Abstract
In this study it is shown that both membrane-bound and soluble forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B cells. Activated B cells express the membrane-bound form of SLAM (mSLAM), the soluble (s) and the cytoplasmic (c) isoforms of SLAM, and the expression levels of mSLAM on B cells are rapidly upregulated after activation in vitro. Importantly, recombinant sSLAM and L cells transfected with mSLAM efficiently enhance B cell proliferation induced by anti-μ mAbs, anti-CD40 mAbs or Staphylococcus aureus Cowan I (SAC) in the presence or absence of IL-2, IL-4, IL-10, IL-12, or IL-15. sSLAM strongly enhances proliferation of both freshly isolated B cells and B cells derived from long-term in vitro cultures, indicating that SLAM acts not only during the initial phase of B cell activation but also during the expansion of preactivated B cells. In addition, sSLAM enhances production of IgM, IgG, and IgA by B cells activated by antiCD40 mAbs. SLAM has recently been shown to be a high affinity self-ligand, and the present data suggest that signaling through homophilic SLAM–SLAM binding during B–B and B–T cell interactions enhances the expansion and differentiation of activated B cells.
Bcell activation is initiated after recognition of specific antigen by cell surface Ig, which in naive, unprimed B cells is of IgM or IgD isotype. Cytokines and membranebound costimulatory molecules expressed by activated CD4+ T cells are required for subsequent proliferation, Ig isotype switching and differentiation of activated B cells. Cytokines produced by Th2-type cells, such as IL-4, IL-5, IL-13, are considered to be primarily involved in providing help for B cells, whereas Th1-type cytokines, IL-2 and IFN-γ, mediate delayed-type hypersensitivity reactions and activate macrophages (1–3). In addition to T cells, monocytes can regulate B cell proliferation and differentiation through release of cytokines, such as IL-6, IL-8, and IL-10 (4–6), and B cell proliferation and differentiation also appear to be regulated by autocrine mechanisms. IL-1, IL-6, IL-10, TNF-α, and lymphotoxin have been shown to be produced, among other cell types, by activated B cells and they enhance proliferation and differentiation of these cells (7–11).
The CD40 ligand (CD40L)1 on activated CD4+ T cells, which interacts with CD40 on B cells (12), was shown to be a potent membrane-bound costimulatory molecule for proliferation and differentiation of both human and murine B cells (13–17). The B cell antigen CD19, the ligand of which remains to be characterized, has also been shown to mediate maturation, proliferation and differentiation of B cells (18). In addition, CD70, a member of the TNF family expressed by activated B and T cells, and the membrane form of TNF-α, expressed by activated and HIV-infected T cells, are involved in productive T–B cell interactions (19–21). Although several surface molecules can mediate T–B cell collaboration, the role of CD40L appears to be crucial, as is clearly demonstrated in patients with hyperIgM syndrome, who are unable to produce IgG, IgA, or IgE because of mutations in their genes encoding CD40L (22–25). However, the patients have normal or elevated levels of IgM and normal numbers of B cells in the circulation indicating that a CD40L-independent pathway of B cell proliferation and differentiation is operational in these patients. Moreover, CD40L-deficient mice have normal Ab responses against thymus-independent antigens (26, 27), and some T cell clones from patients with mutated CD40L genes induce Ig isotype switching even in the presence of neutralizing anti–TNF-α mAbs (28), supporting the conclusion that yet to be characterized costimulatory molecules contribute to the expansion, Ig isotype switching and differentiation of activated B cells.
Signaling lymphocytic activation molecule (SLAM) is a 70-kD glycoprotein expressed by CD45RO+ T cells, immature thymocytes, and a proportion of B cells (29). SLAM is a member of the immunoglobulin gene superfamily and belongs to the CD2 family of cell surface molecules (29, 30). SLAM has limited homology to CD48 (26%), CD58/LFA-3 (17%), and 2B4 (28%), a signaling molecule expressed on murine NK and cytotoxic T cells (31). Activated human T cells express, in addition to membrane-form of SLAM (mSLAM), mRNA encoding a soluble, secreted form of SLAM (sSLAM) lacking 30 amino acids (aa) encompassing the entire 22-aa transmembrane region (29). In addition, a cytoplasmic (c) form lacking the leader sequence and a variant membrane (vm)SLAM with a truncated cytoplasmic tail were identified (29). SLAM was recently shown to be a high-affinity self-ligand with an affinity constant of 0.1 nM (Cocks, B.G., G. Aversa, S. Balasubramanian, C.-C.J. Chang, B. Bennett, D. Peterson, J.M. Carballido, J. Punnonen, and J.E. de Vries, manuscript submitted for publication). SLAM-Ig fusion protein specifically bound to SLAM transfectants and to surface-immobilized SLAM as shown by surface plasmon resonance. Engagement of SLAM by specific mAb or sSLAM-induced proliferation of activated human T cells in a CD28-independent manner, suggesting that the T cell responses observed in CD28-deficient mice may be partially or completely mediated via SLAM (29). In addition, SLAM engagement induced IFN-γ production even in Th2-type T cell clones, suggesting a role for SLAM in directing cytokine synthesis in T helper cells (29).
