Abstract
CD8+ cytotoxic T lymphocytes (CTLs) have the ability to recognize and eliminate virally infected cells before new virions are produced within that cell. Therefore, a rapid and vigorous CD8+ CTL response, induced by vaccination, can, in principle, prevent disseminated infection in vaccinated individuals who are exposed to the relevant virus. There has thus been interest in novel vaccine strategies that will enhance the induction of CD8+ CTLs. In this study, we have tested the hypothesis that targeting an antigen to undergo more efficient processing by the class I processing pathway will elicit a more vigorous CD8+ CTL response against that antigen. Targeting a type I transmembrane protein, the HIV-1 envelope (env) protein, for expression in the cytoplasm, rather than allowing its normal co-translational translocation into the endoplasmic reticulum, sensitized target cells expressing this mutant more rapidly for lysis by an env-specific CTL clone. Additionally, a greatly enhanced de novo env-specific CTL response was induced in vivo after immunization of mice with recombinant vaccinia vectors expressing the cytoplasmic env mutant. Similarly, targeting a cytoplasmic protein, HIV-1 nef, to undergo rapid cytoplasmic degradation induced a greatly enhanced de novo nef-specific CD8+ CTL response in vivo after immunization of mice with either recombinant vaccinia vectors or DNA expression plasmids expressing the degradation targeted nef mutant. The targeting of viral antigens for rapid cytoplasmic degradation represents a novel and highly effective vaccine strategy for the induction of enhanced de novo CTL responses in vivo.
CD8+ CTLs play a vital role in controlling the spread of viral infection (1–3). These cells have the ability to recognize virally infected cells that present peptide epitopes derived from viral proteins on their surfaces. These viral peptides are presented by the MHC class I molecules synthesized within the infected cells. The processing of viral proteins for association with class I molecules can occur as soon as viral proteins are translated (4, 5), and is not dependent upon any subsequent events in the life cycle of the virus. Thus, the peptide epitopes may be presented to and recognized by CD8+ CTLs before the viral life cycle is complete, conferring on these CTLs the ability to lyse virally infected cells before new virions are produced (6).
In HIV-1 infection, the ability of CTLs to recognize and lyse infected cells may be responsible for the dramatic fall in viremia after acute infection, a fall that occurs concomitantly with a rise in HIV-specific CTL activity (7–12). Furthermore, the control of viral replication seen during the prolonged clinically latent stage of the infection has been associated with HIV-specific CTL activity (13). It is possible that if this CTL activity could be enhanced, the total elimination of viral burden could be achieved. In a vaccine setting, the rapid clearance of the initially infected cells by vaccine-induced CTLs could, in principle, result in sterilizing immunity, and would prevent clinical manifestations of the infection. In studies of the immune response to the lymphocytic choriomeningitis virus, a virus that is capable of establishing a persistent infection of mice, it has been shown that a CD8+ CTL response specific for LCMV antigens is able to clear the persistent infection, or if induced by vaccination, to prevent the establishment of a persistent infection (14–16). Indeed, it has been observed that there is a detectable HIV-specific CTL response in HIV-uninfected sexual contacts of HIV-infected individuals suggesting that a strong CTL response against HIV may confer protection from natural infection (17, 18).
There is now considerable interest in the development of vaccine strategies that will enhance the generation of virus-specific CTL responses (9, 19). One potential approach to inducing a more vigorous CTL response involves specific targeting of vaccine antigens into the MHC class I antigen processing pathway. Normally, cytoplasmic degradation of a small fraction of the viral protein made in an infected cell provides the peptide substrates that enter the class I pathway. Cytoplasmic proteins are degraded by the ubiquitin (Ub)1-dependent pathway through the action of proteasomes (6, 20–24) and the resulting peptides are then transported by the Tap1/Tap2 heterodimer (25–32), into the endoplasmic reticulum where they associate with MHC class I molecules and β2-microglobulin. The resulting complexes are then transported via the exocytic pathway to the cell surface where they are recognized by CD8+ CTLs (6, 33).
Several lines of evidence suggest that an APC expressing higher concentrations of MHC class I molecules loaded with the appropriate peptide from a given antigen will stimulate a more vigorous CD8+ CTL response against the relevant antigen (34–36). Given that cytoplasmic degradation of antigens by the 26S proteasome provides the peptide substrate for the class I pathway (21, 37, 38), targeting of vaccine antigens to undergo more efficient degradation in the cytoplasm should yield increased amounts of peptide substrate available to enter the class I pathway. Increased production of peptide substrate should result in a higher concentration of cell surface MHC class I molecules that are loaded with peptides derived from the relevant antigen. Enhancing the entry of viral proteins into the class I pathway may be accomplished by either targeting a noncytoplasmic protein to the cytoplasm for degradation or by targeting a cytoplasmic protein for more rapid degradation.
The factors that regulate the degradation of cytoplasmic proteins by the 26S proteasome have been well described. Unlike the 20S proteasome, which recognizes unfolded proteins, the 26S proteasome can recognize proteins in a folded state, particularly proteins that have been modified by the conjugation of a polyubiquitin chain through an isoamide bond to a conformationally free Lys residue (39). It is the presence of this polyubiquitin chain that is recognized by the proteasome and targets the substrate for degradation. In addition to the conjugation of ubiquitin, the degradation is also regulated by the substrate protein itself. It has been shown that the NH2-terminal amino acid of the substrate protein is responsible for determining the rate of conjugation of the polyubiquitin chain, and thus, the targeting of the protein for degradation by the proteasome (40, 41).
As was initially demonstrated in Saccharomyces cerevisiae and later by the work of Townsend et al. in mammalian cells (42), the degradation of proteins with NH2-terminal residues other than Met can be studied by expressing in cells fusion constructs in which the coding sequence of the substrate protein is fused in frame to the COOH-terminal end of the coding sequence of Ub. Ub is normally made in the cell as a polyprotein chain that is cleaved by Ub hydrolases at the COOH terminus of each Ub subunit, giving rise to individual Ub molecules (43). These same hydrolases will also cleave the Ub–substrate fusion protein at the COOH terminus of Ub, exposing the NH2 terminus of the substrate. In these constructs, the NH2 terminus of the substrate may be mutated to residues other than Met. By expressing such fusion proteins in S. cerevisiae, Bachmair et al. showed that the identity of the NH2 terminal amino acid residue had a dramatic effect on the degradation rate of the substrate protein (44). When the substrate protein had a Met NH2 terminus, the half-life was >10 h; however, if the NH2 terminus was Arg, the half-life of the substrate was <2 min. Townsend et al. then showed that this strategy could be used to overcome a class I processing defect observed late in the course of vaccinia infection (42). They generated a fusion of Ub to influenza nucleoprotein (NP) and showed that if the NH2 terminus was an Arg, the protein was processed and presented to CD8+ CTLs, whereas NP with a Met NH2 terminus was not processed and presented. More recently, Grant et al. demonstrated that the rate of presentation of an endogenous antigen corresponded directly to its rate of degradation via the Ub-mediated pathway, and that the expression of degradation targeted forms of antigen resulted in a more rapid processing of the antigen for presentation by MHC class I (36). Whether the rapid degradation of endogenous antigens affects immunogenicity in vivo is unclear.
