Abstract
Background
Vitamin D is a potent inhibitor of the proinflammatory response and thereby diminishes turnover of leukocytes. Leukocyte telomere length (LTL) is a predictor of aging-related disease and decreases with each cell cycle and increased inflammation.
Objective
The objective of the study was to examine whether vitamin D concentrations would attenuate the rate of telomere attrition in leukocytes, such that higher vitamin D concentrations would be associated with longer LTL.
Design
Serum vitamin D concentrations were measured in 2160 women aged 18–79 y (mean age: 49.4) from a large population-based cohort of twins. LTL was measured by using the Southern blot method.
Results
Age was negatively correlated with LTL (r = −0.40, P < 0.0001). Serum vitamin D concentrations were positively associated with LTL (r = 0.07, P = 0.0010), and this relation persisted after adjustment for age (r = 0.09, P < 0.0001) and other covariates (age, season of vitamin D measurement, menopausal status, use of hormone replacement therapy, and physical activity; P for trend across tertiles = 0.003). The difference in LTL between the highest and lowest tertiles of vitamin D was 107 base pairs (P = 0.0009), which is equivalent to 5.0 y of telomeric aging. This difference was further accentuated by increased concentrations of C-reactive protein, which is a measure of systemic inflammation.
Conclusion
Our findings suggest that higher vitamin D concentrations, which are easily modifiable through nutritional supplementation, are associated with longer LTL, which underscores the potentially beneficial effects of this hormone on aging and age-related diseases.
Keywords: Vitamin D, telomere length, inflammation, aging
INTRODUCTION
Mounting evidence suggests that, in addition to its well-described roles in skin, bone, and muscle physiology (2), the hormone vitamin D acts as an inhibitor of the inflammatory response through several pathways (1). Decreased vitamin D concentrations have been associated with an increased risk of developing autoimmune diseases, such as multiple sclerosis, rheumatoid arthritis, and type 1 diabetes (3–6). Vitamin D administration has been shown to prevent the initiation and to attenuate the severity of immune-mediated diseases, including type 1 diabetes (7, 8) and an animal model for multiple sclerosis (9). In addition, a recent open-label trial showed that vitamin D decreased rheumatoid arthritis disease activity (10).
Subsets of leukocytes have receptors for the active form of vitamin D (1,25-dihydroxyvitamin D3; 11–13) that support the direct effect of vitamin D on these cells (14–16), which explains, in part, the connections between vitamin D and autoimmune disease. Furthermore, an inverse relation has been shown between vitamin D concentrations and C-reactive protein (CRP), a marker of inflammation, in both healthy subjects and patients with rheumatoid arthritis and frailty (17, 18). The inhibitory effect of vitamin D on the inflammatory response also points to a potential link between this vitamin and telomere dynamics (length and attrition rate) in leukocytes.
Telomeres are the ends of chromosomes and undergo attrition with each replication (19, 20), a process that is accelerated by oxidative stress (21, 22). What is more, leukocyte telomere length (LTL) is relatively short in persons with chronic inflammation, because the inflammatory response entails an increase in leukocyte turnover. Consistent with this proposition, both vascular diseases (23–28) and autoimmune diseases such as lupus (29) and arthritis (30, 31) have been associated with shorter LTL. Furthermore, cigarette smoking and obesity, which provoke a proinflammatory milieu, are both a source of oxidative stress (32, 33) and are associated with shortened LTL (34, 35). In fact, several studies have documented associations of indexes of oxidative stress and inflammation with LTL (24, 27, 36). Recently, a randomized case-control analysis showed that shortened LTL was an independent risk factor for coronary heart disease, and the magnitude of risk attributed to shortened LTL was similar to that for conventional risk factors (37). Thus, shortened LTL seems to be a marker of aging-related diseases and conditions associated with an increased burden of oxidative stress and inflammation. Yet, little is known about the environmental factors, other than obesity and smoking, that may affect LTL.
