Abstract
Elevated levels of the p53 protein occur in ∼50% of human malignancies, which makes it an excellent target for a broad-spectrum T cell immunotherapy of cancer. A major barrier to the design of p53-specific immunotherapeutics and vaccines, however, is the possibility that T cells may be tolerant of antigens derived from wild-type p53 due to its low level of expression in normal thymus and lymphohemopoetic cells. The combination of p53 deficient (p53−/−) and p53+/+ HLA-A2.1/Kb transgenic mice was used as a model to explore the possibility that A2.1restricted cytotoxic T lymphocytes (CTL) are functionally tolerant of self peptides derived from the wild-type p53 tumor suppressor protein. A2.1-restricted CTL specific for a naturally processed p53 self-epitope spanning residues 187-197 were completely aborted in p53+/+ as opposed to p53−/− transgenic mice. In contrast, CTL specific for a second self-epitope spanning residues 261-269 of the murine p53 sequence were detected in both p53−/− and p53+/+ A2.1/Kb transgenic mice. However, the avidity of the CTL effectors obtained from p53+/+ mice was 10-fold lower than that obtained from p53−/− mice, again suggesting elimination of CTL with high avidity for the A2.1-peptide complex. The circumvention of functional tolerance of high avidity CTL may therefore be a necessary prerequisite for optimizing immunotherapy against A2.1-restricted wild-type p53 epitopes in humans.
Peptides presented by class I MHC molecules and derived from normal self-proteins that are expressed at elevated levels by cells from a wide variety of human (Hu)1 malignancies provide, in theory, potential target antigens for a broad-spectrum, CTL-based immunotherapy of cancer. The Hu p53 tumor suppressor protein is an excellent source for such general, tumor-associated and class I MHC–bound CTL epitopes as its expression is markedly upregulated in many different types of tumor cells. Such overexpression correlates with the presence of mutation within p53 that inactivates its normal activity in tumor supression (1, 2). Due to the diversity of mutations in p53 that can arise in tumors, if this protein is to serve as a general tumor antigen, it would be necessary to target peptides representative of the wild-type (WT) sequence. However, since it is also expressed at low level in some types of normal tissues, such as thymus, spleen, and lymphohemopoetic cells (3–5), WT p53 derived self-MHC–self-peptide complexes may also represent thymic and/or peripheral tolerogens, thereby preventing immune responses (6–14). This is particularly true for class I MHC–peptide complexes expressed by bone marrow derived cells in the thymus, as such expression would cause negative selection of immature thymic T cells with high avidity for self-MHC–self-peptide complexes (6–11). In the case of p53, this intrathymic deletion of potentially self-p53-reactive T cells could result in a peripheral T cell repertoire purged of CTL precursors with sufficient avidity to recognize natural WT p53 epitopes presented by class I MHC molecules on tumor cells. Several studies in mice and man, however, have provided conflicting results of whether class I MHC–restricted CTL are functionally tolerant of WT p53 (15–22).
We have recently devised an experimental strategy (23) that exploits species differences between Hu and murine (Mu) WT p53 protein sequences (24) to circumvent Hu WT p53-specific T cell tolerance and obtain HLA-restricted CTL specific for epitopes from Hu p53. HLA-A2.1− (A2.1−) transgenic (Tg) mice (25) were used to generate A2.1restricted CTL with high avidity for endogenously processed, nonhomologous Hu WT p53 peptides. The Hu WT p53-specific CTL obtained were able to lyse a broad variety of p53-overexpressing Hu tumor cells yet did not recognize nontransformed Hu targets (23). Hu p53 peptides that were homologous to Mu WT p53 sequences did not induce CTL responses capable of lysing tumor cells, regardless of whether these peptides had high, intermediate, or low binding affinity to A2.1 (23). Considering that not all of the nonhomologous Hu WT p53 peptides that bound A2.1 were capable of inducing a CTL response (23), this left unresolved the issue of whether self tolerance, or some other mechanism, was responsible for nonresponsiveness to homologous p53 peptides.
To explore the possibility that class I MHC–restricted CTL are tolerant of WT p53, we took advantage of p53−/− mice (26) backcrossed onto HLA-Tg mice expressing a chimeric class I molecule, A2.1/Kb, which contains the α-1 and α-2 domains of the HLA-A2.1 molecule, and the α-3 domain of Mu H-2Kb (23, 25, 27, 28). The expression of the chimeric A2.1/Kb molecule allows A2.1-restricted Mu T cells to interact sufficiently with the α-3 domain of the transgene product via their Mu CD8 coreceptor during cognate recognition of A2.1-peptide complexes in thymus and periphery (25, 27–33). Furthermore, the Ag processing and presentation machinery in A2.1/Kb-Tg mice and Hu cells are sufficiently similar to present the same CTL epitopes (23, 25, 28, 34, 35). Also, the TCR repertoire in man and A2.1/Kb-Tg mice has been demonstrated to be sufficiently diverse to respond to the same A2.1-peptide complexes (23, 36–38). The p53−/− A2.1/Kb mice do not express Mu WT p53 protein (26) and therefore would not be tolerant of self-p53 epitopes presented by A2.1. Although p53 is required for thymocyte apoptosis induced by ionizing radiation (26, 39) or adenosine deaminase deficiency (40), apoptotic cell death of thymocytes and mature T cells as a result of exposure to compounds that mimic TCR engagement during negative selection has been shown to be independent of p53 expression (26, 41). Except for a putative effect due to the lack of expression of self-MHC–p53 peptide complexes, repertoire selection of T cells in p53−/− and p53+/+ A2.1/Kb mice is anticipated to be identical. In this report, we compared the capability of p53−/− and p53+/+ A2.1/Kb-Tg mice to mount A2.1-restricted CTL responses after immunization with A2.1-binding WT p53 peptides that were either homologous or nonhomologous in sequence between Hu and Mu WT p53.
Materials and Methods
Mice.
