Abstract
Background
Disease management programs (DMPs) that use multidisciplinary teams and specialized clinics reduce hospital admissions and improve quality of life and functional status. Evaluations of cardiac DMPs delivered by home health nurses are required.
Methods
Between August 1999 and August 2000 we identified consecutive patients admitted to hospital with elevated cardiac enzymes. Patients who agreed were randomly assigned to participate in a DMP or to receive usual care. The DMP included 6 home visits by a cardiac-trained nurse, a standardized nurses' checklist, referral criteria for specialty care, communication with the family physician and patient education. We measured readmission days per 1000 follow-up days for angina, congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD); all-cause readmission days; and provincial claims for emergency department visits, physician visits, diagnostic or therapeutic services and laboratory services.
Results
We screened 715 consecutive patients admitted with elevated cardiac markers between August 1999 and August 2000. Of those screened 71 DMP and 75 usual care patients met the diagnostic criteria for myocardial infarction, were eligible for visits from a home health nurse and consented to participate in the study. Readmission days for angina, CHF and COPD per 1000 follow-up days were significantly higher for usual care patients than for DMP patients (incidence density ratio [IDR] = 1.59, 95% confidence interval [CI] 1.27–2.00, p < 0.001). All-cause readmission days per 1000 follow-up days were significantly higher for usual care patients than for DMP patients (IDR = 1.53, 95% CI 1.37–1.71, p < 0.001). The difference in emergency department encounters per 1000 follow-up days was significant (IDR = 2.08, 95% CI 1.56–2.77, p < 0.001). During the first 25 days after discharge, there were significantly fewer provincial claims submitted for DMP patients than for usual care patients for emergency department visits (p = 0.007), diagnostic or therapeutic services (p = 0.012) and laboratory services (p = 0.007).
Interpretation
The results provide evidence that an appropriately developed and implemented community-based inner-city DMP delivered by home health nurses has a positive impact on patient outcomes.
The World Health Organization has called for the implementation of integrated management approaches to service delivery to reduce the worldwide burden of chronic diseases.1 Disease management2,3,4 is one innovative approach designed to improve the quality and reduce the cost of caring for people. Hunter and Fairfield5 define disease management as “a combination of patient education, provider use of practice guidelines, appropriate consultation, and supplies of drugs and ancillary services.” McAlister and colleagues6 identified 12 randomized clinical trials that examined the efficacy of disease management programs (DMPs). Patients randomly assigned to a DMP that used multidisciplinary teams and specialized clinics were less likely to be admitted to hospital. These programs also improved their quality of life and functional status.
Extrapolation from studies set in specialized clinics to nonclinic settings is not sufficient because some evidence indicates that home-based cardiac programs may have an adverse impact on outcomes. Frasure-Smith and colleagues7 examined the impact of a home-based psychosocial nursing intervention for patients recovering from myocardial infarction (MI). For women, the all-cause mortality rate was 10.3% in the intervention arm and 5.4% in the control arm. For men, the all-cause mortality rate was 3.1% in both the intervention and the control arms.
We have completed a nonblinded comparative study that evaluates the impact of a community-based inner-city DMP for patients recovering from MI, delivered by home health nurses. We report the impact of a community-based DMP for postmyocardial infarction patients on readmission days per 1000 follow-up days for angina, congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD); emergency visits; and provincial claims for physician-office visits, diagnostic or therapeutic services and laboratory services. Angina, CHF and COPD are considered treatable in ambulatory settings; hospitalization for these conditions indicates a lack of access to high-quality primary care.8,9,10 Neither better management of these conditions nor improved access to primary care will eliminate all hospitalizations, but such steps could eliminate many of them.
Method
Patients were enrolled in this study at The Toronto East General and Orthopaedic Hospital (TEGH). TEGH, a member of Partners for Health that serves southeast Toronto, is a 407-bed acute care hospital, affiliated with the University of Toronto. TEGH discharged over 1000 MI patients in the 3 fiscal years between 1994/95 and 1996/97.11 The Partners for Health catchment population (about 330 000) is culturally diverse, and its income and education levels are lower than the average levels for the entire population of Toronto.
