Abstract
Kettin is a high molecular mass protein of insect muscle that in the sarcomeres binds to actin and α-actinin. To investigate kettin's functional role, we combined immunolabeling experiments with mechanical and biochemical studies on indirect flight muscle (IFM) myofibrils of Drosophila melanogaster. Micrographs of stretched IFM sarcomeres labeled with kettin antibodies revealed staining of the Z-disc periphery. After extraction of the kettin-associated actin, the A-band edges were also stained. In contrast, the staining pattern of projectin, another IFM–I-band protein, was not altered by actin removal. Force measurements were performed on single IFM myofibrils to establish the passive length-tension relationship and record passive stiffness. Stiffness decreased within seconds during gelsolin incubation and to a similar degree upon kettin digestion with μ-calpain. Immunoblotting demonstrated the presence of kettin isoforms in normal Drosophila IFM myofibrils and in myofibrils from an actin-null mutant. Dotblot analysis revealed binding of COOH-terminal kettin domains to myosin. We conclude that kettin is attached not only to actin but also to the end of the thick filament. Kettin along with projectin may constitute the elastic filament system of insect IFM and determine the muscle's high stiffness necessary for stretch activation. Possibly, the two proteins modulate myofibrillar stiffness by expressing different size isoforms.
Keywords: connecting filament; titin; muscle mechanics; projectin; PEVK sequence
Introduction
Many insects use a special type of muscle, the indirect flight muscle (IFM),* which is characterized by high resting (or passive) stiffness and stretch activation, a delayed active force response to stretch (Pringle, 1967; Rüegg, 1968; Maughan and Vigoreaux, 1999; Josephson et al., 2000). The stiffness of nonactivated IFM resides in the contractile units, the sarcomeres, and is thought to arise mainly from connecting filaments linking thick filaments to Z-discs (Garamvölgyi, 1966; Trombitas and Tigyi-Sebes, 1974; White, 1983). More recently, two proteins present in the actin-myosin overlap zone, flightin (Vigoreaux et al., 1993; Reedy et al., 2000) and troponin H (Reedy et al., 1994; Clayton et al., 1998), have also been suggested to potentially contribute to passive stiffness. During active contraction of IFM, passive stiffness persists in parallel with actomyosin crossbridge stiffness (White, 1983). Therefore, it is conceivable that strain on the connecting filament affects crossbridge activity and thus enhances the muscle's mechanical performance (Vigoreaux et al., 2000). The nature of connecting filaments is not entirely clear yet, although a main constituent is projectin, an ∼900-kD polypeptide of the titin-like protein family (Bullard et al., 1977; Saide, 1981; Ayme-Southgate et al., 1991; Fyrberg et al., 1992; Benian et al., 1999). In the IFM of Drosophila, projectin was shown to be anchored at the Z-disc and associate with myosin at the A-band edge (Saide et al., 1989).
Another high molecular weight protein in the I-band of Drosophila and Lethocerus sarcomeres is kettin (Lakey et al., 1990, 1993). The kettin sequence predicts a modular structure, with 35 repeating Ig domains separated by short linker sequences (Hakeda et al., 2000; Kolmerer et al., 2000). In contrast to other titin-like proteins, the published kettin sequence (which shows an ∼500-kD polypeptide) contains no fibronectin-like modules, kinase, or PEVK domains. Kettin binds to actin (and α-actinin) with high affinity and a stoichiometry of one Ig linker module to one actin protomer, suggesting that the protein contributes to thin filament stability and anchorage in the Z-disc (van Straaten et al., 1999). Kettin may have an essential function for sarcomere formation because adult fruit flies heterozygous for a kettin mutation cannot fly (Hakeda et al., 2000). Although many members of the titin-like protein family, including projectin and titin, are known for their elasticity, kettin's tight association with actin and lack of myosin-binding propensity, according to an early report (Lakey et al., 1993), made it seem less likely that this protein is also functionally elastic. The work reported here now suggests that kettin does function as an elastic filament in addition to the projectin filaments.
Drosophila kettin could be part of a larger protein, D-titin (Machado et al., 1998; Zhang et al., 2000). The D-titin gene encodes a 1.8-MD polypeptide with homology to the NH2-terminal half of vertebrate titin (Labeit and Kolmerer, 1995). The kettin and D-titin genes are located at the same chromosome position, and the 5′ end of the D-titin genomic sequence contains the sequence of Drosophila kettin (Machado and Andrew, 2000). Thus, the possibility exists that kettin is a cleavage product of D-titin. However, kettin is expressed in several isoforms of different molecular mass. An abundant ∼500-kD variant and a rare ∼700-kD isoform are found in Drosophila IFM (Lakey et al., 1993). Although the precise relationship between kettin and D-titin needs to be established, it is likely that kettin derives from an alternative splice form of D-titin. Alternative splicing has been confirmed for Drosophila projectin (Daley et al., 1998) and vertebrate titin (Labeit and Kolmerer, 1995). In mammals, different titin isoforms are expressed in a muscle type-specific manner. Vertebrate cardiac titin occurs in many size variants that coexist in the same cell (Freiburg et al., 2000). The functional relevance of the titin isoform diversity may lie in a modulation of myofibrillar passive stiffness.
If kettin exists in different isoforms, it could assume roles extending beyond those ascribed to the protein so far. For instance, if kettin connected actin and myosin, just as titin does in vertebrate-muscle sarcomeres, it could act as an elastic filament and contribute to passive stiffness. In the present study, we combined cell biological and biochemical approaches with single myofibril mechanics to elucidate the molecular basis of Drosophila IFM stiffness. The results show that kettin indeed provides a link between thin and thick filaments. A model emerges in which both kettin and projectin are responsible for the high stiffness of relaxed insect IFM necessary to develop stretch activation.
Results
Passive tension of single Drosophila IFM myofibrils
To characterize the sarcomere length (SL)-tension relationship of nonactivated Drosophila IFM, force measurements were performed on single myofibrils (Fig. 1) . The force of three to four myofibrils of similar length was recorded in identical step-stretch protocols, and median-filtered force traces were superimposed to obtain clearer signals (Fig. 1 B). A summary of stress-strain curves obtained from 24 myofibrils is shown in Fig. 1 C. Because stretched myofibrils showed inhomogeneous SLs, Fig. 1 C depicts passive tension related either to the length of the longest sarcomere (solid line) or to mean SL (dotted line). At larger stretches, the difference between the two curves was significant. Passive tension rose steeply on low stretch and reached a first plateau after ∼5% extension. Additional stretch further increased force until at ∼12% extension a second plateau phase (or some force decline) commenced. Higher stretches frequently led to myofibril breakage. Upon release of myofibrils from the stretched SL, large force hysteresis was seen.
Figure 1.
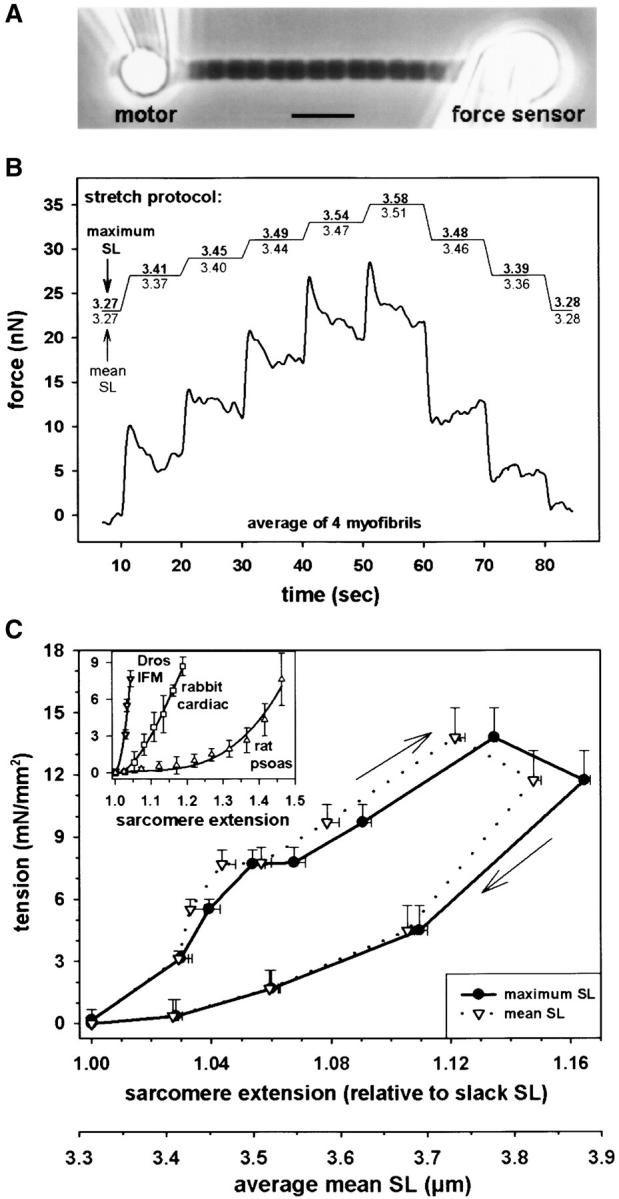
Passive force measurements on single Drosophila IFM myofibrils. (A) Phase–contrast image of myofibril suspended between glue-coated needles. (B) Stretch protocol and averaged force response of four myofibrils. Maximum SL refers to the length of the longest sarcomere, and mean SL refers to an average of all sarcomeres. (C) Passive tension-SL relationship. Data represent quasi steady-state tension (mean ± SEM; n = 24 myofibrils). Average slack SL was 3.32 μm. (Inset) Comparison of stress-strain curves of Drosophila IFM and mammalian myofibrils (Linke, 2000). Bar, 5 μm.