The p53−/− mice (26) were obtained from Tyler Jacks (Massachusetts Institute of Technology) and mated with line 6 A2.1/Kb-Tg (23, 25, 28). Progeny were interbred, and offspring screened for mice that were p53−/− and expressed A2.1/Kb. Line 22 A2.1-Tg mice were H-2b/b and heterozygous for the transgene, which contains the α-1, -2, and -3 domain of A2.1 (23, 25). C57BL/6 mice were purchased from the breeding colony of The Scripps Research Institute (TSRI). Mice were propagated and maintained under specific pathogen free conditions in our vivarium at TSRI. All experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Peptides.
The selection of synthetic peptides representing sequences within the Hu WT p53 protein as well as their binding affinities for A2.1 have been reported (23). Synthetic WT p53 peptides that were homologous and nonhomologous to Mu WT p53 sequences and had either high (Hu p53.65-73, 129-137, 187195, 264-272, and Mu p53.261-269) or intermediate (Hu p53.149157, 187-197, 210-218, 255-264, and 322-330) A2.1-binding affinity were used in these studies (Table 1) (23). Peptides were synthesized by the core facility of TSRI using a synthesizer (model 430A; Applied Biosystems Foster City, CA). Purity was ascertained by mass spectrometry and reverse-phase HPLC analysis on a Vydac C18 column (Hesperia, CA).
Table 1.
Immunogenicity of A2.1-binding WT p53 Peptides in p53− /−A2.1/Kb-Tg and p53+ /+A2.1/Kb-Tg Mice
| E/T | Percent specific lysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT p53 peptide | p53−/− A2.1/Kb-Tg | p53+/+ A2.1/Kb-Tg | ||||||||||
| Position | Sequence | T2A2Kb | T2A2Kb + Pe* | T2A2Kb | T2A2Kb + Pe | |||||||
| Hu 65-73 | R MPE A APPV | 30 | 5 | 44 | 4 | 31 | ||||||
| 10 | 3 | 16 | 2 | 13 | ||||||||
| Hu 129-137 | A LNK M FCQL | 30 | 3 | 2 | 4 | 5 | ||||||
| 3 | 6 | 2 | 3 | 0 | ||||||||
| Hu 149-157 | S TPP P G T RV | 30 | 4 | 57 | 2 | 52 | ||||||
| 3 | 1 | 11 | 1 | 8 | ||||||||
| Hu 187-195 | GLAPPQHLI | 30 | 3 | 3 | 5 | 1 | ||||||
| 3 | 1 | 0 | 1 | 1 | ||||||||
| Hu 187-197 | GLAPPQHLIRV | 30 | 1 | 84 | −1 | 3 | ||||||
| 3 | 1 | 32 | 1 | 0 | ||||||||
| Hu 210-218 | N TFRHSVVV | 30 | 4 | 4 | 4 | 4 | ||||||
| 3 | 0 | 2 | 1 | 1 | ||||||||
| Hu 255-264 | ITLEDSSGNL | 30 | 2 | −1 | 0 | 1 | ||||||
| 3 | 0 | 0 | 2 | 0 | ||||||||
| Hu 264-272 | LLGR N SFEV | 30 | 3 | 42 | 3 | 57 | ||||||
| 3 | 1 | 10 | 1 | 14 | ||||||||
| Mu 261-269 | LLGRDSFEV | 30 | 1 | 82 | 1 | 45 | ||||||
| 3 | 1 | 37 | 0 | 16 | ||||||||
| Hu 322-330 | PLDGEYFTL | 60 | 11 | 12 | ND | ND | ||||||
| 6 | 7 | 1 | ND | ND | ||||||||
Responder spleen cells from peptide-primed p53−/− and p53+/+ A2.1/Kb-Tg mice were restimulated in vitro with the indicated priming peptide. After 6 d, effector cells were assayed for cytotoxicity against T2A2Kb targets and the same cells pulsed with the priming peptide at 10−6 M. Data are representative for at least two independent experiments for each peptide and a total of at least four mice per strain and peptide. Amino acid residues that are homologous to the MuWT p53 sequence are in bold type. Nonhomologous residues are underlined.
Pe, peptide.
Cell Lines.
Previously described cell lines and transfectants used in these studies included T2-A2.1/Kb (T2A2Kb), Jurkat-A2.1 (JA2), the H-2b/b thymoma line EL4, EL4-A2.1/Kb (EA2Kb), the naturally A2.1-expressing, p53-deficient osteosarcoma line Saos-2, and this same line transfected with a Hu mutant p53 gene, Saos2/143 (23, 25, 27, 42, 43). To obtain EA2Kb.1p53 transfectants, EL4 cells were cotransfected with 10 μg of plasmid containing a genomic clone of A2.1/Kb (27) and 2 μg of pC53-4.2N3 containing a Hu mutant p53 cDNA linked to the neomycin resistance gene (44), as described (27). The corresponding parental and p53-transfected lines expressed similar levels of A2.1 (Saos-2, Saos2/143) or A2.1/Kb (EA2Kb, EA2Kb.1p53) as detected by flow cytometry (27). The 10(3) Balb/c fibroblast cell line, which lacks endogenous Mu p53 and a transfectant of the line that expresses a Mu p53 gene containing a mutation at residue 215, were kindly provided by Drs. Dirk Dittmer and Arnold Levine (Princeton University) (42, 45).
Peptide Priming of p53− /− and p53+ /+ A2.1/Kb-Tg Mice and Propagation of CTL.
