Summary
It has become increasingly clear that airway epithelial cells are central participants in innate and adaptive immune responses as well as mucosal inflammation. Epithelial cells produce antimicrobial host defense molecules, proinflammatory cytokines and chemokines in response to activation via pathogen recognition receptors. Recruitment of immune cells including dendritic cells, T cells and B cells into the proximity of epithelium results in the enhancement of adaptive immunity through interactions with epithelial cells. Newly identified epithelial-derived cytokines, including TSLP, IL-33 and BAFF, help to shape the local accumulation and activation of Th2 responses and B cell immunoglobulin production. Epithelial cells are also downstream targets of molecules that activate IL-13R and EGFR and are responsible for mucus production in both protective immune responses and allergic airway inflammatory diseases. Improved understanding of epithelial immune and inflammatory responses will hopefully suggest new strategies for therapeutic intervention.
Introduction
A long-recognized property of airway epithelial cells is their function as a complex physical barrier that defends against exposures to potentially harmful inhaled substances and microbial pathogens. It is now clear that airway epithelial cells also play crucial roles in initiating and augmenting airway host defense mechanisms. Epithelial cells, which are positioned at the line of first exposure to many pathogens, regulate both innate and adaptive immunity through production of functional molecules and via physical interactions with cells of the immune system. Activation of epithelial cells can result in immediate host defense responses that exclude pathogens, as they are induced to produce host defense molecules including antimicrobial and antiviral proteins along with proinflammatory cytokines that can activate other mucosal innate immune cells. Activation of the innate immune response secondarily induces recruitment of immune cells into epithelium to initiate adaptive immunity. In contrast, prolonged and/or robust epithelial activation can result in the release of large quantities of proinflammatory cytokines, growth factors and chemokines that attract inflammatory cells and that initiate and sustain airway inflammatory diseases such as asthma. The role of the airway epithelium in the pathogenesis of airway inflammatory diseases has been extensively studied and is well established.
Bronchial asthma is a classic Th2 disease that is characterized by prolonged epithelial activation associated with exposure to allergens to which the subject has been sensitized. Following activation by T cell cytokines, epithelial cells release large quantities of proinflammatory cytokines, growth factors and chemokines, amplifying the influx of T cells, eosinophils, basophils and other inflammatory cells. This inflammation results in the associated pathological features of airway hyperresponsiveness, hyperplasia/metaplasia of goblet cells and subepithelial fibrosis. The purpose of this review is to discuss several recently recognized functions of epithelial cells in innate and adaptive immune responses that go far beyond the inflammatory role of epithelial cells and to put them in the context of allergic airways disease. Emphasis will be placed on newly identified epithelial innate immune effector responses and regulation of the activation of dendritic cells (DC), T cells and B cells. Finally, we discuss two recently recognized pathways by which the products of infiltrating immune and inflammatory cells activate epithelial cells to induce pathogenic changes in allergic inflammation.
Epithelium and innate immune recognition
The mammalian immune system is comprised of two branches, the innate immune system and the adaptive immune system, that work in tandem to provide resistance to infection. The innate immune response is the first line of host defense and is responsible for immediate recognition and control of microbial invasion. The innate immune response relies on evolutionarily ancient germline-encoded receptors, the pattern-recognition receptors (PRRs), which recognize highly conserved microbial structures [1–3]. This genetically encoded recognition system enables the host to recognize a broad range of pathogens quickly, without the need for time-consuming somatic hypermutation of receptors on T cells or immunoglobulin genes. PRRs recognize microbial components, known as pathogen-associated molecular patterns (PAMPs), which are essential for the survival of the microorganism and relatively invariant. A breakthrough in the understanding of the ability of the innate immune system to rapidly recognize pathogens occurred with the discovery of the Toll-like receptors (TLRs).
