infect Dis. 1994;170:1037–1040. doi: 10.1093/infdis/170.4.1037. [DOI] [PubMed] [Google Scholar]
- 6.Cenini P, Benhe N, Hailu A, McGinnes K, Frommel D. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis. 1993;168:986–993. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 7.Holaday BJ, Pompeu M, Evans T, Braga D, Texeira M, Sousa A, Sadick M, Abrams J, Pearson R, Locksley R. Correlates of Leishmania-specific immunity in the clinical spectrum of infection with Leishmania chagasi . J Infect Dis. 1993;167:411–417. doi: 10.1093/infdis/167.2.411. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho EM, Barral A, Pedral-Sampario D, BarralNetto M, Badaro R, Rocha H. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi . J Infect Dis. 1992;165:535–540. doi: 10.1093/infdis/165.3.535. [DOI] [PubMed] [Google Scholar]
- 9.Kenney, R., S. Sundar, D. Sacks, and H. Murray. 1995. Cytokine responses in interferon-gamma monotherapy for kalaazar. Clin. Infect. Dis. 21:781a. (Abstr.)
- 10.Sundar, S., S.G. Reed, S. Sharma, A. Mehrota, and H.W. Murray. Circulating Th1 cell– and Th2 cell–associated cytokines in Indian patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. In press. [DOI] [PubMed]
- 11.Zwingenberger K, Harms G, Pedrosa C, Omena S, Beate S, Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-γ production. Clin Immunol Immunopathol. 1990;57:242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]
- 12.Liew F, O'Donnell C. Immunology of leishmaniasis. Adv Parasitol. 1993;32:162–192. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 13.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major . Ann Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 14.Doherty T, Kastelein R, Menon S, Coffman RL. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- 15.Barral-Netto M, Barral A, Brownell C, Skeiky Y, Twardizk D, Reed S. Transforming growth factor–β in leishmanial infection. Science (Wash DC) 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon-γ, interleukin-2, interleukin4, and interleukin-10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharton-Kersten T, Afonso L, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 18.Alfonso L, Scott P. Immune response associated with susceptibility of C57BL/10 mice to Leishmania amazonensis . Infect Immun. 1993;61:2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovaniinfection. Infect Immun. 1995;63:4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley DJ, Taylor BA, Blackwell J, Evans EP, Freeman J. Regulation of Leishmaniapopulations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979;37:7–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Crocker P, Blackwell JM, Bradley DJ. Expression of the natural resistance gene Lshin resident liver macrophages. Infect Immun. 1984;43:1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray HW, Stern JJ, Welte K, Rubin BY, Carriero SM, Nathan CF. Experimental visceral leishmaniasis: production of interleukin-2 and interferon-γ, tissue immune reaction, and response to treatment with interleukin-2 and interferon-γ. J Immunol. 1987;138:2290–2296. [PubMed] [Google Scholar]
- 23.Kaye PM, Curry AJ, Blackwell JM. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991;146:2764–2770. [PubMed] [Google Scholar]
- 24.Murray HW, Hariprashad J, McDermott D, Stoeckle MY. Multiple host defense defects in the failure of C57BL/6 ep/ep (pale ear) mice to resolve visceral Leishmania donovaniinfection. Infect Immun. 1996;62:161–166. doi: 10.1128/iai.64.1.161-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson ME, Sandor M, Blum AM, Younbg B, Metwali A, Elliot D, Lynch RG, Weinstock JV. Local suppression of IFN-γ in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi . J Immunol. 1996;156:2231–2239. [PubMed] [Google Scholar]
- 26.Miralles GD, Stoeckle MY, McDermott DF, Finkelman FD, Murray HW. Induction of Th1 and Th2 cell–associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994;62:1058–1062. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squires KE, Schreiber RD, McElrath JJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous interferon-γ in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- 28.Murray, H.W., and J. Hariprashad. 1995. Endogenous and exogenous IL-12 in experimental visceral leishmaniasis: effect of anti–IL-12 and treatment with IL-12 plus antimony. J. Investing. Med. 43:413a. (Abstr.)