Mice were injected s.c. at the base of the tail with 100 μg of the indicated WT p53 peptide and 120 μg of the I-Ab-binding synthetic T helper peptide representing residues 128-140 of the hepatitis B virus core protein (36) emulsified in 100 μl of IFA (23). After 10 d, spleen cells of primed mice were cultured with irradiated A2.1/Kb-Tg LPS-activated spleen cell stimulators that had been pulsed with the priming WT p53 peptide at 5 μg/ml and Hu β2-microglobulin at 10 μg/ml in complete RPMI media (RPMI1640 containing 10% vol/vol fetal calf serum, 25 mM Hepes, 2 mM glutamine, 5 × 10−5 M β-mercaptoethenol and 50 μg/ml gentamycin) (23, 25, 28). After 6 d, the resultant effector cells were assayed in a 4-h 51Cr-release assay (27) at various E/T ratios for lytic activity against T2A2Kb and EL4 targets that had been pulsed with either the priming WT p53 peptide, an unrelated A2.1-binding peptide, or no peptide. Immunization of mice with a given peptide as well as the subsequent effector cell cultures and 51Cr-release assays were performed simultaneously for p53−/− and p53+/+ A2.1/Kb-Tg mice. A polyclonal, A2.1-restricted CTL line (p53−/− A2Kb 187) derived from peptide-primed p53−/− A2.1/Kb mice and specific for Hu WT p53.187-197 was established by weekly restimulation of effector CTL with irradiated JA2 cells that had been pulsed with 5 μg of the indicated p53 peptide, irradiated C57BL/6 spleen filler cells, and 2% (vol/vol) rat Con A supernatant (23, 27). The polyclonal, A2.1restricted CTL line (A2 149) specific for Hu WT p53.149-157 has been established from peptide-primed A2.1-Tg mice as previously reported (23). CTL clones specific for Hu WT p53.187-197 (p53−/− A2Kb 187 clone 4) and 149-157 (A2 149 clone 5) were derived by limiting dilution (46) of effector cell populations originally obtained from peptide primed p53−/− A2.1/Kb and A2.1Tg mice, respectively. Stimulators used for CTL cloning were peptide-pulsed JA2 cells. Lysis of EA2Kb and EA2Kb.1p53 as well as cytokine- and noncytokine-pretreated Saos-2 and Saos-2/143 targets by Hu WT p53-specific CTL lines and clones was determined in a 5-h 51Cr-release assay (27). Cytokine-pretreated targets had been exposed for 20 h to both rIFN-γ (R&D Systems, Minneapolis, MN) at 20 ng/ml and rTNF-α (R&D Systems) at 3 ng/ml (23). Anti-A2.1 inhibition was performed by exposure of 51Cr-labeled target cells to the anti-A2.1 mAb PA2.1 at saturating, nontoxic concentrations (23, 47).
Origin and Use of Vaccinia Recombinants.
The vaccinia recombinant, vv-A2.1, which expresses the HLA-A2.1 molecule (48) was kindly provided by Dr. M. Nishimura (Surgery Branch, National Cancer Institute). The vPE16 (49) vaccinia recombinant, which expresses the gp160 of the human immunodeficiency virus type 1, was kindly provided by Drs. Jack Bennink and Jonathan Yewdell (National Institute of Allergy and Infectious Diseases). Target cells were infected as monolayers with 20 PFU per cell for 1 1/2 h in RPMI containing 0.1% BSA. Virus was removed, and cells incubated 4 h in complete RPMI. Monolayers were disrupted by trypsinization, and cells labeled with 51Cr as previously described (27).
Results
Immunogenicity of A2.1-binding WT p53 Peptides in p53− /− and p53+ /+ A2.1/Kb-Tg Mice.
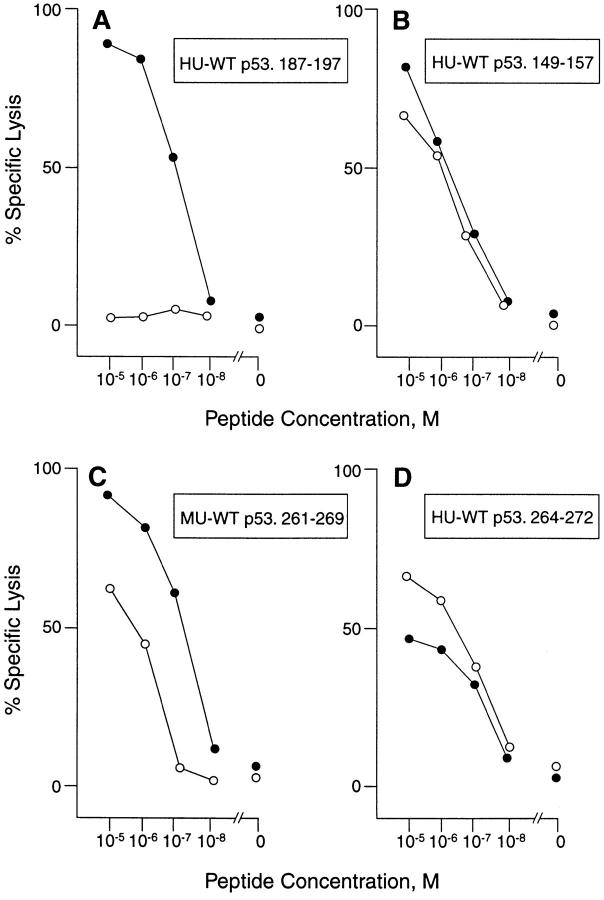
Spleen cells from mice primed with homologous and nonhomologous A2.1-binding WT p53 peptides were restimulated with peptide in vitro and tested for an A2.1-restricted, peptide-specific CTL response. As reported, p53+/+ A2.1/Kb-Tg mice could mount an A2.1restricted CTL response specific for several nonhomologous Hu WT p53 peptides, including 65-73, 149-157, and 264272, the latter two of which have been shown to represent naturally processed CTL epitopes (Table 1) (23). Also as reported, these mice failed to develop A2.1-restricted CTL specific for the remaining homologous and nonhomologous Hu WT p53 peptides tested, including 129-137, 187-195, 187-197, 210-218, and 255-264 (23). As anticipated, p53−/− A2.1/Kb mice responded to the same three nonhomologous Hu p53 peptides recognized by p53+/+ A2.1/Kb mice, demonstrating a comparable level of lytic activity. A lack of A2.1restricted CTL responses by p53−/− A2.1/Kb-Tg mice was also observed with the remaining nonhomologous peptides, as well as with three out of four of the homologous WT p53 peptides (187-195, 255-264, and 322-330) which are identical in sequence in Hu and Mu p53 (Table 1). In contrast, however, a strong A2.1-restricted, peptide-specific CTL reponse was induced in p53−/− as opposed to p53+/+ A2.1/Kb-Tg mice after priming with the homologous WT p53.187-197 peptide (Table 1 and Fig. 1 A). Also, the amount of lysis obtained with responder spleen cells from p53−/− A2.1/Kb-Tg mice immunized with the Mu WT p53.261-269 peptide was about twofold higher as compared with CTL derived from p53+/+ A2.1/Kb-Tg mice (Table 1). Mu WT p53.261-269 is identical with Hu p53.264-272 at all but one amino acid residue, and has an equivalent high affinity for A2.1 (23). Significantly, the concentration of Mu WT p53.261-269 peptide required to obtain half-maximum lysis of T2A2Kb targets by CTL derived from p53−/− versus p53+/+ A2.1/Kb-Tg mice was ∼10-fold lower (5.2 × 10−8 M versus 4.6 × 10−7 M) (Fig. 1 C). This was in contrast to the comparable avidity demonstrated by p53+/+ and−/− A2.1-restricted CTL specific for the nonhomologous Hu WT p53 epitopes 149-157 and 264-272 (Fig. 1, B and D). As may be anticipated from lack of tolerance against the Hu WT 264-272 peptide, there is no cross-reactive recognition of the Mu 261-269 peptide by the Hu WT 264-272–specific CTL, and vice-versa.