In this report, we have analyzed two different approaches for inducing the cytoplasmic degradation of vaccine antigens in an effort to enhance the generation of CTLs, and have examined whether the degradation targeted antigens can induce enhanced primary CTL responses in the vaccine setting. In the first approach, we used a form of the HIV-1 env protein that lacks the signal sequence and is therefore expressed and degraded entirely in the cytoplasm. The second approach involved the generation of Ub fusion constructs in which the Ub coding sequence was fused to the NH2 terminus of mutant forms of the HIV-1 nef protein. In both cases, enhanced cytoplasmic degradation of the vaccine antigen was observed and was associated with markedly enhanced stimulation of murine and human CTLs and enhanced generation of de novo murine CTL responses in vivo.
Materials and Methods
Generation of Ub-nef Fusion Constructs.
The full-length Ub gene was amplified from genomic DNA by PCR using the following primer pair: 5′ primer: GTCAGTCAGTCAGTCAACTAGTATGCAGATCTTCGTGAAGACC, 3′ primer: TTTTGA CCACTTGCCACC (CAT or CCT)ACCCCCCCTCAAGCGCAGGAC. The 5′ primer contains a SpeI site for cloning and the 3′ primer contains the reverse compliment of the 5′ end of the HIV-1 nef gene with either a Met or Arg codon at the NH2 terminus. A 50fold excess of 5′ primer was used to generate a single stranded PCR product. The resulting 271 base product was gel purified from the double stranded PCR product and used as the 5′ primer for the amplification of HIV-1 nef from the plasmid pNL4-3. The 3′ primer for amplifying nef, GTCAGTCAGTCAGTCACTTAAGTCAGCAGTTCTTGAAGTACTC, contained an AflII site. The resulting 894-bp Ub-nef fusion product was gel purified and subcloned into the pCRScript vector (Stratagene Corp., La Jolla, CA).
Generation of Recombinant Vaccinia Viral Vectors.
The Ub-nef fusion gene was cut out of pCRScript and cloned into the pSC11.MCS1 vector using SpeI and AflII sites for the generation of recombinant vaccinia vectors expressing the Ub-nef fusion protein. The recombinant viral vectors were created by infecting CV-1 cells with wildtype (wt) vaccinia virus (vWR-Lvar), followed by co-transfection of the infected cells with 4.4 μg pSC11.MCS1 constructs using the CellPhect transfection kit (Pharmacia LKB Biotechnology Inc., Piscataway, NJ). Recombinant virus was screened by the expression of β-galactosidase (β-gal) and selected by resistance to BrdU. The recombinant viruses were carried through three rounds of plaque purification under selective conditions. Expression of the foreign gene was then assayed by Western blot analysis.
Generation of In Vivo Expression Constructs.
The Ub-nef fusion gene was cut out of the pSC11 vector using NotI and BamHI, and cloned into the pcDNA3 vector (Invitrogen, San Diego, CA). The nef gene was cut out of pCRScript using NotI and SmaI and cloned into the NotI and EcoRV sites in the pcDNA3 vector. Expression by these constructs was checked by Western blot analysis of transiently transfected HeLa cells and CT26 cells.
Cell Lines.
CT26nef cells were generated by transfecting the CT26 tumor line with the pcDNA3nef construct using the mammalian transfection kit (Stratagene Corp.). Stable transfectants were selected by neomycin resistance and maintained in 400 μg/ml G418. Expression of nef in stable transfectants was checked by Western blot analysis.
Pulse-Chase Metabolic Labeling.
5 × 106 human EBV–transformed B cells (B-lymphoblastoid cell line [B-LCL]) per time point were infected with recombinant vaccinia vectors expressing either β-gal (vSC8 control), nef, UbMNef, or UbRNef for 2 h at a multiplicity of infection (MOI) of five. Cells were then washed and incubated for 1 h in Met-free, Cys-free RPMI containing 5% dialyzed FCS and antibiotics. 100 μCi [35S]Cys and 100 μCi [35S]Met were added to the cultures and incubated for 30 min. Cells were washed once with PBS and then incubated in RPMI containing 10% FCS, antibiotics, and 10× Met, 10× Cys for 0, 15, 60, or 240 min. After incubation, cells were washed once in PBS and then pelleted.
Immunoprecipitation of Labeled Cells.
Cell pellets were resuspended in 1 ml lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100) + protease inhibitors and incubated for 30 min at 4°C. Cell debris was pelleted at maximum speed for 15 min at 4°C. Supernatants were transferred to fresh tubes and precleared two times with normal rabbit serum and protein G–Sepharose beads (once overnight [O/N] at 4°C, once for 2 h at 4°C). Nef proteins were immunoprecipitated with anti– Nef-COOH-terminal polyclonal sera (National Institutes of Health AIDS Research and Reference Reagent Program, Rockville, MD) at 1:250 dilution for 1 h. Immune complexes were collected on protein G–Sepharose beads for 1 h and the supernatant was aspirated. Protein G–Sepharose beads were washed two times in lysis buffer + 1% SDS, four times in lysis buffer + 1% SDS + 0.5M NaCl, and again two times in lysis buffer + 1% SDS. Immune complexes were removed from beads by boiling in SDS loading buffer for 10 min. Radiolabeled proteins were separated by SDSPAGE on a 14% gel and visualized by autoradiography.
Western Blot Analysis.
Cells were infected with recombinant vaccinia vectors at an MOI of three for 2 h and then incubated O/N to allow protein expression. Transfected cells were incubated O/N under normal culture conditions. 106 cells per lane were pelleted, resuspended, and lysed in SDS sample buffer for 10 min followed by boiling for 10 min. Cell debris was pelleted at maximum speed for 10 min and proteins were separated by SDSPAGE on a 14% minigel. Bands were transferred to nitrocellulose and the nitrocellulose was blotted according to enhanced chemiluminescence kit protocols (1° Ab anti–Nef-COOH terminus at 1:2,500 dilution, 2° Ab goat anti–rabbit [Bio Rad Labs., Hercules, CA] at 1:3,000 dilution).
In Vitro Stimulation of Bulk PBMC.
Autologous B-LCL were infected with recombinant vaccinia vectors for 2 h, incubated O/N, and treated with psoralen and UV irradiation to inactivate vaccinia. These cells were then used to stimulate nef-specific CTLs from bulk PBMCs. PBMCs were isolated from the blood of an HIV-seropositive donor by Ficoll gradient centrifugation, and then cultured for 10 d in the presence of IL-2 and titrating numbers of stimulating cells.
CTL Assay.