As humans age, both LTL and vitamin D concentrations decrease (17, 38), whereas inflammatory mediators increase (39, 40). In addition, CRP, a marker of systemic inflammation, displays an inverse relation with vitamin D concentrations (17, 18) and LTL (24). Given that vitamin D displays antiinflammatory properties, we hypothesized that it may attenuate the rate of LTL attrition. To this end, we examined the associations between LTL and serum 25-hydroxyvitamin D concentrations and CRP in a population-based cohort of women across a wide age spectrum.
SUBJECTS AND METHODS
Study population
We studied women from the TwinsUK cohort (see www.twinsuk.ac.uk), an ongoing adult twin registry examining several age-related phenotypes, which include osteoporosis, obesity, diabetes, and visual, endocrine, and cardiovascular diseases. The twins involved in the present study were previously shown to represent the general population of the United Kingdom (41). The present study was approved by the Guy’s and St Thomas’ Hospital Ethics Committee and conformed with the Helsinki Declaration. Participants provided written informed consent.
Phenotypic variables
Body mass index, physical activity, smoking, serum insulin, CRP, and leptin concentrations were considered to be potential confounders according to previous studies that showed a relation between these variables and LTL (34, 42). Physical activity was recorded as inactive, light, moderate, or heavy exercise during leisure time. This previously validated measure of activity correlated well with an in-depth measure of physical activity in the Dunbar Health Survey (43). Decreased physical activity was recently shown to be associated with shorter telomere length (44). Those subjects that reported current daily cigarette smoking were classified as daily smokers. To assess the relation between vitamin D supplementation and LTL, subjects were asked if they used vitamin D supplements.
Fasting serum insulin and glucose concentrations were measured by using methods described previously (45). Fasting serum insulin concentrations were assayed by using a chemiluminescent Immulite kit (Diagnostics Products Corp, Los Angeles, CA). Serum leptin concentrations were measured after the subjects had fasted overnight by using a radioimmunoassay (Linco Research, St Louis, MO). 25-Hydroxyvitamin D concentrations were measured by using a radioimmunoassay kit (DiaSorin Inc, Stillwater, MN). This assay has a detection limit of 4 nmol/L, and the analytic CV of the method is 9.1% at 22 nmol/L. Serum CRP concentrations were measured by using an enzyme-linked immunosorbent assay. The lower limit of detection of this assay is 0.15 mg/L, and the assay has a CV of 8.7% at 0.5 mg/L.
Telomere length measurement
LTL was derived from the mean of the terminal restriction fragment length by using the Southern blot method on DNA extracted from peripheral leukocytes, as described elsewhere (26). Each DNA sample was resolved in duplicate (on different gels). If the difference between the duplicates was >5%, a third measurement was performed, and the mean of the 2 results <5% apart was taken. This occurred in <5% of the samples. The CV of the terminal restriction fragment length assay in the present study was 1.5%. The Center of Human Development and Aging at the University of Medicine and Dentistry of New Jersey conducted the terminal restriction fragment length assays and was blinded to the identity of the subjects.