Figure 1.
Efficiency of peptide recognition by p53-specific CTL derived from p53−/− (closed circle) and p53+/+ (open circle) A2.1/Kb-Tg mice. Responder spleen cells from peptide-primed p53−/− and p53+/+ A2.1/ Kb-Tg mice were restimulated in vitro with the indicated priming peptide. After 6 d, effector CTL were assayed at an E/T ratio of 30:1 for lytic activity against T2A2Kb targets and the same cells pulsed with (A) Hu WT p53.187-197, (B) Hu WT p53.149-157, (C) Mu WT p53.261-269, and (D) Hu WT p53.264-272 at the indicated concentrations.
The peptide specificity and the A2.1 restriction of the CTL reponses shown in Table 1 and Fig. 1 were evidenced by the failure of these CTL both to lyse T2A2Kb cells pulsed with unrelated A2.1-binding peptides, and to respond to A2.1/Kb-negative targets pulsed with the priming peptide (data not shown). It is of interest that A2.1-restricted CTL derived from p53−/− A2.1/Kb-Tg mice and specific for the homologous 11-mer peptide p53.187-197 did not recognize the 9-mer peptide p53.187-195, even though the 9-mer had an 1.4-fold higher binding affinity for A2.1 (23) (data not shown).
The Homologous WT p53.187-197 Peptide Is Naturally Processed and Presented by A2.1-expressing Cells from Mice and Man.
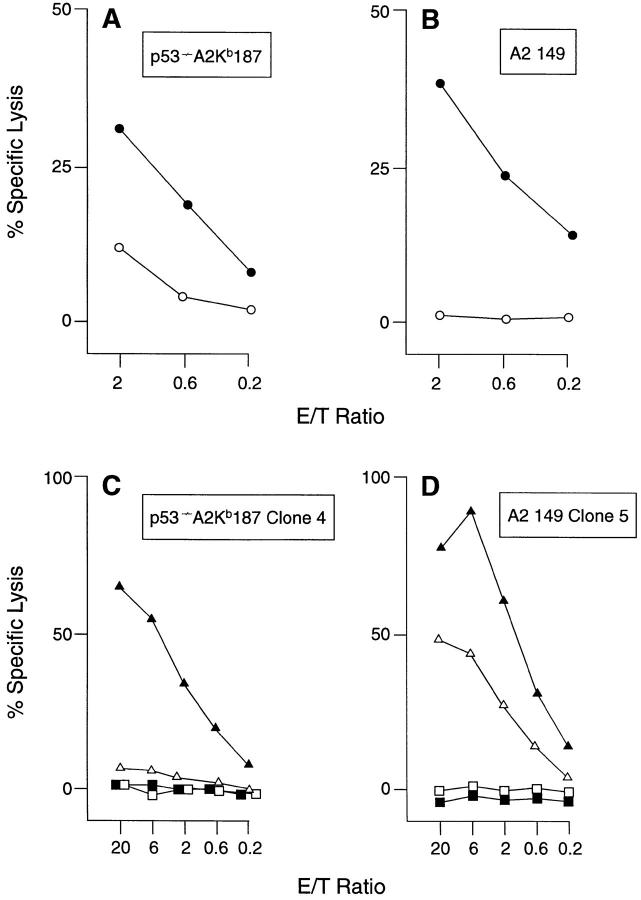
A prerequisite for tolerance induction by any self peptide is its ability to be endogenously processed and transported into the endoplasmic reticulum for association with class I MHC molecules (50). To determine whether the homologous WT p53.187-197 peptide is actually presented as naturally processed T cell epitope by A2.1 on the surface of Mu and Hu cells, both a peptide-specific, polyclonal CTL line (p53−/− A2Kb 187) and a CTL clone (p53−/− A2Kb 187 clone 4) were established from p53−/− A2.1/KbTg mice and tested for p53-specific recognition of Mu and Hu cell lines transfected with Hu p53, EA2Kb.1p53 and Saos-2/143, respectively (Fig. 2, A and C). As a positive control for p53-specific, A2.1-restricted lysis, the polyclonal CTL line A2 149 and the A2 149 CTL clone 5, both of which were derived from A2.1-Tg mice and specific for the natural CTL epitope Hu WT p53.149-157 (23), were included in these studies (Fig. 2, B and D). Comparison of the levels of lysis of the p53 transfectants relative to the parental target lines indicated that both WT p53.187-197 and 149-157 were endogenously processed and presented by Mu and Hu cells. Recognition was A2.1-restricted, as lysis was inhibited by an A2.1-specific antibody (data not shown). To obtain substantial Ag-specific lysis of A2.1-expressing Saos-2/143 cells by p53−/− A2Kb 187 CTL clone 4 as opposed to A2 149 CTL clone 5, targets had to be pretreated by IFN-γ and TNF-α (Fig. 2, C and D), a method that is known to facilitate TCR-mediated Ag recognition and target cell lysis by increasing the numbers both of MHC-peptide complexes and of adhesion molecules expressed on the cell surface (51, 52). The failure of the particular p53−/− A2Kb 187 CTL clone 4 to lyse untreated Saos-2/143 cells was consistent with the previous finding that CTL from A2.1/Kb-Tg mice are at a disadvantage in recognition of cells expressing A2.1 as compared with A2.1/Kb, due to the inability of Mu CD8 to interact with the Hu α-3 domain of the A2.1 molecule (23, 25, 27, 28, 31). However, due to their in vivo selection and stimulation in the absence of a sufficient participation by Mu CD8, CTL from A2.1Tg mice, such as A2 149 clone 5, express TCRs with unusually high affinity for the relevant A2.1-peptide complex and thus require less peptide Ag for their CD8-independent target cell recognition (23, 25, 53). A2 149 CTL clone 5 as opposed to p53−/− A2Kb 187 CTL clone 4 was therefore able to lyse untreated Saos-2/143 targets.