Those included in this study were admitted to TEGH between August 1999 and August 2000 with a confirmed diagnosis of MI,12 resident in the catchment area, assessed by a care coordinator as eligible for a visit from a home health nurse at no cost to the patient and continued to meet these criteria on discharge. Eligibility generally implied that the services were necessary to enable the patient to remain at home.
Patients who were transferred to an acute care or long-term care institution, who moved out of the catchment area after discharge or who withdrew their consent before discharge were no longer eligible for inclusion in the study. They were excluded from the analysis.
An analysis of historical data indicated that the number of postmyocardial readmission days for angina, CHF and COPD was 7 per 1000 follow-up days. We calculated that 65 patients were required for each treatment arm to detect a 50% reduction in readmission days per 1000 follow-up days (i.e., from 7.0 to 3.5 readmission days per 1000 follow-up days) for patients with angina, CHF and COPD, with an α of 0.05 and a β of 0.2.13
An independent consultant used a random-number generator to create the randomization sequence. An administrative assistant inserted cards reading “usual care” or “experimental care” into sequentially numbered envelopes and then sealed the envelopes. To ensure that the envelopes were opaque so that the care the next patient would receive could not be determined, blank index cards were inserted in front of and behind these cards. Research staff members were unaware of the random assignment before enrolling the patients. Once the patient's written informed consent was obtained the sealed envelope was opened and the patient was shown the card.
The treatment of the patients randomly assigned to the experimental care group was a DMP. The 4 components of the DMP were the standardized pathway, labelled “The nursing checklist,” the referral criteria for specialty care management, the communication systems, including the discharge summary and the nurses' visit report and patient education. DMP patients were eligible to receive a minimum of 6 home care visits from a cardiac-trained nurse. A complete description of the protocol is published elsewhere.14,15
Patients randomly assigned to receive usual care were referred to a noninvasive cardiac laboratory for diagnostic testing, followed up by their cardiologist and given information on TEGH's cardiac teaching class as well as cardiac rehabilitation at the Toronto Rehabilitation Centre. Usual care patients, if referred to home care, received the currently practised home care.
As part of hospital policy, the primary care nurse was responsible for the completion of the standardized discharge summary for all patients, and the unit clerk was responsible for faxing it to their family physicians.
Baseline data, and the number of inpatient and emergency department encounters between August 1999 and July 2001 were obtained from the hospital. Provincial claims for emergency visits, physician-office visits, diagnostic or therapeutic services and laboratory services were obtained for the fiscal years 1999/2000 and 2000/01 from the Ministry of Health and Long Term-Care. The Ontario Drug Benefit databases for the fiscal years 1999/2000, 2000/01 and the year-to-date up until July 2001 were used to identify patients admitted to a long-term care institution. Home care data between August 1999 and August 2001 were obtained from Community Care Access Centres. Population-based provincial datasets have been shown to be complete.16
The primary outcome measure was readmission days per 1000 follow-up days for angina, CHF and COPD. The censoring date was the end of the follow-up period (July 19, 2001), the date of death or the date of admission to a long-term care institution. Follow-up days were defined as the censoring date minus the discharge date on the index admission. Secondary measures included all-cause readmission days per 1000 follow-up days, emergency department visits, and provincial claims for physician visits, diagnostic or therapeutic services and laboratory services.