Statistical analyses
The normality of the variables was assessed, and 25-hydroxyvitamin D, CRP, and leptin concentrations were subsequently natural log–transformed. The relation between 25-hydroxyvitamin D concentrations and age-adjusted LTL was assessed by using a scatter plot with a fitted regression line. A Pearson’s correlation coefficient was calculated between 25-hydroxyvitamin D concentrations, LTL, age-adjusted LTL, and CRP concentrations. The relation between 25-hydroxyvitamin D concentrations and LTL was further assessed by using standard linear regression techniques, after control for multiple covariates. These covariates included age, body mass index, fasting insulin and serum leptin concentrations, smoking status, CRP, physical activity level, season of 25-hydroxyvitamin D measurement, menopausal status, and use of hormone replacement therapy. Model selection was carried out by using the Bayesian Information Criterion, by which the potential confounders were analyzed by assessing the top 10 models as generated by the Bayesian Information Criterion and by including the variables that predicted LTL. If a variable had no effect on the relation between LTL and vitamin D, then the variable was removed from the model; however, if a variable altered the relation between LTL and vitamin D, that variable was included in the final model. The resultant linear regression residual plots were checked for violations of linear relations. A quadratic term was computed for age and was included in the regression analysis to further test for nonlinearity. This quadratic term did not affect the relation between 25-hydroxyvitamin D and LTL, nor did it predict LTL, and it was thus discarded from further analysis. To further assess the relation between multiply adjusted LTL and 25-hydroxyvitamin D concentrations, the study population was divided into tertiles of 25-hydroxyvitamin D, and the average LTL for each tertile and nonparametric test for trend across tertiles was calculated. This analysis was also repeated after the study population was divided into quintiles of 25-hydroxyvitamin D concentrations. Because vitamin D influences inflammation and LTL is reduced by increased levels of systemic inflammation, we divided the study population into those subjects with a CRP concentration ≥2.0 or <2.0 mg/L, which is regarded as the lower limit for clinically detectable inflammation (46). Moreover, some authors (47) have suggested that a CRP concentration ≥10.0 or <10.0 mg/L more appropriately defines inflammation, and we therefore repeated our analysis by using this alternate threshold. A statistical interaction term between CRP and 25-hydroxyvitamin D was computed but was not statistically significant in linear regression models and was therefore not included in subsequent analyses. The difference in multiply adjusted LTL between current users of vitamin D supplements and nonusers was then calculated by using a 2-tailed Student’s t test. Because of the nonindependence of twins, we controlled for familial aggregation by treating twin-pairs as clusters of information by using the robust regression cluster option in STATA software (version 9.2; Stata Corp, College Station, TX). All analyses were carried out with the use of STATA/SE software (version 9.2; Stata Corp).
RESULTS
General characteristics
We identified a total of 2160 women with data on both 25-hydroxyvitamin D concentrations and LTL (Table 1). The mean age of the sample was 49.4 y (range: 18–80 y). Most of the subjects were nonsmokers, and approximately one-half of the sample reported moderate or heavy physical activity.
TABLE 1.
Selected characteristics of the study population (n = 2160)1
| Characteristic | Value |
|---|---|
| Age (y) | 49.4 ± 12.92 |
| Leukocyte telomere length (kb) | 7.0 ± 0.7 |
| Serum 25-hydroxyvitamin D (nmol/L) | 78.9 ± 41.3 |
| Mean serum 25-hydroxyvitamin D by tertile (nmol/L) | |
| Lowest tertile | 40.9 ± 11.0 |
| Middle tertile | 72.7 ± 9.0 |
| Highest tertile | 124 ± 37.3 |
| Serum CRP (mg/L) | 3.2 ± 6.1 |
| BMI (kg/m2) | 25.3 ± 4.5 |
| Fasting serum insulin (μU/mL) | 9.9 ± 11.4 |
| Fasting serum glucose (mmol/L) | 4.8 ± 1.1 |
| Physical activity [n (%)] | |
| Inactive or light | 1062 (49.2) |
| Moderate or heavy | 1098 (50.8) |
| Menopausal status [n (%)] | |
| Premenopausal | 553 (25.6) |
| Menopausal and never HRT | 1045 (48.4) |
| Menopausal and former HRT | 301 (13.9) |
| Menopausal and current HRT | 261 (12.1) |
| Smoking status [n (%)] | |
| Nonsmoker | 1763 (81.6) |
| Smoker | 397 (18.4) |
CRP, C-reactive protein; HRT, hormone replacement therapy.
x̄ ± SD (all such values).