The passive SL-tension curve of single Drosophila IFM myofibrils was compared with that of vertebrate cardiac and skeletal myofibrils established previously (Linke, 2000). Fig. 1 C, inset, demonstrates that Drosophila IFM has by far the steepest stress-strain curve; the stiffness (approximated linear slope at modest extension) is about one order of magnitude higher than in rabbit cardiac myofibrils. Much lower stiffness is found in rat psoas myofibrils.
Stretch-dependent behavior of I-band proteins in normal IFM myofibrils
Kettin
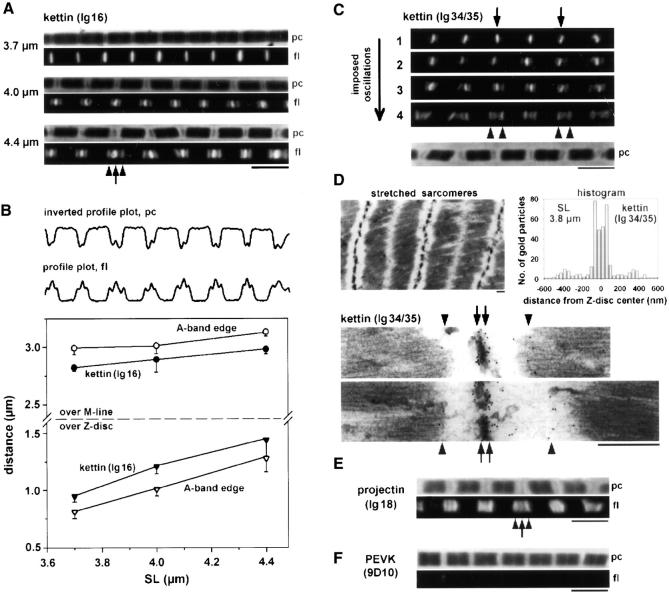
The position of kettin in relaxed Drosophila IFM sarcomeres was analyzed by immunofluorescence (IF) and immunoelectron microscopy (IEM). Two different antibodies were used, one against the central part (Ig16) and the other against the COOH terminus (Ig34/35) of the published kettin sequence (Fig. 2 A). By IF, kettin epitopes appeared as a single stripe in the Z-disc region of sarcomeres independent of the stretch state (Fig. 2, B and C). IEM showed that α-Ig34/35 labels both nonstretched and modestly stretched sarcomeres at the periphery of the Z-disc (Fig. 2 D). In extremely stretched myofibrils, some rearrangement of kettin could be seen: IF with α-Ig16 or α-Ig34/35 revealed a “smear” near some Z-discs (Fig. 2, B and C, arrows).
Figure 2.
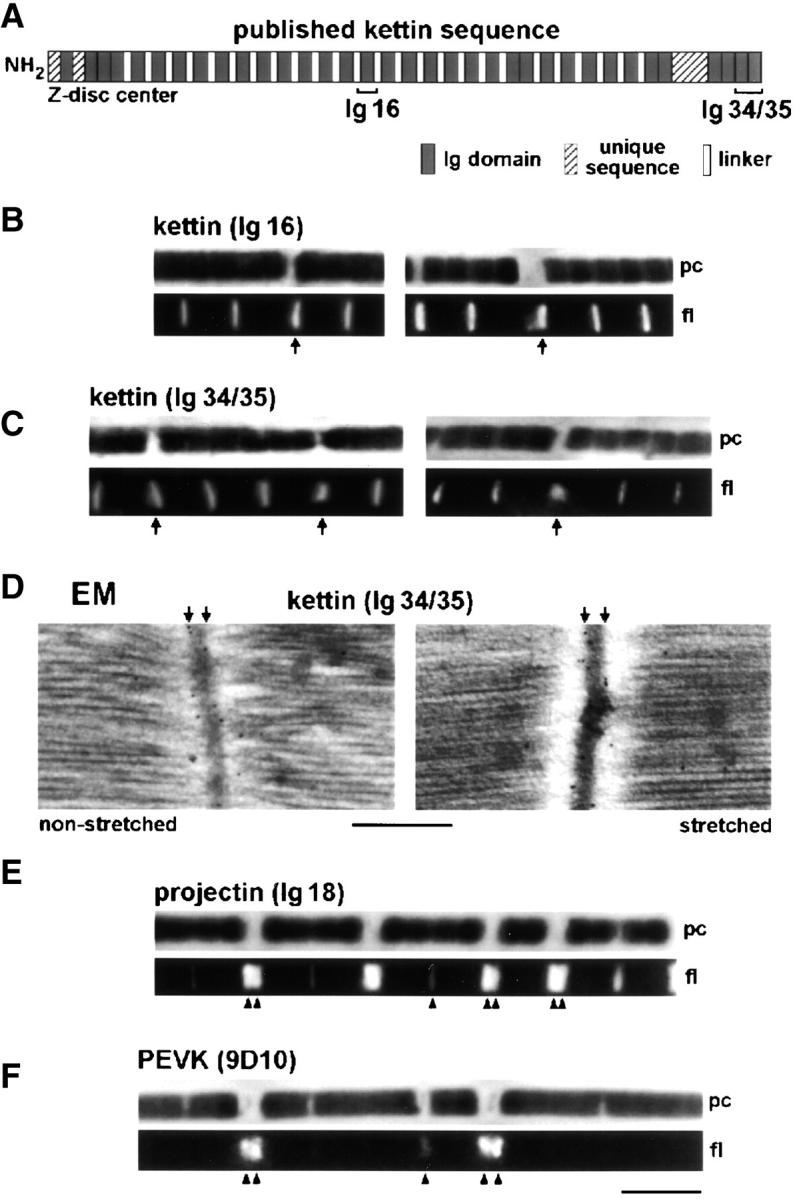
Immunolabeling of relaxed Drosophila IFM sarcomeres with various antibodies. (A) Domain structure of kettin (Kolmerer et al., 2000) and position of kettin antibodies used. (B and C) IF microscopy of stretched single myofibrils stained with kettin antibodies and Cy-3–conjugated IgG. (D) IEM of sarcomeres at rest length and after small stretch, stained with α-kettin Ig34/35. (E and F) IF of stretched single myofibrils stained with α-projectin (E) and α-PEVK antibodies (F). pc, phase–contrast image; fl, fluorescence image. Bars: (D) 0.5 μm; (F, IF images) 5 μm.
Projectin
We also investigated the staining pattern of antibodies to projectin (Ig18). Antiprojectin labeled the Z-discs in short sarcomeres but fuzzy bands at the A-band edges in stretched sarcomeres (Fig. 2 E).
PEVK-sequence
Antibodies (9D10) to the PEVK region, a unique titin sequence of obscure secondary/tertiary structure, did not stain unstretched myofibrils but labeled the Z-disc region in modestly stretched sarcomeres and two distinct epitopes near the Z-disc in long sarcomeres (Fig. 2 F). Analysis of the intensity profiles (Linke et al., 1999) revealed that in stretched myofibrils the PEVK epitopes are located in the I-band.
Damage to Drosophila IFM sarcomeres upon extension
Low extensibility and stretch-induced high SL inhomogeneity were consistently observed in relaxed Drosophila IFM myofibrils. Somewhat surprisingly, when nonactivated myofibrils were stretched by ∼10% from slack SL and stained with rhodamine-phalloidin, actin always appeared to be broken at the Z-disc (Fig. 3 A); it seemed impossible to pull the actin filaments out of the A-band. Myofilament breakage at the Z-disc could be observed also on electron micrographs of stretched IFM sarcomeres (Fig. 3 B). In addition, actin-filament breakage was seen in stretched myofibrils stained with α-actin antibodies (unpublished data). A possible explanation of this result is that some actin-myosin connections remained in the A-band, despite the presence of 20 mM BDM (an active force-suppressing agent) in the relaxing buffer. Alternatively, some additional structure(s) could link actin and myosin filaments thereby stiffening the sarcomere.
Figure 3.
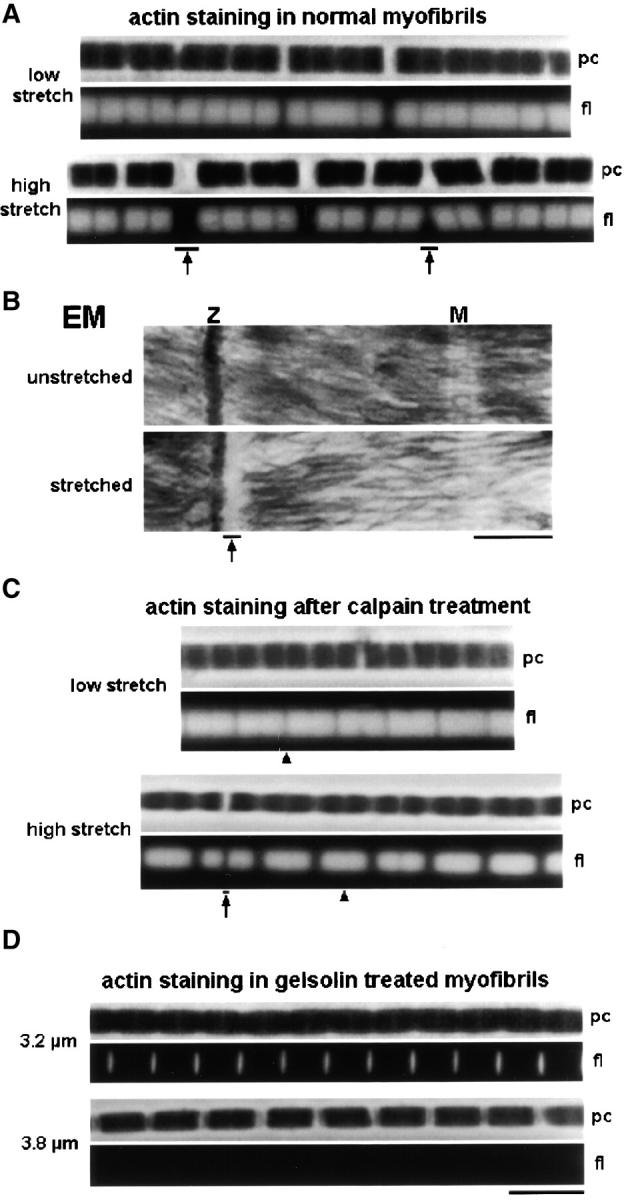
Actin filaments in stretched Drosophila IFM sarcomeres. (A) Single myofibrils stained with rhodamine-phalloidin. Note actin filament breakage at Z-discs (arrows). (B) Electron micrographs of IFM sarcomeres. Myofilament breakage at the Z-disc (Z) was observed at modest stretch (arrow). M, M-line; (c) Actin staining of stretched single myofibrils after digestion with μ-calpain. Actin filaments of opposing half sarcomeres usually remained connected at the Z-disc (arrowheads); actin was seen rarely to be broken (arrow). (D) Actin staining of sarcomeres after extraction with 0.2–0.3 mg/ml (top) and >0.3 mg/ml (bottom) gelsolin fragment. Bars: (B) 0.5 μm; (all IF images) 5 μm.