- 29.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (Lond) 1993;362:245–247. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 30.Muller W, Kuhn R, Rajewsky K. Major histocompatibility complex class II hyperexpression on B cells in interleukin 4–transgenic mice does not lead to B cell proliferation and hypergammaglobulinemia. Eur J Immunol. 1991;21:921–925. doi: 10.1002/eji.1830210410. [DOI] [PubMed] [Google Scholar]
- 31.Leal LM, Kuhn R, Muller W, Liew FY. Interleukin-4 transgenic mice of resistant background are susceptible to Leishmania majorinfection. Eur J Immunol. 1993;23:566–569. doi: 10.1002/eji.1830230241. [DOI] [PubMed] [Google Scholar]
- 32.Finkelman F, Madden K, Morris S, Katoma I, Maliszewki C. Anti-cytokine antibodies as carrier proteins: prolongation of in vitro effects of exogenous cytokines by injection of cytokine–anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1242. [PubMed] [Google Scholar]
- 33.Heinzel FP, Rerko RM, Ahmed F, Perlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 34.Sypek J, Chung C, Mayor S, Goldman S, Sieburth D, Wolf S, Schwab R. Resolution of cutaneous leishmaniasis: IL-12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liew FY, Hale C, Howard JG. Prophylactic immunixation against experimental leishmaniasis. IV. Subcutaneous immunization prevents induction of protective immunity against fatal Leishmania majorinfection. J Immunol. 1985;135:2095–2101. [PubMed] [Google Scholar]
- 36.Abrams JS, Roncarolo MG, Yssel H, Anderson U, Gleich GJ, Silver JE. Strategies of anticytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–16. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 37.Murray HW, Hariprashad J. Interleukin 12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray HW. Effect of continuous administration of interferon-gamma in experimental visceral leishmaniasis. J Infect Dis. 1990;161:992–994. doi: 10.1093/infdis/161.5.992. [DOI] [PubMed] [Google Scholar]
- 39.Heinzel F, Schoenhaut D, Rosser L, Gately M. Recombinant interleukin-12 cures mice infected with Leishmania major . J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 41.Mocci S, Coffman RL. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol. 1995;154:3779–3787. [PubMed] [Google Scholar]
- 42.Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major–infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 43.Sadick MD, Street N, Mosmann TR, Locksley RM. Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect Immun. 1991;59:4710–4712. doi: 10.1128/iai.59.12.4710-4714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin 12: a proinflammatory cytokine with immunoregulatory functions that bridges innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–262. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 45.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon-γ or interleukin-4 during the resolution or progression of murine leshmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bretscher P, Wei G, Menon J, Bielefeldt H. Establishment of stable cell-mediated immunity that makes susceptible mice resistant to Leishmania major . Science (Wash DC) 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 47.McElrath JJ, Murray HW, Cohn ZA. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988;167:1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray HW, Hariprashad J, Aguero B, Yeganeyi H. Intracellular antimicrobial response of T cell–deficient host to cytokine therapy: IFN-γ in experimental visceral leishmaniasis in nude mice. J Infect Dis. 1995;171:1309–1314. doi: 10.1093/infdis/171.5.1309. [DOI] [PubMed] [Google Scholar]
- 49.Ghalib H, Whitle J, Kubin M, Hashim F, Grabstein K, Trinchieri G, Reed S. IL-12 enhances Th1-type responses in human Leishmania donovaniinfections. J Immunol. 1995;154:4623–4629. [PubMed] [Google Scholar]
- 50.Powrie F, Menon S, Coffman RL. IL-4 and IL-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- 51.Sadick MD, Heinzel FP, Holaday BJ, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti–interleukin-4 monoclonal antibody. Evidence for a T celldependent, interferon-γ–independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabors GS, Afonso L, Farrell J, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania majorinfection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wynn T, Oswald I, Cheever A, Sher A. Endogenous interleukin 12 regulates granuloma formation induced by Schistosoma mansoniand exogenous IL-12 both inhibits and immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gazzinelli R, Giese N, Morse H. In vivo treatment with IL-12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome. J Exp Med. 1994;180:2199–2208. doi: 10.1084/jem.180.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urban J, Madden K, Cheever A, Trutta P, Finkelman F. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus . J Immunol. 1993;151:7086–7094. [PubMed] [Google Scholar]
- 56.Pearlman, E., F. Hazlett, F. Heinzel, S. Wolf, and J. Kazura. 1994. IL-12 modulation of T helper responses to the filarial helminth, Brugia malayi Am. J. Trop. Med. Hyg. 51:221a. (Abstr.). [PubMed]
- 57.Renz H, Jujo K, Bradley K, Domenico J, Gelfand E, Leung D. Enhanced IL-4 production and IL-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J Invest Dermatol. 1993;99:403–408. doi: 10.1111/1523-1747.ep12616114. [DOI] [PubMed] [Google Scholar]
- 58.Marshall J, Secrist H, DeKruyff R, Wolf S, Umetsu D. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- 59.Donnelly R, Freeman SL, Hayes MP. Inhibition of IL-10 expression by IFN-γ upregulates transcription of TNF-α in human monocytes. J Immunol. 1995;155:1420–1427. [PubMed] [Google Scholar]
- 60.Sundar S, Murray HW. Effect of treatment with interferon-γ alone in Indian visceral leishmaniasis. J Infect Dis. 1995;172:1627–1630. doi: 10.1093/infdis/172.6.1627. [DOI] [PubMed] [Google Scholar]