Figure 2.
Recognition of naturally processed WT p53 epitopes by A2.1-restricted CTL. The responder CTL line and clone specific for WT p53.187-197 and derived from p53−/− A2.1/Kb-Tg mice was (A) p53−/− A2Kb 187 and (C) p53−/− A2Kb 187 clone 4, respectively. The responder CTL line and clone specific for Hu WT p53.149-157 and derived from (p53+/+) A2.1-Tg mice was (B) A2 149 and (D) A2 149 clone 5, respectively. CTL lines and clones were assayed for cytotoxicity against the following targets: EA2Kb (open circles), EA2Kb.1p53 transfectants (closed circles), untreated p53-deficient Saos-2 (open squares), Saos-2 pretreated with IFN-γ and TNF-α (closed squares), untreated Saos-2/143 p53-transfectants (open triangle), and Saos-2/143 pretreated with IFN-γ and TNF-α (closed triangles).
The Mu p53.261-269 Self-peptide is Presented by A2.1 on Murine Cells.
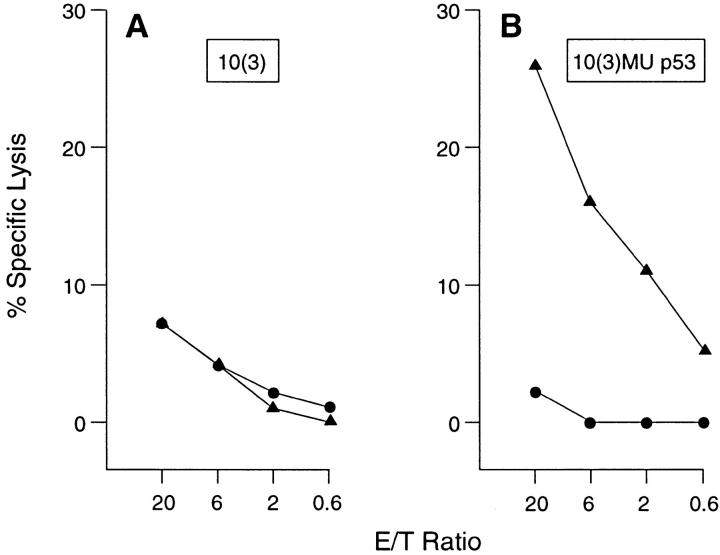
Previous studies had demonstrated the Hu analogue of the Mu p53.261-269 peptide was endogenously processed and presented in association with A2.1 (23). However, as the Mu and Hu peptides differed at one residue (Table 1) it was possible this difference affected the presentation of the Mu peptide, resulting in incomplete tolerance induction. This could explain its recognition in both p53-deficient and -sufficient animals. To determine if the Mu WT p53.261-269 peptide was presented by A2.1 on Mu cells, a transfectant of the mouse 10(3) cell line which expresses high levels of Mu p53, was infected with a recombinant strain of vaccinia virus that expresses the A2.1 molecule, vv-A2.1. As a control for the effect of viral infection, cells were infected by a recombinant strain encoding the gp160 of HIV-1 (vPE16). As presented in Fig. 3, the 10(3) Mu p53 transfectant expressing the A2.1 molecule was specifically lysed by a CTL population specific for the Mu 261-269 peptide epitope. Neither the p53-deficient parental line, 10(3), nor the 10(3) Mu p53 transfectant infected with vPE16 rather than vv-A2.1 were lysed by these same effectors. Therefore, the Mu WT p53.261269 peptide is presented by cells that express both Mu p53 and A2.1.
Figure 3.
The Mu WT p53.261-269 peptide is endogenously processed and presented in association with HLA-A2.1 on the surface of vvA2.1–infected murine cells. The A2.1 restricted, Mu WT p53.261-269 peptide specific CTL were used as effectors in a 5 h 51Cr-release assay in which 10(3) tumor cells (A), or 10(3) Mu p53 transfectants (B) were infected with a vaccinia recombinant expressing either the HLA-A2.1 molecule (closed triangles), or gp 160 (closed circles).
Discussion
The combination of p53−/− and p53+/+ A2.1/Kb-Tg mice was used as a model to determine if normal levels of expression of WT p53 self-peptides presented by A2.1 result in functional tolerance. The studies demonstrate that the A2.1-restricted CTL response to the homologous WT p53.187-197 self-peptide was eliminated by self tolerance in p53+/+ A2.1/Kb-Tg mice. Also, comparison of the levels of lysis by effector cells specific for the Mu WT p53.261269 self-peptide indicates that CTL with a significantly lower avidity were recruited in p53+/+ relative to the p53−/− strain of A2.1/Kb-Tg mice. These findings are consistent with a scenario in which functional tolerance of class I MHC–restricted T cells to WT p53 results in either elimination of all p53-specific CTL (as with p53.187-197) or elimination of CTL with high avidity for the relevant class I MHC–peptide complexes (as with p53.261-269), while those with low avidity persist. Recent studies demonstrate that low avidity T cells can escape the negative selection process and emerge into the periphery (54–57).
However, the data further suggest that gaps in the T cell repertoire that are independent of p53 expression also occur, as not all of the homologous and nonhomologous WT p53 peptides with high or intermediate A2.1-binding affinity were capable of inducing a CTL response in either strain of Tg mice. Such gaps may reflect lack of expression or positive selection of TCRs of the relevant specificity, or deletion attributable to the presence of crossreactive epitopes contributed by other proteins (10, 11, 58–60).
