Abstract
Granzyme B (GraB) induces apoptosis in the presence of perforin. Perforin polymerizes in the cell membrane to form a nonspecific ion pore, but it is not known where GraB acts to initiate the events that ultimately lead to apoptosis. It has been hypothesized that GraB enters the target cell through a perforin channel and then initiates apoptosis by cleaving and activating members of the ICE/Ced-3 family of cell death proteases. To determine if GraB can enter the cell, we treated YAC-1 or HeLa cells with FITC-labeled GraB and measured intracellular fluorescence with a high sensitivity CCD camera and image analyzer. GraB was internalized and found diffusely dispersed in the cell cytoplasm within 10 min. Uptake was inhibited at low temperature (4°C) and by pretreatment with metabolic inhibitors, NaF and DNP, or cytochalasin B, a drug that both blocks microfilament formation, and FITC-GraB remained on the cell membrane localized in patches. With the simultaneous addition of perforin and FITC-GraB, no significant increase in cytoplasmic fluorescence was observed over that found in cells treated only with FITC-GraB. However, FITC-GraB was now detected in the nucleus of apoptotic cells labeling apoptotic bodies and localized areas within and along the nuclear membrane. The ability of GraB to enter cells in the absence of perforin was reexamined using anti-GraB antibody immunogold staining of ultrathin cryosections of cells incubated with GraB. Within 15 min, gold particles were detected both on the plasma membrane and in the cytoplasm of cells with some gold staining adjacent to the nuclear envelope but not in the nucleus. Cells internalizing GraB in the absence of perforin appeared morphologically normal by Hoechst staining and electron microscopy. GraB directly microinjected into the cytoplasm of B16 melanoma cells induced transient plasma membrane blebbing and nuclear coarsening but the cells did not become frankly apoptotic unless perforin was added. We conclude that GraB can enter cells autonomously but that perforin initiates the apoptotic process and the entry of GraB into the nucleus.
CTL and NK cells induce apoptosis through granule- or Fas-dependent pathways (1–5). Initiation of apoptosis by granule exocytosis is the result of the action of two types of molecules, the pore-forming protein perforin and the lymphocyte-specific granule serine esterase granzyme B (GraB)1, which together can reproduce all of the features of CTL-induced apoptosis (6–8). In mice made deficient in perforin or GraB as a result of a directed gene targeting, CTL/NK cytotoxicity and apoptosis do not proceed normally (1–5, 9). The exact mechanism by which these molecules interact to produce apoptosis is not understood. Perforin polymerizes in the plasma membrane in the presence of calcium and allows the nonspecific entry of ions (10–12). At high doses of perforin the cell membrane is damaged as measured by the loss of cytoplasmic proteins, however, perforin by itself does not induce apoptosis when incubated with target cells of different types (6, 7). Similarly, purified GraB and other granzymes induce apoptosis in the presence of perforin, yet the protease has no effect when incubated with a target cell alone (6, 7).
GraB cleaves proteins after aspartic acid (7, 13) and this proteolytic specificity is shared with members of the cysteine protease interleukin-1β–converting enzyme (ICE) family (14), which are homologues of the CED-3 cell death gene of Caenorhabditus elegans (15). Recent work suggests that GraB can proteolytically cleave and activate several members of ICE family in vitro including CPP32 (16– 19), MCH3/ICE-LAP3 (18, 19), MCH4 (18), FLICE/ Mach1/MCH5 (20, 21), ICE-LAP6 (22), and ICH-3 (23). There is also increasing evidence that ICE homologues are required for GraB- and perforin-induced apoptosis. For example, inhibition of ICE family protease activity using tetrapeptide inhibitors Ac-DEVD-CHO or Ac-YVAD-CHO, which react with different ICE protease catalytic sites (24, 25), and overexpression of a dominant negative mutant of ICE (25) suppress GraB apoptosis. Furthermore, fibroblasts and B cells from mice deficient in ICE on the basis of directed gene deletion (26) show high levels of resistance to GraB-mediated apoptosis (25).
ICE is a cytoplasmic protease in monocytes, however, the exact subcellular localization of this protease or other members of the family is not known. Thus, to initiate apoptosis after its release by CTL, GraB would likely need to cross the target cell plasma membrane. Currently, there is no direct evidence that GraB penetrates the target cell at any time during the induction of apoptosis. GraB can enter isolated nuclei (27), although the functional significance is unclear since GraB does not cause apoptotic morphological changes to isolated nuclei in the absence of cytosol (24).
In this study, we report that GraB is able to rapidly enter the cell cytoplasm via an energy-dependent mechanism in the absence of perforin. Perforin does not significantly affect GraB intracellular concentration, but it does initiate apoptosis and GraB translocation to the nucleus. Thus, we conclude that GraB has the capacity to autonomously enter the cell cytoplasm where it remains inactive, and requires some critical signal from perforin to initiate apoptosis and nuclear translocation.
Materials and Methods
Reagents and Cells.
Rat and human GraB and rat perforin were purified as described in earlier publications (6, 7, 16). YAC-1 or Rat-1 cells were cultured in RPMI 1640 supplemented with 10% FCS and penicillin-streptomycin, while B16 melanoma cells were cultured in α-MEM supplemented with 10% FCS, penicillin-streptomycin and 2 mM l-glutamine. Hoechst 332385, cytochalasin B, NaF, DNP, rhodamine-conjugated transferrin, digitonin, and Triton X-100 were obtained from Sigma Chem. Co. (St. Louis, MO).
Monoclonal 2C5 anti-GraB antibody has been described previously (28). The mAb recognizes a 32-kD protein in cells transfected with GraB cDNA but not H, A, or Met-ase and the same 32-kD protein in human peripheral blood lymphocyte and cytotoxic lymphocyte cell line YT. It also does not recognize granzymes A, H, or Met-ase determined by reactivity with GST fusion proteins. The antibody immunoprecipitates asp-ase but not tryptase, Met-ase or chymase activities from LAK cells.
For fluorescenation, human GraB (60 μg/ml) was equilibrated in 0.2 M NaCl/Na borate buffer, pH 9.2. FITC (1 mg/ml in the same buffer) was then added for 2 h at ambient temperature. Uncoupled FITC was removed by dialysis.
Apoptosis Assays.
Apoptosis was assayed as described in an earlier publication (6). In experiments in which GraB was preincubated with target cells, YAC-1 cells were first labeled with 125IUdR for 90 min, washed, and then 2 × 104 cells were incubated with GraB at increasing concentrations in 80 μl of HBSS with 2 mg/ml of BSA at 37°C. After an incubation of 15–60 min, cells were washed two times with HBSS and then 5 × 103 cells were placed in a V-bottomed microtiter plate with perforin at the concentrations indicated for an additional 2–4 h. Cells were analyzed as described previously. Experiments in which apoptosis was analyzed by chromatin condensation with Hoechst dye used in previously published methods (25).
Fluorescence Microscopy.
HeLa or YAC-1 cells were washed twice in HBSS and then resuspended at 2.5 × 106 cells/ml in HBSS containing 10 mM Hepes, 2 mM CaCl2, and 4 mg/ml BSA, pH 7.2. Aliquots of 2 × 105 cells in 80 μl were added to 80 μl of FITC-GraB (final concentration 0.25–2 μg/ml), with or without perforin diluted in buffer containing 140 mM NaCl, 10 mM Hepes, 1 mM EGTA, pH 7.2. In some experiments cells were preincubated for 15 min at room temperature with 100 μM DNP and 1 mM NaF, or 2 μg/ml cytochalasin B, and then the cells incubated with FITC-GraB. The mixture was incubated at 37°C for 10 min to 3 h and then fixed for 10 min in 3.4% formaldehyde by adding 16 μl of 37% formaldehyde stock. Then the mixture was washed twice in PBS containing 3.4% formaldehyde. 20 μl of anti-bleach was added to the cell pellet and the cells were mounted on a glass slide and analyzed.
Image analysis was performed using a Zeiss Axiophot microscope equipped with a cooled CCD camera CH250/a (Photometrics Inc., Woburn, MA) with a KAF-1400-50 sensor chip (1317 × 1035 pixels) driven by IPLabs Spectrum H-SU2 (version 3.0; Signal Analytics Corp., Vienna, VA) and Multiprobe 1.1 E (Signal Analytics) software on a Power Macintosh 8100. The intensity of the fluorescence was calculated using the above software in which the x-axis represented a line starting outside the cell (the background) then extending through the cytoplasm and nucleus of the cell. The y-axis represents the relative fluorescence intensity per pixel. All cell images were examined at the same magnification and at the same light exposure time within a given experiment. The value of intracellular fluorescence represents the difference between the peak intracellular and the extracellular fluorescent intensity. Between 15 and 30 cells in a minimum of 5 randomly chosen fields were analyzed for each experimental point.
Immunoelectronmicroscopy.