Target cells expressing Ag or a control Ag are labeled with 51Cr for 2 h and then washed three times to remove excess Cr. Targets are mixed with stimulated effector cells at varying E/T ratios and incubated for 4 h at 37°C in 200 μl RPMI + 10% FCS supplemented with IL-2 and antibiotics. Plates are spun to pellet cells and 100 μl media is removed from each well and counted. Percent specific lysis is defined as ([counts experimental lysis − counts media lysis] / [counts NP40 lysis − counts media lysis]) × 100%.
Generation of Envelope-specific CTL In Vivo by Immunization.
Mice were immunized intraperitoneally with either 107 or 108 PFU of recombinant vaccinia vectors expressing either β-gal (control), wild-type envelope (env), or signal sequence minus forms of the env protein (ss− env) (gifts of Dr. Patricia Earl, National Institutes of Health). 3 wk after immunization, the mice were killed and their spleens were harvested. Splenocytes were isolated by homogenizing the spleen, and then passing the suspension through a nylon mesh followed by Ficoll gradient centrifugation. Splenocytes were then incubated in the presence of IL-2 and either the immunodominant H-2 Ld peptide epitope P18, or media alone for 5 d. Stimulated splenocytes were then used as effectors in a standard 51Cr release assay for CTL activity.
Precursor CTL Frequency Analysis.
BALB/c mice were immunized intraperitoneally with 107 PFU of vaccinia (vac) control, vac-env, or vac-ss− env in 0.1 ml sterile PBS. At 21 d after immunization, mice were killed and spleens were harvested. Splenocytes were isolated as described above and counted. Titrating numbers of splenocytes were plated in 96-well plates (48 wells/ dilution) in the presence of 105 irradiated naive syngeneic splenocytes that had been pulsed with the P18 peptide (50 μM) for 2 h. These cells were cultured for 7 d in media containing 10% supernatant from Con A–stimulated rat splenocytes, 50 μM methylα-d-mannopyranoside, 10 U/ml IL-2, and 50 μM 2-ME. After culture, each well was split into two equal volumes and assayed for CTL activity against 51Cr-labeled, P18- or media-pulsed P815 cells (3,000 targets/well). The natural log of the fraction of negative cultures was plotted against the number of splenocytes/well. The frequency of precursor CTLs (f ) was obtained from the slope of the resulting lines and is given by the formula f = (lnb − lny) / x where b is the y-intercept for each line, y is the fraction of negative wells, and x is the number of splenocytes/well.
Stimulation of a Primary env-specific CTL Response.
BALB/c mice were immunized intraperitoneally with 107 PFU of vac control, vac-env, or vac-ss− env in 0.1 ml sterile PBS. At 7 d after immunization, mice were killed and spleens harvested. Splenocytes were isolated as described earlier. Isolated splenocytes were counted and used as effectors directly in a 51Cr release assay (as described above) for primary CTL activity. Target cells were MHC class I matched, MHC class II negative P815 cells that had been pulsed with the P18 peptide of HIV-1 env or media and labeled with 51Cr for 2 h.
Generation of nef-specific CTLs In Vivo by Immunization.
Two different immunization protocols were used. Mice were immunized with either 107 PFU recombinant vaccinia vectors expressing either β-gal (control), UbMNef, or UbRNef intraperitoneally, or they were immunized with 50 μg pcDNA3 expressing no insert, nef, UbMNef, or UbRNef at four sites intramuscularly. 3 wk after immunization, the mice were killed and their spleens were harvested. Splenocytes were isolated by resuspending the spleen, and passing it through a nylon mesh followed by Ficoll gradient centrifugation. Splenocytes were then incubated in the presence of IL-2 and psoralen/UV-treated vVnef-infected CT26 or P815 (MHC matched) stimulator cells for 5 d. Stimulated splenocytes were then used as effectors in a standard 51Cr release assay for CTL activity.
Anti-CD8 Blocking of CTL Activity.
BALB/c mice were immunized with 200 μg pcDNA3 constructs and splenocytes were isolated and stimulated as described above. Stimulated splenocytes were then preincubated with the anti-CD8 antibody 2.43 (American Type Culture Collection, Rockville, MD) or an isotype matched control Ab at 1:250 dilution for 2 h. Splenocytes were then used, without additional washing, as effectors in a standard 51Cr release assay for CTL activity.
Results
Enhanced Presentation of a Cytoplasmic Form of the HIV-1 env Protein to env-specific CTLs.
To determine whether targeting vaccine antigens for rapid cytoplasmic degradation enhances the generation of antigen-specific CTLs, we first examined the effects of targeting the HIV-1 env protein, a type I transmembrane protein, for synthesis and degradation in the cytoplasm. Targeting of the env protein into the cytoplasm was accomplished by expressing in cells an ss− env. Previous studies have shown that ss− form of the hemagglutinin (HA) protein of influenza virus (45) and the env protein of HIV-1 (46, 47) expressed using vaccinia vectors can be processed for presentation to specific CTLs. When a ss− form of the HIV-1 env protein is expressed in cells using a vaccinia vector (vac-ss− env), steady state levels of env are very low or undetectable by Western blot despite the fact that these cells are readily recognized by envspecific CTLs (46). To determine whether ss− env protein is subject to rapid degradation, pulse chase experiments were carried out as described in Materials and Methods. However, the protein remained undetectable, even when cells infected with vac-ss− env were lysed immediately after 35Slabeling. Two different sources of pooled polyclonal sera from HIV seropositive individuals were used in attempts to immunoprecipitate the ss− env protein. Under conditions where a very strong 160-kD band is detected in immunoprecipitates of cells infected with a vector carrying the wild-type env gene, no band in the molecular weight range expected for the nonglycosylated ss− env was seen in cells infected with a vaccinia vector carrying the ss− env gene (data not shown). These results suggest that the protein undergoes extremely rapid degradation, resulting in the inability to detect any full-length protein in these cells.
To compare the processing of wt and ss− forms of the env protein, we used the env-specific human CD8+ CTL clone A42.46, which recognizes a 9–amino acid env epitope in association with HLA A3.1 (48). As shown in Fig. 1, this clone recognized an autologous B-LCL infected with recombinant vaccinia virus expressing the HIV-1 env protein (vac-env). The clone also lysed target cells infected with a vaccinia vector expressing the vac-ss− env in a standard 51Cr release assay. However, when target cells were assayed at 2 h after infection with vaccinia expression vectors, rather than after the standard overnight incubation, only the vac-ss− env infected cells were recognized by this clone. The recognition of these cells by clone A42.46 was dependent on de novo protein synthesis, as cells infected with vaccinia expression vectors in the presence of cycloheximide were not lysed. This result suggests that targeting of the env protein for expression in the cytoplasm results in more rapid and/or efficient processing and presentation of the antigen by the class I pathway.
Figure 1.