TLRs were originally identified as homologues of Drosophila Toll. To date, 10 human TLRs have been identified [1–3]. TLRs are type I integral membrane glycoproteins characterized by a recognition domain containing varying numbers of leucine-rich-repeat (LRR) motifs and a transmembrane signaling domain homologous to that of the interleukin 1 receptor (IL-1R), termed the Toll/IL-1R homology (TIR) domain. TLR can be located on the plasma membrane or on intracellular vesicles, depending on the type of pathogen that is to be recognized (Figure 1). TLR signaling can lead to activation of several transcription factors, including NF-κB and interferon (IFN) regulatory factors (IRFs), especially IRF-3, IRF-5 and IRF-7. The activation of TLR signaling pathways originates from the cytoplasmic TIR domains of TLR. Four TIR domain containing adaptors that play an important role in TLR signaling have been identified; myeloid differentiation factor 88 (MyD88), TIR-associated protein (TIRAP)/MyD88-adaptor-like (MAL), TIR-domain-containing adaptor protein-inducing IFN-β (TRIF)/TICAM1 and TRIF-related adaptor molecule (TRAM)/TICAM2. MyD88 is critical for signaling by all TLRs except TLR3. Upon stimulation, MyD88 associates with the cytoplasmic portion of TLRs and then recruits IL-1R-associated kinase 4 (IRAK-4), IRAK-1, TNFR-associated factor 6 (TRAF6) and TGF-β-activated kinase-1 (TAK1) that activate NF-κB. TLR3 directly associates with TRIF, whereas TLR4 requires another adapter, TRAM, for the association with TRIF. TRIF recruits TAK1 and TANK-binding kinase 1 (TBK1) that activate NF-κB and IRF-3 respectively. The activation of NF-κB induces proinflammatory genes such as TNF, IL-6 and IL-12. In contrast, the activation of IRF-3 induces production of type I IFN and induces an anti-viral response. Airway epithelial cells have been shown to express mainly TLR2-6 [4,5]. TLR2, which is heterodimerized with TLR1 or TLR6, recognizes bacterial components such as lipoprotein, peptidoglycan (PGN) and lipoteichoic acid, GPI anchor from protozoa and some viral envelope proteins such as those expressed by measles virus and human cytomegalovirus. TLR3 recognizes viral double-stranded RNA (dsRNA) and a synthetic analog of dsRNA, polyinosine-polycytidylic acid. TLR4 recognizes lipopolysaccharide (LPS) from gram-negative bacteria and a protein that mediates membrane fusion from respiratory syncytial virus. TLR5 recognizes flagellin, which is a component of bacterial flagella. These particular TLRs are thus very strategically positioned on epithelial cells to enable recognition of organisms that are frequently encountered on the mucosal surface and may threaten the host upon initial exposure. The responses that they initiate can usually result in elimination of the organism without the need for the participation of the adaptive immune response (see below).
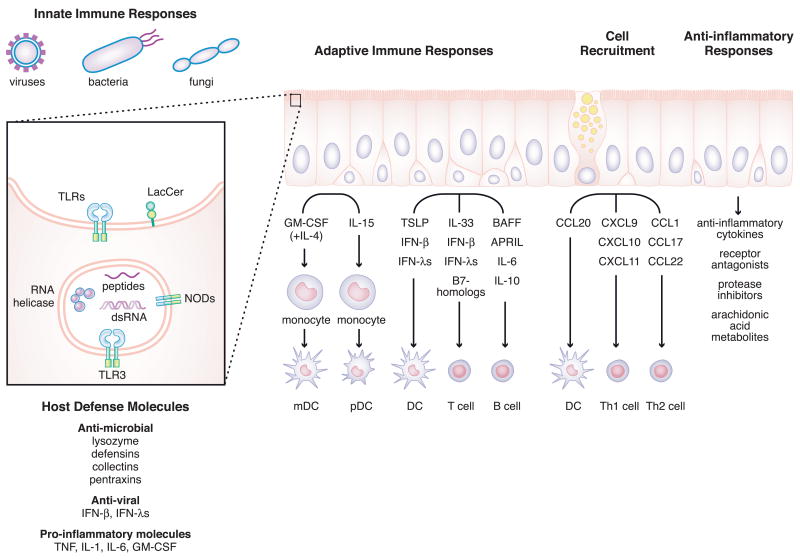
Figure 1. Model summarizing the influence of epithelial cells on innate and adaptive immune responses as well as anti-inflammatory processes in the airways.
The left part of figure shows the expression profiles of PRRs in the epithelium. TLRs and lactosylceramide recognize pathogens on the cell surface. In contrast, TLR3, NODs and RNA helicases recognized pathogens intracellularly. After activation of PRRs, epithelial cells produce a wide range of molecules which enhance innate and adaptive immune responses, cell recruitment and mediate anti-inflammatory responses.
Cytosolic pathogen recognition receptors distinct from the TLR have been recently identified [1–3]. Two cytoplasmic RNA helicases, melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-inducible gene I (RIG-I), are expressed in airway epithelial cells and are thought to detect viral dsRNA independent of TLR3 (Figure 1) [6,7]. IFN-β stimulator 1 (IPS-1) is a critical adapter molecule that can interact with RIG-I and MDA5 that activates NF-κB and IRF-3 through FADD/RIP1 and TBK1 respectively. In addition, some members of the NACHT-LRR proteins (NLRs) family, which include both nucleotide-binding oligomerization domain (NOD) proteins and NACHT-LRR- and pyrin-domain-containing proteins (NALPs), recognize microbial components in the cytosol (Figure 1) [1,2,8]. NOD1 and NOD2, which recognize PGN-derived peptides,γ-D-glutamyl-meso-diaminopimelic acid and muramyl dipeptide (MDP) respectively, have been reported to be expressed in airway epithelial cells [9]. Ligand recognition by the NODs induces recruitment of the CARD-containing serine/threonine kinase RICK (also called RIP2), which leads to NF-κB activation to induce proinflammatory genes. Synergy between NOD ligands and the cytokine IL-32, a recently identified epithelial-derived cytokine, has been observed [10]. Dectin-1, which recognizes β-glucans from fungi, is known to be an important PRR for antifungal immunity, although some TLRs also recognize fungal products [11]. Although it has been shown that β-glucan activates alveolar epithelial cells involving the participation of lactosylceramide (CDw17), dectin-1 was not found to be expressed (Figure 1) [12]. Epithelial cells are frequently the first, or only, target of infection by respiratory viruses, bacteria and fungi. While it is clear that epithelial cells express a host of PRR, more information is sorely needed on this topic, as expression of multiple viral, bacterial and fungal recognition systems in epithelium provides great potential for local protection of the epithelial lining as well as the respiratory system and the circulation of the host.