We tested the null hypothesis that there was no reduction in readmission days per 1000 follow-up days using the incidence density ratio (IDR).17 The IDR was calculated as the number of readmission days for angina, CHF and COPD per 1000 follow-up days among usual care patients, divided by the number of readmission days for angina, CHF and COPD per 1000 follow-up days among DMP patients. When the IDR is greater than 1, it indicates that the intervention has a protective effect. Confidence intervals around the IDR were calculated with the formula provided by Kleinbaum and colleagues.17
We had a sufficient number of emergency department encounters in each group to use the Anderson–Gill extension of Cox's proportional hazards model. The variables included in this model were based on the 2-stage analysis strategy for variable selection described by Kleinbaum and colleagues.17 This strategy combines knowledge of the disease origins based on the literature and an understanding of the statistical procedures. Initial variable selection was based on current thought on risk factors.18
The second part of the analysis strategy was variable retention. Kleinbaum and others17 recommend the forward selection procedure. Only those covariates with a significance level of p = 0.25 were included in the base regression model; the final multivariate model included covariates with a significance level of p = 0.05. The difference between the log-likelihood without assignment to treatment was compared to the log-likelihood with assignment by means of the likelihood ratio test. Since the difference is distributed as the χ2 statistic, the difference was assessed for statistical significance with the standard χ2 significance levels. The degree of freedom for the test was 1, the difference in the number of parameters being estimated with and those without treatment.
Differences in number of claims were examined with the Wilcoxon's rank-sum test.
Results