Lysis of targets expressing wt or ss− env protein by the envspecific CD8+ CTL clone A42.46. Autologous B-LCL were infected with the indicated vaccinia expression vectors 2 h before assay (diamonds) or were infected for 2 h and then incubated overnight at 37°C before assay (squares). Infected cells were then used as targets for the env-specific CD8+ CTL clone A42.46 in a standard 51Cr release assay at an E/T ratio of 10:1. One set of targets was infected in the presence of 0.1 mM cycloheximide (CHX, circles). The experiment was repeated three times with similar results. Data from a representative experiment are shown.
Effect of Cytoplasmic Targeting on the Generation of an envspecific CTL Response In Vivo.
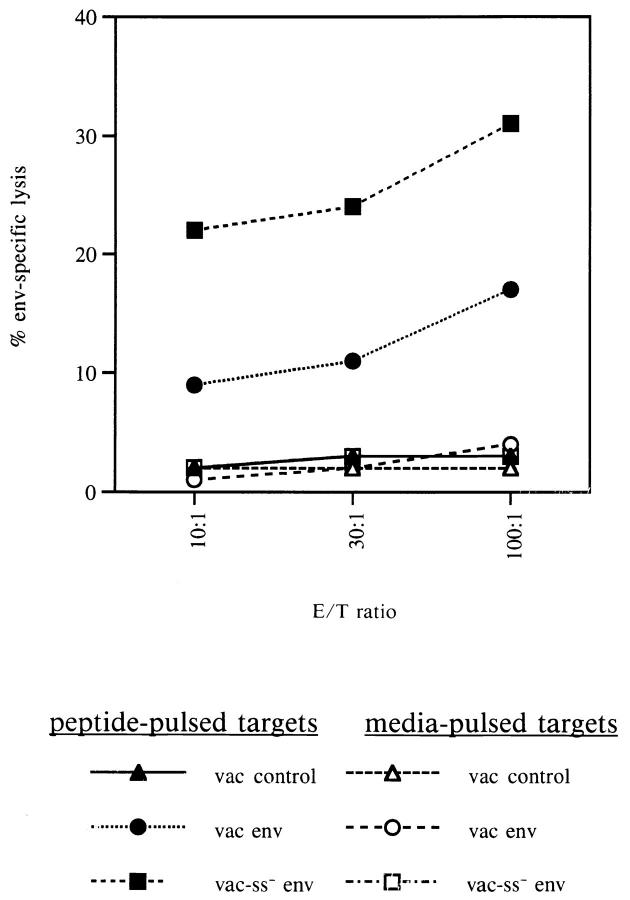
To determine whether the cytoplasmic targeting of the env protein would result in enhanced generation of de novo CTL responses in vivo, BALB/c mice were immunized with the control vaccinia vector, vac-ss− env, or with vac-env as described in Materials and Methods. Splenocytes from immunized mice were cultured in vitro in the presence or absence of a peptide representing an immunodominant H-2 Ld–restricted epitope within the env protein, RGPGRAFVTI (49). As seen in Fig. 2, stimulated splenocytes from mice immunized with the vac-ss− env vector were able to recognize and efficiently lyse target cells pulsed with the env peptide. Class II negative P815 target cells were used in this assay so that only class I–restricted lysis could be observed. Most importantly, the lysis by CTLs from mice immunized with vacss− env was much greater than the lysis mediated by stimulated splenocytes from mice immunized with the vac-env vector. Splenocytes from mice immunized with a control vaccinia vector did not lyse targets presenting the relevant env epitope. When mice were immunized with a higher dose (108 PFU) of recombinant vaccinia vectors, the immune response to both constructs was blunted (data not shown). However, this dose exceeds the standard dose for immunization with vaccinia virus, and the diminished immune response is most likely a result of other secondary effects. Taken together, these results suggest that targeting the env protein for translation on free ribosomes in the cytoplasm, rather than for co-translational translocation into the endoplasmic reticulum, results in more rapid and efficient processing for presentation to class I–restricted T cells and the more efficient stimulation of de novo CTL responses in vivo.
Figure 2.

Enhanced induction of env-specific CTLs by immunization with vac ss− env. BALB/c mice were immunized with 107 PFU of vac control, vac-env, or vac-ss− env. Splenocytes from immunized mice were stimulated for 5 d in IL-2 containing media in the presence of the H-2Ld– restricted env peptide (RGPGGRAFVTI). Stimulated splenocytes were then assayed for env-specific CTL activity against P815 cells pulsed with the H-2Ld–restricted env peptide or media alone. The data shown are representative of three separate experiments.
Effect of Cytoplasmic Targeting on CTL Precursor Frequency.
To determine whether the increased CTL response observed in mice immunized with recombinant vaccinia expressing the cytoplasmic ss− env reflects a quantitative increase in the number of env-specific CTL precursors in those mice, we assayed the env-specific CTL precursor frequency in mice immunized with either vac control, vac-env, or vac-ss− env. As shown in Fig. 3, the precursor frequency of CTLs specific for the P18 env peptide was approximately fourfold greater in mice immunized with vac-ss− env (1/69,618) compared to that observed in mice immunized with vac-env (1/267,980). The frequency of env-specific CTL precursors in mice immunized with vac control was 1/1,154,338. Thus, the enhanced CTL response that is seen in mice immunized with vac-ss− env after in vitro stimulation of splenocytes correlates with a fourfold increase in precursor CTL frequency in these mice relative to mice that were immunized with vac-env.
Figure 3.
Precursor CTL (pCTL) frequency analysis. BALB/c mice were immunized with 107 PFU vac control, vac-env, or vac-ss− env. After 21 d, splenocytes from immunized mice were cultured (48 wells/titration) in the presence of 105 naive splenocytes that had been pulsed with the H-2Ld–restricted env peptide for 2 h and then irradiated for 7 d. Culture media contained IL-2, T cell growth factor (from Con A–stimulated rat splenocytes), and methyl-α-d-mannopyranoside. Each well was then split into two equal parts and assayed for env-specific CTL activity against P815 targets pulsed with the H-2Ld–restricted env peptide or with media for 2 h. Positive wells were scored as >3 standard deviations above the percent specific lysis without effectors. The fraction of negative wells is plotted on a log scale versus cell number/well and from this line the pCTL frequency was determined (see Materials and Methods).
Effect of Cytoplasmic Targeting on the Induction of a Primary CTL Response.
Both the analysis of bulk env-specific CTL responses in stimulated cultures of splenocytes from immunized mice and the analysis of env-specific precursor CTL frequency involve a secondary antigen-specific stimulation in vitro. To determine whether the cytoplasmic targeting of env affects the generation of a primary CTL response in vivo, we assessed the ability of splenocytes from immunized mice to lyse peptide-pulsed target cells without earlier stimulation in vitro. In the experiment shown in Fig. 4, when BALB/c mice were immunized with vac-ss− env, a vigorous env-specific primary CTL response was detected. In the same experiment, mice immunized with vac-env yielded a detectable, but reduced env-specific primary CTL response, whereas mice that were immunized with the control vaccinia vector showed no env-specific primary CTL activity. In other experiments, the levels of primary CTL activity were much lower, consistent with the general finding that detection of virus-specific CTL usually requires an in vitro restimulation. Nevertheless, the vac-ss− env construct was consistently superior to vac-env. These results indicate that the enhanced CTL responses to vac-ss− env detected in bulk culture and limiting dilution studies reflect enhanced stimulation of de novo CTL responses in vivo by this degradation targeted construct.