In the present study, the expression and function of SLAM on B cells was investigated. We demonstrate that mSLAM is rapidly induced on B cells after activation with anti-μ or anti-CD40 mAbs or Staphylococcus aureus Cowan I (SAC). A recombinant form of sSLAM and murine L cells transfected with mSLAM induced B cell proliferation in the absence of other stimuli, and they strongly enhanced B cell proliferation induced by anti-μ or anti-CD40 mAbs or SAC in the presence or absence of cytokines IL-2, IL-4, IL-10, IL-12, IL-13, or IL-15. These results indicate that SLAM is a novel B cell growth and differentiation promoting molecule.
Materials and Methods
Reagents.
Purified human recombinant (r) IL-4 and IL-10 were provided by Schering Plough Research Institute (Kenilworth, NJ). Purified rIL-2, rIL-13, and rIL-15 were kindly provided by Drs. Gerard Zurawski (DNAX), Satish Menon (DNAX) and Robert Kastelein (DNAX), respectively. rIL-12 was purchased from R&D Systems, Inc. (Minneapolis, MN). Unconjugated mAbs specific for CD3, CD4, CD8, CD14, CD16, and CD56, PE- and FITC-conjugated mAb specific for CD20, and control antibodies with irrelevant specificities were obtained from Becton-Dickinson (San Jose, CA). The purified anti-CD40 mAb 89 (IgG1) (32) was kindly provided by J. Banchereau (ScheringPlough France, Dardilly, France). mAb A12 specific for SLAM (IgG1) was generated and purified as described (29). Beads coated with anti-μ mAbs were obtained from Irvine Scientific (Santa Ana, CA), and Staphylococcus aureus Cowan I cells (Pansorbin Cells) were purchased from Calbiochem Novabiochem (La Jolla, CA). FITCconjugated mAb anti-IgD was obtained from Nordic Immunology (Tilburg, The Netherlands).
Generation of sSLAM.
Recombinant sSLAM was expressed in insect cells using the baculovirus expression system as described (33). In brief, Spodoptera frugiperda (Sf 9) cells were transfected with cDNA encoding the soluble secreted form of SLAM (pSECslam) (29), which was subcloned into the vector pVL1393 (Invitrogen, San Diego, CA) using PCR and primers 5′-GAC TCA GAC GAA TTC ATG GAT CCC AAG GGG CTC-3′ and 5′-AGC TAG ATC AGA TCT GCT CTC TGG AAG TGT CAC-3′ followed by a Bg1I/EcoRI digestion. A Flag sequence was fused to the carboxy terminus of the protein, and the expressed recombinant sSLAM was purified from the supernatants using an M2 antiFlag mAb affinity matrix (Eastman Kodak Company, New Haven, CT) and glycine elution according to the manufacturer's instructions.
Preparation of sSLAM-coated Beads.
Polystyrene latex beads with a mean diameter of 0.6 μm (Interfacial Dynamics Corporation, Portland, OR) were first incubated with anti-Flag mAb M2 for 30 min at room temperature (0.3 μg/108 beads), and then an additional 30 min on ice. The beads were washed with PBS. Recombinant sSLAM with a Flag tail was added to the beads at 0.3 μg/108 beads, and the suspension was incubated at room temperature for 30 min. These sSLAM-coated beads were then washed three times with PBS supplemented with 2% FCS, and finally resuspended in PBS plus 2% FCS.
Cell Preparations.
Blood samples and spleens were obtained from healthy volunteers or from patients undergoing splenectomy due to trauma, respectively. PBMC were isolated by centrifugation over Histopaque-1077 (Sigma Chem. Co., St. Louis, MO).