Epithelium and Host defense
Airway epithelial cells secrete a host of molecules from several families that are involved in the protection against infection by bacteria, viruses and fungi. Major antimicrobial products secreted constitutively and/or inducibly by epithelial cells include lysozyme, lactoferrin, defensins, collectins, pentraxins, LL-37, secretory leukocyte protease inhibitor (SLPI) and serum amyloid A (SAA) (Figure 1) [13]. Mechanistically, these molecules can be divided into enzymes, permeabilizing peptides, opsonins, protease inhibitors, toxic small molecules and macromolecules that bind and neutralize potential pathogens. The pathologic deficiency or inappropriate regulation of the anti-microbial properties of airway secretions may contribute to epithelial colonization by microorganisms. Allergic inflammation (i.e. that involving activation of Th2 cells and release of IL-4, IL-5 and IL-13) is linked with impaired antibacterial responses. For example, nearly all patients with atopic dermatitis have colonization of the skin by staphylococcus aureus (S. aureus), whereas this is rare in people with normal skin [14]. Likewise, the vast majority of patients with severe polypoid chronic rhinosinusitis have S. aureus colonization in their upper airways while only a fourth of normal subjects carry nasal S. aureus [15]. Experimentally, recent findings show that exposure of airway epithelial cells to the Th2 cytokines IL-4 and IL-13 resulted in a significant decrease in antimicrobial activity of the cells and suppressed mRNA levels of the antimicrobial peptide human β-defensin 2 but not human β-defensin 1 or LL-37 [16]. Furthermore, mice with allergic airway inflammation had significantly more viable bacteria in their lungs after infection. Some variability in susceptibility to infection might reflect the association of Th2 responses with anti-parasite immunity, Th1 responses with antiviral and intracellular pathogen responses and Th17 responses with extracellular bacterial infections and the complex counter-regulation among these responses.
Disease exacerbations of asthma (“asthma attacks”) are usually triggered by infection with airway-targeting viruses. The most common pathogens associated with asthma exacerbations are rhinoviruses, which can induce intense inflammation in both upper and lower airways and often increase bronchial responsiveness to bronchoconstrictors. Expression of the type I IFN, IFN-β, and type III IFNs, IFN-λ1 (IL-29) and IFN-λ2/3 (IL-28A/B), is important in the anti-viral response of the epithelium. TLR3 and RNA helicases are important sensors for detection of viral infection and induce production of IFN-β and IFN-λ in epithelial cells [4–7]. Type II IFN, IFN-γ, which is produced from activated T cells and NK cells, also activates IFN-λ production in epithelial cells, probably amplifying the antiviral effects of specific T cells. Induction of IFN-β and IFN-λs by rhinovirus infection is deficient in the airway epithelial cells of asthmatics and the extent of the deficiency is highly correlated with the severity of rhinovirus induced asthma exacerbations [17,18]. While the mechanism is still unknown, the epithelial defect in production of IFN-β and IFN-λs in response to rhinovirus is likely to play a role in the pathogenesis of asthma exacerbations.
Interactions of epithelial cells with DC
Airway epithelial cells not only mediate and activate innate immune responses but also regulate adaptive immune responses through interactions with DC, T cells and B cells. Local and infiltrating DC have a vital role in the initiation of adaptive immune responses to inhaled foreign antigens. Airway epithelial cells are capable of inducing DC migration into epithelium via CCL20 (MIP-3α) production (Figure 1) [19]. CCL20 is the only chemokine known to interact with CCR6 that is expressed by immature DC and Langerhans cells; this chemokine also has antimicrobial properties that it shares with defensins [20]. CCL20 production by epithelial cells is induced by several stimuli, including the cytokines TNF, IL-1, IL-4, IL-13 and IL-17, PAMPs including dsRNA, PGN and KpOmpA, allergens such as house dust mite allergen Der p1, and extracellular nucleotides [4,21–25]. Recruitment of DC to the airway is essential in the adaptive B, T and NK cell-mediated immune responses that are important in defense against infection by viruses and bacteria and in inflammation of the airway [26,27]. Infection of CCR6−/− mice with A. fumigatus leads to a more severe infection than in wild type mice, associated with reduced accumulation of DC in the lung following challenge [27]. CCR6−/− mice also have reductions in airway hyperreactivity, peribronchial DC and eosinophils, and serum IgE levels after antigen challenge, supporting the role of DC-expressed CCR6 (and CCL20 from epithelial cells and other cells) in allergic inflammation [26].