Relations between 25-hydroxyvitamin D, age, leukocyte telomere length, and C-reactive protein
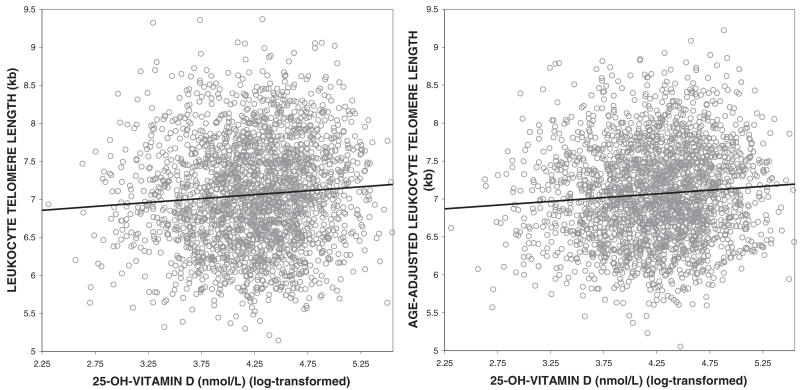
Age was negatively correlated with LTL (Pearson’s correlation coefficient: −0.40; P < 0.0001), with an extrapolated annual rate of decrease of 21.5 base pairs/y. 25-Hydroxyvitamin D concentrations were positively correlated with LTL, and this relation was strengthened after LTL was adjusted for age (Figure 1). CRP concentrations were negatively associated with age-adjusted LTL (Pearson’s correlation coefficient: −0.05; P = 0.0009) and with 25-hydroxyvitamin D concentrations (Pearson’s correlation coefficient: −0.05; P = 0.0016).
FIGURE 1.
Relations between 25-hydroxyvitamin D (25-OH-vitamin D) concentrations and leukocyte telomere length (n = 2160, Pearson’s correlation coefficient = 0.07, P = 0.0010) and between 25-hydroxyvitamin D concentrations and age-adjusted leukocyte telomere length (n = 2160, Pearson’s correlation coefficient = 0.09, P < 0.0001)
The covariates adjusted for in the regression model after consideration of the Bayesian Information Criterion were age, season of vitamin D measurement, menopausal status, use of hormone replacement therapy, and physical activity. We divided the study population by tertiles of 25-hydroxyvitamin D concentrations and observed that increasing tertiles of 25-hydroxyvitamin D were associated with increased multiply adjusted LTL [P for nonparametric trend = 0.003; mean LTL in lowest tertile of 25-hydroxyvitamin D = 6.97 (95% CI: 6.93, 7.01), mean LTL in middle tertile of 25-hydroxyvitamin D = 7.02 (95% CI: 6.98, 7.07), mean LTL in highest tertile of 25-hydroxyvitamin D = 7.08 (95% CI: 7.03, 7.12)]. The multiply adjusted difference in LTL between the highest and lowest tertile of 25-hydroxyvitamin D concentrations was 107.1 base pairs (P for difference between means = 0.0009), which was equivalent to 5.0 y of telomeric aging. The study population was also divided by quintiles of 25-hydroxyvitamin D concentrations to investigate whether this approach altered the results. In the analysis by quintiles of 25-hydroxyvitamin D, the relation between 25-hydroxyvitamin D and multiply adjusted LTL (adjusted for the same variables) did not change (P for nonparametric trend = 0.007). Furthermore, LTL increased with each increasing quintile of vitamin D.