After incubation with GraB, cells were fixed in suspension with an equal volume of 8% paraformaldehyde in 0.1 M PBS for 10 min at room temperature. Then cells were centrifuged and resuspended in fresh paraformaldehyde for 1 h. The pelleted cells were cut into small blocks and placed into a cryoprotectant mixture of 20% polyvinylpyrolidine, molecular weight 10,000 (Sigma), and 1.84% sucrose for 4 h at room temperature. The specimen was mounted and frozen in liquid nitrogen, then 75-nM sections were prepared on an RMC CR21 Cryosectioning system (EMLAB Equipment, Inc.,Whitby, Ontario). Sections were mounted on a Formvar/carbon-coated grid, then inverted and placed on droplets of mouse anti–human GraB (1/100 in 0.1% BSA in 0.1 M PBS) for 60 min at room temperature. The grids were rinsed four times for 5 min in 0.1 M PBS and then transferred to diluted 10 nM gold-labeled goat anti–mouse IgG (Amersham Corp., Arlington Heights, IL) (1/10 in 0.1% BSA in 0.1 M PBS) for 30 min at room temperature. Grids were then washed four times as above in 0.1 M PBS, then four more times in distilled H20. Sections were then protected by embedding in a mixture of 20% polyvinyl alcohol, molecular weight 10,000 (Sigma), and 0.3% uranyl acetate (JB EM Services Inc., Montreal, Quebec) for 10 min at room temperature for counterstaining sections. Then excess solution was wicked off with a filter paper. Grids were dried and then examined with an electron microscope (EM 400; Philips Technologies, Cheshire, CT).
Cell Microinjection.
Baxter glass cover slips 22 × 22 mm (Canlab) were precleaned with chromic acid and preincubated with FCS for 18 h at 37°C to prepare for tissue culture. The coverslips were glued into a window cut from the base of a 60-mm Nunclon petri dish with silicone sealant. B16 or Rat-1 cells were cultured on the cover slips in α-MEM and 10% FCS and allowed to adhere for several hours. Culture medium was then removed and replaced with phenol red-free HBSS containing 20 mM Hepes and 2 mg/ml BSA. GraB was adjusted to 0.15 M NaCl in 20 mM sodium phosphate. Cells were microinjected manually with continuous pressure of 20 hPa with an Eppendorf microinjector and Femptotips (Eppendorf, Hamburg, Germany) or by automated injection of a fixed volume using a Leitz micromanipulator (Leitz, Wetzlar, Germany) on a heated stage. For image analysis, cells were viewed through a Zeiss IM35 microscope and analyzed at 30-s intervals by an Image-1 computer imaging system (Empix Imaging, Mississauga, Ontario). In other experiments, 2 μl of 20 mM Hoechst dye was added at various times after injection, and the cells were examined 3–4 min later by fluorescent microscopy and photographed with a Contax 167MT camera with Tri-X pan 400 film. Nuclear staining was rapid in cells after microinjection whereas longer incubation periods were required in intact B16 cells (see below).
In some experiments, cells were incubated on 22-mm round glass coverslips, treated as above, in 24-well Nunclon plates for 24–48 h in α-MEM and 10% FCS and then transferred to another 24-well plate where they were incubated in 50 μl of HBSS containing 20 mM Hepes, 1 mM EGTA, and 4 mM CaCl2. GraB and perforin were diluted in 0.15 mM NaCl, 1 mM EGTA, and 20 mM Hepes to the appropriate concentration and then added to the incubation medium for the indicated times at 37°C. The coverslips were then incubated with 1 μl of 20 mM Hoechst 33258 dye for 1 h and inverted onto a Zeiss Axiovert 35M microscope slide for photomicroscopy.
Results
FITC-GraB Crosses the Plasma Membrane.
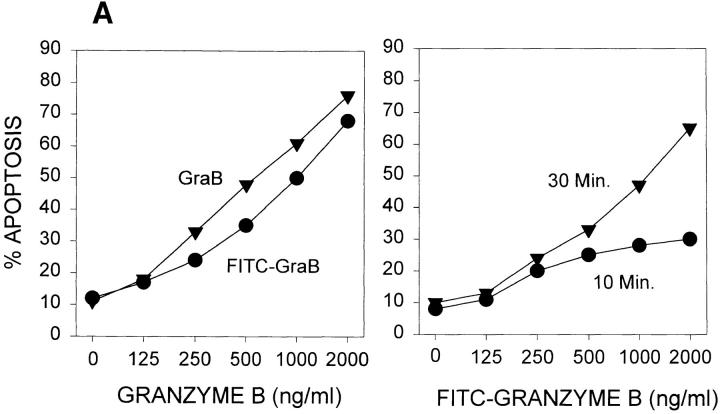