Figure 4.
Enhanced stimulation of primary env-specific CTL response by immunization with vac-ss− env. BALB/c mice were immunized with 107 PFU vac control, vac-env, or vac-ss− env. After 6 d, splenocytes were isolated and assayed for direct CTL activity against peptide-pulsed (filled) or media-pulsed (open) P815 cells.
Targeting of the HIV-1 nef Protein for Rapid Cytoplasmic Degradation.
The env protein was targeted for enhanced class I processing by intentionally inducing the mislocalization of the protein to the cytoplasm. To enhance the class I processing of proteins that are normally localized to the cytoplasm, we made use of the N-end rule that states that the identity of the NH2-terminal residue of a protein determines its half-life (44). We generated fusion protein constructs in which the Ub coding sequence was fused to the codon for the NH2-terminal residue of the HIV-1 nef protein. Constructs with a stabilizing Met residue (UbMNef) or a destabilizing Arg residue (UbRNef) at the NH2-terminus of the nef coding sequence were compared. Preliminary Western blot analysis established that the steady state level of the nef protein in cells expressing the UbRNef construct was much lower than the steady state level of nef in cells expressing the UbMNef or the wt nef, suggesting that the UbRNef construct is subject to rapid degradation (data not shown). The synthesis and degradation rates of the Ub-Nef fusion proteins as well as wt nef were investigated in pulse chase experiments, as described in the Materials and Methods section. Fig. 5 shows that in cells infected with a vaccinia vector carrying the wt nef gene (vVnef), the nef protein was stable, with a slow rate of degradation (t1/2 ∼10 h). In cells infected with a vaccinia vector carrying the Ub-Met-nef fusion construct (vVUbMNef), nef was again slowly degraded. A band at the expected relative molecular mass of a Ub-nef fusion protein, 35 kD, was not observed, indicating the rapid cleavage at the COOH terminus of Ub by the endogenous Ub hydrolases. The resulting nef band derived from the UbMNef construct had a t1/2 slightly less than 10 h. In cells infected with a vaccinia vector carrying the Ub-Arg-nef fusion construct (vVUbRNef), the fusion construct was again rapidly cleaved to give Ub and the nef protein, in this case with an Arg residue at the NH2 terminus. The rate of synthesis of this protein was similar to that of UbMNef, as judged from the intensity of the 0 min chase time bands. However, the UbRNef construct displayed a rapid rate of degradation (t1/2 = 15 min), suggesting that the exposed Arg residue at the NH2 terminus of nef does indeed confer instability and rapid degradation on this protein in mammalian cells.
Figure 5.
Pulse-chase analysis and immunoprecipitation of nef. P815 cells infected with vaccinia vectors expressing β-gal (lane 1), nef (lanes 2–5), UbMNef (lanes 6–9), and UbRNef (lanes 10–13) were pulse labeled with 35S-Met and 35S-Cys for 30 min and then chased with an excess of unlabeled Met and Cys for 0 min (lanes 1, 2, 6, 10), 15 min (lanes 3, 7, 11), 60 min (lanes 4, 8, 12) or 240 min (lanes 5, 9, 13), followed by extraction, immunoprecipitation, and electrophoretic analysis of nef and MHC class I expression (see Materials and Methods).
In Vitro Studies of the Processing of UbRNef.
Preliminary studies demonstrated that B-LCL infected with vVUbRNef, vVUbMNef, and vVnef were all lysed to comparable extents by the previously described nef-specific human CTL clone 4N225 (50). For other nef-specific human CTL clones, different patterns were observed, with the degradation targeted construct giving preferential lysis in some cases (data not shown). However, the critical test of this strategy is to determine whether cells expressing the degradation targeted construct are better able to stimulate primary and secondary CTL responses. To determine whether the rapid degradation of the UbRNef construct permits enhanced stimulation of secondary human CTL responses, autologous B-LCL infected with recombinant vaccinia vectors expressing either UbMNef or UbRNef were used to stimulate nef-specific memory CTLs present in PBMCs obtained from an HIV-1 seropositive donor. Stimulation of PBMCs with large numbers of either vVUbMNef-infected B-LCL or vVUbRNef-infected B-LCL led to the induction of a nef-specific secondary CTL response, as seen in Fig. 6. However, the number of vVUbMNef-infected stimulating cells required to obtain a positive CTL response was >30-fold higher than the number of vVUbRNef-infected stimulating cells required to induce a comparable response. Thus, on a per cell basis, cells expressing the rapidly degraded UbRNef construct are >30-fold more effective at stimulating a secondary CTL response.
Figure 6.
Stimulation of secondary nef-specific CTLs by UbMNef and UbRNef constructs. PBMCs were isolated from an HIV-1 seropositive donor and stimulated in the presence of IL-2 with titrating numbers of vVUbMNef (squares) or vVUbRNef (diamonds) infected, psoralen/UV–treated autologous B-LCL. Stimulated PBMCs were assayed for nef-specific CTL activity against vVnef infected (filled) or vac control infected (open) autologous B-LCL at an E/T ratio of 10.
Effect of Targeted nef Degradation on the Generation of nefspecific CTLs In Vivo.
To determine whether the targeting of nef for Ub-dependent degradation could enhance the generation of de novo CTL responses in vivo, BALB/c mice were immunized with vVUbMNef and vVUbRNef as described in Materials and Methods. As shown in Fig. 7, a vigorous nef-specific CTL response was seen after stimulation of splenocytes from mice immunized with vVUbRNef by vVnef-infected CT26 cells. This response was detected using the MHC class I+, MHC class II− cell line, CT26, indicating that the lysis was class I restricted. In contrast, stimulated splenocytes from mice immunized with vVUbMNef exhibited a detectable, but much reduced nefspecific CTL response. Despite this difference in nef-specific CTL activity, splenocytes that were stimulated with vVnef-infected CT26 cells had statistically indistinguishable levels of vaccinia-specific CTL activity, as was seen when vac control–infected CT26 cells were used as targets in the CTL assay (29, 27, and 21% vaccinia-specific lysis by splenocytes from mice immunized with vac control, vVUbMNef, and vVUbRNef, respectively). When splenocytes were stimulated with CT26 cells transfected to express nef, nef-specific CTL activity was detectable in splenocytes from mice immunized with vVUbRNef and was virtually lost in splenocytes from mice immunized with vVUbMNef. This lower level of stimulation is presumably due to the greater than fivefold lower level of expression of nef in the stably transfected CT26 cells than in the vVnef infected CT26 cells as seen by Western blot analysis (data not shown). As expected, stimulated splenocytes from mice immunized with a control vaccinia vector failed to recognize and lyse target cells expressing nef.