Although epithelial DCs are recruited from the blood, circulating DC numbers are low and it is likely that attraction and differentiation of monocytes from the blood is the main mechanism of expansion of DC in the lung. This process is driven by cytokines secreted by resident pulmonary cells including epithelial cells. The mechanisms of DC differentiation from monocytes are incompletely understood. Based on in vitro studies, it is reasonable to expect that immature myeloid DCs are generated from monocytes in the epithelium under the influence of GM-CSF derived from epithelial (and other) cells and IL-4 from Th2 cells, NKT cells or basophils. One recent study shows that IL-15 is produced by epithelial cells, and that it induces the differentiation of DC from monocytes without participation of GM-CSF [28]. Based on the cell surface expression of CD123, BDCA2 and BDCA4, IL-15 dependent epithelial cell culture medium-induced DC have phenotypic characteristics of plasmacytoid DC. The available data suggest that epithelial cells, in collaboration with lymphocytes, influence the local differentiation of monocytes into both myeloid and plasmacytoid DC (Figure 1).
Recent studies have shown that airway epithelial cells also influence DC control of Th differentiation locally via production of the DC activator thymic stromal lymphopoietin (TSLP) (Figure 1) [29–32]. TSLP is an IL-7-like cytokine molecule that was first isolated from a murine thymic stromal cell line and was shown to support B cell development [31–33]. The TSLP receptor is a heterodimeric receptor consisting of the IL-7 receptor alpha chain (IL7RA) and a common γ-like TSLP specific receptor (TSLP receptor (TSLPR)) which is expressed on DC in human and on early stages of B cells and T cells in mouse [32,34]. TSLP has recently been shown to be an important cytokine that induces DC mediated Th2 differentiation. In fact, TSLP-stimulated DC induce naive CD4+ T cells to differentiate into Th2 cells that produce IL-4, IL-13 and TNF, but not IL-10 and IFN-γ. TSLP strongly induces expression of major histocompatibility class I and II and costimulatory molecules such as CD40, CD80 and CD86 on DC. A key regulator of Th2 differentiation may be OX40L, which is a TNF ligand family member that is induced by TSLP but not by other DC stimulators including TLR ligands and IFNs [29,30]. Recent studies show that epithelial-derived TSLP also activates mast cells to produce cytokines including IL-5, IL-6, IL-13 and GM-CSF and chemokines including CCL1 (I-309) and CXCL8 (IL-8) only in the presence of IL-1β and TNF [35]. Taken together, these data suggest that epithelium may enhance allergic airway inflammation by inducing DC-mediated Th2 differentiation and also mast cell activation via the production of TSLP.
Considerable evidence implicates TSLP in the pathogenesis of inflammatory airway diseases [32]. TSLP has been found to be increased in the airways of asthmatic subjects [36]. High amounts of TSLP have been found in BAL fluid in a mouse asthma model and lung-specific expression of TSLP in transgenic mice induces airway inflammation including massive infiltration of eosinophils and other inflammatory cells, goblet cell hyperplasia and airway hyperresponsiveness, whereas mice lacking the TSLPR exhibit strong Th1 responses and fail to develop an inflammatory lung response to antigen [37,38].
Due to the potential importance of TSLP in airway disease, the regulation of TSLP production in airway epithelial cells has recently been investigated by several laboratories. TLR3 ligation by dsRNA and infection with the common cold respiratory virus rhinovirus are strong stimuli for TSLP production, working through NF-κB and IRF-3 activation in epithelial cells [39]. In addition, inflammatory cytokines (IL-1β and TNF), Th2 cytokines (IL-4 and IL-13) and some other TLR including TLR2 also activate TSLP induction in the epithelium [35,39–41]. Importantly, IL-4 synergistically enhances dsRNA- and rhinovirus-dependent TSLP production in airway epithelial cells [39]. This implies that respiratory viral infection in an asthmatic subject who already has activated Th2 cytokine-producing cells in the airway mucosa may further amplify Th2 inflammation via induction of TSLP. This finding may help explain why respiratory viruses exacerbate allergic inflammation despite the fact that they induce Th1-type immune responses that can theoretically counterbalance Th2 mediated inflammation.
Interaction of epithelial cells with T cells
Airway epithelial cells induce migration of Th1 cells into mucosae via production of the CXCR3 ligands, CXCL9 (Mig), CXCL10 (IP-10) and CXCL11 (I-TAC), and induce migration of Th2 cells via production of the CCR8 ligand CCL1 (I-309) and the CCR4 ligands CCL17 (TARC) and CCL22 (MDC) (Figure 1) [42–47]. CXCR3 is expressed on activated T cells, predominantly of the Th1 phenotype, as well as on NK cells and a subset of circulating memory CD4+ and CD8+ T cells. CXCR3 ligands are well known to be induced by the Th1 cytokine IFN-γ and epithelial cells are the main source of these ligands in the airways. CXCR3 ligands are also induced in epithelial cells by type I IFNs, type III IFNs, dsRNA, respiratory virus and bacterial infection. In addition to chemoattractant activity, CXCL9, CXCL10 and CXCL11 possess antibacterial activity [20,48]. These data suggest the presence of a feed-forward mechanism in which CXCR3 ligands are induced in epithelium by pathogens, leading to recruitment of Th1 cells which release IFN-γ that induces more CXCR3 ligands in epithelium, amplifying the Th1-type immune response.