To determine whether GraB entered cells, we examined the localization of FITC-labeled human GraB using a high-sensitivity CCD camera and image analysis to detect and quantify intracellular fluorescence. We first evaluated the apoptotic activity of FITC-GraB to determine whether the fluorescenation of the granzyme had ablated its biological activity. YAC-1 cells were incubated with increasing concentrations of FITC-GraB (0.0625–2.0 μg/ml) in the presence of a constant amount of perforin (125 ng/ml). After 10 min of incubation a low level of activity was detected on the basis of Hoechst staining of apoptotic nuclei and by 30 min ∼70% of the cells had undergone apoptosis by the highest GraB concentrations (Fig. 1 A, right). The apoptosis produced by FITC-GraB was equivalent to that induced by unlabeled GraB and perforin (Fig. 1 A, left).
Figure 1.
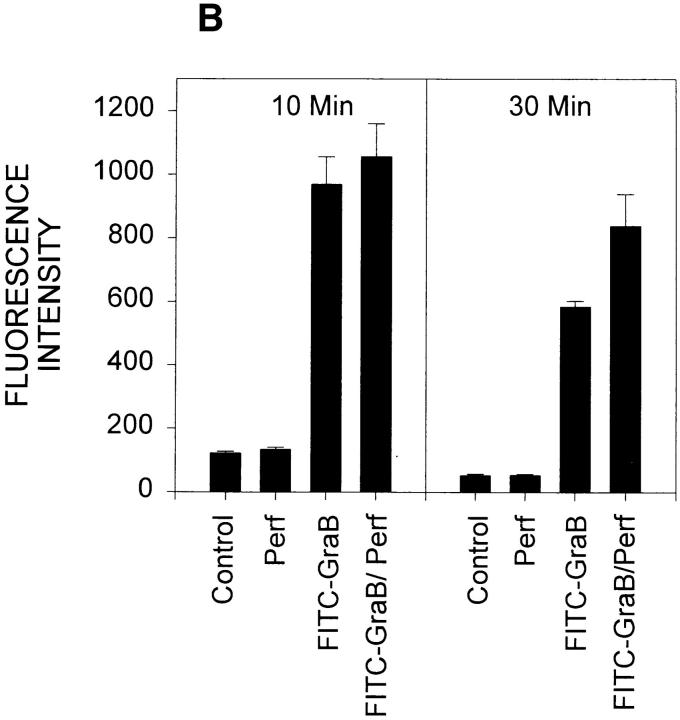
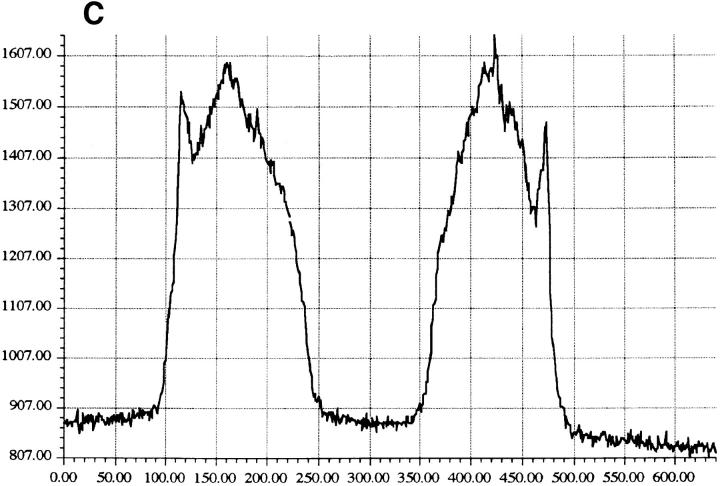
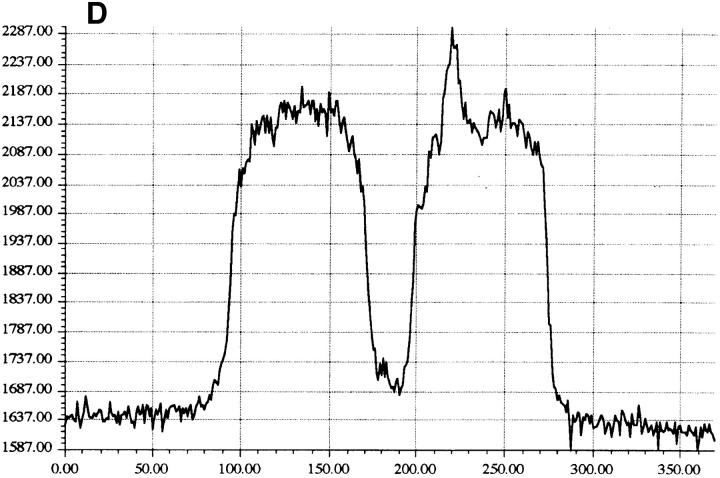
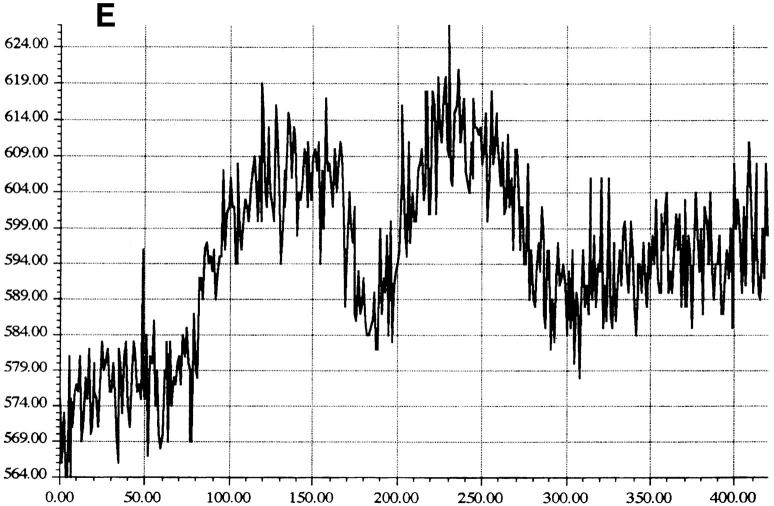
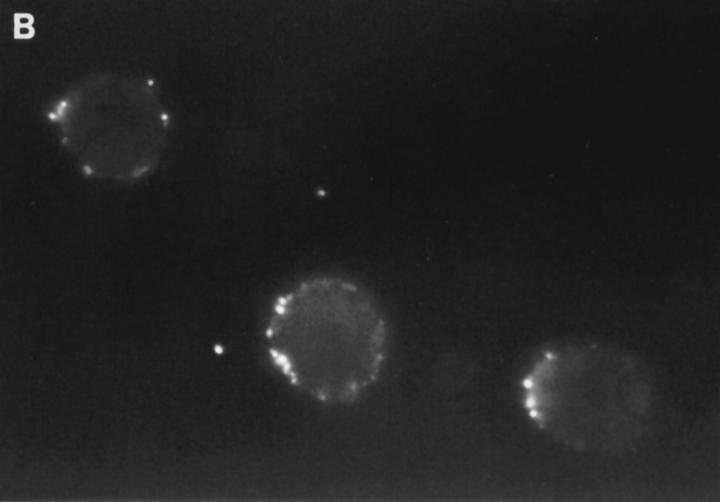
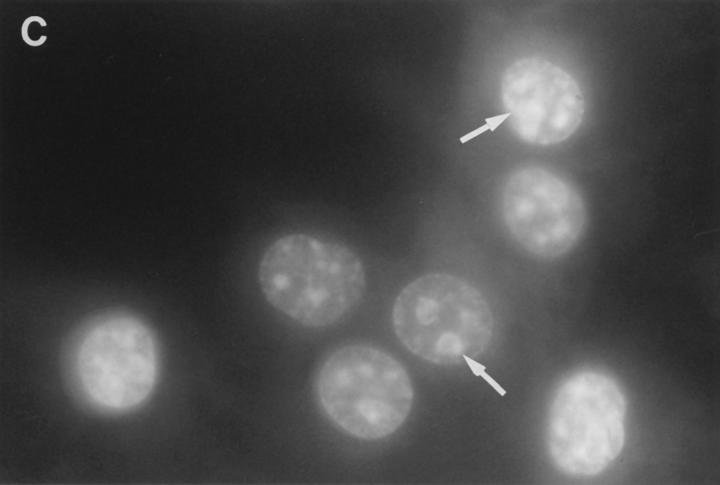
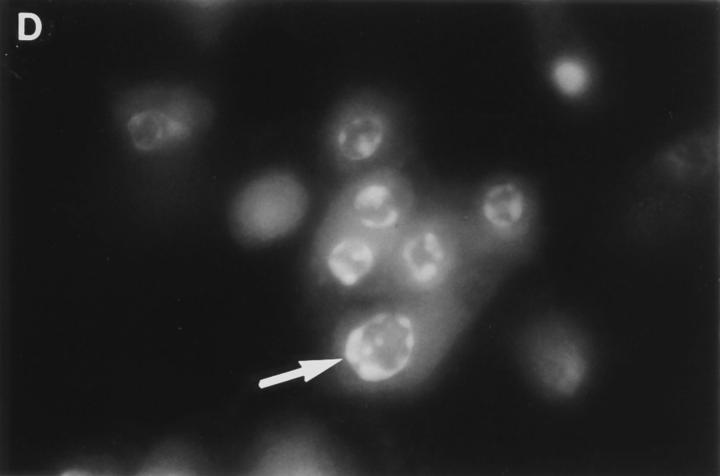
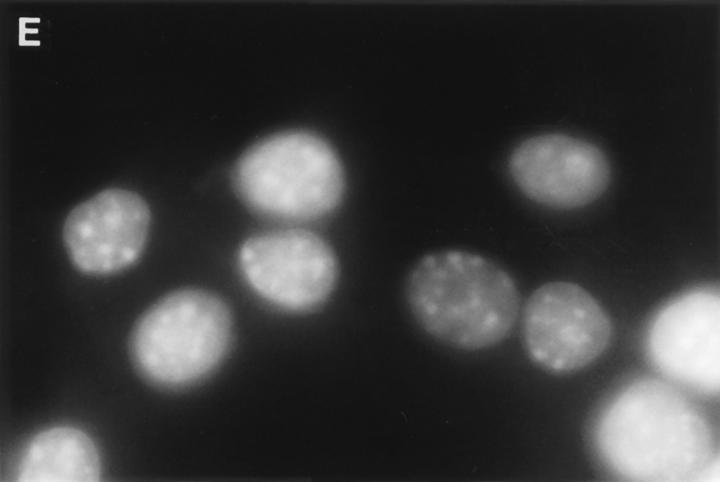
Cytoplasmic uptake of FITC-GraB. (A, left) Apoptosis of YAC-1 cells comparing GraB (filled triangles) to FITC-labeled GraB (filled circles)with a constant amount of perforin (125 ng/ml). Apoptosis was measured by the release of 125IUdR and the results are expressed as percentage of total label incorporated into the target cells. (Right) Apoptosis of YAC-1 cells by increasing concentrations of FITC-GraB in the presence of perforin (125 ng/ml) incubated for 10 min (filled circles) or 30 min (filled triangles) as indicated. Apoptosis was measured by the condensation of chromatin visualized by Hoechst staining. (B) Quantitation of fluorescence label in YAC-1 cells treated with FITC-GraB (GraB) with or without perforin (Perf). Cells were incubated in 1 μg/ml of FITC-GraB for 10 or 30 min then fixed and visualized with a CCD camera. Intracellular fluorescence was quantitated using an IPLabs Spectrum H-SU2 image analysis program. At least 30 cells were measured for each condition and the difference between extracellular and a maximum plateau of intracellular fluorescence calculated and scored. Data is expressed as the mean and SEM. The experiment was repeated several times with YAC-1 or HeLa cells with the same result. (C) Fluorescence of two YAC-1 cells treated for 30 min with FITC-GraB and analyzed using an IPLabs Spectrum image analysis program. Intracellular fluorescence was calculated for each cell by the difference between the background surrounding the cell and the mean peak of intracellular fluorescence on the y-Axis. (D) Image analysis of two cells treated with FITC-GraB and perforin for 30 min. (E) Image analysis of the autofluorescence of two YAC-1 cells used as background.
We then determined if the FITC-GraB entered YAC-1 or HeLa cells after incubation with 1 μg/ml of the protease for 10 or 30 min in the presence or absence of perforin (Fig. 1 B). This dose of GraB produced ∼50% apoptosis after 30 min in the presence of perforin (Fig. 1 A). Cells were fixed and fluorescence distribution examined then quantitated using a CCD camera with IPLabs Spectrum H-SU2 and Multiprobe 1.1 E (Signal Analytics) software (see Materials and Methods). Cells treated with FITC-GraB exhibited an 8–12-fold increase in fluorescence within 10 min with no further increase and perhaps a small decrease by 30 min of incubation (Fig. 1 B). Cells treated with both FITC-GraB and perforin exhibited similar levels of intracellular fluorescence compared with cells treated with FITCGraB alone at 10 min with a slight increase at 30 min (Fig. 1 B). Fig. 1, C–E illustrates the image analysis of intracellular fluorescence of two cells represented by peaks of fluorescence after treatment with FITC-GraB (Fig. 1 C ), with FITC-GraB and perforin (Fig. 1 D), and the autofluorescence of untreated controls (Fig. 1 E ).