Figure 7.
Enhanced induction of primary nef-specific CTLs by immunization with vVUbRNef. BALB/c mice were immunized with 107 PFU of vac control (open), vVUbMNef (stippled), or vVUbRNef (filled). Splenocytes from immunized mice were stimulated for 5 d in the presence of IL-2 with either CT26nef transfectants treated with mitomycin C or with CT26 cells which had been infected with vVnef and then treated with psoralen/UV light to inactivate vaccinia virus. The ratio of responders to stimulators was 50:1. Stimulated splenocytes were then assayed for nefspecific CTL activity against vVnef-infected CT26 cells or vac control– infected CT26 cells. The percentage nef-specific lysis reported is the percentage specific lysis of vVnef-infected targets − the percentage specific lysis of vac control–infected targets. The data shown are from a single experiment which is representative of three experiments conducted with similar results.
To confirm the results obtained with vaccinia vectors, we used DNA immunization to express the UbMNef and UbRNef constructs in mice as described in Materials and Methods, and evaluated the responses induced by this form of immunization. As shown in Fig. 8, after antigen-specific stimulation, splenocytes from mice immunized a single time with an expression plasmid encoding UbRNef were able to recognize and lyse nef-expressing CT26 target cells at low E/T ratios. Stimulated splenocytes from mice immunized with plasmid encoding UbMNef, wt nef, or with plasmid alone were unable to recognize and lyse CT26 target cells expressing nef even at E/T ratios as high as 100:1. Nef-expressing P815 target cells were also recognized and lysed by stimulated splenocytes from UbRNef immunized mice at low E/T ratios. Stimulated splenocytes from mice immunized with a plasmid expressing UbMNef or wt nef were also able to recognize and lyse these targets, but only at high E/T ratios and at reduced levels relative to the cells from UbRNef immunized mice. Both target cells used in these experiments are MHC class II−, indicating that the lysis mediated by CTLs from mice immunized with pcDNA3UbRNef was MHC class I restricted. In addition, lysis of nef-expressing targets by antigen simulated splenocytes from immunized mice was completely blocked by antiCD8 antibodies (data not shown). Taken together, these results indicate that when host cells are induced to express the rapidly degraded UbRNef, either by immunization with recombinant vaccinia virus vectors or with a DNA expression plasmid, BALB/c mice mount a strong primary CTL response against nef. However, when immunized with a stable form of nef, either UbMNef or wt nef, the CTL response against nef that they mount is greatly reduced or undetectable.
Figure 8.
Enhanced induction of nef-specific CTLs by immunization with DNA expression vectors encoding UbRNef. BALB/c mice were immunized once with 4 × 50 μg pcDNA3 (open), pcDNA3nef (cross hatched), pcDNA3UbMNef (stippled), or pcDNA3UbRNef (filled). Splenocytes from immunized mice were stimulated with psoralen/UV–treated, vVnefinfected CT26 cells at a ratio of 50 responders/stimulator cell for 5 d in the presence of IL-2. Stimulated splenocytes were assayed for nef-specific CTL activity against CT26 cells infected with vVnef or with vac control or against P815 cells infected with vVnef or vac control at an E/T ratio of 10:1 or 100:1. The data shown are from one experiment. The experiment was repeated a total of four times, with similar results.
Discussion
In this paper, we describe a vaccine strategy that is designed specifically to induce an enhanced CD8+ CTL response. This strategy is based on current understanding of the MHC class I antigen processing pathway. The source of peptide substrates for the class I pathway is the cytoplasmic degradation of antigen by the proteasome. By targeting antigens for rapid cytoplasmic degradation mediated by the proteasome, we have been able to stimulate enhanced CD8+ CTL responses against the antigens.
Using a ss− form of the HIV-1 env protein, we have shown that the rapid degradation of env in the cytoplasm results in the rapid sensitization of cells expressing the ss− env to lysis by an env-specific human CD8+ CTL clone, as seen by the ability of targets expressing this protein, but not the wt env, to be lysed by CTLs shortly after inducing expression of the env protein. This rapid sensitization is due to the presence of peptide-loaded MHC class I molecules on the cell's surface, indicating that not only is the protein rapidly degraded, but that the peptide products of that degradation do serve as substrate peptides for the class I processing pathway. This enhanced degradation, and thus enhanced processing, also leads to the induction of a more vigorous CD8+ CTL response in vivo as is seen in the greater CTL activity observed in splenocytes from mice immunized with the rapidly degraded env as compared to those from mice immunized with the wt env. This effect is observed both in secondary CTL responses in which splenocytes are stimulated before being assayed for CTL activity and in primary CTL assays in which splenocytes received no prior in vitro stimulation. Furthermore, immunization of mice with the ss− env results in a quantitative increase in the induction of memory CTL of almost fourfold relative to immunization with the wt env. These results are consistent with previous mechanistic studies showing that for some TAP-dependent epitopes in the extracellular domain of the env protein, processing for class I restricted recognition involves degradation of a nonglycosylated form of the protein which presumably arises by failure of a small fraction of the newly synthesized env protein to engage the translocation apparatus, resulting in mislocalization to the cytoplasm (47). These findings are also consistent with those of Townsend et al. (45) who showed that influenza HA protein that lacked a signal sequence was efficiently processed for presentation to HAspecific CTLs. It was further observed that deletion of the HA signal sequence overcame an antigen processing defect observed late in the course of vaccinia infection (42). Our results extend previous studies showing that the rapid cytoplasmic degradation of ss− env protein enables enhanced stimulation of both primary and secondary env-specific CTL responses in vivo.
Townsend et al. also showed that the same defect in antigen processing observed late in vaccinia infection could be overcome by generating degradation targeted forms of the influenza nucleoprotein. The nature of the defect was not elucidated, but may be related to the shutoff of host protein synthesis by vaccinia. These investigators showed that a rapidly degraded form of influenza NP expressed by a late vaccinia promoter was efficiently processed and presented to NP-specific CTLs, whereas a stable NP was not presented to those CTLs (42). We have expanded upon that work by investigating the use of these Ub fusion proteins in the context of a fully competent antigen processing pathway. Rather than attempting to rescue the defective processing of an Ag, we attempted to enhance the processing of targeted forms of the antigen relative to the wild-type forms of the antigen to enhance the induction of primary and secondary CTL responses against that antigen. Expression of nef as a Ub-nef fusion protein in which the NH2terminal residue of nef was mutated to Arg (UbRNef) conferred rapid degradation to nef. When this construct was used to stimulate a secondary CTL response, it was >1 log more effective than the stable UbMNef at stimulating nefspecific memory CTL from an HIV-1 infected donor. This result could have implications for the development of therapeutic vaccines that stimulate memory T cells as well as naive T cells. In a prophylactic vaccine setting, however, it is the stimulation of naive T cells, not memory T cells, that is important. When the UbRNef construct was used to immunize naive mice, either in the form of a recombinant vaccinia vector or a naked DNA expression vector, the stimulated splenocytes from immunized mice were able to recognize and lyse target cells expressing the wt nef protein. Splenocytes from the UbMNef immunized mice, however, were less able to recognize and lyse these targets. Thus, in addition to the secondary stimulation of memory CTLs, the rapidly degraded UbRNef construct is also able to stimulate the primary response of naive CTLs in vivo with much greater efficacy than does the stable UbMNef construct.