Proteins potentially involved in the passive stiffening were extracted from IFM myofibrils by various procedures. Actin was removed with a Ca2+-independent gelsolin fragment, which extracted all but the Z-disc actin when used at concentrations of 0.2–0.3 mg/ml (Fig. 3 D, top). At concentrations >0.3 mg/ml, Z-disc actin was removed also (Fig. 3 D, bottom). Even after complete actin extraction, myofibrils did not disintegrate; rather, they could be stretched highly while showing reasonable SL homogeneity (Fig. 3 D and Fig. 4) . Removal of actin allowed the myofibrils to be extended reversibly over a wider SL range.
Figure 4.
Actin-extracted, stretched, Drosophila IFM myofibrils. (A) Images of single myofibrils at different SLs stained with α-kettin Ig16. Both Z-discs (arrow) and A-band edges (arrowheads) are labeled. (B) Examples of intensity profiles and graph showing the SL-dependent spacing (mean ± SD; n = 22) of kettin-Ig16 epitopes at the A-band edge measured across the M-line (•) or Z-disc (▴). For comparison, the distance between A-band edges is also shown (○ and ▵). (C) Sequence of fluorescence images of a single myofibril at ∼4.0 μm SL stained with α-Ig34/35. Upon exposure of the myofibril to 20 Hz sinusoidal length oscillations, the intensity of Z-disc epitopes gradually decreased (arrows), whereas that of epitopes at the A-band edge increased (arrowheads). (D) IEM of actin-extracted fibers stained with α-Ig34/35. A larger area is shown in the top left image. Images at bottom depict sarcomeres at two different degrees of stretch: ∼3.8 and ∼4.2 μm SL. Nanogold particles indicate the position of kettin epitopes at the Z-disc periphery (arrows) and A-band edge (arrowheads). The histogram shows the nanogold particle distance from the center of the Z-disc measured in ∼3.8-μm-long sarcomeres. A major peak is at ∼60 nm, a minor peak at 360–400 nm, out from the Z-disc center. (E) IF of single myofibril stained with α-projectin antibody. The A-band edge is stained strongly (arrowheads) and the Z-disc faintly (arrow). (F) α-PEVK antibody did not stain actin-extracted myofibrils. Bars: (D) 0.5 μm; (all IF images) 5 μm.
Myosin was extracted by application of a buffer containing 400 mM KCl. Within seconds of application, thick filaments became depolymerized and sarcomeres broke apart at the M-line (unpublished data). The remaining myofibril fragments (sometimes only one or a few half sarcomeres long) were so fragile that they could not be used for stretch experiments.
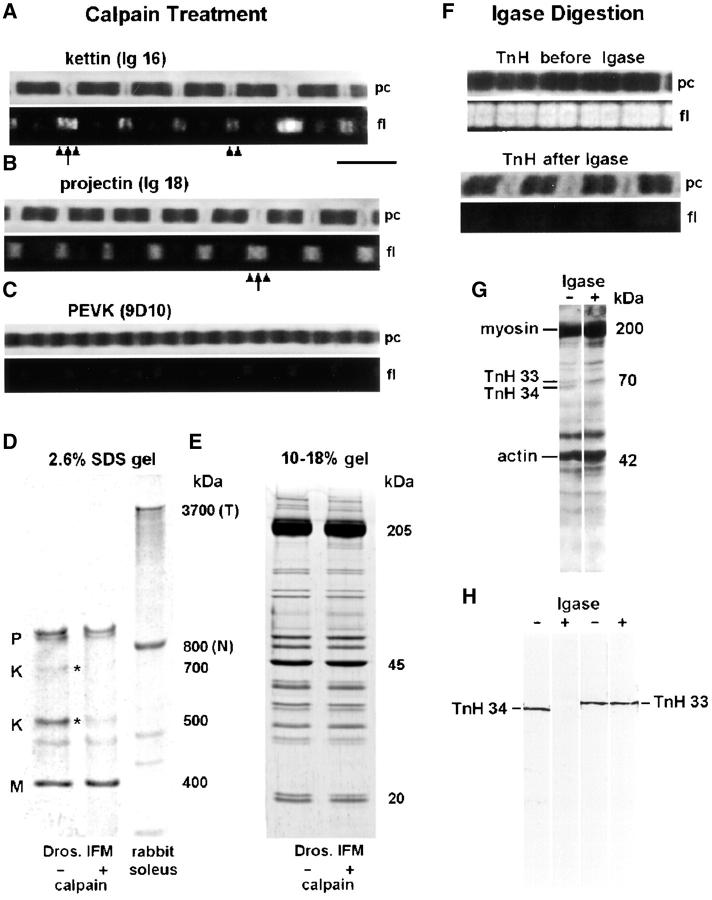
A Ca2+-dependent protease, μ-calpain (3 μg/ml), selectively digests kettin in Drosophila IFM sarcomeres (Fig. 5, D and E) . After treatment of IFM myofibrils with μ-calpain, SL homogeneity upon stretch in relaxing buffer was much improved compared with control myofibrils (Fig. 3 C). More importantly, rhodamine-phalloidin staining revealed that the actin filaments could now be pulled out of the A-bands. Only rarely did actin still break at the Z-disc (Fig. 3 C, arrow); usually, the actin filaments of two adjacent half sarcomeres remained connected at the Z-band (Fig. 3 C, arrowhead at bottom). Thus, cleavage of kettin removed the elements that normally prevent Drosophila IFM sarcomeres from tolerating larger stretches. The leftover sarcomere structure might be held together by the remaining projectin molecules. (Projectin is cleaved only slightly at the μ-calpain concentration of 3 μg/ml chosen [Fig. 5 D].)
Figure 5.
Effect of treatment with μ-calpain (A–E) and Igase (F–H) on Drosophila IFM myofibrils. (A) IF of single myofibril stained with α-kettin Ig16 after calpain treatment and stretch; the A-band edge is labeled (arrowheads), and the Z-disc is labeled also in some sarcomeres (arrow). (B) α-Projectin staining of stretched sarcomeres showed a fuzzy epitope at the A-band edge (arrowheads) and also Z-disc labeling (arrow). (C) PEVK sequence was not stained by 9D10 antibody in calpain-treated, stretched, myofibrils. (D) Low percentage SDS-gel to show the effect of μ-calpain (3 μg/ml, 25°C, 45 min) on high molecular weight proteins. Only kettin (K) isoforms are substantially digested, whereas projectin (P) and M-line protein (M) remain largely intact. Nebulin (N) and titin (T) from rabbit soleus muscle are used as standards. (E) Lower molecular weight proteins are not affected by μ-calpain treatment as detectable on 10–18% SDS-gradient gels. (F) Igase-mediated digestion of troponin H effectively decreased the staining intensity of α-TnH34 antibodies (MAC 143) on myofibrils. (G) 12% SDS-gel electrophoresis of washed IFM myofibrils to separate TnH33 and TnH34 isoforms. Igase treatment eliminated the TnH34 isoform. (H) Western blot with antibodies to either TnH33 or TnH34 confirms that Igase treatment removed TnH34 but left TnH33 intact.
Stretch-dependent kettin epitope position in actin-extracted IFM myofibrils
The epitope positions of kettin antibodies were investigated in single Drosophila IFM myofibrils from which actin was completely extracted (Fig. 4). Actin removal was confirmed at the end of each experiment by rhoda-mine-phalloidin staining. Interestingly, actin-free sarcomeres showed a kettin labeling pattern much different from that of nonextracted sarcomeres (Fig. 2, B–D). Whereas α-kettin Ig16 was still seen by IF to stain the Z-disc region (Fig. 4 A, arrow), stretched myofibrils exhibited two additional stripes per sarcomere (Fig. 4 A, arrowheads). These stripes were located on either side of the Z-disc and separated during myofibril stretch but moved toward one another (unpublished data) during release. The segment flanking the Z-disc and the Ig16 kettin epitope thus showed reversible extensibility.
The stretch-dependent position of kettin Ig16 epitopes was measured from intensity profiles recorded along the myofibril axis (Fig. 4 B, bottom trace) by using a custom-written peak detection program (Linke et al., 1999). Also, the A-band edges were detected on corresponding phase images (Fig. 4 B, top trace) to obtain the position of “out-of-Z-disc” kettin epitopes in the sarcomere. The graph in Fig. 4 B shows the SL-dependent separation of Ig16 kettin epitopes compared with that of A-band edges measured across the M-line (top) or the Z-disc (bottom). The graph (a) confirms that the Ig16 kettin position varies with the stretch state, (b) indicates that the length of the A-band (normally ∼3.0 μm) increases upon extreme stretch by ∼0.15 μm (Trombitas and Tigyi-Sebes, 1977), and (c) demonstrates that the Ig16 kettin epitopes are positioned somewhat inside the A-band.