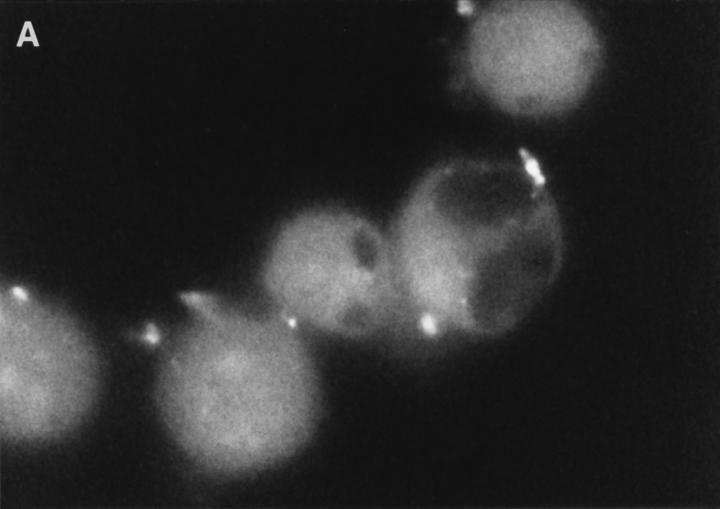
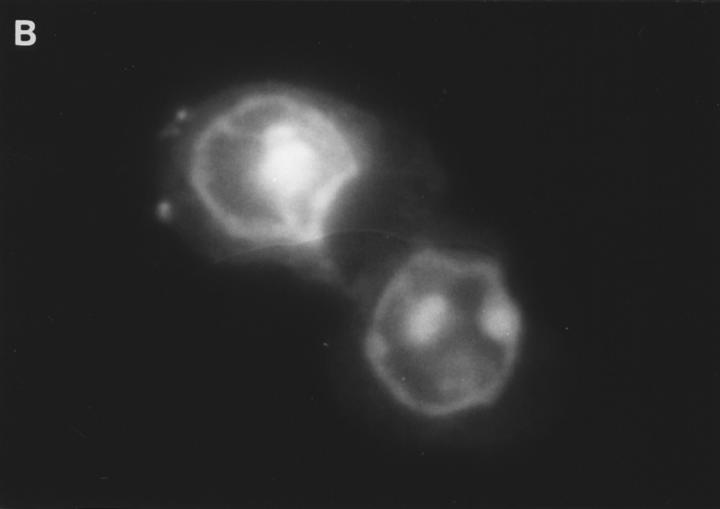
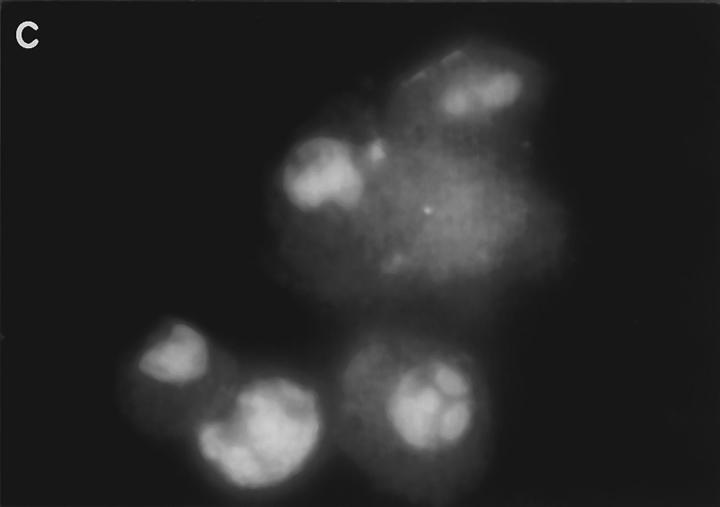
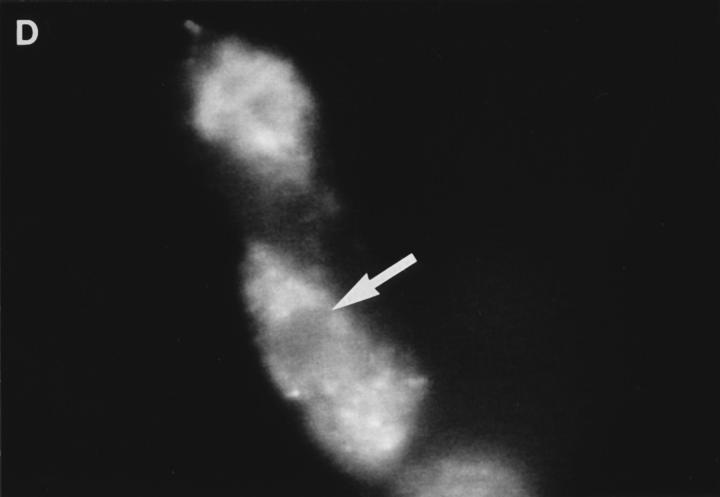
Examination of the distribution of fluorescence in FITCGraB–treated cells, showed the most intense fluorescence in the cytoplasm with a shadow evident over the nucleus suggesting that the granzyme had not crossed the nuclear membrane (Fig. 2, A and B). However, in perforin- and GraB-treated cells the fluorescence pattern had shifted dramatically. Here we observed that the nucleus of cells that were undergoing apoptosis now stained strongly for FITCGraB (Fig. 2, B and C ). In HeLa cells, after 90 min of FITCGraB and perforin treatment, staining was evident around the nuclear membrane with localized areas of fluorescence in the nucleus (Fig. 2 B). In YAC-1 cells treated in the same way, intense staining was evident in areas of chromatin margination and in apoptotic bodies with fainter residual staining in the cytoplasm (Fig. 2 C ). To determine if proteins other than FITC-GraB cross the nuclear membrane as a result of apoptosis induced damage, we examined the fate of rhodamine-conjugated transferrin administered to cells with GraB and perforin. Under these conditions, transferrin was excluded from the nucleus (Fig. 2 D). Cells were also analyzed after treatment with different doses of FITC-GraB ranging from 62.5 to 1,000 ng/ml in the presence or absence of perforin and showed the identical staining pattern but with proportionately lower levels of fluorescence (not shown).
Figure 2.
(A) Intracellular fluorescence of cells treated with FITCGraB in the presence and absence of perforin. HeLa cells are shown 30 min after incubation with FITC-GraB (1 μg/ml). The fluorescence is distributed diffusely throughout the cytoplasm and a shadow indicating lower levels is seen over the nucleus. After treatment with FITC-GraB and perforin for 30 min both HeLa cells (B) or YAC-1 cells (C) showed a dramatic redistribution with the FITC-GraB now seen in the nucleus with some cytoplasmic staining. In HeLa cells (B) the fluorescence was distributed around the nuclear membrane and in localized areas in the nucleus while in YAC-1 cells (C) the fluorescence was detected in areas of condensed chromatin or in apoptotic bodies. YAC-1 cells were treated with GraB and perforin in the presence of rhodamine-conjugated transferrin (D). Transferrin was not detected in the nucleus in cells undergoing apoptosis (arrow). Cells from GraB and perforin treatment in the presence of rhodamine-conjugated transferrin shown in D were stained with Hoechst dye to demonstrate the condensed chromatin in the apoptotic nucleus (E). The experiments described above were repeated at least three times with each target cell.
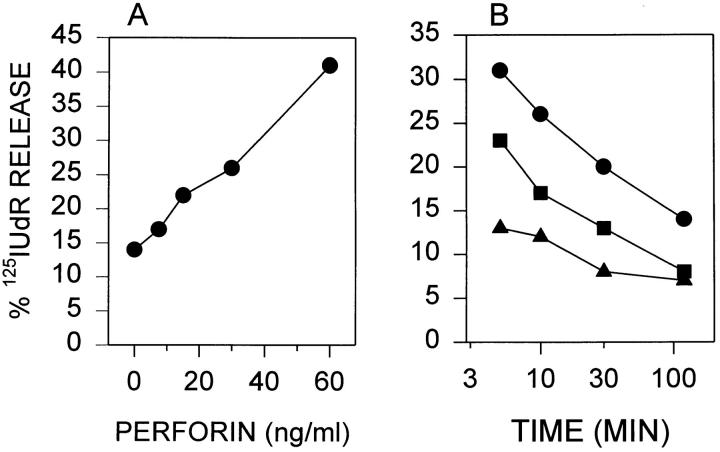
To determine if the cell-associated GraB detected by fluorescence was sufficient to induce apoptosis, YAC-1 cells were labeled with [125I]UdR then incubated with GraB (2 μg/ml) for 15 min and then washed to remove extracellular protease. This was immediately followed by the addition of perforin at increasing concentrations. These cells underwent apoptosis efficiently and in a dose-dependent manner (Fig. 3 A). If the cells were first treated with GraB as above and then washed and incubated for increasing times, the addition of perforin became gradually less efficient at inducing DNA damage suggesting that the cell associated GraB had been progressively eliminated from the cells (Fig. 3 B).