B cells were purified either by depleting non–B cells from human splenic MC by magnetic beads or sorting CD20+ cells using flow cytometry. In brief, to deplete non–B cells from splenic MC the cells were first stained with mAbs specific for CD3, CD4, CD8, CD14, CD16, and CD56 at 4°C for 30 min. Then, the cells were washed twice and incubated with magnetic beads coated with sheep anti–mouse IgG mAbs (Dynal A.S., Oslo) at 4°C for 30 min. Positive cells were removed using a magnetic field. The remaining cells were washed, counted, and an aliquot of the cells was stained with FITC- or PE-conjugated anti–CD20 mAb or control Ab to analyze the purity of the cells. Always >97% of these negatively selected B cells were CD20+ (data not shown). To positively sort total B cells, splenic MC were first stained with FITC-conjugated mAb specific for CD20, and to sort naive CD20+, sIgD+ B cells the cells were stained with antiCD20-PE and anti–IgD-FITC mAbs. Thereafter, the cells were washed twice and single or double positive cells were sorted using a FACStar® Plus or Vantage cell sorter (Becton Dickinson). The purity of the sorted cells also was analyzed, and it was always 98.5–100% (data not shown).
Murine L cells were transfected with cDNA encoding mSLAM by electroporation and positive cells were selected by three rounds of cell sorting. These L cell transfectants and normal L cells used as controls were cultured in RPMI 1640 supplemented with 10% FCS. The cells were harvested using a cell scraper, and the cells were washed twice. Then, the cells were irradiated 7,300 rads and washed again twice before adding them to B cell cultures. The ratio of L cells to B cells at the onset of the culture was 1:6.
Culture Conditions.
Purified B cells were cultured in roundbottomed 96-well plates (Linbro, McLean, VA) in 0.2 ml Yssel's medium supplemented with 10% FCS and incubated at 37°C in a humidified atmosphere containing 5% CO2. Cultures were performed in triplicate when proliferation of B cells was studied and in quadruplicate when Ig synthesis was studied. Total negatively selected B cells purified by magnetic beads were cultured at 105 cells/well, whereas sorted CD20+ or CD20+, sIgD+ B cells were cultured at 3 × 104 cells/well.
Phenotypic Analysis of Cultured Cells.
B cells were cultured as described above and they were harvested and washed after culture periods varying from 6 to 72 h. FITC- and PE-conjugated mAbs were added to the cell pellet at saturating concentrations and the cells were then incubated at 4°C for 30 min. B cells cultured in the presence of SAC were preincubated with 5% mouse serum at 4°C for 30 min due to nonspecific staining otherwise observed on these cells. FITC- and PE-conjugated mAbs with irrelevant specificities were used as negative controls. Propidium iodide (PI) (2 μg per 5 × 105 cells) was added to all samples to exclude dead cells. A total of 104 cells with light scatter characteristics of lymphocytes of each sample were analyzed using FACScan® flow cytometer and CellQuest software (Becton Dickinson).
Isolation of RNA and Reverse-Transcriptase PCR.
Cultured cells were harvested and washed in PBS. RNA was isolated using RNAzol B (CNNA; Biotech, Friendswood, TX) according to manufacturer's instructions. The samples were stored at −80°C until used. Reverse transcription was performed using Superscript R/T enzyme, and the Superscript system protocol I (GIBCO BRL, Gaithersburg, MD). The resulting cDNA was diluted 10-fold in water and stored at −70°C until used. 1–5 ng of cDNA was amplified with 1 U of Taq polymerase (Perkin Elmer Cetus, Norwalk, CT) and using 40 cycles of PCR (initial denaturation at 94°C for 2 min, then 30 s at 94°C; annealing 30 s at 55°C; extension 1 min, 72°C). To detect various isoforms of SLAM, specific primers were used as follows: 5′-ATC ACT GGA GAA CAG TGT-3′ and 5′-CCC AGC ATA CAC TGC CC-3′, to amplify mSLAM, cSLAM and vmSLAM; 5′-ATC ACT GGA GAA CAG TGT-3′ and 5′-TTC GTT TTA CCT GAG GGG TCT G-3′ to amplify sSLAM; 5′-ACT TTG GAC TGC CTG TGT GAG-3′ and 5′-CAG CCC AGT ATC AAG GTG CA-3′, to amplify cSLAM; and 5′-CAG CTG GAG TGA AAA GGC-3′ and 5′-CCG CAC CGG TCT TGG CGG-3′, to amplify vmSLAM.
Primers for detection of hypoxanthine phosphoribosyltransferase (HPRT) transcripts were 5′-GTA ATG ATC AGT CAA CGG GGG AC-3′ and 5′-CCA GCA AGC TTG CAA CCT TAA CCA-3′. Each PCR product was electophoresed through 1.2% agarose gel, and visualized as ultraviolet fluorescence after staining with ethidium bromide.