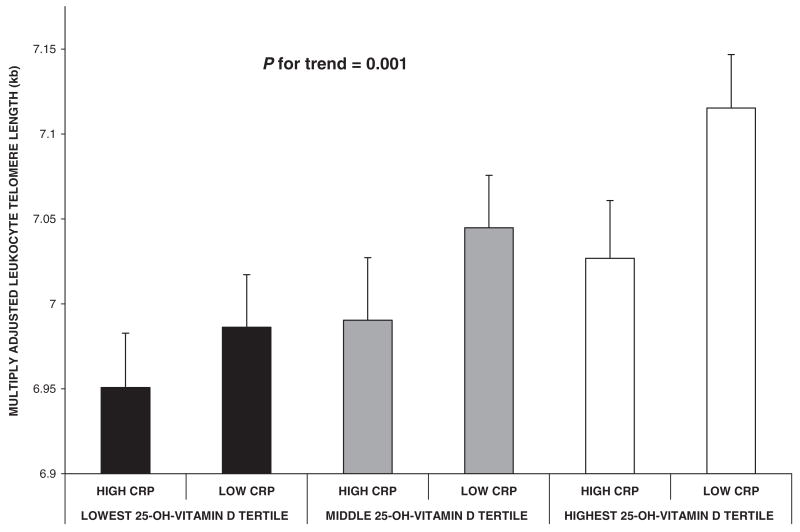
We also stratified the study population by level of systemic inflammation by using a CRP concentration of 2.0 mg/L to designate the minimal inflammation status that may have clinical relevance (46). We observed that, within each tertile of serum 25-hydroxyvitamin D, LTLs were longer in those with lower serum CRP concentrations (adjusted for age and level of physical activity; P for trend = 0.001) than in subjects with higher CRP concentrations (Figure 2). When assessing the most extreme groups, the difference in LTL between those with the lowest 25-hydroxyvitamin D concentrations and highest CRP concentrations and those with the highest 25-hydroxyvitamin D concentrations and lowest CRP concentrations was 164.6 base pairs, which was equivalent to 7.6 y of telomeric aging (P = 0.0003 for difference in mean LTL between groups). To assess the sensitivity of the chosen CRP cutoff, we changed the CRP threshold to 10.0 mg/L. This did not change the relation between 25-hydroxyvitamin D and multiply adjusted LTL once stratified by CRP (P for nonparametric trend = 0.001). In addition, for each tertile of 25-hydroxyvitamin D concentrations, those with higher CRP had shorter LTL (results not shown). The statistical interaction term between CRP and 25-hydroxyvitamin D was not significant.
FIGURE 2.
Multiply adjusted associations between tertiles of 25-hydroxyvitamin D (25-OH-vitamin D) and leukocyte telomere length were stratified by serum C-reactive protein (CRP) concentrations (n = 2160) and adjusted for age, season of vitamin D measurement, menopausal status, use of hormone replacement therapy, and physical activity. High and low CRP concentrations were delineated by a CRP value of 2.0 mg/L. Error bars indicate SE. P value was derived from the nonparametric trend test across all 6 means. There was no significant interaction between CRP and vitamin D.
Vitamin D supplement information was available on a subset of the study population (n = 700). Vitamin D supplement users had longer LTLs, despite adjustment for age, season of vitamin D measurement, menopausal status, use of hormone replacement therapy, and physical activity, than did nonusers. Mean adjusted LTL in subjects who did not use vitamin D supplements was 6.95 kb, whereas that of current users of vitamin D supplements was 7.06 kb; however, this difference was not statistically significant (P for 2-tailed Student’s t test = 0.06).
DISCUSSION
In the large population of women in the present study, higher serum 25-hydroxyvitamin D concentrations were associated with longer LTL. Moreover, in every tertile of vitamin D, LTL was shorter in individuals with higher CRP concentrations. The difference in multiply adjusted LTL between the highest and lowest serum vitamin D tertiles was similar in magnitude to the difference in LTL associated with 5 y of chronologic age. Although these associations do not prove causality, they do suggest that vitamin D may play an important role in the modulation of LTL, which is related to aging and age-related diseases. Previous studies indicated that shortened LTL is an independent risk factor for coronary heart disease (37), and our results suggest that vitamin D, which is easily modifiable through supplementation, may possibly attenuate LTL degradation.
Vitamin D supplement users were also found to have longer LTLs than did nonusers, which provides support for this hypothesis. The reduction in total sample size by 1460 subjects in this subset analysis of vitamin supplement use possibly explains why this difference was only of borderline significance. However, direct measurement of serum 25-hydroxyvitamin D concentrations is likely a better proxy for vitamin D status that is the use of supplements alone.
There are several mechanisms that may explain the association between LTL and vitamin D concentrations. Vitamin D decreases the mediators of systemic inflammation, such as interleukin-2 and tumor necrosis factor-α (48), and, confirming this, in our study population, vitamin D concentrations were negatively correlated with levels of CRP. Vitamin D receptors are ubiquitously expressed in T and B lymphocytes, natural killer cells, and monocytes (49, 50), and through the down-regulation of cytokines and other proinflammatory factors, vitamin D exerts profound antiinflammatory and antiproliferative actions, which would affect the turnover rate of leukocytes (1). It follows that vitamin D would attenuate the rate of LTL attrition.