A similar staining pattern was seen with α-kettin Ig34/35. An example is shown in Fig. 4 C. This actin-extracted stretched myofibril initially exhibited strong Z-disc staining but faint labeling of the A-band edges. However, when the myofibril (held at constant SL) was exposed to small amplitude sinusoidal length oscillations (20 Hz), the fluorescence intensity of the Z-disc epitopes decreased with time (Fig. 4 C, arrows), whereas that of Ig34/35 epitopes at the A-band edge increased strongly (Fig. 4 C, arrowheads). These findings clearly show that the Ig34/35 kettin epitopes are connected to both the Z-disc and the A-band edge.
IEM was employed to investigate Ig34/35 kettin staining in actin-extracted Drosophila IFM fibers. Fig. 4 D demonstrates that in contrast to nonextracted fibers (unpublished data) sarcomeres could be readily stretched to reveal a wide I-band region. After gelsolin treatment and high stretch, the specimens showed lower structural preservation than normal IFM sarcomeres (Fig. 2 D). The IEM measurements on actin-extracted fibers again showed that α-Ig34/35 labels both the periphery of the Z-disc (Fig. 4 D, arrows) and the A-band edges (Fig. 4 D, arrowheads). Additional confirmation comes from a histogram showing the distribution of nanogold particles (indicating α-Ig34/35 binding) in the I-Z-I region of ∼3.8-μm-long sarcomeres (Fig. 4 D, graph).
For comparison, IF images were recorded from Drosophila IFM myofibrils that had been actin extracted, stretched, and then stained with either α-projectin (Ig18) or α-PEVK (9D10) antibodies. The projectin staining pattern was similar to that of nonextracted sarcomeres (Fig. 2 E): fuzzy epitopes appeared near the A-band edge and faintly at the Z-disc (Fig. 4 E). In contrast, α-PEVK 9D10 failed to label myofibrils even at high stretch (Fig. 4 F). Apparently, some structure connecting the PEVK site to the Z-disc had been damaged or removed by the actin extraction procedure.
Digestion of sarcomere proteins: effect on staining pattern and myofibril stiffness
Exposure of IFM myofibrils to μ-calpain (3 μg/ml) digested both the 500 and 700 kD kettin isoforms to a large degree (Fig. 5 D). Projectin was barely affected, whereas no effect was seen on low molecular weight sarcomere proteins (Fig. 5 E). Single Drosophila IFM myofibrils were treated with 3 μg/ml μ-calpain, stretched, and investigated by IF for their reactivity to α-kettin Ig16, α-projectin Ig18, and α-PEVK 9D10 antibodies (Fig. 5, A–C). Antikettin Ig16 stained the Z-disc in a few sarcomeres but a distinct stripe at the A-band edge in all sarcomeres (Fig. 5 A). Antiprojectin Ig18 antibody stained broader stripes at the A-band edge and the Z-disc (Fig. 5 B). Thus, projectin labeling was not much different than in normal or actin-extracted myofibrils. Anti-PEVK 9D10 antibody did not label stretched myofibrils in which kettin had been cleaved (Fig. 5 C), resembling the result obtained after actin removal.
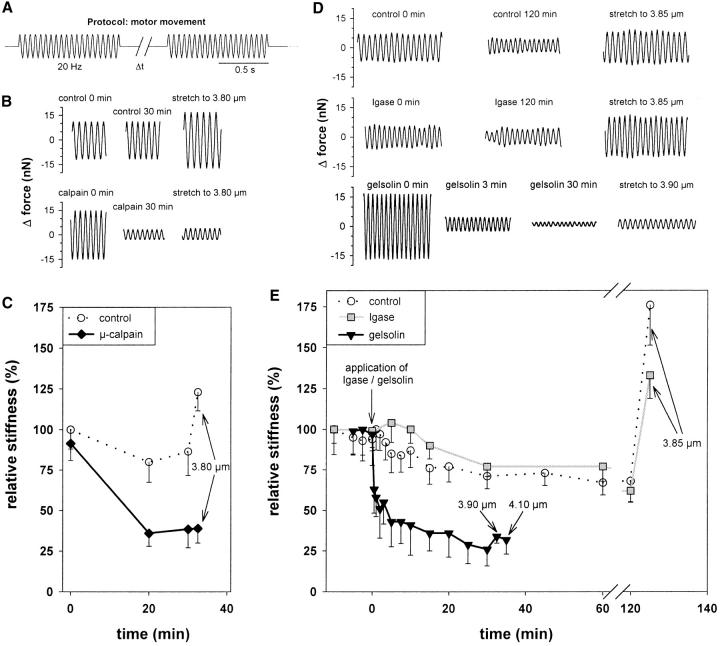
To investigate how μ-calpain and gelsolin may affect passive stiffness, we measured the force response of single nonactivated Drosophila IFM myofibrils to sinusoidal motor movement (Fig. 6 A). Calpain-treated myofibrils were stretched in relaxing buffer to 3.7 μm SL, and stiffness was recorded immediately after the stretch and after a waiting period of 20 and 30 min, respectively (Fig. 6 B). Fig. 6 C shows that stiffness of μ-calpain–treated myofibrils dropped by 50–60% within 20–30 min, whereas that of control specimens decreased only by ∼20% (normal stress relaxation). In contrast to controls, digested myofibrils exhibited no stiffness increase upon further stretch. Thus, cleavage of kettin significantly depresses myofibril stiffness. Whereas passive stiffness was recorded after μ-calpain digestion (because digestion requires Ca2+), it was measured during treatment with gelsolin fragment in relaxing buffer (Fig. 6 D, bottom panels). As shown in Fig. 6 E (black solid curve), stiffness decreased greatly within the first minute of gelsolin application. This initial stiffness drop could be as large as 50%. Then, stiffness decreased only a little and after 20–30 min reached values comparable to those measured in calpain-treated myofibrils (Fig. 6, C and E). Further stretch of actin-extracted specimens failed to increase stiffness appreciably.
Figure 6.
Stiffness measurements on single Drosophila IFM myofibrils. (A) Protocol: 20 Hz sinusoidal oscillations were applied for 1–2 s; the rest interval (Δt) was 1–20 min. (B) Examples of oscillatory force response of control specimens and calpain-treated myofibrils. (C) Stiffness (mean ± SD; n = 3) versus time after calpain treatment (solid line) in comparison with control stiffness (dotted line). SL was 3.7 μm except after 30 min when myofibrils were stretched to 3.8 μm. (D) Examples of force response of control specimens, igase-digested, and gelsolin-treated single myofibrils. (E) Stiffness (mean ± SD; n = 3) versus time in control myofibrils (dotted curve), during igase digestion (gray solid curve), and during actin extraction (black solid curve). SL before the stretch at the end of the experimental protocol was 3.7 μm.
Part of the IFM stiffness could potentially be due to cross-linking of actin and myosin by a thin filament-associated protein, troponin H, which exists in two isoforms, TnH33 and TnH34. To test whether some stiffening can be ascribed to TnH, we tried to digest the protein with Igase (Clayton et al., 1998). Digestion was monitored by IF in myofibrils stained with α-TnH34 antibody on SDS-gels and Western blots (Fig. 5, F–H). The fluorescence intensity of α-TnH34–stained myofibrils disappeared almost entirely upon Igase treatment (Fig. 5 F). Western blots revealed that TnH33 was little affected by Igase, whereas TnH34 was digested completely (Fig. 5 H). Since both isoforms are normally present in more or less equal amounts (Fig. 5 G), we assumed that digestion of one isoform should significantly decrease stiffness if these proteins played some role for myofibril stiffening. However, Fig. 6 E shows that stiffness recorded over a 2-h period of Igase treatment (gray solid curve) remained at a high level not significantly different from that of control myofibrils (dotted curve). Thus, TnH34 is unlikely to contribute much to the passive stiffness of Drosophila IFM.
Protein composition of insect muscles: in search of large proteins
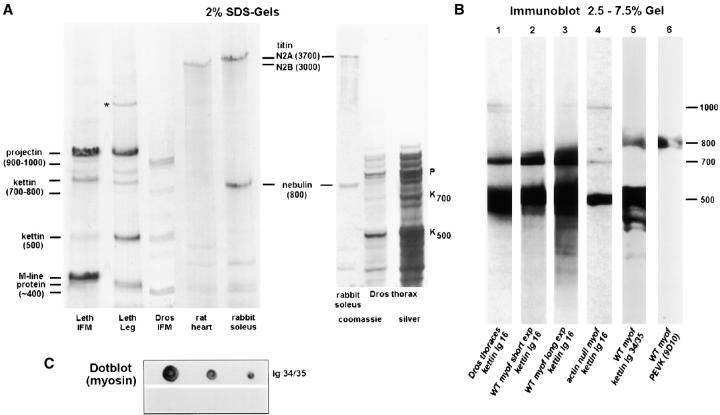
In a more systematic effort to detect high molecular mass proteins, 2% SDS-PAGE was carried out on myofibrils from both Drosophila and Lethocerus IFM and Lethocerus leg muscle (Fig. 7 A). Vertebrate titin and nebulin served as size markers. The analysis confirmed that all insect muscle types contain various large proteins (Fig. 7 A, left). In Drosophila IFM, stronger protein bands likely correspond to 400-kD M-line protein, 500-kD kettin, and ∼900-kD projectin (Fig. 7 A, lane 3). A faint band appearing at ∼700 kD may correspond to another kettin isoform (see next paragraph). The possibility exists that larger titin-like proteins were not visualized because of degradation during myofibril preparation. (The ORFs from the genome sequence of D-titin predict that the maximum size of the protein is 1.8 MD [Machado and Andrew, 2000; Zhang et al., 2000].) Therefore, Drosophila thoraces were frozen in liquid nitrogen immediately after dissection and were also tested for their protein composition (Fig. 7 A, right). No protein larger than ∼1,100–1,200 kD was detectable, even on SDS-gels stained with silver to improve sensitivity. Thus, Drosophila flight muscles, which make up a majority of the thoracic muscles, may not express a protein of 1.8-MD size corresponding to a full-length D-titin. Lethocerus leg muscle has a protein of high molecular mass not present in the IFM.