There were 715 patients with elevated cardiac enzymes admitted to TEGH between August 1999 and August 2000 (see Fig. 1). Of these, 509 were excluded. These patients were excluded because they were nonresident (n = 128), had transferred to another institution (n = 128), had an unconfirmed MI diagnosis (n = 103) or died (n = 81), or because of some other reason (n = 69). Forty-four (21.4%) of the remaining 206 patients refused to participate in the study. Sixteen patients (8 in each group) became ineligible for home visits after randomization. These patients were institutionalized (n = 13), moved (n = 2) or withdrew consent (n = 1). Of the 715 admissions screened, 146 (20.4%) patients — 75 usual care patients and 71 DMP patients — met the final inclusion criteria.
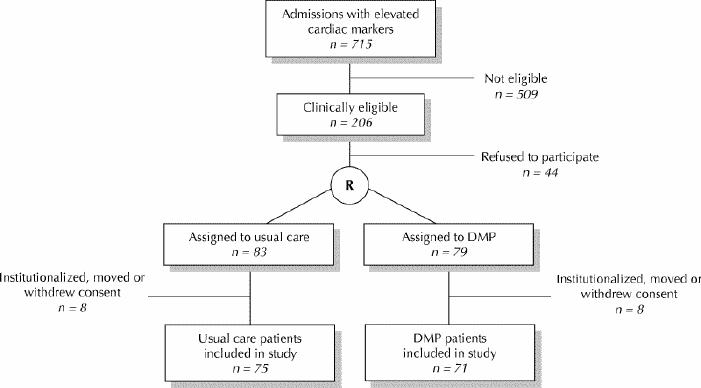
Fig. 1: Selection and assignment of study population.
Follow-up was 100% complete. There were 19 deaths after discharge between August 1999 and August 2001: 11 usual care patients and 8 DMP patients (p > 0.05). Six people were admitted to a long-term care institution (3 from each group). The number of study follow-up days did not differ significantly between groups (mean 453.6 days for usual care patients v. 434.1 days for DMP patients, p > 0.05).
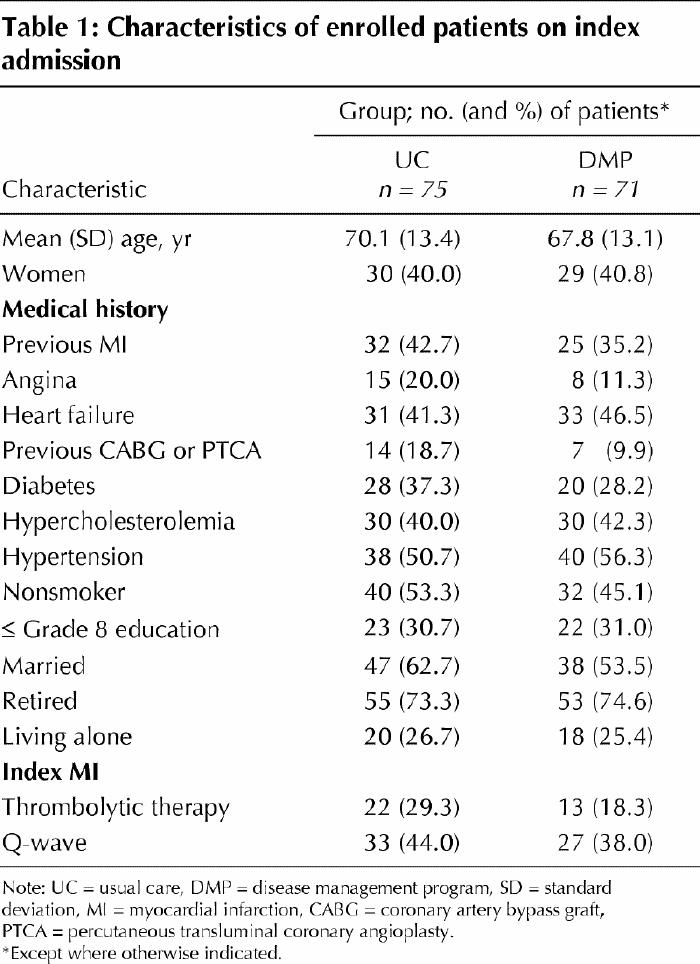
The baseline characteristics of enrolled patients are given in Table 1. Usual care and DMP patients were similar in age, sex, medical history and index MI. Thirty-eight usual care patients and 33 DMP patients were born in Canada, which was similar to the immigrant status of the catchment area.
Table 1
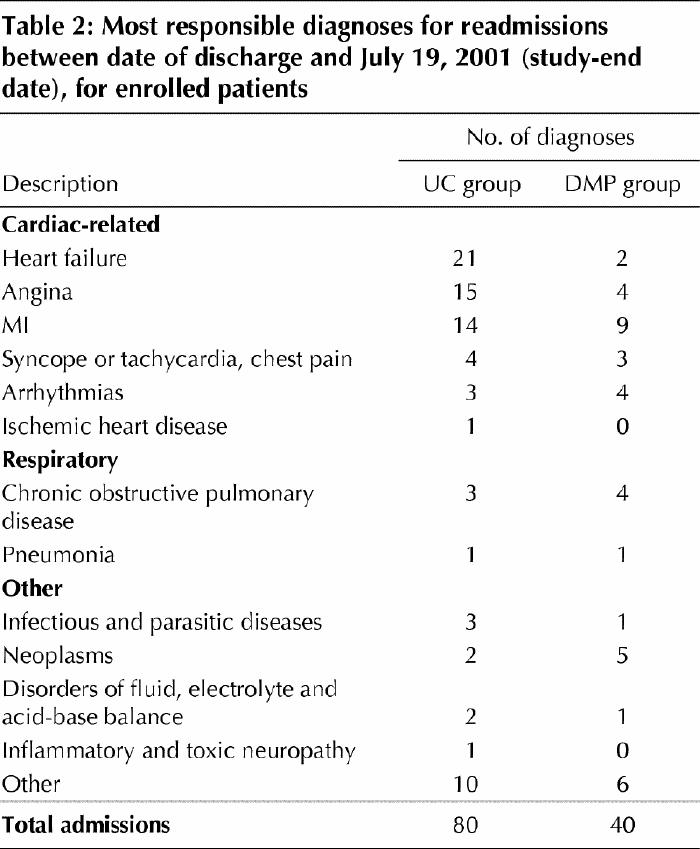
During the follow-up period, there were a total of 120 patients readmitted to hospital: 80 readmissions for usual care patients and 40 for DMP patients (Table 2). In the usual care group 36 patients were readmitted for angina or CHF and 3 for COPD. In the DMP group 6 patients were readmitted for angina or CHF and 4 for COPD.
Table 2
The number of readmission days for angina, CHF and COPD was 200 during 34 021 follow-up days in the usual care group and 114 during 30 823 follow-up days in the DMP group (IDR = 1.59, 95% confidence intervals [CI] 1.27–2.00, p < 0.001).
One hospital day per patient enrolled was prevented. Usual care patients were more likely than the DMP patients to have more than 1 readmission for angina, CHF or COPD (12.0% v. 1.3%, p = 0.05).