Figure 3.
Cell associated GraB induces apoptosis with the addition of perforin. (A) 125IUdR-labelled YAC-1 cells were preincubated in GraB (2 μg/ml) for 15 min, then washed and perforin added at increasing concentrations and the percent apoptotic cells calculated after incubation for 3 h. (B) GraB (2 μg/ml) was incubated with YAC-1 cells for 15 min, washed, and then incubated for increasing periods of time as shown. Cells were then washed again and perforin added for an additional 3 h. (Filled circles) 60 ng/ml, (filled squares) 30 ng/ml, (filled triangles) 15 ng/ml.
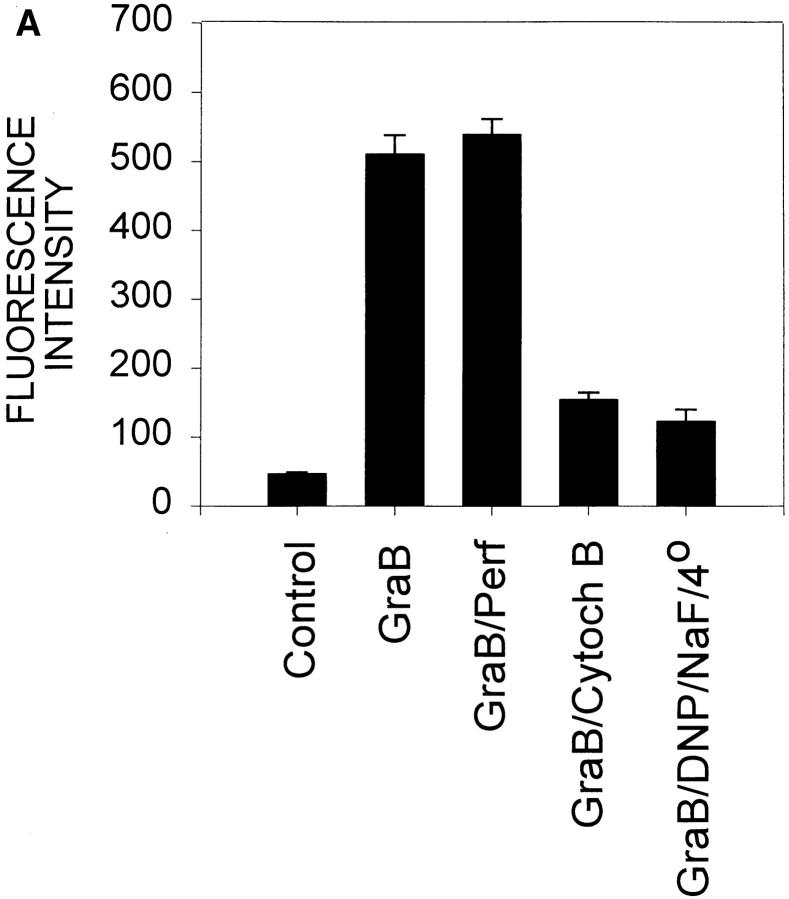
Next we determined whether GraB transit of the plasma membrane was an active energy-dependent process. FITCGraB was incubated with cells that had been pretreated either with NaF an inhibitor of glycolysis and DNP, which uncouples oxidative phosphorylation, or incubated at 4°C. Pretreatment with NaF and DNP in combination or incubation at 4°C reduced fluorescence by 30–50% in either YAC-1 or HeLa cells (not shown). When cells were treated with both inhibitors at 4°C then fluorescence was reduced by over 80% (Fig. 4 A). Cells examined under these conditions revealed GraB in a patchy plasma membrane distribution, indicating that it had bound to but had not crossed the cell membrane (Fig. 4 B). In earlier studies we had shown that pretreatment of target cells with cytochalasin B, an inhibitor of actin polymerization and microfilament formation (29), blocked apoptosis induced by GraB and perforin (7). When cytochalasin B was incubated with cells, we found that the amount of FITC-GraB that entered the cell was also profoundly suppressed (Fig. 4 A).
Figure 4.
GraB crosses the plasma membrane by an energy-dependent mechanism. (A) HeLa cells were treated with NaF (1 mM) an inhibitor of glycolysis and DNP (100 μM) which uncouples oxidative phosphorylation at 4°C or cytochalasin B (Cytoch B) (2 μg/ml) and then incubated with FITC-GraB (GraB) or GraB and perforin (Perf) for 30 min then analyzed as described in Fig. 2. (B) Cells treated with DNP/NaF at 4°C then FITC GraB showed fluorescence localized to the plasma membrane in discreet patches or clumps.
Detection of Cytoplasmic GraB by Immunoelectronmicroscopy.
To confirm the observation that GraB can enter cells in the absence of perforin by an independent method, we next used colloidal gold labeling of GraB using anti-GraB antibody. YAC-1 cells were treated with 2 μg/ml of GraB and then harvested and fixed at increasing time intervals of 10, 20, 45, and 60 min as described in Materials and Methods. Ultrathin cryosections were prepared and then incubated with anti-human GraB antibody or normal IgG control followed by goat anti–mouse IgG colloidal gold (10 nM), and then the cells were then examined by transmission electron microscopy. Anti-GraB antibody colloidal gold staining was found on the external surface of the plasma membrane associated with areas of increased electron density (Fig. 5 A). Gold particles were also identified in the cell cytoplasm of GraB-treated but not control cells treated with primary and secondary antibody but without GraB preincubation (Fig. 5, B–D). Colloidal gold was not seen associated with any particular cellular structure but was distributed throughout the cytoplasm and was sometimes observed near the nuclear envelope as illustrated in Fig. 5, B and C. No gold particles were detected in the nucleus. At later time points the amount of GraB in the cell cytoplasm was either equivalent to the 15-min time point or somewhat decreased. The nucleus of the cells and the overall cell morphology was not significantly altered (Fig. 5 C ).
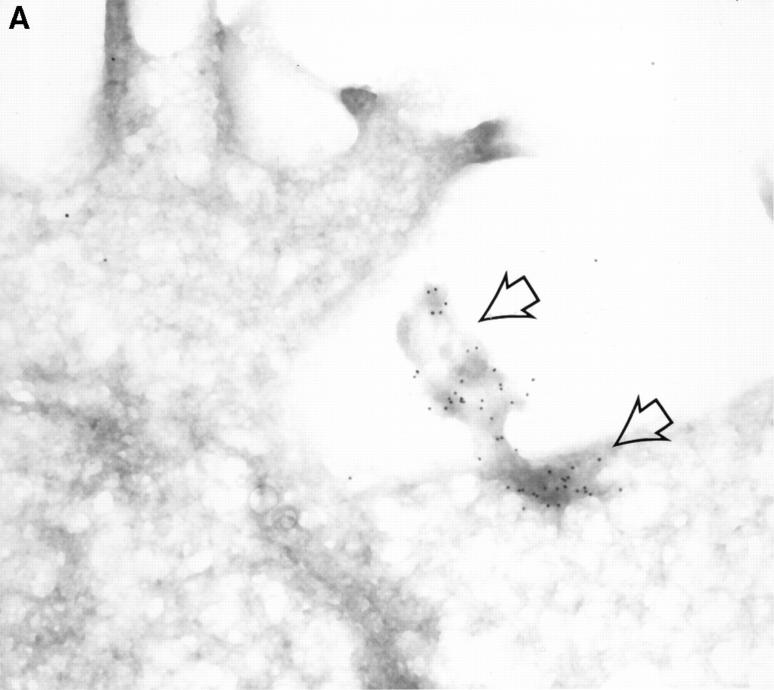
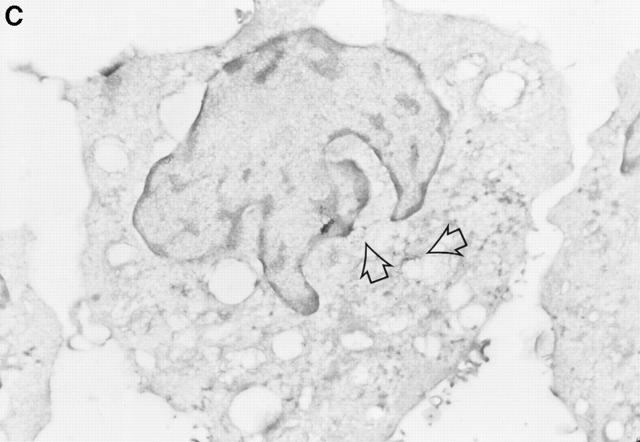
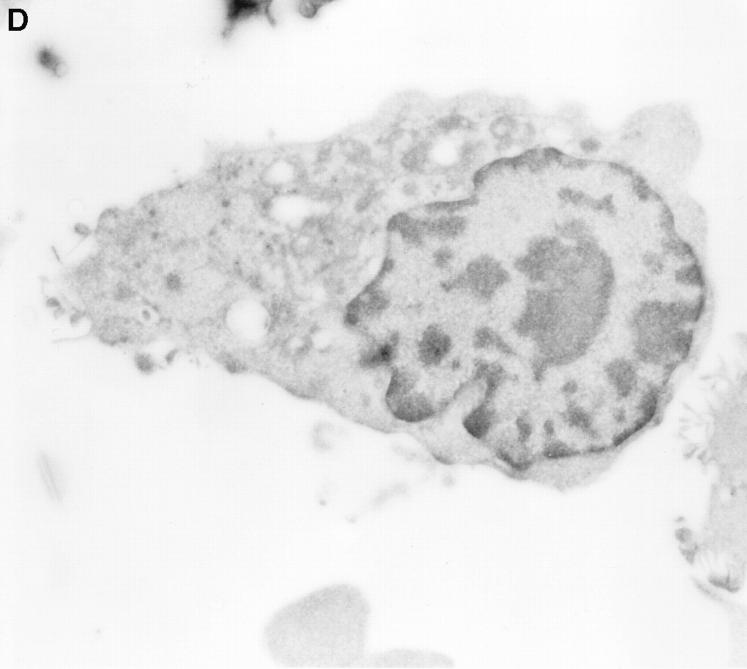
Figure 5.