In a recent report by Goth et al. (51), it was shown that targeting OVA for rapid degradation via the generation of Ub fusion proteins did not result in improved recognition of cells expressing this construct by a T cell clone. This suggests that rapid degradation may not lead to enhanced recognition by all T cell clones, at least as far as can be determined in in vitro assays. Using a panel of human nefspecific clones, we have observed that whereas some clones were able to recognize cells expressing the UbRNef construct more efficiently than cells expressing wt nef, other clones showed equivalent lysis of the targets. Thus, rapid degradation does not necessarily lead to enhanced presentation of all CTL epitopes. Because of this variation in recognition that occurs on a clonal level, it is important to examine the overall effects of targeting antigens for rapid degradation on the induction of polyclonal CTL responses. In addition, from the point of view of vaccine development, the critical question is whether degradation targeted constructs show enhanced immunogenicity in vivo. We provide here direct evidence that this is the case for two antigens, HIV-1 env and nef.
Schwartz et al. have recently demonstrated that expression of the HIV-1 nef protein induces the downregulation of cell surface MHC class I expression (52). The authors suggest that this would result in the diminished ability of cells expressing nef to present peptide epitopes to CD8+ CTLs. It is possible that expression of a form of the nef protein that is targeted for rapid degradation would not show this effect on MHC class I, and thus cells expressing this protein would not have diminished abilities to present peptide epitopes to CTLs. It is unlikely that this effect is responsible for the enhanced immunogenicity of the vVUbRNef construct, since the ability to stimulate vaccinia-specific CTLs is unaffected by the degradation rate of nef. Mice immunized with vaccinia expressing wt nef have indistinguishable vac-specific CTLs from mice immunized with vaccinia expressing rapidly degraded nef. Furthermore, the numerous studies of the nef-specific CTL response, in both natural infection with HIV-1 and in animal studies cited suggest that cells expressing nef are able to efficiently process and present nef-derived peptide epitopes to CD8+ CTLs.
Whereas the majority of early HIV-1 vaccine efforts focused on eliciting an immune response against HIV-1 env, particularly a neutralizing Ab response, the results from these studies have been disappointing. One of the major obstacles to these approaches has been the wide genetic variability of env observed across strains of HIV-1. Therefore, vaccine strategies targeted against other HIV-1 antigens are now being actively pursued. Since these other antigens (i.e., gag, pol, and nef) are not present on the surface of the virion or of infected cells, the antibody response against these antigens is essentially irrelevant. However, these antigens do represent excellent targets for CD8+ CTLs. Indeed, it has been demonstrated in the simian immunodeficiency virus (SIV) model that vaccine-induced nef-specific CTLs are able to suppress the early replication of an SIV challenge in rhesus macaques (9). Also in the SIV model, it was observed that nef-specific precursor CTLs were detectable within days of acute infection of rhesus macaques with SIV (12). For HIV-1, nef-specific CTL activity has been observed in HIV-uninfected children of HIV-infected mothers (53, 54). Finally, in HIV-uninfected heterosexual contacts of HIV-infected patients, nef-specific CTLs are observed at a much higher frequency than CTLs specific for other HIV antigens (18). Taken together, these data suggest that nef-specific CTLs may play a very important role in the clearance of HIV, particularly immediately after acute infection, and that this nef-specific CTL response may be capable of mediating clearance of the virus. Therefore, augmenting these very important nef-specific CTL responses by targeting the antigen for rapid cytoplasmic degradation represents a very attractive strategy for vaccination against HIV.
Acknowledgments
The authors wish to thank Dr. Patricia Earl, National Institutes of Health, for the generous gift of the env construct vaccinia vectors and Drs. Alex Huang and Michele Keane for technical advice on the animal studies.
This work was supported by National Institutes of Health grants AI37924 and AI28108, and a grant from the Mobil Corporation.
Footnotes
1 Abbreviations used in this paper: β-gal, β-galactosidase; env, envelope; HA, hemagglutinin; MOI, multiplicity of infection; NP, nucleoprotein; O/N, overnight; SIV, simian immunodeficiency virus; ss−, signal sequence minus; Ub, ubiquitin; vac, vaccinia; wt, wild type.
References
- 1.Klavinskis LS, Tishon A, Oldstone MB. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in vivo. J Immunol. 1989;143:2013–2016. [PubMed] [Google Scholar]
- 2.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science (Wash DC) 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 4.Braciale TJ, Braciale VL. Viral antigen presentation and MHC assembly. Semin Immunol. 1992;4:81–84. [PubMed] [Google Scholar]
- 5.Yewdell JW, Bennink JR. Antigen processing: a critical factor in rational vaccine design. Semin Hematol. 1993;30:26–32. [PubMed] [Google Scholar]
- 6.Monaco JJ. Pathways for the processing and presentation of antigens to T cells. J Leukocyte Biol. 1995;57:543–547. doi: 10.1002/jlb.57.4.543. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, et al. Early suppression of SIV replication by CD8+ nefspecific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 10.Letvin NL, Reimann KA, Yasutomi Y, Ringler DJ, Yamamoto H. The SIVmac specific cytotoxic T lymphocyte response in the acutely infected rhesus monkey. Curr Top Microbiol Immunol. 1994;188:175–184. doi: 10.1007/978-3-642-78536-8_10. [DOI] [PubMed] [Google Scholar]
- 11.Reimann KA, Tenner RK, Racz P, Montefiori DC, Yasutomi Y, Lin W, Ransil BJ, Letvin NL. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasutomi Y, Reimann KA, Lord CI, Miller MD, Letvin NL. Simian immunodeficiency virus–specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalams SA, Walker BD. The cytotoxic T-lymphocyte response in HIV-1 infection. Clin Lab Med. 1994;14:271–299. [PubMed] [Google Scholar]
- 14.Oldstone MB, Tishon A, Eddleston M, de la Torre JC, McKee T, Whitton JL. Vaccination to prevent persistent viral infection. J Virol. 1993;67:4372–4378. doi: 10.1128/jvi.67.7.4372-4378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klavinskis LS, Whitton JL, Joly E, Oldstone MB. Vaccination and protection from a lethal viral infection: identification, incorporation, and use of a cytotoxic T lymphocyte glycoprotein epitope. Virology. 1990;178:393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 16.Tishon A, Eddleston M, de la Torre JC, Oldstone MB. Cytotoxic T lymphocytes cleanse viral gene products from individually infected neurons and lymphocytes in mice persistently infected with lymphocytic choriomeningitis virus. Virology. 1993;197:463–467. doi: 10.1006/viro.1993.1613. [DOI] [PubMed] [Google Scholar]