It is of interest that the WT p53.187-197 specific CTL obtained from p53−/− A2.1/Kb mice do not lyse EA2Kb targets unless they are transfected with p53 and contain high levels of the protein. Similarly, the EA2Kb targets were not lysed by the Mu 261-269–specific CTL (data not shown). As is the case in normal lymphohemopoetic cells, EL4 cells express only low levels of WT p53 (Hernandez, J., and L.A. Sherman, unpublished observation). Therefore, these results support our previous findings that normal levels of expression of p53 are insufficient for recognition by CTL raised against p53 peptides (23). Nevertheless, these same low numbers of class I MHC–peptide complexes are sufficient to achieve tolerance of high avidity CTL, as observed in these studies. Previous reports have demonstrated that the amount of Ag required for negative selection is less than that required for recognition by effector T cells (61, 62).
Several Hu WT p53 peptides, such as 149-157, 187-197, and 264-272, that have been shown in this and a previous study (23) to be immunogenic in Tg mice and to represent naturally processed CTL epitopes, have also been demonstrated to stimulate Hu PBL primarily in vitro and to induce an A2.1-restricted, peptide-specific CTL response (19–21). Whereas CTL from Tg mice were able to lyse Hu p53 transfectants and/or Hu tumor cells (23), this has not been reported for CTL from Hu PBL (19–21), thereby raising the possibility that the latter represent low avidity T cells that have survived p53-specific tolerance induction. This hypothesis is supported by our observation that A2.1restricted CTL with apparently higher avidity for the Mu WT p53.261-269 self-peptide were aborted in p53+/+ A2.1/Kb-Tg mice, whereas low avidity CTL could be induced. It should be emphasized that Mu WT p53.261-269 differs by only one conservative amino acid exchange from the naturally processed Hu WT p53.264-272 T cell epitope and is bound by A2.1 with an equivalent high affinity (23).
In contrast to our results with Mu WT p53.261-269 and to studies with Hu PBL (19–21), we could not detect A2.1restricted CTL with even low avidity for the WT p53.187197 T cell epitope in p53+/+ A2.1/Kb-Tg animals. The molecular and biological reasons for these discrepancies are as yet unclear. As demonstrated by the data in Figs. 2 and 3, both peptides are endogenously presented in association with A2.1 on the surface of Mu cells. However, we cannot exclude the possibility that they are endogenously presented at different densities. It is possible the Mu WT p53.261269 epitope is processed and/or presented less efficiently than the p53.187-197 epitope. This could result in less efficient tolerance induction, but would not necessarily explain the difference in responsiveness to p53.187-197 by mouse and human CTL. Alternatively, the lower A2.1 binding affinity of p53.187-197 relative to Mu p53.261-269 (23) in conjunction with the low level of expression of the A2.1/Kb transgene relative to the level of expression of conventional MHC molecules (28), could be directly or indirectly involved. For example, the primary in vitro selection of Hu PBL derived, peptide-specific CTL involved repeated cycles of restimulation with Hu stimulators expressing high levels of A2.1 and pulsed with high concentrations of synthetic peptide in the presence of exogenous T cell growth promoting cytokines (19–21). Such conditions may promote expansion of low avidity CTL specific for peptides with intermediate A2.1-binding affinity, such as p53.187-197 (19–21, 63). However, the in vivo priming of A2.1/Kb-Tg mice expressing low levels of A2.1/Kb, followed by one cycle of in vitro restimulation of responder spleen cells with peptide-pulsed A2.1/Kb stimulators (in the absence of exogenous cytokines) may not be sufficient to induce T cells with low avidity for peptides that represent intermediate (WT p53.187-197) as opposed to high (Mu WT p53.261-269) A2.1 binders. It may be anticipated that low avidity T cells would be the ones most dependent for their stimulation on large numbers of MHC–peptide complexes, which in turn, would be fewer for peptides with lower affinity for A2.1. Future experiments will determine if altered experimental conditions can lead to induction of murine CTL specific for p53.187-197. Alternatively, A2.1-positive humans and these A2.1/Kb-Tg mice that have been demonstrated to be sufficiently similar in selecting A2.1-restricted T cells specific for peptides with high binding affinity to A2.1 could differ considerably from each other in their selection of low avidity T cells specific for self-peptides with intermediate A2.1-binding affinity (38).
In several recent papers, the immune response of BALB/c mice to a Mu p53 peptide was contrasted with that same peptide containing a point mutation (Mu p53.234-240 and 236 M to I) (15–17). Although immunization with peptide in adjuvant resulted in responsiveness only to the mutated sequence (15, 16), a response to the WT peptide was obtained when these same mice were injected with dendritic cells pulsed with peptide (17). These results indicated that it was more difficult to obtain a response to the WT versus the mutant p53 sequence, consistent with an effect on the repertoire due to self tolerance. By using p53−/− mice, our results have directly demonstrated the influence of self tolerance on T cell recognition of p53.
In summary, this study shows functional tolerance of high avidity CTL specific for naturally processed WT p53 peptides presented by A2.1. The data also demonstrate the flexibility of WT p53-specific tolerance, as the extent by which low avidity CTL survived the induction of self-tolerance varied between the particular class I MHC–p53 peptide combinations. These residual low avidity CTL, however, could provide an opportunity for immunotherapy of tumors that express high levels of p53 (17, 18). Given the variability of the effect observed on the repertoire by selftolerance to different peptides, as well as variability of responsiveness due to different modes of immunization, it is likely that the success of the immunotherapy directed towards self-proteins will require careful examination of responses to each MHC-peptide complex. Furthermore, given the fact that low level expression of p53, such as that observed in normal cells and tumors expressing nonmutant p53, such as EL4, is insufficient for cell lysis, it is further likely that the level of presentation of p53 peptides by individual tumors will also determine the success of such immunotherapy.
Acknowledgments
We thank Tyler Jacks for providing p53−/− mice, and Arnold J. Levine (Princeton University) for providing Saos-2/143 transfectants and the 10(3) cell lines. We thank Patty Krier and Carol Wood for excellent secretarial assistance.