Inflammation and oxidative stress are key determinants in the biology of aging (51), and LTL dynamics appear to chronicle the accruing burden of these variables (52). The present study further supports the concept that LTL may serve as a cumulative index of an individual’s lifelong burden of oxidative stress and inflammation (52). Some of the factors that heighten oxidative stress and inflammation are genetic, but others are clearly environmental in nature, and a few may be easily modifiable. For instance, cigarette smoking (34, 35), obesity (34), and sedentary lifestyle (44) are associated with shortened LTL. Whereas these lifestyle habits may be difficult to change, vitamin D concentrations are easily modifiable through nutritional supplementation or sunshine exposure.
We note the limitation of the cross-sectional approach of studying LTL, which may represent cumulative lifetime exposure to oxidative stress and inflammatory burden. In contrast, serum vitamin D concentrations represent the status of the vitamin at a certain time point. Therefore, longitudinal studies with multiple time points would provide a much more coherent account of the effect of different variables, including vitamin D, on leukocyte telomere dynamics. For example, longitudinal studies indicate that insulin resistance explains 28% (53) of the variation in leukocyte telomere attrition, whereas cross-sectional studies of this relation show that insulin resistance explains only 2.5% of the variation in LTL (27). The reason for this is that LTL is highly variable at birth (54) and is as variable afterward. Thus, cross-sectional studies of LTL may not always capture reliably the effect of a given factor on LTL attrition rate. On the basis of these considerations, our findings suggest that the effect of vitamin D on leukocyte telomere attrition may not be at all trivial.
We also note that the observations afforded by twin pairs in our cohort are not necessarily independent, and we have thus controlled for nonindependence by using robust statistical methods. Furthermore, our twin cohort was shown to be similar to the general population of the United Kingdom (41). Finally, our results are cross-sectional and therefore may only suggest causality.
In conclusion, our study provides evidence that a longer LTL is associated with increased serum vitamin D concentrations in women. Although both LTL and serum vitamin D concentrations decrease with age and are thus possible markers of aging in general, we have shown that the positive association between LTL and vitamin D concentrations is independent of age and many other covariates. Vitamin D exerts immunomodulatory effects that may attenuate LTL attrition rate. Longitudinal studies or randomized controlled trials of supplementation exploring the effect of vitamin D on LTL will be necessary to unequivocally establish the relation between vitamin D and leukocyte telomere dynamics; but for the moment, our data suggest another potential benefit of vitamin D—on the aging process and age-related disease.
Acknowledgments
The authors’ responsibilities were as follows—JBR and AMV: participated in the concept, analysis, and drafting of the manuscript; JPG, DP, MK, AN, XL, GLS, and RS: participated in data acquisition; TDS and AA: participated in obtaining funding and in the concept, analysis, and drafting of the manuscript. The funding sources had no role in the concept, design, analysis, or drafting of this manuscript. The authors reported no conflicts of interest.
Footnotes
Supported by the Wellcome Trust (TDS and AV), the Arthritis Research Campaign (TDS and AV), the Chronic Disease Research Foundation (TDS and AV), the Canadian Institutes of Health Research (JBR), the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (JBR), NIH grants AG021593 and AG020132, and The Healthcare Foundation of New Jersey (AA).