Figure 7.
Isoforms of large insect muscle proteins studied by SDS-PAGE and immunoblotting and dotblot to probe kettin-myosin binding. (A) Coomassie-stained 2% SDS-gels to separate projectin and kettin of Lethocerus IFM/leg muscle or Drosophila IFM myofibrils. Drosophila thoraces frozen in liquid nitrogen immediately after dissection were also included in the analysis (right two lanes; right lane is silver stain). For size comparison and to construct a calibration curve for high molecular weight proteins, large rat cardiac and rabbit psoas proteins were used. P, projectin; K, kettin; *unidentified band. (B) Immunoblots. Proteins in Drosophila thoraces (lane 1) or washed IFM myofibrils (lanes 2–6) were separated on 2.5–7.5 SDS-gradient gels. Lane 4 was run with IFM myofibrils from the Drosophila actin-null mutant, KM88. Immunoblots in lanes 1–4 were incubated with α-kettin Ig16; lane 3 is a longer exposure of lane 2, whereas lanes 3 and 4 were exposed for the same time to compare relative amounts of 500-kD kettin in wild-type and actin-null. Lane 5 was incubated with α-kettin Ig34/35 and lane 6 with 9D10 antibody to PEVK. Molecular masses are estimated relative to kettin (500 kD) and projectin (900 kD). (C) Dotblot to show binding of expressed kettin Ig34/35 domains to myosin. Upper strip, dots of 2.0, 1.0, and 0.5 μg of Lethocerus myosin incubated in Ig34/35 followed by anti-His and second antibody; lower strip, myosin dots incubated with anti-His and second antibody only.
Kettin binds to thick filaments
If kettin were important for myofibril stiffness, the protein should bind not only to thin filaments (van Straaten et al., 1999) but also directly or indirectly to thick filaments. We therefore probed kettin for a possible interaction with thick filaments. Immunoblots with α-kettin Ig16 on Drosophila IFM myofibrils or whole thoraces demonstrated the presence of two major isoforms of 500- and 700-kD size (Fig. 7 B, lanes 1–3). In addition, two lesser species estimated to be ∼1,000 kD were identified (Fig. 7 B, lane 1). We then reasoned that myofibrils from a Drosophila actin-null mutant, Act88FKM88, which contains neither thin filaments nor Z-discs in IFM, should not react with kettin antibodies unless kettin is associated with the thick filaments. (Thin filament proteins, for example, troponin and tropomyosin, are not accumulated in actin-null myofibrils [Clayton et al., 1998].) Fig. 7 B, lane 4, demonstrates that α-kettin Ig16 did label the actin-null mutant, detecting the abundant 500-kD form and the 700- and the ∼1,000-kD variants. Further, isoform composition is altered in the actin-null species: KM88 IFM has less 700-kD and more 1,000-kD isoform compared with wild-type IFM (Fig. 7 B, lanes 2 and 4). The presence of kettin in actin-null mutant is consistent with attachment of kettin to thick filaments.
Western blots with normal IFM myofibrils were also prepared using α-kettin Ig34/35 and α-PEVK antibodies. α-kettin Ig34/35 detected the 500-kD isoform but failed to react with the 700-kD variant; instead, an ∼800-kD isoform was labeled (Fig. 7 B, lane 5). The 800-kD isoform was detected by α-PEVK, but this antibody did not react with the 500- and 700-kD variants (Fig. 7 B, lane 6). These findings strongly support the idea that Drosophila IFM myofibrils contain several kettin isoforms arising from alternative splicing.
To more directly test for kettin-myosin binding, a dotblot method was used (Fig. 7 C). When IFM myosin was incubated with kettin fragment Ig34/35 (followed by anti-His and secondary antibody), a signal was detected whose intensity depended on the myosin concentration. Incubation with only anti-His and secondary antibody revealed no signal. Thus, the COOH terminus of the published kettin sequence may interact with myosin filaments.
Discussion
Connecting filaments were clearly identified in insect flight muscles in the late 1960s and early 1970s (Garamvölgyi, 1966; Pringle, 1967; Reedy, 1971; Trombitas and Tigyi-Sebes, 1974; White, 1983), but the protein(s) constituting these structures at first remained unknown. With the discovery of projectin, a large polypeptide in the I-band of IFM sarcomeres (Bullard et al., 1977; Saide, 1981; Ayme-Southgate et al., 1991), the protein making up part or all of the connecting filament structure was found. In this study, we provide evidence that the elastic filament system of Drosophila IFM sarcomeres also contains kettin as a major constituent.
Partial sequence analysis showed that kettin is composed of Ig domains interspersed with short unique sequences (Lakey et al., 1993). Kettin was found to bind to both α-actinin and actin in the center and at the periphery of the Z-disc (Lakey et al., 1993; van Straaten et al., 1999). Association with thick filaments initially was considered unlikely because an Ig domain from the middle part of kettin did not interact with myosin in vitro (Lakey et al., 1993). When more sequence information on kettin became available (Hakeda et al., 2000; Kolmerer et al., 2000), the presence of additional Ig domains at the protein's COOH terminus was revealed. Currently, it is unresolved whether the published kettin sequence could be part of a larger protein, D-titin, in Drosophila muscles (Hakeda et al., 2000; Kolmerer et al., 2000; Machado and Andrew, 2000; Zhang et al., 2000). Possibly, kettin is a splice form of D-titin. We showed that, at least in Drosophila thoraces D-titin is not expressed as a 1,800-kD protein, the maximum size expected from the genome sequence data (Fig. 7 A). Support for the idea that kettin exists in isoforms of ≤1,000 kD now comes from our results on the reactivity of kettin-specific antibodies with different molecular size bands on Western blots (Fig. 7 B).
With kettin's NH2 terminus known to be positioned within the Z-disc (Kolmerer et al., 2000), we hypothesized that the protein may span the half I-band of an IFM sarcomere and at the COOH terminus be attached to thick filaments. If kettin indeed connected Z-discs and thick filament ends, it could act in parallel with projectin to contribute to passive stiffness. Therefore, we investigated whether stiffness is affected by removal of one anchorage point of kettin, the actin filaments near the Z-disc. We also studied the effect of proteolytic digestion of kettin on myofibrillar stiffness. Along the way, we established the passive SL-tension relationship of single Drosophila IFM myofibrils. The combined results of mechanical, IF, and IEM measurements, using antibodies to kettin, projectin, and PEVK-titin, led us to propose a model as to how kettin may function in the I-band under the different experimental conditions. This model, shown in Fig. 8 , also considers the presence of different size kettin isoforms.
Figure 8.
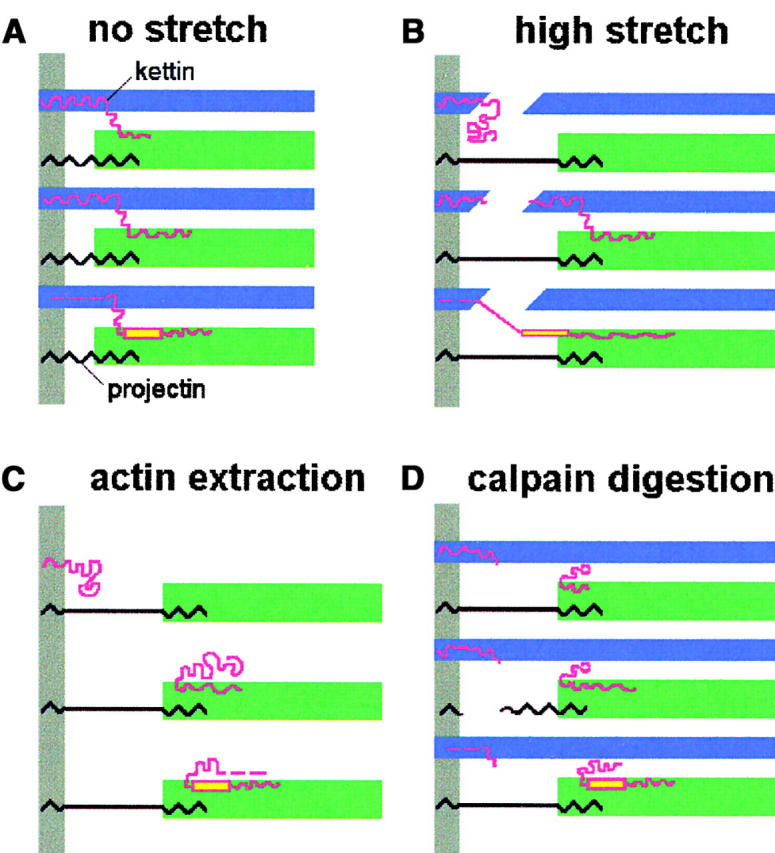
Model of the arrangement of kettin (red), projectin (black), actin (blue), and myosin (green) in Drosophila IFM sarcomeres. The model attempts to explain this study's results obtained under the respective experimental conditions indicated above each panel. Kettin is drawn in different length variants to take into account the presence of 500-, 700-, and 800-kD isoforms (from top to bottom in each panel). Yellow color indicates PEVK domain (position of PEVK domain was tentatively induced from the genome sequence of D-titin; Machado and Andrew, 2000; Zhang et al., 2000). Gray bars indicate Z-disk. For further explanation, see text.