A similar mechanism regulates Th2 responses; airway epithelial cells produce chemoattractants for Th2 cells that are further induced by Th2 cytokines. Some of these ligands are highly expressed in the epithelial cells from patients with atopic asthma [44,45]. One of these chemokines, CCL1, is the only chemokine known to interact with CCR8, a receptor that is expressed on Th2 cells and T regulatory (Treg) cells. CCL1 production is induced in airway epithelial cells by Th2 cytokines, IL-4 and IL-13, and also by IFN-γ [45]. CCR4, a receptor for the epithelial products CCL17 and CCL22, is also expressed on Th2 cells. CCL17 production is induced by IL-4, IL-13, TGF-β and the house dust mite allergen Der p in airway epithelial cells [46,47]. In addition, synergistic induction of CCL17 is induced by the combination of TNF with either Th2 cytokines, TGF-β or Der p antigen. It is notable that TNF is able to amplify both Th1 and Th2 inflammatory responses, and several recent studies have demonstrated the value of TNF antagonists in asthma [49]. Taken together, in vitro and in vivo studies suggest that epithelial cells may control and amplify both Th1- and Th2-type inflammatory responses locally.
Airway epithelial cells not only recruit T cells into epithelium but also interact with T cells via expression of cell surface molecules and production of cytokines (Figure 1). Epithelial cells weakly express cell surface molecules typically associated with T cell interactions including HLA-DR, CD40, Fas and Fas ligand and most of these are induced by T cell cytokines. Epithelial cells also constitutively express costimulatory molecules, B7-H1, B7-H2, B7-H3 and B7-DC [50]. Levels of expression of B7-H1 and B7-DC are increased by exposure to T cell cytokines including IFN-γ. Although the effect of costimulatory molecules on epithelial cells is not fully understood, expression of B7-H1 and B7-DC on epithelial cells affects T cell responses. Recent studies suggest that one important function of B7-H1 is the induction of T regulatory cells, and the strong expression of B7-H1 in activated epithelium in vivo may reflect part of a feedback inhibitory mechanism [50–52].
Recently, IL-33, an IL-1 family member, has been identified as a ligand for ST2 [53]. ST2, an IL-1R-related protein specifically expressed on Th2 cells, has been shown to function as an important effector molecule of Th2 responses in a number of experimental settings including mouse asthma models. IL-33 is produced in a precursor form by airway epithelial cells, smooth muscle cells and fibroblasts, and can be cleaved by caspase-1. IL-33 enhances the production of IL-5 and IL-13 by Th2 cells but not by Th1 cells in vitro. Intraperitoneal administration of IL-33 to mice results in an allergic phenotype including blood eosinophilia, and increases in serum concentration of IL-5, IL-13, IgA and IgE. Administration of IL-33 also induces phathological changes in the lung and the digestive tract, including eosinophilic and mononuclear infiltrates, increased mucus production, and epithelial cell hyperplasia and hypertrophy [53].
Interaction of epithelial cells with B cells
Mucosal surfaces are continually exposed to potentially harmful substances including microbial pathogens. Local immunoglobulin class switch recombination (CSR) and production of IgA and IgE in the upper airway have been proposed to be important events in both protection from pathogens and the pathogenesis of airway allergic diseases in response to otherwise innocuous antigens. Although CSR is generally thought to be highly dependent on CD40-CD40L ligation, it is also reported that viral and bacterial products can induce immunoglobulin production in the absence of T cells or CD40-dependent signaling [54,55].
B cell-activating factor of the TNF family (BAFF; also known as BLyS) and a proliferation-inducing ligand (APRIL) are recently identified members of the TNF ligand superfamily that play important roles in B cell maturation, survival and proliferation. BAFF and APRIL also promote CD40-independent, T cell-independent CSR and immunoglobulin production via their receptors transmembrane activator and CAML interactor (TACI) and BAFF specific receptor (BAFF-R) [55–58]. While early reports suggested that BAFF is produced mainly by myeloid cells, airway epithelial cells also produce BAFF following stimulation with the TLR3 ligand dsRNA, interferons and TNF (Figure 1) [59].
In the tonsillar mucosa, epithelial cells as well as DC make BAFF in the presence of microbial products [40]. Epithelial cell-derived BAFF and IL-10 stimulate B cells to induce CSR and ultimately secrete polyreactive IgG and IgA against multiple microbial determinants. According to recent studies, epithelial cell-dependent B cell CSR is reinforced by DC production of BAFF induced by TSLP (derived from epithelial cells) and is restrained by SLPI, an epithelial homeostatic protein that inhibits BAFF signaling in B cells [40]. Once IgA and IgM are produced by mucosal B cells, mucosal epithelial cells act as transporters of the polymeric forms of these immunoglobulins via the polymeric Ig receptor (pIgR) [60]. Taken as a whole, these recent findings suggest that epithelial products can activate B cell CSR and production of IgG and IgA during the host response to pathogens. In view of the fact that IL-4 and IL-13 are increased in asthmatic airways, epithelial BAFF may also contribute to local production of IgE and may therefore be involved in the pathogenesis of allergic airway inflammatory diseases.
STAT6-dependent airway inflammation and mucus production
In vivo studies in mice have indicated that allergen- and IL-13-induced airway inflammation are dependent on the activation of signal transducer and activator of transcription 6 (STAT6) in the airway epithelial cells. Although epithelial cells are involved in regulation of recruitment and activation of DC, T cells and B cells (see above) and eosinophils, neutrophils and basophils (not discussed), other important responses of epithelium in immunity and disease are the formation of fibrogenic responses in the lamina reticularis adjacent to the airway basement membrane and the differentiation to a mucus-secreting phenotype, notably goblet cells.