Footnotes
M. Theobald is a fellow of the Stipendienprogramm Infektionsbiologie provided by the German Cancer Research Center (DKFZ) and funded by the German Ministry for Education and Research (BMBF). J. Hernandez is the recipient of a fellowship funded by the Spanish Ministry for Education and Science (EX 94 05269499). J. Lustgarten is the recipient of an American Cancer Society fellowship. These studies were supported by National Institutes of Health Grants CA 57855 and CA 25803 to L.A. Sherman.
1 Abbreviations used in this paper: A2.1, HLA-A2.1; Hu, human; Mu, murine; Tg, transgenic; WT, wild-type.
References
- 1.Levine AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature (Lond) 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 2.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science (Wash DC) 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 3.Rogel A, Popliker M, Webb CG, Oren M. p53 cellular tumor antigen: Analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985;5:2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner J. Different forms of p53 detected by monoclonal antibodies in non-dividing and dividing lymphocytes. Nature (Lond) 1984;310:143–145. doi: 10.1038/310143a0. [DOI] [PubMed] [Google Scholar]
- 5.Terada N, Lucas JJ, Gelfand EW. Differential regulation of the tumor suppressor molecules, retinoblastoma susceptibility gene product (Rb) and p53, during cell cycle progression of normal human T cells. J Immunol. 1991;147:698–704. [PubMed] [Google Scholar]
- 6.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald HR, Schneider R, Lees RK, Howe RC, Acha-Orbea H, Festenstein H, Zinkernagel RM, Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature (Lond) 1988;332:40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 8.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 9.Sprent J, Schaefer M. Antigen-presenting cells for CD8+T cells. Immunol Rev. 1990;117:213–234. doi: 10.1111/j.1600-065x.1990.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 10.Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76:593–596. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 11.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller JFAP, Morahan G, Allison J, Hoffmann M. A transgenic approach to the study of peripheral T-cell tolerance. Immunol Rev. 1991;122:103–116. doi: 10.1111/j.1600-065x.1991.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 13.Hmmerling GJ, Schnrich G, Ferber I, Arnold B. Peripheral tolerance as a multi-step mechanism. Immunol Rev. 1993;133:93–104. doi: 10.1111/j.1600-065x.1993.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi Y, Chen Y-T, Old LJ. A mouse mutant p53 product recognized by CD4+ and CD8+T cells. Proc Natl Acad Sci USA. 1994;91:3171–3175. doi: 10.1073/pnas.91.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi Y, Richards EC, Chen Y-T, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci USA. 1995;92:2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, Storkus WJ, Appella E, DeLeo AB. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med. 1996;183:1357–1365. doi: 10.1084/jem.183.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth J, Dittmer D, Rea D, Tartaglia J, Paoletti E, Levine AJ. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor challenge. Proc Natl Acad Sci USA. 1996;93:4781–4786. doi: 10.1073/pnas.93.10.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houbiers JGA, Nijman HW, van der Burg SH, Drijfhout JW, Kenemans P, van de Velde CJ, Brand A, Momburg F, Kast WM, Melief CJM. In vitroinduction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993;23:2072–2077. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- 20.Nijman HW, Houbiers JGA, van der Burg SH, Vierboom MPM, Kenemans P, Kast WM, Melief CJM. Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immunother. 1993;14:121–126. [PubMed] [Google Scholar]
- 21.Nijman HW, van den Burg SH, Vierboom MPM, Houbiers JGA, Kast WM, Melief CJM. p53, a potential target for tumor-directed T cells. Immunol Lett. 1994;40:171–178. doi: 10.1016/0165-2478(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 22.Tilkin A-F, Lubin R, Soussi T, Lazar V, Janin N, Mathieu M-C, Lefrere I, Carlu C, Roy M, Kayibanda M, et al. Primary proliferative T cell response to wild-type p53 protein in patients with breast cancer. Eur J Immunol. 1995;25:1765–1169. doi: 10.1002/eji.1830250642. [DOI] [PubMed] [Google Scholar]
- 23.Theobald, M., J. Biggs, D. Dittmer, A.J. Levine, and L.A. Sherman. 1995. Targeting p53 as a general tumor antigen. Proc. Natl. Acad. Sci. USA. 1995. 92:11993–11997. [DOI] [PMC free article] [PubMed]
- 24.Harlow E, Williamson NM, Ralston R, Helfman DM, Adams TE. Molecular Cloning and in vitroexpression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985;5:1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman LA, Hesse SV, Irwin MJ, La D, Face, Peterson P. Selecting T cell receptors with high affinity for self-MHC by decreasing the contribution of CD8. Science (Wash DC) 1992;258:815–818. doi: 10.1126/science.1439792. [DOI] [PubMed] [Google Scholar]
- 26.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation induced apoptosis in mouse thymocytes. Nature (Lond) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 27.Irwin MJ, Heath WR, Sherman LA. Speciesrestricted interactions between CD8 and the α3 domain of class I influence the magnitude of the xenogeneic response. J Exp Med. 1989;170:1091–1101. doi: 10.1084/jem.170.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitiello A, Marchesini D, Furze J, Sherman LA, Chesnut RW. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samberg NL, Scarlett EC, Stauss HJ. The α3 domain of major histocompatibility complex class I molecules play a critical role in cytotoxic T lymphocyte stimulation. Eur J Immunol. 1989;19:2349–2354. doi: 10.1002/eji.1830191225. [DOI] [PubMed] [Google Scholar]
- 30.Kalinke U, Arnold B, Hmmerling GJ. Strong xenogeneic HLA response in transgenic mice after introducing an α3 domain into HLA B27. Nature (Lond) 1990;348:642–644. doi: 10.1038/348642a0. [DOI] [PubMed] [Google Scholar]
- 31.Engelhard VH, Lacy E, Ridge JP. Influenza A-specific, HLA-A2.1-restricted cytotoxic T lymphocytes from HLA-A2.1 transgenic mice recognize fragments of the M1 protein. J Immunol. 1991;146:1226–1232. [PubMed] [Google Scholar]