Detection of intracellular GraB by immunogold staining. YAC-1 cells incubated in GraB (2 μg/ml) for 10 min were washed and fixed, then thin cryosections incubated with murine anti-GraB antibody or colloidal gold goat anti–mouse IgG. (A) Colloidal gold anti-GraB (arrows) localized to an area of increased electron density on the external leaf of the plasma membrane. (B) High power magnification of gold particles (arrows) in the cytoplasm near the nuclear membrane. (C) A lower magnification of B showing the position of the immunogold in the whole cell (arrows). (D) YAC-1 cell treated with anti-GraB antibody and immunogold in the absence of GraB had no detectable gold particles.
GraB Microinjection into the Cytoplasm.
It was evident from the above experiments that GraB crossed the cell membrane and was found within the cytoplasm in the absence of perforin but produced no obvious damage to the cells. If GraB can enter the target cell but fails to initiate apoptosis then it is possible that it is unable to enter the cell in sufficient quantity on its own to cleave and activate its substrates. Another possibility is that GraB is in some way segregated from the substrates that it uses to initiate apoptosis. To test these hypotheses, we directly microinjected GraB into the cell cytoplasm. B16 melanoma cells, which are highly susceptible to GraB and perforin-induced apoptosis within 2 h, were injected with either GraB (2 μg/ml) or buffer control and observed by DIC microscopy using an Image 1 time lapse image analysis system over a 2–3-h period. We observed changes to both the nucleus and plasma membrane within a few minutes of placing the protease in the cytoplasm. In 4–6 min the nuclear membrane and nucleolus appeared coarse and more prominent compared to control injections (Fig. 6, A and B). The plasma membrane began blebbing starting ∼5–7 min after injection which became pronounced by 8–12 min. However, in no instance did we observe progression to obvious membrane or nuclear disruption. Membrane blebs were usually transient in that they appeared and disappeared quickly. Nuclear changes also did not progress over the observation period. The rate at which the blebbing and nuclear changes occurred was dependent on the concentration of the GraB in the injection buffer. At high concentrations the changes were evident by 4–12 min, while at lower concentrations the effects were delayed to 20 min or longer. The experiment was repeated using the Rat-1 cell, microinjecting GraB at a 10-fold high concentration (20 μg/ml) using an automated injection protocol in which 1.2 × 10−12 ml was injected per cell containing 0.2–0.4 femtograms. Cells were again followed for 2–3 h and no apoptosis was observed.
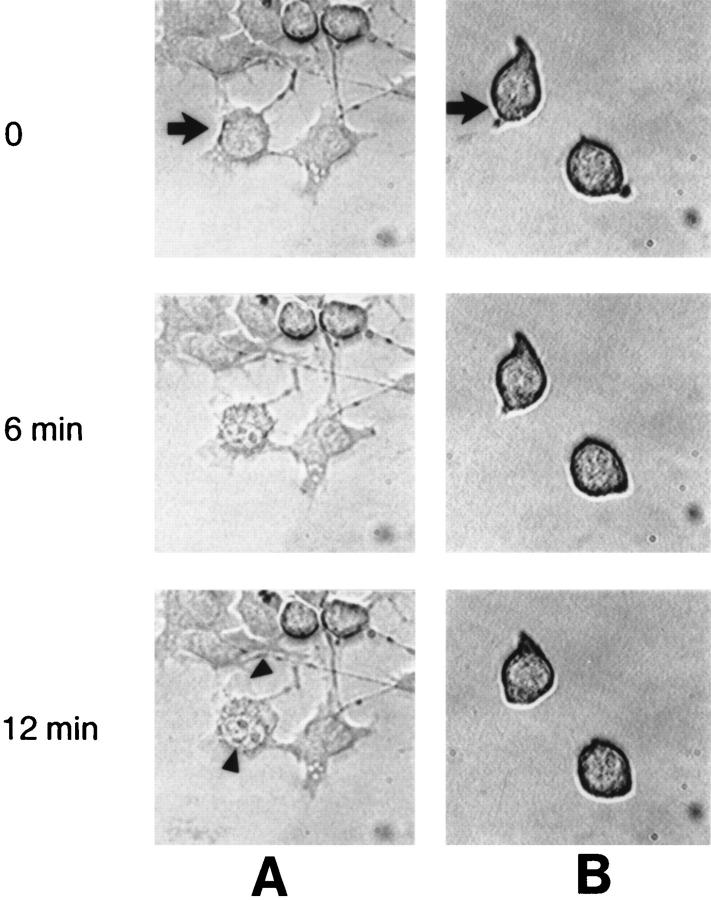
Figure 6.
Microinjection of GraB into the cytoplasm of B16 melanoma. After GraB (2 μg/ml) (A) or medium (B) injection into B16 cells (injected cells indicated by arrows), the B16 cells were examined by DIC microscopy using image analysis. After 6 min the cell nucleoli became prominent and by 12 min plasma membrane blebbing was noted (arrowheads). No further changes were observed over the next 60 min. Plasma membrane and nuclear changes were transient. (C) Hoechst dye staining of GraB microinjected B16 melanoma cells. A group of cells were injected with GraB (1 μg/ml) and after 45 min stained with Hoechst dye. Similar to the DIC microscopy (Fig. 3) the only nuclear changes observed was the increasing prominence of the nucleoli (arrows). Microinjection experiments were repeated at different doses and times in at least five separate experiments. (D) Cells incubated in GraB (1 μg/ml) and perforin (60 ng/ml) for 45 min display the typical chromatin condensation pattern of apoptotic cells (arrows). (E) Control cells incubated in medium.
To more directly visualize whether nuclear apoptotic changes occurred in microinjected cells we used Hoechst 33258 dye. The cytoplasm of B16 melanoma cells (Fig. 6 C ) was microinjected with GraB and then stained at different time intervals with Hoechst dye. The cells exhibited nuclear changes that were mainly characterized by prominent nucleoli or localized areas of chromatin condensation but they did not display the intense chromatin condensation observed in cells incubated with GraB and perforin (Fig. 6 D). Control B16 cells (Fig. 6 E ) or those treated with either perforin or GraB alone (not shown) were unchanged and stained in a diffuse punctate pattern.
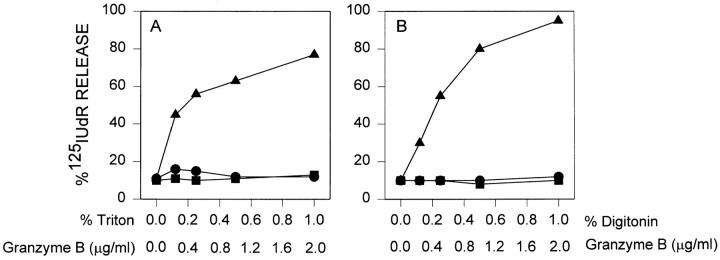
A second method of determining if cytoplasmic introduction of GraB was sufficient to induce apoptosis was to solubilize the plasma membrane with the detergents digitonin or Triton X-100 to allow free entry of the granzyme into the cell. We determined if the detergents over a wide concentration range could support GraB induced apoptosis of YAC-1 cells. At none of the doses of either detergent were we able to see evidence of DNA damage measured by 125IUdR release compared to perforin in the same experiment (Fig. 7, A and B).
Figure 7.