Allergen- and IL-13-induced mucus production in mouse airways are critically dependent upon the expression of both the IL-13 receptor and the IL-13 signaling molecule STAT6 in airway epithelial cells. Thus, many researchers screened IL-4/IL-13-inducible genes in airway epithelial cells over the past few years to identify new pathways and factors involved in goblet cell metaplasia, a key event in the pathogenesis of asthma. One group searched for genes that are induced by allergen and by direct effects of IL-13 on airway epithelial cells and then analyzed expression of these same genes in airway epithelial cells derived from patients with asthma [61]. They identified four genes, chloride channel calcium activated 1, intelectin, 15-lipoxygenase and trefoil factor 2, that were increased in both mouse models and in epithelial cells from asthmatic patients. In another study it was found that the adipocyte fatty acid-binding protein aP2 is strongly upregulated by IL-4 and IL-13 in airway epithelial cells (Figure 2) [62]. Allergen-induced airway eosinophilia and pulmonary inflammatory cytokine production are significantly reduced in aP2−/− mice. Recent studies demonstrate that aP2 can bind the 5-lipoxygenase product leukotriene A4 (LTA4) and markedly extend its half-life [62,63]. Thus, epithelial aP2 may regulate allergic airway inflammation and may provide a link between fatty acid metabolism and asthma. SAM pointed domain-containing ETS transcription factor (SPDEF) is a newly identified key regulator of Th2 cytokine-dependent goblet cell hyperplasia that transdifferentiates clara cells to goblet cells [64]. IL-13 treatment caused goblet cell hyperplasia in association with increased SPDEF staining in control but not in STAT6−/− mice. Goblet cell hyperplasia and increased SPDEF staining were observed following repeated intratracheal administration of dust mite allergen to wild-type mice but were not observed in treated IL-13−/−mice. SPDEF interacted with thyroid transcription factor 1 (TTF-1) and then additively or synergistically activated gene transcription of FOXJ1, SRY-box containing gene 17 (SOX17), secretoglobin 1a1 (SCGB1A1, also known as CC10), and surfactant protein A (SP-A) but did not activate the MUC5AC gene promoter (Figure 2) [64]. Thus, the transcription factor SPDEF regulates STAT6-dependent mucus production, but it requires the participation of at least one additional molecule to induce MUC5A/C expression.
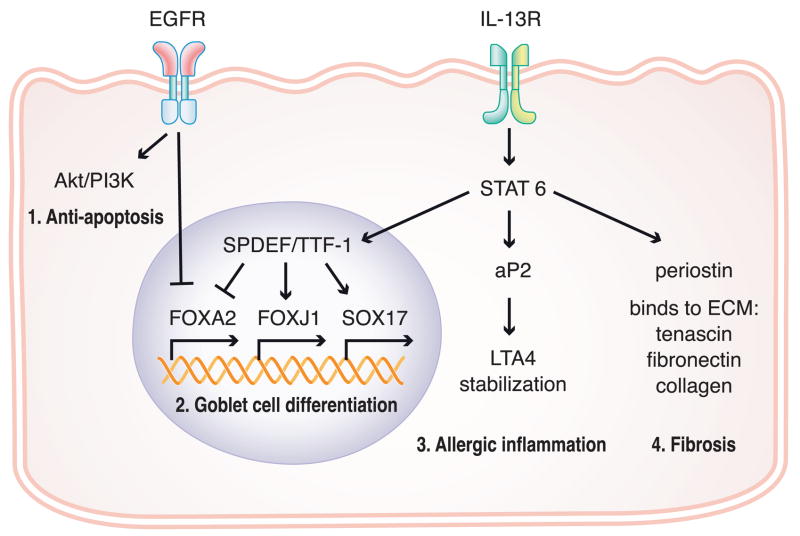
Figure 2. Schematic of recently identified pathways of airway inflammation and goblet cell differentiation in epithelial cells.
1. EGFR signaling induces the activation of PI3K/Akt to inhibit ciliated cell and Clara cell apoptosis. 2. EGFR signaling and IL-13 signaling regulate goblet cell differentiation. 3. IL-13 signaling induces adipocyte fatty acid-binding protein aP2 that regulates allergic inflammation. 4. IL-13 signaling induces periostin which can bind to the extracellular matrix proteins to induce fibrosis.
In addition to the IL-13-STAT6 pathway, epidermal growth factor receptor (EGFR) signaling is also known to be a key regulator for mucus production. Recent studies showed that both IL-13 and EGFR signaling are necessary for mucus production but differentially regulate the various mucus producing cell types [65,66]. EGFR signaling induces phosphorylation and activation of PI3K/Akt to inhibit ciliated cell and Clara cell apoptosis (Figure 2). In addition, the transcription factor FOXA2, which is known to prevent mucus production, is down-regulated by both IL-13 signaling and EGFR signaling (Figure 2). As far as fibrosis is concerned, periostin, a secreted protein originally isolated from an osteoblast cell line, was identified to be an IL-4 and IL-13 inducible gene in airway epithelial cells [67]. Periostin can bind to the extracellular matrix proteins tenascin-C, fibronectin and collagens, which are involved in fibrosis (Figure 2). Periostin−/− mice have a significant reduction in collagen fibril diameter [68]. These studies suggest that periostin is a novel component of subepithelial fibrosis in bronchial asthma.