References
- 1.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Munger KL, Zhang SM, O’Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 4.Hillman L, Cassidy JT, Johnson L, Lee D, Allen SH. Vitamin-D metabolism and bone mineralization in children with juvenile rheumatoid-arthritis. J Pediatr. 1994;124:910–6. doi: 10.1016/s0022-3476(05)83179-8. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–6. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu C, Waer M, Casteels K, Laureys J, Bouillon R. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology. 1995;136:866–72. doi: 10.1210/endo.136.3.7867594. [DOI] [PubMed] [Google Scholar]
- 8.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–74. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 9.Cantorna MT, Hayes CE, Deluca HF. 1,25-Dihydroxyvitamin D-3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andjelkovic Z, Vojinovic J, Pejnovic N, et al. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol. 1999;17:453–6. [PubMed] [Google Scholar]
- 11.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–2. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 12.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 13.Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, di-hydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Tobler A, Gasson J, Reichel H, Norman AW, Koeffler HP. Granulocyte-macrophage colony-stimulating factor. Sensitive and receptor-mediated regulation by 1,25-dihydroxyvitamin D3 in normal human peripheral blood lymphocytes. J Clin Invest. 1987;79:1700–5. doi: 10.1172/JCI113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manolagas SC, Provvedini DM, Tsoukas CD. Interactions of 1,25-dihydroxyvitamin D3 and the immune system. Mol Cell Endocrinol. 1985;43:113–22. doi: 10.1016/0303-7207(85)90074-7. [DOI] [PubMed] [Google Scholar]
- 17.Puts MTE, Visser M, Twisk JWR, Deeg DJH, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol. 2005;63:403–11. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 18.Oelzner P, M A, Deschner F, et al. Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int. 1998;62:193–8. doi: 10.1007/s002239900416. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 20.Artandi SE. Telomeres, telomerase, and human disease. N Engl J Med. 2006;355:1195–7. doi: 10.1056/NEJMp068187. [DOI] [PubMed] [Google Scholar]
- 21.von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000;908:99–110. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–26. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60:174–80. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 25.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 26.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging - The gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 27.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Kurosaka D, Yasuda J, Yoshida K, et al. Telomerase activity and telomere length of peripheral blood mononuclear cells in SLE patients. Lupus. 2003;12:591–9. doi: 10.1191/0961203303lu426oa. [DOI] [PubMed] [Google Scholar]
- 30.Schonland SO, Lopez C, Widmann T, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–6. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steer SE, Williams FM, Kato B, et al. Reduced telomere length in rheumatoid arthritis is independent of disease activity and duration. Ann Rheum Dis. 2007;66:476–80. doi: 10.1136/ard.2006.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernhard D, Rossmann A, Henderson B, Kind M, Seubert A, Wick G. Increased serum cadmium and strontium levels in young smokers: effects on arterial endothelial cell gene transcription. Arterioscler Thromb Vasc Biol. 2006;26:833–8. doi: 10.1161/01.ATV.0000205616.70614.e5. [DOI] [PubMed] [Google Scholar]
- 33.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 34.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 35.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–10. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 36.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 38.Aviv A. Chronology versus biology: telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40:229–32. doi: 10.1161/01.hyp.0000027280.91984.1b. [DOI] [PubMed] [Google Scholar]
- 39.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 40.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–8. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 41.Andrew T, Hart DJ, Snieder H, de Lange M, Spector TD, MacGregor AJ. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 2001;4:464–77. doi: 10.1375/1369052012803. [DOI] [PubMed] [Google Scholar]
- 42.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 43.Etherington J, Harris PA, Nandra D, et al. The effect of weight-bearing exercise on bone mineral density: A study of female ex-elite athletes and the general population. J Bone Miner Res. 1996;11:1333–8. doi: 10.1002/jbmr.5650110918. [DOI] [PubMed] [Google Scholar]
- 44.Cherkas LF, Hunkin JL, Kato BS, et al. Physical acitivity in leisure time is associated with longer telomeres in leukocytes. Arch Intern Med. doi: 10.1001/archinternmed.2007.39. in press. [DOI] [PubMed] [Google Scholar]
- 45.De Lange M, Snieder H, Ariens RAS, Andrew T, Grant PJ, Spector TD. The relation between insulin resistance and hemostasis: pleiotropic genes and common environment. Twin Res. 2003;6:152–61. doi: 10.1375/136905203321536281. [DOI] [PubMed] [Google Scholar]
- 46.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 47.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–18. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 48.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49:26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 49.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 50.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 51.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 52.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006;61:871–3. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 53.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–7. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 54.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–81. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]