The number of readmission days was 814 during 34 021 follow-up days in the usual care group and 483 during 30 823 follow-up days in the DMP group (IDR = 1.53, 95% CI 1.37–1.71, p < 0.001).
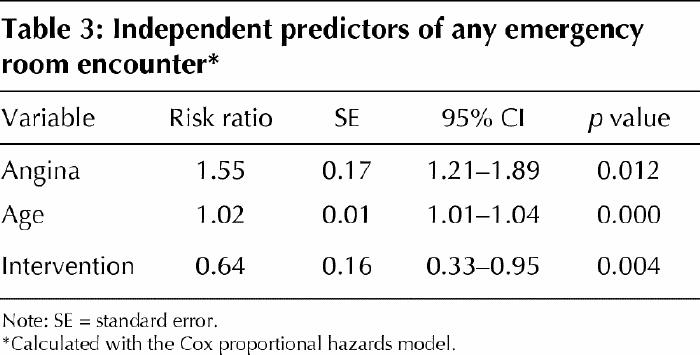
During the follow-up period, the number of emergency department encounters was 211: 147 emergency department encounters for usual care patients and 64 for DMP patients (IDR = 2.08, 95% CI 1.56–2.77, p < 0.001). Thirty-one (41.3%) of 75 usual care patients had more than 1 emergency department visit during the follow-up period compared to 16 (22.5%) of 71 DMP patients (p = 0.05). The mean number of emergency visits for usual care patients was 1.96 compared to 0.90 for DMP patients (p = 0.007). The log-likelihood in the model with age and angina but without random assignment was 26.55 (2 degrees of freedom [df]). The log-likelihood in the model with age, angina and random assignment was 35.00 (3 df). The difference in the log-likelihoods was significant (χ2 = 8.45, 1 df, p < 0.004). The Andersen–Gill extension of Cox's proportional hazards model showed that those who received the DMP program had a significantly longer time between emergency department visits (p < 0.001). When the covariates (age, angina) were considered simultaneously, the relative risk of having an emergency department visit was 0.64 (95% CI 0.33–0.95), similar to that of a usual care patient (see Table 3).
Table 3
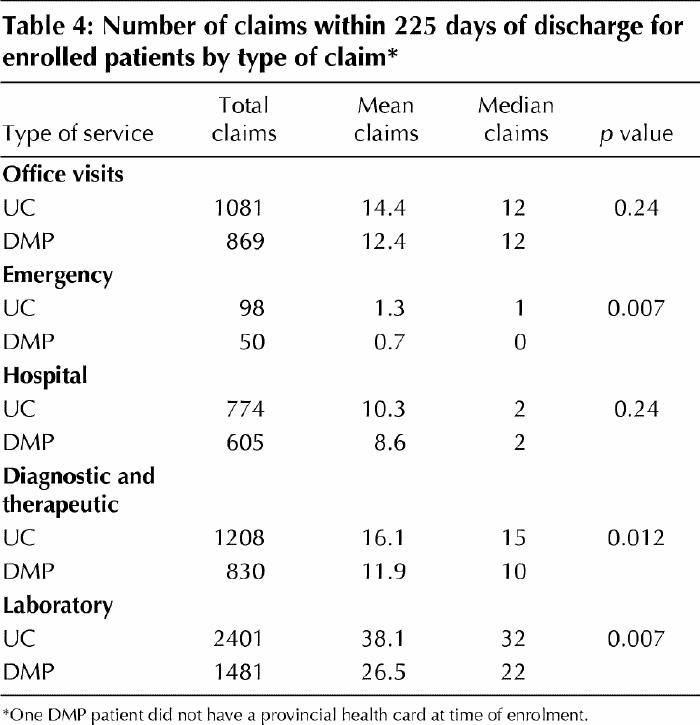
A total of 16 400 physician claims was submitted during 1999/2000 and 2000/01 and discharge: 11 370 (69.3%) during the first 225 days after the index discharge, the shortest follow-up period available. Of these 11 370 claims, 6639 (58.4%) were submitted for usual care patients and 4731 (41.6%) for DMP patients. During the first 225 days after discharge, DMP patients had significantly fewer claims for emergency department visits (p = 0.007), diagnostic or therapeutic services (p = 0.012) and laboratory services (p = 0.007) than usual care patients (Table 4). The number of claims was not significantly different between groups for physician-office visits (p = 0.24) or for hospital claims (p = 0.24).