Measurement of Ig Production.
Purified B cells were cultured in Yssel's medium supplemented with 10% FCS as described above for 12 d. IgM, IgG, IgA, and IgE levels in culture supernatants were determined by ELISA as described previously (34). The sensitivities of these ELISAs were 0.5 ng/ml.
Results
Expression of mSLAM on PB and Splenic B Cells.
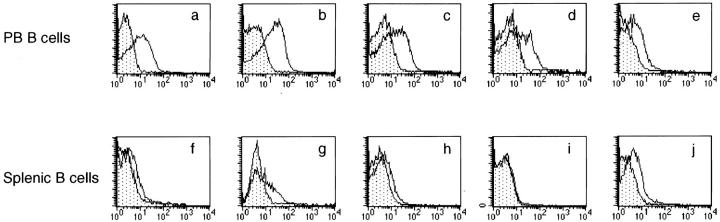
The expression of mSLAM on peripheral blood (PB) and splenic B cells was studied by double immunofluorescence using mAbs specific for CD20 and SLAM (29). The expression level of SLAM was generally higher on PB B cells than on splenic B cells (Fig. 1). The percentages of SLAM+ cells among PB B cells ranged between 20 and 50%, whereas those among splenic B cells were generally less than 20%. B cells were rather homogenous in terms of their SLAM expression, and staining with SLAM-specific mAb generally caused a shift in the staining pattern rather than resulted in the detection of two separate populations of positive and negative cells (Fig. 1). Because of the low proportion of B cells in PB mononuclear cells (MC), in some experiments B cells were first enriched by depleting T cells, NK cells and monocytes by magnetic beads. The results using total splenic MC or PBMC were comparable to those using B cell enriched cell populations (Fig. 1 and data not shown), indicating that cell purification method did not affect the results.
Figure 1.
Expression of mSLAM on PB and splenic B cells. PB (a–e) or splenic (f–j) B cells were stained with FITC-conjugated anti-CD20 mAbs and PE-conjugated anti-SLAM mAb A12 (open histograms) or PE-conjugated mouse IgG1 Ab with irrelevant specificity (stippled histograms). CD20+ cells were gated and expression of mSLAM was studied by flow cytometry. All histograms represent stainings of cells derived from different donors. PB B cells in a–d were first enriched for B cells by depleting T cells, NK cells and monocytes by magnetic beads. Dead cells were always excluded by gating out PI+ cells.
mSLAM Is Upregulated on B Cells after Activation, and Activated B Cells Express Soluble, Cytoplasmic and Membrane Forms of SLAM.
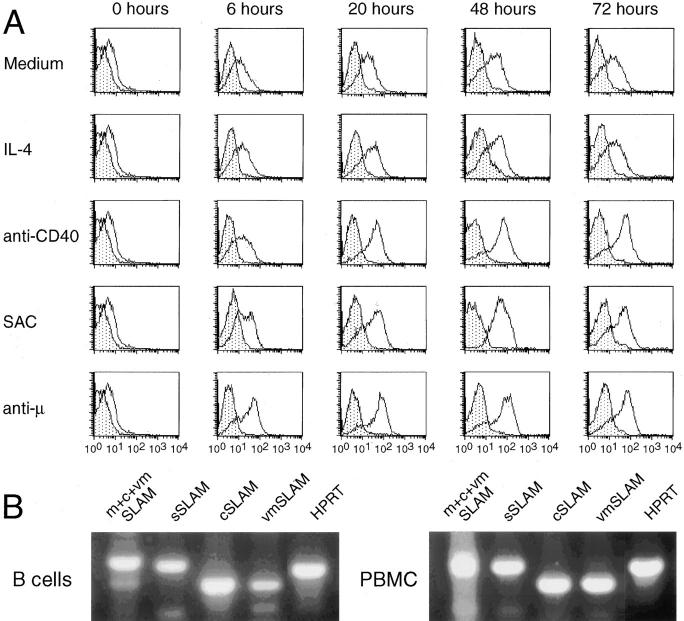
We next studied the effect of activation on SLAM expression on B cells. As shown in Fig. 2 A, mSLAM expression was moderately upregulated on B cells cultured in the presence of medium alone. The expression level of mSLAM was not significantly modulated by IL-2, IL-4, or IL-10 (100 U/ml each), when compared to B cells cultured in the presence of medium alone for up to 72 h (Fig. 2 A and data not shown). In contrast, SAC, anti-μ mAbs and anti-CD40 mAbs induced high levels of mSLAM expression on >85% of the B cells during a culture period of 20 h and the high expression levels were sustained for up to 3 d. The most rapid effect was observed in response to anti-μ mAbs, which induced high levels of mSLAM expression on B cells already during a culture period of 6 h, whereas optimal enhancement of mSLAM expression by SAC and anti-CD40 mAbs was observed after 20 h. These data indicate that mSLAM is strongly upregulated on B cells after activation, and suggest that mSLAM is rapidly induced after recognition of antigen by surface Ig.