GraB is inactive against YAC-1 target cells with detergent permeabilized plasma membrane. (A) GraB was titered against a constant dose of perforin (40 ng/ml). (Filled triangles) Increasing amounts of Triton X-100 were added to cells with either a constant dose of GraB (1 μg/ml) (filled circles) or medium control (filled squares). (B) GraB titered with perforin as in Fig. 1 A (filled triangles). GraB (1 μg/ml) was incubated with increasing amounts of digitonin (filled circles) and compared to incubation with digitonin only (filled squares).
Discussion
In the granule exocytosis model of killing it was proposed that after contact between the CTL and target cell, perforin and granzymes are released into the intercellular space (30). Perforin then assembles into a pore-like structure in the target cell plasma membrane under the influence of calcium (30, 31), however, the fate of the released granzymes is not known. CTL and NK cells of mice deficient in GraB are unable to fully induce apoptosis (9), and GraB apoptotic activity can be blocked by the tripeptide inhibitor Boc-Ala-Ala-Asp-cmk pretreatment (6), indicating that a proteolytic active GraB is required for apoptosis. The cleavage and activation of one or more members of the ICE family proteases has been proposed as a mechanism for initiation of GraB action (16–23). At least two possibilities can be considered that would allow GraB to activate these intracellular cysteine proteases after being released by CTL at the external face of the target cell membrane. The granzyme hydrolyses a cell surface protein that triggers the activation of ICE family proteases through a second messenger similar to the model proposed for Fas/TNF-R1 activation (21, 22), or GraB crosses the plasma membrane to initiate the activation by direct proteolysis of ICE family proteases (16–23). In this study we have shown that GraB is capable of crossing the plasma membrane and entering the target cell and it does this in the absence of perforin. However, it is only in the presence of perforin that apoptosis is induced and GraB is then also found in the target cell nucleus.
GraB may first attach to the cell surface and then be translocated across the plasma membrane into the cytoplasm. We detected GraB on the cell membrane by immunogold staining with anti-GraB antibody and by direct fluorescence of FITC-GraB in cells in which membrane activity was blocked. How the GraB attaches to the plasma membrane is not known. Although it is possible that there is a receptor for GraB, its highly cationic nature suggests that this may be nonspecific perhaps similar to its interaction with chondroitin sulfate proteoglycan in the granule matrix (32). Once bound, movement across the membrane appears to be energy dependent as the addition of NaF and DNP and the maintenance of low temperature prevents accumulation of FITC-GraB in the cytoplasm.
Once in the cytoplasm, there were no cellular structures identified with which GraB was associated. FITC-GraB stained the cytoplasm diffusely. Although some immunogold staining was detected along the nuclear membrane and a some gold particles were found within and along the edges of micropinocytic vesicles, most gold staining was not associated with any structure in the cytoplasm. In the absence of perforin, GraB appears to be excluded from the nucleus at least at the limits of our detection systems with either the localization of FITC-GraB or colloidal gold antiGraB antibody staining. This contrasts with earlier observations that GraB is taken up by isolated nuclei from YAC-1 cells (27). In this isolated nucleus model, GraB crosses the nuclear membrane by an energy-independent nuclear import mechanism that may use a cytoplasmic transport factor. The uptake exhibits some specificity as another serine protease, chymotrypsin, is not imported to the nucleus under the same conditions. One might reconcile the observation that intact cell nuclei do not take up GraB proposing that the nuclear transport mechanism has been abnormally activated in isolated nuclei, and that it remains inactive in intact cells. However, further work on the nature of the nuclear transport mechanism will be needed to resolve this issue. In addition, other experiments indicate that isolated nuclei do not undergo chromatin condensation and DNA fragmentation with GraB in the absence of cytosol and that an ICE family substrate must be cleaved before nuclear apoptosis is initiated (24). These experiments support a model in which GraB initiates apoptosis by a cytoplasmic ICE family protease signal that leads to nuclear changes. However, it appears that GraB is unable to initiate nuclear apoptotic changes in the absence of perforin signaling.
Data from this current study demonstrate that the autonomous entry of GraB into the cytoplasm or its direct microinjection does not initiate apoptosis until perforin is delivered to the cell. Although the current model of initiation of a cytoplasmic apoptotic signal by GraB is the cleavage and activation of ICE family proteases, the presence of the active GraB protease in the cell cytoplasm appears to be insufficient to cleave ICE proteases. We have shown that incubation of GraB with YAC-1 or HeLa cells in the absence of perforin for 2 h or more does not result in the hydrolysis of either p45 ICE or CPP32 and its substrate poly-ADP ribose polymerase (PARP) (25). In the presence of perforin the same dose of GraB induces cleavage and activation of both ICE proteases and the hydrolysis of PARP to p85 within 45 min (25). The simplest explanation of why the GraB that autonomously enters the cell does not cleave ICE proteases is that an insufficient amount has entered the cell in the absence of perforin. Although we cannot detect an increase in the cytoplasmic levels by our methods, perforin may rapidly move GraB from the cell membrane to the nucleus and a transient rise sufficient for apoptosis may have occurred. Similarly, we may not have been able to microinject sufficient GraB to activate cell death. The level of cytoplasmic GraB that must be reached to activate apoptosis is not known, so we can conclude that the amount we microinject (4 femtograms per cell) is not sufficient.
We have considered several other possible explanations for why cytoplasmic GraB does not initiate CPP32 or ICE cleavage and apoptosis in the absence of perforin: (a) ICE family proteases are sequestered and not available to GraB, (b) ICE proteases are not the primary target of GraB, (c) GraB is not active without further posttranslational processing, or (d ) GraB is blocked or suppressed. Although ICE is found as a cytoplasmic protein in monocytes (33), the precise intracellular localization of ICE family proteases in other cell types has not been determined. CPP32 can cleave nuclear substrates including the DNA repair enzymes PARP and DNA-PK early in apoptosis (34). Thus, CPP32 most probably enters the nucleus during apoptosis to access these substrates. It is possible that GraB may cleave ICE proteases such as CPP32 in the nucleus after their movement across the nuclear membrane. However, since granzyme can induce apoptotic membrane and cytoplasmic changes in the absence of a nucleus (35), the ICE family protease processing almost certainly can take place in the cytoplasm. Another possibility is that the internalized GraB may be sequestered within vesicles after transport across the plasma membrane. However, we do not see any evidence of this by electronmicroscopy and the cytoplasmic distribution of FITC-GraB is quite diffuse. Thus, we can find no evidence to support the physical sequestration of this granzyme. However, the possibility remains that GraB may enter the cell in a microvesicle then rapidly translocates to the cytoplasm making it difficult to detect within a vesicle by the methods we have employed.
The second possibility, that ICE proteases in the cytoplasm are not the primary target of GraB, would be surprising since there are so many ICE family proteases that can be activated by GraB processing (16–23). However, GraB may hydrolyze another protein that initiates the activation of many different ICE family proteases. The availability of this substrate would also have to be restricted in some way in the absence of perforin.
The third possibility is that GraB must be further posttranslationally processed. The protease does exist in several glycosylated forms which have unknown function but are not accessible to the nucleus (27), thus, deglycosylation may be necessary for activity. The precursor of GraB is processed by a dipeptidyl peptidase I and remains inactive until the removal of an NH2-terminal dipeptide (36). The GraB preparations used in our study are quite active against synthetic peptide substrates and do not require any further processing. However, we cannot rule out other types of intracellular processing that are required for GraB apoptotic activity.
The last possibility is that GraB activity is blocked or suppressed until perforin releases this inhibition. In that regard, we found that the microinjection of GraB had an identifiable although transient effect on the cell (Fig. 6, A and B). The changes that were seen were similar to the early stages of apoptosis with plasma membrane blebbing and nuclear coarsening even though they did not proceed to intense chromatin condensation. This inability to progress to apoptosis might be explained by the inactivation or inhibition of GraB after injection. Consistent with this idea was the observation that cells that were pretreated with GraB and washed became progressively less able to induce apoptosis with time before perforin addition (Fig. 3 B), again suggesting inactivation. The existence of molecules that inhibit GraB is limited to the cowpox serine protease inhibitor crmA (37), and no equivalent molecule has been identified in eukaryotic cells. It is not known whether other inhibitors of apoptosis exist in mammalian cells that are active against GraB.
Although a great deal is known about the structure of perforin and its requirement for CTL and NK activity, its function at the cellular level remains obscure. The hypothesis that it acts as a membrane channel to allow granzymes to cross the cell membrane is not supported by the experiments presented in this paper. GraB enters cells without perforin, and the addition of perforin had little effect on cytoplasmic levels of GraB in YAC-1 or HeLa cells. It is estimated that up to 20 perforin monomers form a functional pore, which is a transmembrane tubular structure (10, 38–43). Studies in artificial bilayer membranes and resealed erythrocyte ghosts indicate that the functional pore radius is 3.1–6 nm. Pores have been shown to allow the movement of various monovalent and divalent ions regardless of charge and small molecules such as glucosamine with a Stokes diameter of 8 Å (40, 41, 43). Perforin pores formed by granules from cytotoxic lymphocytes allow the release of an 8,000 dalton polypeptide α-bungerotoxin (41). To this point, no one has directly shown that a molecule the size of GraB, which is 32 kD, is able to move through a perforin pore, and the data from this study argues that a perforin pore is not required for it to cross the plasma membrane.