Anti-inflammatory effects of epithelium
Epithelial cells play roles in the regulation of inflammation by production of several families of anti-inflammatory molecules, including cytokines (IL-10, TGF-β), soluble cytokine receptors/receptor antagonists (sIL-1RN, sIL-13RA2, sTNFR1), protease inhibitors (SLPI, SERPINA1 (α1-antitrypsin), SERPINB1, TIMP-1), inhibitory arachidonic acid metabolites (PGE2, PGI2, lipoxin A4) and others (CC10, SP-A, etc). Epithelial cells also express anti-inflammatory or immunosuppressive cell surface molecules including B7-H1, B7-DC, IL-13RA2 and FASL (Figure 1) [50,64,69–76]. Many of these molecules are induced by proinflammatory cytokines and Th2 cytokines, suggesting that they may be regulated by negative feedback pathways that dampen inflammatory signaling [69,71]. An imbalance of pro-inflammatory responses and anti-inflammatory responses in the epithelium may induce several inflammatory diseases.
Conclusions
Airway epithelial cells act first as a physical barrier that protects against inhaled substances and pathogens. It is now becoming increasingly clear that airway epithelial cells express PRRs that recognize microbial pathogens and activate innate host defense mechanisms in the airway. Activation of innate immunity in the epithelium secondarily induces recruitment and activation of DC, T cells and B cells that amplify antigen recognition, antibody production and other adaptive immune responses. Local T cell responses and immunoglobulin production are quite important both for protection from pathogens and in the pathogenesis of various types of inflammatory diseases of the airways. These responses involve mechanisms that include epithelial production of chemokines and cytokines such as BAFF, TSLP, SLPI and IL-33, products that allow epithelial cells to communicate with B cells, T cells and DC. In allergic airway diseases, epithelial anti-bacterial and anti-viral functions may be compromised, enhancing the tendency for pathogens to induce adaptive immune and inflammatory responses. Continuous activation of epithelial cells promotes differentiation to mucus producing cells and stimulates fibrogenic processes via activation of signaling pathways involving receptors for IL-13 and EGF. Considering the accessibility of epithelium to inhaled drugs, further elucidation of these mechanisms holds promise for the development of novel strategies for therapeutic intervention.
Acknowledgments
This research is supported in part by NIH grants, R01 HL068546, R01 HL078860 and 1R01 AI072570 and by a grant from the Ernest S. Bazley Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1••.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. This review describes the mechanism of pathogen recognition by PRRs including TLRs. [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–80. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 4.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 5.Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 2007;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 9.Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 12.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol. 2005;32:490–497. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schleimer RP, Lane AP, Kim J. Innate and acquired immunity and epithelial cell function in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:51–78. [PubMed] [Google Scholar]
- 14.Cardona ID, Cho SH, Leung DY. Role of bacterial superantigens in atopic dermatitis : implications for future therapeutic strategies. Am J Clin Dermatol. 2006;7:273–279. doi: 10.2165/00128071-200607050-00001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Gevaert P, van Zele T, Perez-Novo C, Patou J, Holtappels G, van Cauwenberge P, Bachert C. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology. 2005;43:162–168. [PubMed] [Google Scholar]
- 16.Beisswenger C, Kandler K, Hess C, Garn H, Felgentreff K, Wegmann M, Renz H, Vogelmeier C, Bals R. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J Immunol. 2006;177:1833–1837. doi: 10.4049/jimmunol.177.3.1833. [DOI] [PubMed] [Google Scholar]
- 17.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 18.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upham JW, Stick SM. Interactions between airway epithelial cells and dendritic cells: implications for the regulation of airway inflammation. Curr Drug Targets. 2006;7:541–545. doi: 10.2174/138945006776818647. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 21.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 22.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 23.Pichavant M, Taront S, Jeannin P, Breuilh L, Charbonnier AS, Spriet C, Fourneau C, Corvaia N, Heliot L, Brichet A, et al. Impact of bronchial epithelium on dendritic cell migration and function: modulation by the bacterial motif KpOmpA. J Immunol. 2006;177:5912–5919. doi: 10.4049/jimmunol.177.9.5912. [DOI] [PubMed] [Google Scholar]
- 24.Pichavant M, Charbonnier AS, Taront S, Brichet A, Wallaert B, Pestel J, Tonnel AB, Gosset P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J Allergy Clin Immunol. 2005;115:771–778. doi: 10.1016/j.jaci.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Marcet B, Horckmans M, Libert F, Hassid S, Boeynaems JM, Communi D. Extracellular nucleotides regulate CCL20 release from human primary airway epithelial cells, monocytes and monocyte-derived dendritic cells. J Cell Physiol. 2007;211:716–727. doi: 10.1002/jcp.20979. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med. 2001;194:551–555. doi: 10.1084/jem.194.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phadke AP, Akangire G, Park SJ, Lira SA, Mehrad B. The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis. Am J Respir Crit Care Med. 2007;175:1165–1172. doi: 10.1164/rccm.200602-256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regamey N, Obregon C, Ferrari-Lacraz S, van Leer C, Chanson M, Nicod LP, Geiser T. Airway epithelial interleukin-15 transforms monocytes into dendritic cells. Am J Respir Cell Mol Biol. 2007;37:75–84. doi: 10.1165/rcmb.2006-0235OC. [DOI] [PubMed] [Google Scholar]