- 32.LaFace DM, Vestberg M, Yang Y, Srivastava R, DiSanto J, Flomenberg N, Brown S, Sherman LA, Peterson PA. Human CD8 transgene regulation of HLA recognition by murine T cells. J Exp Med. 1995;182:1315–1325. doi: 10.1084/jem.182.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Leahy DJ, Kavathas PB. Interaction between CD8 and major histocompatibility complex (MHC) class I mediated by multiple contact surfaces that include the α2 and α3 domains of MHC class I. J Exp Med. 1995;182:1275–1280. doi: 10.1084/jem.182.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man S, Ridge JP, Engelhard VH. Diversity and dominance among TCR recognizing HLA-A2.1+influenza matrix peptide in human MHC class I transgenic mice. J Immunol. 1994;153:4458–4467. [PubMed] [Google Scholar]
- 35.Lehner PJ, Wang ECY, Moss PAH, Williams S, Platt K, Friedman SM, Bell JI, Borysiewicz LK. Human HLA-A0201-restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the V beta 17 gene segment. J Exp Med. 1995;181:79–91. doi: 10.1084/jem.181.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM, Melief CJM, Oseroff C, Yuan L, Ruppert J, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 37.Shirai M, Arichi T, Nishioka M, Nomura T, Ikeda K, Kawanishi K, Engelhard VH, Feinstone SM, Berzofsky JA. CTL responses of HLA-A2.1-transgenic mice specific for hepatitis C viral peptides predict epitopes for CTL of humans carrying HLA-A2.1. J Immunol. 1995;154:2733–2742. [PubMed] [Google Scholar]
- 38.Wentworth PA, Vitiello A, Sidney J, Keogh E, Chesnut RW, Grey H, Sette A. Differences and similarities in the A2.1-restricted cytotoxic T cell repertoire in humans and human leukocyte antigen-transgenic mice. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- 39.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature (Lond) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 40.Benveniste P, Cohen A. p53 expression is required for thymocyte apoptosis induced by adenosine deaminase deficiency. Proc Natl Acad Sci USA. 1995;92:8373–8377. doi: 10.1073/pnas.92.18.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehme SA, Lenardo MJ. TCR-mediated death of mature T lymphocytes occurs in the absence of p53. J Immunol. 1996;156:4075–4078. [PubMed] [Google Scholar]
- 42.Dittmer D, Pati S, Zambetti G, Chu S, Teresky AK, Moore M, Finlay C, Levine AJ. Gain of function mutations in p53. Nat Genet. 1993;4:42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- 43.Masuda H, Miller C, Koeffler HP, Battifora H, Cline MJ. Rearrangements of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci USA. 1987;84:7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinds PW, Finlay CA, Quartin RS, Baker SJ, Fearon ER, Vogelstein B, Levine AJ. Mutant p53 cDNAs from human colorectal carcinomas can cooperate with ras in transformation of primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1990;1:571–580. [PubMed] [Google Scholar]
- 45.Harvey D M, Levine A J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:23752385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 46.Vitiello A, Sherman LA. Recognition of influenza-infected cells by cytolytic T lymphocyte clones: Determinant selection by class I restriction elements. J Immunol. 1983;131:1635–1640. [PubMed] [Google Scholar]
- 47.Parham P, Bodmer WF. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature (Lond) 1978;276:397–398. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 48.O'Neil BH, Kawakami Y, Restifio NP, Bennink JR, Yewdell JW, Rosenberg SA. Detection of shared MHC-restricted human melanoma antigens after vaccinia virus-mediated transduction of genes coding for HLA. J Immunol. 1993;151:1410–1418. [PMC free article] [PubMed] [Google Scholar]
- 49.Earl P L, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schild H, Rtzschke O, Kalbacher H, Rammensee HG. Limit of T cell tolerance to self proteins by peptide presentation. Science (Wash DC) 1990;247:1587–1589. doi: 10.1126/science.2321019. [DOI] [PubMed] [Google Scholar]
- 51.Nistico P, Tecce R, Giacomini P, Cavallari A, D'Agnano I, Fisher PB, Natali PG. Effect of recombinant human leukocyte, fibroblast, and immune interferons on expression of class I and II major histocompatibility complex and invariant chain in early passage human melanoma cells. Cancer Res. 1990;50:7422–7429. [PubMed] [Google Scholar]
- 52.Mortarini R, Belli F, Parmiani G, Anichini A. Cytokine-mediated modulation of HLA-class II, ICAM-1, LFA-3 and tumor-associated antigen profile of melanoma cells. Comparison with anti-proliferative activity by rIL1-β, rTNF-α, rIFN-γ, rIL4 and their combinations. Int J Cancer. 1990;45:334–341. doi: 10.1002/ijc.2910450221. [DOI] [PubMed] [Google Scholar]
- 53.Alexander MA, Damico CA, Wieties KM, Hansen TH, Connolly JM. Correlation between CD8 dependency and determinant density using peptide-induced, Ld-restricted cytotoxic T lymphocytes. J Exp Med. 1991;173:849–858. doi: 10.1084/jem.173.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heath WR, Allison J, Hoffmann MW, Schnrich G, Hmmerling GJ, Arnold B, Miller JFAP. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature (Lond) 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 55.von Herrath MG, Dockter J, Oldstone MBA. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 56.von Herrath MG, Dockter J, Nerenberg M, Gairin JE, Oldstone MBA. Thymic selection and adaptability of cytotoxic T lymphocyte responses in transgenic mice expressing a viral protein in the thymus. J Exp Med. 1994;180:1901–1910. doi: 10.1084/jem.180.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu GY, Fairchild PJ, Smith RM, Prowie JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 58.Schaeffer EB, Sette A, Johnson DL, Bekoff MC, Smith JA, Grey HM, Buus S. Relative contribution of “determinant selection” and “holes in the T-cell repertoire” to T-cell responses. Proc Natl Acad Sci USA. 1989;86:4649–4653. doi: 10.1073/pnas.86.12.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T-cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 60.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 61.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature (Lond) 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 62.Karjalainen K. High sensitivity, low affinity-paradox of T-cell receptor recognition. Curr Opin Immunol. 1994;6:9–12. doi: 10.1016/0952-7915(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 63.Speiser DE, Kyburz D, Stuebi U, Hengartner H, Zinkernagel RM. Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivoprotection. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]