Table 4
Interpretation
This study provides evidence that a multifaceted community-based inner-city DMP delivered by home health nurses successfully reduced hospitalization days per 1000 follow-up days for CHF and angina, emergency department encounters, and claims for diagnostic or therapeutic services and laboratory services. It had no impact on COPD. Brown and colleagues,19 in a study published after we chose our primary outcome, reported that 3 panels of Canadian physicians identified CHF and angina, but not COPD, as conditions treatable in ambulatory settings.
Our study has several strengths. First, we used a rigorous method to systematically develop, implement and evaluate an innovative community-based DMP. Second, our 8-week intervention was of relatively long duration. Third, our intervention was delivered within usual medical practice. It was designed to increase patients' access to existing outpatient services. Fourth, we evaluated the DMP using provincial administrative data, which ensured 100% follow-up.
Our study has some limitations. First, for practical reasons we could not enrol patients on discharge. We therefore excluded 16 patients after randomization. Our comparative study does not provide as strong evidence as a randomized trial. Second, our study could not be blinded for practical reasons. However, emergency department physicians and the consultant specialists were unaware of the study, which removes possible motivation for bias. Third, there may have been some inconsistency in the readmission diagnostic coding. However, all-cause readmission days and emergency department encounters, regardless of specific coding type, were also significantly lower in the DMP group. Fourth, we did not have a sufficient number of readmissions in each group to use the Anderson–Gill extension of Cox's proportional hazards model. However, we did have a sufficient number of emergency department encounters to adjust for imbalances in baseline variables. The results of this multivariable analysis suggest that it is unlikely that imbalances caused the outcome differences between groups. Fifth, the income and education levels of residents in this multi-ethnic area are low.20 This community-based DMP may be appropriate only for inner-city areas or for those areas with documented suboptimal usual care.6 Sixth, our primary outcome measure did not include repeat MI or acute coronary syndrome. Future studies should consider the impact of DMPs on all recurrent cardiovascular events. Finally, given the current financial climate, it will be difficult to find permanent and dedicated funding for this DMP. The current emphasis on integrated service delivery offers the potential for its sustainability.
Overall, the results of this study are similar to those of published studies that have examined multifaceted interventions. Our effect size was lower than that reported in 2 trials21,22 that involved a specially trained nurse. The diagnostic inclusion criteria period may account for the smaller effect size.
Our study supports the augmentation of community care infrastructure, strengthening the communication links between family physicians and cardiac-trained nurses, and reimbursing the providers for these activities. Further research is required to delineate which component or components of the DMP accounted for the improved cardiac-related outcomes. On each visit the cardiac-trained nurse assessed the patient's understanding and compliance with medications, checked that refills had been filled and assessed any side effects. These activities may have contributed to the outcomes.
Acknowledgments
We would like to acknowledge the substantial contribution made by Drs. Fountas and Gupta to the study design and the acquisition of data.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Young is the guarantor of the paper. Drs. Young, Rewa, Goodman, Jaglal, Cash, Lefkowitz and Coyte conceived and designed the study. Dr. Young, enrolled the patients, analyzed the data and drafted the paper. All authors contributed to the interpretation of the data and the critical revision of the article, and approved the final version of the manuscript.
Dr. Wendy Young was the recipient of a Health Canada National Health Research and Development Program/Canadian Institutes for Health Research Ph.D. Award at the time the research was conducted and is currently a Canadian Health Services Research Foundation/Canadian Institutes for Health Research Post Doctoral Fellow.
Dr. Susan Jaglal is a Career Scientist of the Ministry of Health and Long Term Care, Toronto, Ont.
Dr. Peter Coyte is the recipient of the Canadian Health Services Research Foundation/Canadian Institutes for Health Research Chair in Health Care Settings.