- 29.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. This is a review compiling all the available data about TSLP in the regulation of immunity and in the pathogenesis of inflammatory diseases. [DOI] [PubMed] [Google Scholar]
- 33.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 34.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 35.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 37.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. This article describes the role of TSLP in allergic airway inflammation using TSLPR deficient mice and lung specific TSLP transgenic mice. [DOI] [PubMed] [Google Scholar]
- 39•.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. This article describes the regulation of TSLP in airway epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, Ding A, Knowles DM, Santini PA, Cerutti A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. This article describes the first observation that mucosal CSR is triggered by epithelial cytokines, BAFF, TSLP and SLPI in tonsils. [DOI] [PubMed] [Google Scholar]
- 41.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 43.Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- 44.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montes-Vizuet R, Vega-Miranda A, Valencia-Maqueda E, Negrete-Garcia MC, Velasquez JR, Teran LM. CC chemokine ligand 1 is released into the airways of atopic asthmatics. Eur Respir J. 2006;28:59–67. doi: 10.1183/09031936.06.00134304. [DOI] [PubMed] [Google Scholar]
- 46.Terada N, Nomura T, Kim WJ, Otsuka Y, Takahashi R, Kishi H, Yamashita T, Sugawara N, Fukuda S, Ikeda-Ito T, et al. Expression of C-C chemokine TARC in human nasal mucosa and its regulation by cytokines. Clin Exp Allergy. 2001;31:1923–1931. doi: 10.1046/j.1365-2222.2001.01152.x. [DOI] [PubMed] [Google Scholar]
- 47.Heijink IH, Marcel Kies P, van Oosterhout AJ, Postma DS, Kauffman HF, Vellenga E. Der p, IL-4, and TGF-beta cooperatively induce EGFR-dependent TARC expression in airway epithelium. Am J Respir Cell Mol Biol. 2007;36:351–359. doi: 10.1165/rcmb.2006-0160OC. [DOI] [PubMed] [Google Scholar]
- 48.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 49.Berry M, Brightling C, Pavord I, Wardlaw A. TNF-alpha in asthma. Curr Opin Pharmacol. 2007;7:279–282. doi: 10.1016/j.coph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, McDyer JF, Fortuno L, Schleimer RP. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;33:280–289. doi: 10.1165/rcmb.2004-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 52.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. This article describes the discovery and characterization of the novel cytokine IL-33 which is a ligand for ST2. [DOI] [PubMed] [Google Scholar]
- 54.Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 55•.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. This article describes T cell- and CD40-independent CSR mediated by BAFF and APRIL derived from DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. This review updates the biological function of BAFF and APRIL in B cells and highlights the pathological role of BAFF and APRIL in various diseases. [DOI] [PubMed] [Google Scholar]
- 59•.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. This article describes the first observation that airway epithelial cells are capable of producing BAFF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 61.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmer JS, Dyckes DF, Bernlohr DA, Murphy RC. Fatty acid binding proteins stabilize leukotriene A4: competition with arachidonic acid but not other lipoxygenase products. J Lipid Res. 2004;45:2138–2144. doi: 10.1194/jlr.M400240-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. This article describes how IL-13 and EGFR regulate differentiation of mucus-producing cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 68.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 70.Salvi S, Holgate ST. Could the airway epithelium play an important role in mucosal immunoglobulin A production? Clin Exp Allergy. 1999;29:1597–1605. doi: 10.1046/j.1365-2222.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 71.Levine SJ, Wu T, Shelhamer JH. Extracellular release of the type I intracellular IL-1 receptor antagonist from human airway epithelial cells: differential effects of IL-4, IL-13, IFN-gamma, and corticosteroids. J Immunol. 1997;158:5949–5957. [PubMed] [Google Scholar]
- 72.Matsumura M, Inoue H, Matsumoto T, Nakano T, Fukuyama S, Matsumoto K, Takayama K, Saito M, Kawakami K, Nakanishi Y. Endogenous metalloprotease solubilizes IL-13 receptor alpha2 in airway epithelial cells. Biochem Biophys Res Commun. 2007;360:464–9. doi: 10.1016/j.bbrc.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 73.Levine SJ, Adamik B, Hawari FI, Islam A, Yu ZX, Liao DW, Zhang J, Cui X, Rouhani FN. Proteasome inhibition induces TNFR1 shedding from human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L233–243. doi: 10.1152/ajplung.00469.2004. [DOI] [PubMed] [Google Scholar]
- 74.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O’Donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L619–627. doi: 10.1152/ajplung.00507.2005. [DOI] [PubMed] [Google Scholar]
- 75.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]