- 17.Rowland JS, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 18.Langlade DP, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J Clin Invest. 1994;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasutomi Y, Robinson HL, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins JI, Voss G, Manson K, Wyand M, et al. Simian immunodeficiency virus–specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belich MP, Trowsdale J. Proteasome and class I antigen processing and presentation. Mol Biol Rep. 1995;21:53–56. doi: 10.1007/BF00990971. [DOI] [PubMed] [Google Scholar]
- 21.Michalek MT, Grant EP, Gramm C, Goldberg AL, Rock KL. A role for the ubiquitin-dependent proteolytic pathway in MHC class I–restricted antigen presentation. Nature (Lond) 1993;363:552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- 22.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 23.Harding CV, France J, Song R, Farah JM, Chatterjee S, Iqbal M, Siman R. Novel dipeptide aldehydes are proteasome inhibitors and block the MHC-I antigen-processing pathway. J Immunol. 1995;155:1767–1775. [PubMed] [Google Scholar]
- 24.Restifo NP, Bacik I, Irvine KR, Yewdell JW, McCabe BJ, Anderson RW, Eisenlohr LC, Rosenberg SA, Bennink JR. Antigen processing in vivo and the elicitation of primary CTL responses. J Immunol. 1995;154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 25.Deverson E, Gow IR, Coadwell WJ, Monaco JJ, Butcher GW, Howard JC. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature (Lond) 1990;348:738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- 26.Trowsdale J, Hanson I, Mockridge I, Beck S, Townsend A, Kelly A. Sequences encoded in the class II region of the MHC related to the “ABC” superfamily of transporters. Nature (Lond) 1990;348:741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- 27.Spies T, Breshnan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature (Lond) 1990;348:744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 28.Monaco JJ, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science (Wash DC) 1990;250:1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- 29.Kelly A, Powis SH, Kerr L-A, Mockridge I, Elliot T, Bastin J, Uchanska-Ziegler B, Ziegler A, Trowsdale J, Townsend A. Assembly and function of the two ABC transporter proteins encoded in the human major histocompatibility complex. Nature (Lond) 1992;355:641–644. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- 30.Van Kaer L, Ashton-Rickardt PG, Pleogh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd JC, Schumacher TNM, Ashton-Rickardt PG, Imaeda S, Pleogh HL, Janeway CA, Tonegawa S. Tap1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993;74:577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- 32.Neefjes JJ, Momburg F, Hammerling GJ. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science (Wash DC) 1993;261:769. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 33.Murray N, McMichael A. Antigen presentation in virus infection. Curr Opin Immunol. 1992;4:401–407. doi: 10.1016/s0952-7915(06)80030-0. [DOI] [PubMed] [Google Scholar]
- 34.Milligan GN, Morrison LA, Gorka J, Braciale VL, Braciale TJ. The recognition of a viral antigenic moiety by class I MHC-restricted cytolytic T lymphocytes is limited by the availability of the endogenously processed antigen. J Immunol. 1990;145:3188–3193. [PubMed] [Google Scholar]
- 35.Villanueva MS, Fischer P, Feen K, Pamer EG. Efficiency of MHC class I antigen processing: a quantitative analysis. Immunity. 1994;1:479–489. doi: 10.1016/1074-7613(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 36.Grant EP, Michalek MT, Goldberg AL, Rock KL. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol. 1995;155:3750–3758. [PubMed] [Google Scholar]
- 37.Gaczynska M, Rock KL, Goldberg AL. Role of proteasomes in antigen presentation. Enzyme Protein. 1993;47:354–369. doi: 10.1159/000468693. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg AL, Rock KL. Proteolysis, proteasomes and antigen presentation. Nature (Lond) 1992;357:375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- 39.Bachmair A, Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989;56:1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 40.Varshavsky A. The N-end rule. Cell. 1992;69:725–735. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- 41.Johnson ES, Bartel B, Seufert W, Varshavsky A. Ubiquitin as a degradation signal. EMBO (Eur Mol Biol Organ) J. 1992;11:497–505. doi: 10.1002/j.1460-2075.1992.tb05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G. Defective presentation to class I–restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozkaynak E, Finley D, Varshavsky A. The yeast ubiquitin gene: head-to-tail repeats encoding a polyubiquitin precursor protein. Nature (Lond) 1984;312:663–666. doi: 10.1038/312663a0. [DOI] [PubMed] [Google Scholar]
- 44.Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science (Wash DC) 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 45.Townsend ARM, Bastin J, Gould K, Brownlee GG. Cytotoxic T lymphocytes recognise influenza hemagglutinin that lacks a signal sequence. Nature (Lond) 1986;234:575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi K, Dai LC, Fuerst TR, Biddison WE, Earl PL, Moss B, Ennis FA. Specific lysis of human immunodeficiency virus type 1–infected cells by a HLAA3.1–restricted CD8+ cytotoxic T-lymphocyte clone that recognizes a conserved peptide sequence within the gp41 subunit of the envelope protein. Proc Natl Acad Sci USA. 1991;88:10277–10281. doi: 10.1073/pnas.88.22.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammond SA, Johnson RP, Kalams SA, Walker BD, Takiguchi M, Safrit JT, Koup RA, Siliciano RF. An epitope-selective, transporter associated with antigen presentation (TAP)-1/2-independent pathway and a more general TAP-1/2-dependent antigen-processing pathway allow recognition of the HIV-1 envelope glycoprotein by CD8+ CTL. J Immunol. 1995;154:6140–6156. [PubMed] [Google Scholar]
- 48.Hammond SA, Bollinger RC, Tobery TW, Siliciano RF. Transporter-independent processing of HIV-1 envelope protein for recognition by CD8+ T cells. Nature (Lond) 1993;364:158–161. doi: 10.1038/364158a0. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Cohen J, Hosmalin A, Cease KB, Houghten R, Cornette JL, DeLisi C, Moss B, Germain RN, Berzofsky JA. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility molecule–restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1988;85:3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig S, Fuerst TR, Wood LV, Woods RM, Suzich JA, Jones GM, de la Cruz VF, Davey RJ, Venkatesan S, Moss B, et al. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990;145:127–135. [PubMed] [Google Scholar]
- 51.Goth S, Nguyen V, Shastri N. Generation of naturally processed peptide/MHC class I complexes is independent of the stability of endogenously synthesized precursors. J Immunol. 1996;157:1894–1904. [PubMed] [Google Scholar]
- 52.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 53.Cheynier R, Langlade DP, Marescot MR, Blanche S, Blondin G, Wain HS, Griscelli C, Vilmer E, Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus1–infected mothers. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 54.De MA, Cirillo C, Moretta L. Occurrence of human immunodeficiency virus type 1 (HIV-1)–specific cytolytic T cell activity in apparently uninfected children born to HIV-1–infected mothers. J Infect Dis. 1994;170:1296–1299. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]