This study was supported by The Change Foundation (grant no. 98048). This study was also supported by the University of Toronto Home and Community Care Evaluation and Research Centre, the East York Access Centre and Partners for Health.
This study was approved by the University of Toronto's Ethics Committee.
Competing interests: None declared.
Correspondence to: Dr. Wendy Young, 2115 Davebrook Rd., Mississauga ON L5J 3M4; fax 905 855-3637; wendy.young@utoronto.ca
References
- 1.World Health Organization. Innovative care for chronic conditions: building blocks for action. Geneva: The Organization; 2002.
- 2.Cheah J. Chronic disease management: a Singapore perspective. BMJ 2001;323(7319):990-3. [DOI] [PMC free article] [PubMed]
- 3.Bodenheimer T. Disease management in the American market. BMJ 2000;320(7234):563-6. [DOI] [PMC free article] [PubMed]
- 4.Hunter DJ. Disease management: Has it a future? It has a compelling logic, but needs to be tested in practice. BMJ 2000;320(7234):530. [DOI] [PMC free article] [PubMed]
- 5.Hunter DJ, Fairfield G. Disease management. BMJ 1997;315(7099):50-3. [DOI] [PMC free article] [PubMed]
- 6.McAlister FA, Lawson FME, Teo KK, Armstrong PW. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. BMJ 2001;323(7319):957-62. [DOI] [PMC free article] [PubMed]
- 7.Frasure-Smith N, Lesperance F., Prince RHVPGRA, Junien C. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997;350:473-9. [DOI] [PubMed]
- 8.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA 1992;268(17):2388-94. [PubMed]
- 9.Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care 1998;36(6):804-17. [DOI] [PubMed]
- 10.Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, et al. Preventable hospitalizations and access to health care. JAMA 1995; 274(4):305-11. [PubMed]
- 11.Tu JV, Austin P, Naylor CD, Iron K, Zhang H. Acute myocardial infarction outcomes in Ontario. In: Naylor CD, Slaughter PM, editors. Cardiovascular health and services in Ontario: an ICES atlas. Toronto: Institute for Clinical Evaluative Sciences; 1999. p. 83-122.
- 12.Myocardial infarction redefined - a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36(3):959-69. [DOI] [PubMed]
- 13.Cohen J. A power primer. Psychol Bull 1992;112(1):155-9. [DOI] [PubMed]
- 14.Young W, Coyte PC, Jaglal S, De Boer D, Naylor CD. Home care utilization following a hospitalization for cardiovascular disease. In: Naylor CD, Slaughter PM, editors. Cardiovascular health and services in Ontario: an ICES atlas. Toronto: Institute for Clinical Evaluative Sciences; 1999. p. 319-33.
- 15.Young WI, Rewa G, Coyte P, Jagal SB, Goodman S, Bentley-Taylor M, et al. The development of Partners for Health's integrated community pathway for postmyocardial infarction patients. Can J Cardiol 2003;19(3):231-5. [PubMed]
- 16.Williams JI, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Willians JI, Anderson GM, Blackstein-Hirsch P, Fooks C, Naylor CD, editors. Patterns of health care in Ontario. Ottawa: Canadian Medical Association; 1996. p. 339-45.
- 17.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research. Belmont, CA: Lifetime Learning Publications; 1982.
- 18.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med 2001;345(12):892-902. [DOI] [PubMed]
- 19.Brown AD, Goldacre MJ, Hicks N, Rourke JT, McMurtry RY, Brown JD, et al. Hospitalization for ambulatory care-sensitive conditions: a method for comparative access and quality studies using routinely collected statistics. Can J Public Health 2001;92(2):155-9. [DOI] [PMC free article] [PubMed]
- 20.Glazier RH, Badley EM, Gilbert JE, Rothman L. The nature of increased hospital use in poor neighbourhoods: findings from a Canadian inner city. Can J Public Health 2000;91(4):268-73. [DOI] [PMC free article] [PubMed]
- 21.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial [see comments]. JAMA 1999;281(7):613-20. [DOI] [PubMed]
- 22.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet 1999;354(9184):1077-83. [DOI] [PubMed]