The scheme of Fig. 8 A depicts kettin in normal unstretched sarcomeres where the protein may provide a short link from the actin filaments over to the A-band edge. The kettin thick filament interaction involves myosin (Fig. 7 C) but could include other thick filament-associated proteins also. For example, one could speculate that some kettin domains also bind to projectin at the A-band edge. Such ternary complexes might have their structural counterparts in the blob-like structures just outside the A-bands frequently seen on high resolution electron micrographs of insect IFM (Reedy et al., 1988; Trombitas, 2000). At this point, it is unclear whether all kettin isoforms can interact with the thick filament. Since we demonstrated myosin binding for the COOH-terminal Ig domains of the published kettin sequence (Ig34/35), association with thick filaments is likely for the 500- and 800-kD isoforms; both variants are recognized by α-kettin Ig34/35 (Fig. 7, B and C). However, this does not exclude that 700-kD kettin can also interact with A-band proteins, perhaps via other Ig domains. What is clear is that even the shortest of these kettin isoforms (500 kD) should be able to attach to the Z-disc and still reach over to the A-band edge, because at physiological SLs the I-band of Drosophila IFM sarcomeres is very short (Kolmerer et al., 2000).
Fig. 8 B shows the situation in a highly stretched sarcomere. Although under these conditions, no clearly defined kettin epitopes appeared outside the Z-disc, a “smear” in the I-band region seen on IF images indicated that at least some kettin had moved toward the A-band (Fig. 2, B and C). Moreover, only in stretched (nonextracted) sarcomeres did we observe PEVK staining, most likely because the PEVK epitope was pulled out of its A-band location. This scenario implies that the PEVK epitope is connected to some structure located upstream (toward the Z-disc). Then, if kettin isoforms are considered alternatively spliced forms of D-titin whose genomic sequence encodes two major PEVK regions (Machado and Andrew, 2000; Zhang et al., 2000) some kettin variants, such as the 800-kD isoform, may in fact contain PEVK domain(s). This conclusion is supported by the results of immunoblot analysis with α-PEVK antibody, which labels 800-kD kettin; the same isoform is recognized by kettin antibody Ig34/35 (Fig. 7 B). A striking observation on relaxed stretched sarcomeres was that the actin filaments appeared to be broken at the Z-line (Fig. 3), for example, in the region of actin-kettin interaction. In Fig. 8 B, we suggest that the actin breakage may also cause damage to kettin. Possibly, it is this kind of damage which causes the first plateau in the passive SL-tension curve of single Drosophila IFM myofibrils already after 5% stretch (Fig. 1 C). However, such a degree of extension may not be reached in situ because the length change of Drosophila IFM fibers in vivo is estimated to be 3.5% (Chan and Dickinson, 1996). In any case, we showed that Drosophila IFM myofibrils are at least one order of magnitude stiffer than vertebrate myofibrils (Fig. 1 C). In comparison, Lethocerus IFM fibers are also relatively stiff and reach an elastic limit after 6–7% stretch (Granzier and Wang, 1993). In contrast, bee flight muscle appears to be more extensible (Trombitas and Tigyi-Sebes, 1977); it can be stretched highly while retaining a greater SL homogeneity than Drosophila IFM fibers. In this context, we note that the great fragility of single Drosophila IFM myofibrils should be considered when such preparations want to be probed for their stretch activation characteristics (Maughan and Vigoreaux, 1999).
Treatment of single IFM myofibrils with gelsolin fragment immediately reduced passive stiffness and effectively extracted the actin filaments. These results are similar to those reported by Granzier and Wang (1993) on Lethocerus IFM fibers. Fig. 8 C schematically shows part of an actin-extracted stretched sarcomere by taking into account the results of Fig. 4. Projectin did not seem to be much affected by the actin extraction procedure, which is not unexpected because the protein does not bind to actin (Bullard et al., 2000). In contrast, gelsolin treatment strongly influenced the position of kettin, which was freed from its association with actin. Some kettin filaments apparently still remained attached to the Z-disc, probably via interaction with α-actinin. However, many kettin molecules moved to the edge of the A-band, indicating their connection to thick filaments. IF and IEM images suggested that the thick filament interaction is just inside the A-band. We speculate that the 800-kD kettin isoform, which might contain a PEVK domain (Fig. 7 B, lanes 5 and 6), may also lose connection to the Z-disc upon actin extraction. Sarcomere stretch then fails to pull out the PEVK epitope from its A-band location thus explaining the observed lack of PEVK staining.
Fig. 8 D depicts the myofilament arrangement in stretched sarcomeres after treatment with μ-calpain to cleave mainly kettin and to a much lesser degree projectin (Fig. 5 D). Remarkably, the digestion made it possible to pull the actin filaments out of their overlap with myosin (Fig. 3 C). We propose that this reflects a loosening up of the tight connection between actin filaments and the structural elements of the I-band that link actin and myosin. Because projectin does not interact with actin (Bullard et al., 2000), kettin may be the component responsible for the linkage. This conclusion is supported twofold. First, projectin staining in calpain-treated specimens was not significantly different from that in normal myofibrils, whereas kettin labeling was altered with distinct epitopes now located mainly at the A-band edge (Fig. 5, A and B). Second, after μ-calpain treatment the PEVK epitope was not stained even at high stretch (Fig. 5 C), suggesting damage to the connection upstream, which might be a kettin isoform. Moreover, myofibrillar stiffness dropped by a similar amount in calpain-digested and gelsolin-treated specimens (Fig. 6), which demonstrates that the passive stiffness decrease on actin extraction is not primarily due to the removal of weakly bound actin-myosin crossbridges in the overlap region as suggested earlier (Granzier and Wang, 1993).
Although the main conclusions of this study are based on results obtained with IFM myofibrils of Drosophila, they may bear more general significance. IFM sarcomeres are more extensible (Lakey et al., 1990) and somewhat less stiff (unpublished data) in Lethocerus than in Drosophila. Consistent with this is that in Lethocerus IFM the major kettin isoform is 700 kD, whereas in Drosophila it is 500 kD (Lakey et al., 1993; van Straaten et al., 1999; Fig. 7 A). Because kettin is present in the I-band region of both flight and leg muscle, some kettin isoforms may also be constituents of the elastic filaments of the latter muscle type. Possibly, larger isoforms from the D-titin gene are expressed in leg muscle where the I-bands are longer than in IFM. Consistent with this is the presence of higher molecular mass bands in gels and on the Western blots of wild-type Drosophila thoraces (which contain both IFM and leg muscle) compared with those of IFMs. Further, the other I-band protein contributing to IFM stiffness, projectin, is larger in Lethocerus than in Drosophila IFM (Fig. 7 A). Projectin of IFM is somewhat smaller than that of other muscle types (Vigoreaux et al., 1991). Interestingly, whereas projectin spans the I-band of insect IFM sarcomeres, the protein is found in the A-band of leg muscle sarcomeres (Hu et al., 1990; Lakey et al., 1990; Nave and Weber, 1990; Vigoreaux et al., 1991). Consistent with this is the observation that resting stiffness is high in IFM but lower in the leg muscle of Drosophila (Peckham et al., 1990). It remains to be seen whether in insect leg muscle the protein determining passive sarcomere stiffness could indeed be kettin. Taken together, we suggest that the stiffness of insect myofibrils may be modulated by the particular length isoforms of projectin and kettin expressed. This concept will need further proof, but it is similar to the concept proposed for vertebrate titin where differential expression of I-band segments underlies myofibrillar elastic diversity (Freiburg et al., 2000).
These results suggest that kettin and projectin together determine most of the high passive stiffness of insect IFM necessary for stretch activation. However, it is possible that other proteins are also involved in the stiffening. A protein from the actin-myosin overlap zone, troponin H, hypothesized to participate in the stiffening (Clayton et al., 1998), was shown here to play no significant role for passive stiffness. The Drosophila A-band–associated protein, flightin (Vigoreaux et al., 1993; Reedy et al., 2000), may contribute to the passive mechanical properties to some degree. However, flightin is cleaved by Igase, like troponin H (Clayton et al., 1998), which suggests it is also unlikely to contribute to passive stiffness. Finally, a novel member of the titin/myosin light chain kinase (MLCK) family has been described recently in Drosophila and was named stretchin-MLCK (Champagne et al., 2000). Its genomic sequence predicts a nearly 1-MD size polypeptide containing a kinase domain (like projectin but unlike D-titin/kettin), 32 Ig domains, two fibronectin type 3 modules, and two highly repetitive PEVK domains. The function of stretchin-MLCK in the sarcomere is unclear; expression at the protein level has not yet been studied. Hence, we conclude that, according to current knowledge, mainly projectin and kettin in the I-band and perhaps flightin in the A-band represent the proteins involved in the passive stiffening of insect IFM.
Materials and methods
Fly stocks
Drosophila melanogaster Oregon-R wild-type strain were maintained at 25°C on yeast-sucrose-agar medium. The Drosophila mutant Act88FKM88 (Hiromi and Hotta, 1985), which lacks actin in IFMs, was obtained from Dr. J. Sparrow (University of York, York, UK).
Myofibril preparation and solutions
Half thoraces from 20–30 flies were dissected (Peckham et al., 1990) and skinned in rigor buffer (20 mM Na-Pi, pH 7.0, 2 mM MgCl2, 2 mM EGTA, 2 mM DTT, 1 mM PMSF, 10 μM leupeptin and antipain) with 0.5% Triton X-100 and 50% glycerol for 4 h at 0°C and then at −20°C for 16 h. IFMs were dissected and myofibrils washed twice in rigor buffer with Triton, then twice in rigor buffer without Triton, and stored in the same buffer at 4°C for <1 wk. Relaxing solution used during experiments had an ionic strength of 200 mM in a MOPS buffer, pH 7.1, and contained 4 mM Na2ATP, 6 mM total magnesium, 15 mM EGTA, methane sulfonate as the major anion, 40 μg/ml leupeptin, and 20 mM BDM (Linke et al., 1997). Experiments were performed at room temperature.