Although perforin has little effect on the level of GraB we detect in the cell, it resulted in dramatic localization of the protease to the nucleus. It is not clear from these experiments whether nuclear localization is a consequence or cause of apoptotic changes in the nucleus. The exact role of perforin in apoptosis induction by GraB is also obscure. As noted above, perforin can promote Ca2+ influx into cells. One possibility is that a perforin-induced increase in intracellular calcium triggers the activity of GraB or proteins that are hydrolyzed by GraB (10–12). We attempted to answer this question by replacing perforin with ionomycin, a calcium ionophore, either when GraB is applied to the external cell surface of intact cells or microinjected, but we were not able to reproduce the effect of perforin (L. Shi and A.H. Greenberg, unpublished data). Another possibility is that perforin initiates a signal from the cell membrane, such as the release of a second messenger, and this molecule is critical to activating GraB-induced apoptosis. At the moment, there is no data to support or refute this hypothesis, so it remains only a speculative possibility. However, understanding the role of perforin in triggering GraB activity may have direct relevance to the regulation of other initiators of apoptosis.
In conclusion, we have demonstrated that GraB can autonomously enter the cytoplasm of target cells where it remains until perforin initiates apoptosis and its translocation to the nucleus.
Acknowledgments
We thank Eileen McMillan for her assistance with the immunogold staining.
This work was supported by grants from the Medical Research Council of Canada (MRC) and National Cancer Institute of Canada (NCIC). A.H. Greenberg is an NCIC Terry Fox Scientist.
Footnotes
1 Abbreviations used in this paper: GraB, granzyme B; ICE, interleukin-1β– converting enzyme; PARP, poly-ADP ribose polymerase.
References
- 1.Kaegi D, Vignaux F, Ledermann B, Buerki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 2.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaegi D, Ledermann B, Buerki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 4.Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MTF, Young JDE, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kojima H, Shinohara N, Hanaoka S, Someya Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 6.Shi L, Kam CM, Powers JC, Aebersold R, Greenberg AH. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinctsubstrate and target cell interactions. J Exp Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima H, Park HL, Henkart PA. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med. 1995;181:1037–1046. doi: 10.1084/jem.181.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heusel JW, Wesselschmidt RL, Shresta S, Russell J H, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis inallogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 10.Young JD-E, Cohn ZA, Podack ER. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: structural, immunological and functional similarities. Science (Wash DC) 1986;233:184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]
- 11.Poenie M, Tsien RY, Schmitt-Verhulst A-M. Sequential activation and lethal hit measured by (Ca2+)i in individual cytolytic T cells and targets. EMBO (Eur Mol Biol Organ) J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraut RP, Bose R, Cragoe EJ, Jr, Greenberg AH. The Na+/Ca2+ exchanger regulates cytolysin/perforininduced increases in intracellular Ca2+and susceptibility tocytolysis. J Immunol. 1992;148:2489–2496. [PubMed] [Google Scholar]
- 13.Poe M, Blake JT, Boulton DA, Gammon M, Sigal NH, Wu JK, Zweerink HJ. Human cytotoxic lymphocyte granzyme B. Its purification from granules and the characterization of substrate and inhibitor specificity. J Biol Chem. 1991;266:98–103. [PubMed] [Google Scholar]
- 14.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 15.Ellis R E, Yuan J Y, Horvitz H R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 16.Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cellderived granzyme B. Nature (Lond) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 17.Quan LT, Tewari M, O'Rourke K, Dixit V, Snipas SJ, Poirier GG, Ray C, Pickup DJ, Salvesen GS. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci USA. 1996;93:1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, Alnemri ES. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnaiyan AM, Hanna WL, Orth K, Duan HJ, Poirier GG, Froelich CJ, Dixit VM. Cytotoxic T-cellderived granzyme B activates the apoptotic protease ICELAP3. Curr Biol. 1996;6:897–899. doi: 10.1016/s0960-9822(02)00614-0. [DOI] [PubMed] [Google Scholar]
- 20.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 21.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/ CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 22.Duan HJ, Orth K, Chinnaiyan AM, Poirier GG, Froelich CJ, He WW, Dixit VM. ICE-LAP6, a novel member of the ICE/Ced-3 gene family, is activated by the cytotoxic T cell protease granzyme B. J Biol Chem. 1996;271:16720–16724. doi: 10.1074/jbc.271.28.16720. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Miura M, Jung Y-K, Ahu H, Gagliardini V, Shi L, Greenberg AH, Yuan J. Identification and characterization of ICH-3, a member of the ICE/Ced-3 family and an upstream regulator of ICE. J Biol Chem. 1996;271:5112–5117. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- 24.Martin SJ, Amarante-Mendes GP, Tewari M, Shi L, Chuang TH, Casciano C, Fitzgerald P, Tan EM, Bokoch GM, Dixit VM, et al. The cytotoxic protease granzyme B initiates apoptosis by proteolytic processing and activation of the ICE/CED-3-family protease, CPP32. EMBO (Eur Mol Biol Organ) J. 1996;15:2407–2416. [PMC free article] [PubMed] [Google Scholar]
- 25.Shi, L., G. Chen, G. MacDonald, L. Bergeron, H. Li, M. Miura, R. Rotello, D.K. Miller, P. Li, T. Seshadri et al. 1996. Activation of an ICE-dependent apoptosis pathway by granzyme B. Proc. Natl. Acad. Sci. USA. 93:11002–11007. [DOI] [PMC free article] [PubMed]
- 26.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 27.Trapani JA, Browne KA, Smyth MJ, Jans DA. Localization of granzyme B in the nucleus—A putative role in the mechanism of cytotoxic lymphocyte-mediated apoptosis. J Biol Chem. 1996;271:4127–4133. doi: 10.1074/jbc.271.8.4127. [DOI] [PubMed] [Google Scholar]
- 28.Trapani JA, Browne KA, Dawson M, Smyth MJ. Immunopurification of functional Asp-ase (natural killer cell granzyme B) using a monoclonal antibody. Biochem Biophys Res Commun. 1993;195:910–920. doi: 10.1006/bbrc.1993.2131. [DOI] [PubMed] [Google Scholar]
- 29.MacLean-Fletcher S, Pollard T D. Mechanism of action of cytochasin B on actin. Cell. 1980;20:329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- 30.Henkart PA. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- 31.Tschopp J, Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- 32.Stevens RL, Kamada MM, Serafin WE. Structure and function of the family of proteoglycans that reside in the secretory granzules of natural killer cells and othr effector cells of the immune system. Curr Topics Microbiol Immunol. 1989;140:93–108. doi: 10.1007/978-3-642-73911-8_8. [DOI] [PubMed] [Google Scholar]
- 33.Miller DK, Ayala JM, Egger LA, Raju SM, Yamin TT, Ding GJ, Gaffney EP, Howard AD, Palyha OC, Rolando AM, et al. Purification and characterization of active human interleukin-1 beta-converting enzyme from THP.1 monocytic cells. J Biol Chem. 1993;268:18062–18069. [PubMed] [Google Scholar]
- 34.Casciola-Rosen L, Nicholson DW, Chong T, Rowan KR, Thornberry NA, Miller DK, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima H, Golstein P, Henkart PA. The target cell nucleus is not required for cell-mediated granzyme- or Fas-based cytotoxicity. J Exp Med. 1995;181:1905–1909. doi: 10.1084/jem.181.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smyth MJ, McGuire MJ, Thia KYT. Expression of recombinant human granzyme B: A processing and activation role for dipeptidyl peptidase I. J Immunol. 1995;154:6299–6305. [PubMed] [Google Scholar]
- 37.Quan LT, Caputo A, Bleackley RC, Pickup DJ, Salvesen GS. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- 38.Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podack ER, Konigsberg PJ. Cytolytic T cell granules: isolation, structural, and biochemical, and functional characterization. JExp Med. 1984;160:695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young JD-E, Hengartner H, Podack ER, Cohn ZA. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer cell activity. Cell. 1986;44:849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- 41.Craido M, Lindstrom CG, Dennert G. Cytotoxic granules from natural killer cells: specificity of granules and insertion of channels of defined size into target membrane. J Immunol. 1985;135:4245–4251. [PubMed] [Google Scholar]
- 42.Sauer H, Pratsch L, Tschopp J, Bhakdi S, Peters R. Functional size of complement and perforin pores compared by confocal laser scanning microscopy and fluorescence microphotolysis. Biochim Biophys Acta. 1991;1063:137–146. doi: 10.1016/0005-2736(91)90363-d. [DOI] [PubMed] [Google Scholar]
- 43.Bashford CL, Menestrina G, Henkart PA, Pasternak CA. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988;141:3965–3974. [PubMed] [Google Scholar]