Single myofibril mechanics
Experimental apparatus
A setup for single myofibril force measurements has been described (Linke et al., 1997). In brief, under a ZEISS Axiovert 135 microscope a myofibril is suspended between micromanipulator-positioned glass needles attached to a piezoelectric micromotor (Physit Instrumente) and a force transducer with nanonewton resolution. Data collection and motor control are done with a PC, DAQ board, and custom-written LabView software (National Instruments). Myofibril images are recorded either with a CCD camera (Völker), frame grabber, and Scion Image software (National Institutes of Health), or with a linear photodiode array, PC, DAQ board, and LabView algorithms (Linke et al., 1998). If desired, force data were related to myofibrillar cross-sectional area inferred from the diameter of the specimens (Linke et al., 1997).
Protocols
Passive force was recorded (sampling rate was 5 kHz) in stretch protocols with relaxed single myofibrils extended step-wise from slack SL. The stretch amplitude per step usually was 0.05 μm/sarcomere, and a given stretch step was completed within 1 s. After each step, the specimen was held at a constant SL for 10 s to wait for stress relaxation. Myofibrils were released in steps back to slack SL. Only forces recorded during the first stretch-release cycle were used for data analysis.
To measure passive stiffness, stretched single myofibrils were subjected to bursts of sinusoidal length oscillations (20 Hz; 20 nm/half-sarcomere). Force was sampled at 1 kHz. The motor sweeps were imposed at variable time intervals before, during, and after application of various substances tested for their effect on stiffness. The average force amplitude (mean ± SD) during the sweep was calculated from Butterworth-filtered data with a LabView program and taken as an indicator of stiffness.
Actin extraction with gelsolin fragment
A Ca2+-independent gelsolin fragment was prepared by proteolytic digestion of pig stomach smooth muscle gelsolin with thermolysin (Hellweg et al., 1993). After purification by anion exchange chromatography, small units were stored at −80°C and thawed just before use. Rhodamine-phalloidin (Sigma-Aldrich) was used to visualize actin in myofibrils and to routinely test for actin extraction.
Cloning and expression of kettin Ig34/35 and preparation of antibody
A cDNA fragment corresponding to nucleotide positions 19068–19765 of the Drosophila kettin sequence (sequence data available at GenBank/EMBL/DDBJ under accession no. AJ245406) coding for Ig domains 34 and 35 of the published kettin sequence (Fig. 2 A) was obtained by PCR and subcloned into a pET9d vector, which expresses the insert fused to a His6tag at the NH2 terminus (Studier et al., 1990). The sequence of the insert was confirmed and the expression vector transformed into Escherichia coli BL21(DE3)pLysS cells (Stratagene). Cultures were grown overnight in M9 minimal medium, diluted fourfold in SB medium, and induced with 0.2 mM IPTG; the cells were lysed, and the soluble overexpressed protein was purified by Ni-NTA chromatography (QIAGEN) then by FPLC on a MonoQ column (Amersham Pharmacia Biotech) (van Straaten et al., 1999).
Polyclonal antibody to recombinant Ig34/35 was raised in rabbits, and specific Ig was isolated from sera on an affinity column of the protein coupled to CH-Sepharose (Amersham Pharmacia Biotech).
Immunofluorescence microscopy
Single myofibrils held in relaxing buffer were labeled with monoclonal IgG α-kettin Ig16 (MAC 155) (Lakey et al., 1990) or polyclonal IgG α-kettin Ig34/35 and Cy3-conjugated secondary IgG (Rockland). Antibody dilution in relaxing buffer was ∼1:50. Other antibodies used were monoclonal IgG α-projectin Ig18 (MAC 150) (Lakey et al., 1990; Daley et al., 1998), monoclonal hybridoma supernatant 9D10 to PEVK sequence (Developmental Studies Hybridoma Bank; Wang and Greaser, 1985), monoclonal IgG α-TnH34 (MAC 143 and MAC 390), and α-TnH33 (MAC 81) (Bullard et al., 1988). In a typical experimental protocol, the myofibril was stretched in relaxing solution and then bathed in antibody-containing buffer, either before or after extraction of a specific sarcomere protein (actin, kettin, or TnH; see below). After repeated washouts with relaxing buffer, myofibrils were analyzed by IF microscopy. If the experiment involved actin extraction, rhodamine-phalloidin fluorescence was recorded at the very end of the experimental protocol. Normally, myofibrils were not chemically fixed before staining (Linke et al., 1999). IF images shown are representative examples of at least five different myofibrils analyzed per antibody under a given experimental condition.
Immunoelectron microscopy
Bundles of three to four Drosophila IFM fibers dissected from glycerinated half thoraces were stretched in a custom-built apparatus composed of two finely sharpened metal tweezers movable by mechanical micromanipulators. Stretching the extremely stiff fibers was facilitated by extracting actin filaments with gelsolin fragment. Preparations were fixed in buffer containing 4% paraformaldehyde and frozen in liquid N2 while still held by the tweezers; they were processed for cryosectioning and immunolabeling with kettin antibodies (α-Ig34/35) and 10 nm protein A gold as described (Linke et al., 1999; van Straaten et al., 1999).
Proteolysis of myofibrillar proteins by calpain and Igase
A protease, μ-calpain from bovine skeletal muscle, digests kettin in IFM myofibrils (Lakey et al., 1993). Other myofibrillar proteins are not affected, except projectin, which is degraded only slightly (Fig. 5); projectin is more strongly digested at higher calpain concentrations (Lakey et al., 1993). Here, myofibrils were incubated with low μ-calpain concentrations (3 μg/ml) in a buffer containing 10 μM free Ca2+ for up to 1 h at 25°C. Digestion was stopped by infusing relaxing buffer containing 15 mM EGTA. μ-Calpain was a gift from Dr. D.E. Goll (University of Arizona, Tucson, AZ). TnH proteolysis was induced by incubating myofibrils for 2 h at 25°C in relaxing buffer containing 20 μg/ml Igase (MoBiTec), a proteinase specific to a Pro-Pro-Y-Pro peptide sequence present in TnH (Clayton et al., 1998).
SDS-gel electrophoresis and immunoblotting
Large proteins of Drosophila and Lethocerus IFM and leg myofibrils, Drosophila thoraces, and for comparison rat heart and rabbit psoas myofibrils were analyzed by 2 or 2.6% SDS-PAGE (Linke et al., 1997). Whole Drosophila thoraces were frozen in liquid N2 immediately after dissection to avoid proteolysis. If not indicated otherwise, gels were stained with Coomassie brilliant blue R. Proteins in whole Drosophila thoraces or washed IFM myofibrils were separated on 2.5–7.5% polyacrylamide gradient gels and transferred to nitrocellulose by electrophoresis at 900 mA for 3 h in a buffer containing 0.1% SDS (Lakey et al., 1990). Immunoblots were incubated with α-kettin Ig16 and α-Ig34/35 or 9D10. Kettin-Ig16 antibody was also used on IFM myofibrils from a Drosophila actin-null mutant, Act88FKM88. Antibody binding was detected by incubating in peroxidase-conjugated secondary antibody (Sigma-Aldrich) followed by ECL chemiluminescent reagents (Amersham Pharmacia Biotech). Variable exposure times were selected to compare relative amounts of kettin in wild-type and actin-null species. For analyzing TnH digests, Drosophila IFM myofibrils were run on 12% gels and immunoblotted (Clayton et al., 1998).
Binding of kettin fragment Ig34/35 to myosin was estimated by dotblot. Lethocerus myosin (2.0–0.05 μg) was spotted onto nitrocellulose membrane, and after blocking in milk buffer (Harlow and Lane, 1988) strips were incubated in recombinant Ig34/35 (0.1 mg/ml) then in anti-His tag antibody (Dianova) diluted 1:500 followed by peroxidase-conjugated second antibody (Sigma-Aldrich). Antibody binding was detected by chemiluminescence as above.
Acknowledgments
We thank C. Ferguson and H. Hosser for help with IEM, M. Ahman for immunoblotting, and R. Dussel for technical assistance with SDS-gel electrophoresis. We are grateful to Dr. K. Leonard for improving the design of the apparatus for stretching fibers for cryoEM and for generous support.
The work was supported by grants from the Deutsche Forschungsgemeinschaft (Li 690/2-2; Li 690/5-1) and European Union (BIO4-CT98-0269).
Footnotes
Abbreviations used in this paper: IEM, immunoelectron microscopy; IF, immunofluorescence; IFM, indirect flight muscle; MLCK, myosin light chain kinase; SL, sacromere length.
References
- Ayme-Southgate, A., P. Lasko, C. French, and M.L. Pardue. 1991. Drosophila has a twitchin/titin-related gene that appears to encode projectin. Proc. Natl. Acad. Sci. USA. 88:7973–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benian, G.M., A. Ayme-Southgate, and T.L. Tinley. 1999. The genetics and molecular biology of the titin/connectin-like proteins of invertebrates. Rev. Physiol. Biochem. Pharmacol. 138:235–268. [DOI] [PubMed] [Google Scholar]
- Bullard, B., K.S. Hammond, and B.M. Luke. 1977. The site of paramyosin in insect flight muscle and the presence of an unidentified protein between myosin filaments and Z-line. J. Mol. Biol. 115:417–440. [DOI] [PubMed] [Google Scholar]
- Bullard, B., K. Leonard, A. Larkins, G. Butcher, C. Karlik, and E. Fyrberg. 1988. Troponin of asynchronous flight muscle. J. Mol. Biol. 294:621–637. [DOI] [PubMed] [Google Scholar]
- Bullard, B., D. Goulding, C. Ferguson, and K. Leonard. 2000. Links in the chain: the contribution of kettin to the elasticity of insect muscles. Adv. Exp. Med. Biol. 481:207–218. [DOI] [PubMed] [Google Scholar]
- Champagne, M.B., K.A. Edwards, H.P. Erickson, and D.O. Kiehart. 2000. Drosophila stretchin-MLCK is a novel member of the titin/myosin light chain kinase family. J. Mol. Biol. 300:759–777. [DOI] [PubMed] [Google Scholar]
- Chan, W.P., and M.H. Dickinson. 1996. In vivo length oscillation of indirect flight muscles in the fruit fly Drosophila virilis. J. Exp. Biol. 199:2767–2774. [DOI] [PubMed] [Google Scholar]
- Clayton, J.D., R.M. Cripps, J.C. Sparrow, and B. Bullard. 1998. Interaction of troponin-H and glutathione S-transferase-2 in indirect flight muscles of Drosophila melanogaster. J. Muscle Res. Cell Motil. 19:117–127. [DOI] [PubMed] [Google Scholar]
- Daley, J., R. Southgate, and A. Ayme-Southgate. 1998. Structure of the Drosophila projectin protein: isoforms and implication for projectin filament assembly. J. Mol. Biol. 279:201–210. [DOI] [PubMed] [Google Scholar]
- Freiburg, A., K. Trombitas, W. Hell, O. Cazorla, F. Fougerousse, T. Centner, B. Kolmerer, C. Witt, J.S. Beckmann, C.C. Gregorio, et al. 2000. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ. Res. 86:1114–1121. [DOI] [PubMed] [Google Scholar]
- Fyrberg, C.C., S. Labeit, B. Bullard, K. Leonard, and E. Fyrberg. 1992. Drosophila projectin: relatedness to titin and twitchin and correlation with lethal (4) 102 CDa and bent-Dominant mutants. Proc. R. Soc. 249:33–40. [DOI] [PubMed] [Google Scholar]
- Garamvölgyi, N. 1966. Elongation of the primary myofilaments in highly stretched insect flight muscle fibrils. Biochem. Biophys. Acta. 1:89–100. [Google Scholar]
- Granzier, H.L., and K. Wang. 1993. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J. Gen. Physiol. 101:235–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda, S., S. Endo, and K. Saigo. 2000. Requirements of kettin, a giant protein highly conserved in overall structure in evolution, for normal muscle function, viability, and flight activity of Drosophila. J. Cell Biol. 148:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 726 pp.
- Hellweg, T., H. Hinssen, and W. Eimer. 1993. The Ca2+-induced conformational change of gelsolin is located in the carboxy-terminal half of the molecule. Biophys. J. 65:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi, Y., and Y. Hotta. 1985. Actin gene mutations in Drosophila: heat shock activation in the indirect flight muscles. EMBO J. 4:1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, D.A., A. Matsuno, K. Terakado, T. Matsuura, S. Kimura, and K. Maruyama. 1990. Projectin is an invertebrate connectin (titin): isolation from crayfish claw muscle and localization in crayfish claw muscle and insect flight muscle. J. Muscle Res. Cell Motil. 11:497–511. [DOI] [PubMed] [Google Scholar]
- Josephson, R.K., J.G. Malamud, and D.R. Stokes. 2000. Asynchronous muscle: a primer. J. Exp. Biol. 203:2713–2722. [DOI] [PubMed] [Google Scholar]
- Kolmerer, B., J. Clayton, V. Benes, T. Allen, C. Ferguson, K. Leonard, U. Weber, M. Knekt, W. Ansorge, S. Labeit, et al. 2000. Sequence and expression of the kettin gene in Drosophila melanogaster and Caenorhabditis elegans. J. Mol. Biol. 296:435–448. [DOI] [PubMed] [Google Scholar]
- Labeit, S., and B. Kolmerer. 1995. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 270:293–296. [DOI] [PubMed] [Google Scholar]
- Lakey, A., C. Ferguson, S. Labeit, M. Reedy, A. Larkins, G. Butcher, K. Leonard, and B. Bullard. 1990. Identification and localization of high molecular weight proteins in insect flight and leg muscle. EMBO J. 9:3459–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey, A., S. Labeit, M. Gautel, C. Ferguson, D.P. Barlow, K. Leonard, and B. Bullard. 1993. Kettin, a large modular protein of the Z-disc of insect muscles. EMBO J. 12:2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, W.A. 2000. Stretching molecular springs: elasticity of titin filaments in vertebrate striated muscle. Histol. Histopathol. 15:799–811. [DOI] [PubMed] [Google Scholar]
- Linke, W.A., M. Ivemeyer, S. Labeit, H. Hinssen, J.C. Rüegg, and M. Gautel. 1997. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys. J. 73:905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, W.A., M. Ivemeyer, P. Mundel, M.R. Stockmeier, and B. Kolmerer. 1998. Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA. 95:8052–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke, W.A., D.E. Rudy, T. Centner, M. Gautel, C. Witt, S. Labeit, and C.C. Gregorio. 1999. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J. Cell Biol. 146: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, C., and D.J. Andrew. 2000. D-titin: a giant protein with dual roles in chromosomes and muscles. J. Cell Biol. 151:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, C., C.E. Sunkel, and D.J. Andrew. 1998. Human autoantibodies reveal titin as a chromosomal protein. J. Cell Biol. 141:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan, D.W., and J.O. Vigoreaux. 1999. An integrated view of insect flight muscle: genes, motor molecules, and motion. News Physiol. Sci. 14:87–92. [DOI] [PubMed] [Google Scholar]
- Nave, R., and K. Weber. 1990. A myofibrillar protein of insect muscle related to vertebrate titin connects Z band and A band: purification and molecular characterization of invertebrate mini-titin. J. Cell Sci. 95:535–544. [DOI] [PubMed] [Google Scholar]
- Peckham, M., J.E. Molloy, J.C. Sparrow, and D.C.S. White. 1990. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J. Muscle Res. Cell Motil. 11:203–215. [DOI] [PubMed] [Google Scholar]
- Pringle, J.W. 1967. The contractile mechanism of insect fibrillar muscle. Prog. Biophys. Mol. Biol. 17:1–60. [DOI] [PubMed] [Google Scholar]
- Reedy, M.K. 1971. Electron microscope observations concerning the behavior of the cross-bridge in striated muscle. Contractility of Muscle Cells and Related Processes. R.J. Podolsky, editor. Prentice Hall, Inc., Englewood Cliffs, NJ. 229–246.
- Reedy, M.C., M.K. Reedy, and R.T. Tregear. 1988. Two attached non-rigor crossbridge forms in insect muscle. J. Mol. Biol. 204:357–383. [DOI] [PubMed] [Google Scholar]
- Reedy, M.C., M.K. Reedy, K.R. Leonard, and B. Bullard. 1994. Gold/Fab immuno electron microscopy localization of troponin H and troponin T in Lethocerus flight muscle. J. Mol. Biol. 239:52–67. [DOI] [PubMed] [Google Scholar]
- Reedy, M.C., B. Bullard, and J.O. Vigoreaux. 2000. Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscles. J. Cell Biol. 151:1483–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg, J.C. 1968. Oscillatory mechanism in fibrillar insect flight muscle. Experientia. 24:529–536. [DOI] [PubMed] [Google Scholar]
- Saide, J.D. 1981. Identification of a connecting filament protein in insect fibrillar flight muscle. J. Mol. Biol. 153:661–679. [DOI] [PubMed] [Google Scholar]
- Saide, J.D., S. Chin-Bow, J. Hogan-Sheldon, L. Busquets-Turner, J.O. Vigoreaux, K. Valgeirsdottir, and M.L. Pardue. 1989. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster. J. Cell Biol. 109:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier, F.W., A.H., Rosenberg, J.J. Dunn, and J.W. Dubenhoff. 1990. Use of T7RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60–89. [DOI] [PubMed] [Google Scholar]
- Trombitas, K. 2000. Connecting filaments: a historical prospective. Adv. Exp. Med. Biol. 481:1–23. [DOI] [PubMed] [Google Scholar]
- Trombitas, K., and A. Tigyi-Sebes. 1974. Direct evidence for connecting C filaments in flight muscle of honey bee. Acta Biochim. Biophys. Acad. Sci. Hung. 9:243–253. [PubMed] [Google Scholar]
- Trombitas, K., and A. Tigyi-Sebes. 1977. Fine structure and mechanical properties of insect muscle. Insect Flight Muscle. R.T. Tregear, editor. Elsevier Science Publishing Co., Inc., Amsterdam, Netherlands. 79–90.
- van Straaten, M., D. Goulding, B. Kolmerer, S. Labeit, J. Clayton, K. Leonard, and B. Bullard. 1999. Association of kettin with actin in the Z-disc of insect flight muscle. J. Mol. Biol. 285:1549–1562. [DOI] [PubMed] [Google Scholar]
- Vigoreaux, J.O., J.D. Saide, K. Valgeirsdottir, and M.L. Pardue. 1993. Flightin, a novel myofibrillar protein of Drosophila stretch-activated muscles. J. Cell Biol. 121:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigoreaux, J.O., J.D. Saide, and M.L. Pardue. 1991. Structurally different Drosophila striated muscles utilize distinct variants of Z-band associated proteins. J. Muscle Res. Cell Motil. 12:340–354. [DOI] [PubMed] [Google Scholar]
- Vigoreaux, J.O., J.R. Moore, and D.W. Maughan. 2000. Role of the elastic protein projectin in stretch activation and work output of Drosophila flight muscles. Adv. Exp. Med. Biol. 481:237–247. [DOI] [PubMed] [Google Scholar]
- Wang, S.M., and M.L. Greaser. 1985. Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J. Muscle Res. Cell Motil. 6:293–312. [DOI] [PubMed] [Google Scholar]
- White, D.C. 1983. The elasticity of relaxed insect fibrillar flight muscle. J. Physiol. 343:31–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., D. Featherstone, W. Davis, E. Rushton, and K. Broadie. 2000. Drosophila D-titin is required for myoblast fusion and skeletal muscle striation. J. Cell Sci. 113:3103–3115. [DOI] [PubMed] [Google Scholar]