Figure 2.
Effect of B cell activation on mSLAM expression, and expression of SLAM isoforms in activated B cells. (A) B cells negatively selected by magnetic beads were cultured in the presence of medium only, IL-4 (100 U/ml), anti-CD40 mAbs (10 μg/ml), SAC (0.005%), or anti-μ mAbs (10 μg/ ml) for 6, 20, 48, or 72 h as indicated. Thereafter, the cells were harvested, washed and stained with mAb CD20-FITC and PE-conjugated SLAM-specific mAb A12 or PE-conjugated mouse IgG1 Ab with irrelevant specificity. Open and stippled histograms represent stainings with mAb A12 and control Ab, respectively. PI was always added to exclude dead cells. mSLAM expression on CD20+ cells was analyzed using FACScan® flow cytometer and CellQuest software. A representative of three experiments is shown. (B) B cells purified by cell sorting were cultured in the presence of anti-CD40 mAbs and IL-4 for 24 h, whereas PBMC were cultured in the presence of PMA (1 ng/ml) and Ca2+ ionophore A23187 (500 ng/ml) for 4 h. The cells were harvested and washed, and expression of different isoforms of SLAM was analyzed by RT-PCR using primers specific for each isoform as described in experimental procedures (m, membrane; s, soluble; c, cytoplasmic; vm, variant membrane). HPRT mRNA was amplified as a positive control.
Activated human T cells were previously shown to express two alternatively spliced isoforms of mSLAM (29). In addition, they expressed a cytoplasmic and a soluble, secreted form of SLAM (29). Sorted, highly purified B cells activated by anti-CD40 mAbs and IL-4 transcribed the same isoforms of SLAM that are detectable in PBMC activated with PMA and Ca2+ ionophore (Fig. 2 B), including the secreted soluble form of SLAM. These data indicate that, in addition to T cells, B cells are capable of producing sSLAM, and suggest that the expression of SLAM on B and T cells are regulated by similar mechanisms.
sSLAM Induces Proliferation of Highly Purified B Cells.
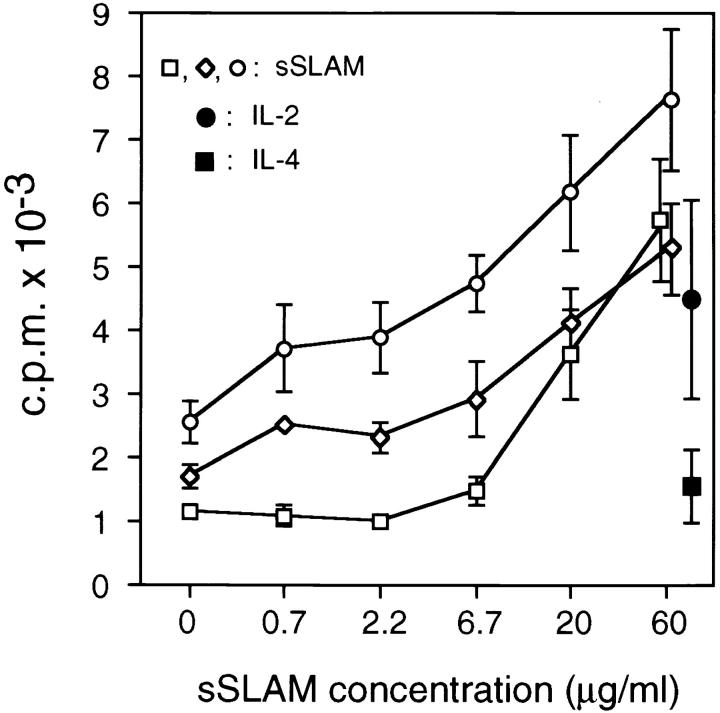
Since SLAM is a high-affinity self-ligand, we investigated the role of SLAM–SLAM interactions in the regulation of B cell function. As shown in Fig. 3, recombinant sSLAM directly induced B cell proliferation of freshly isolated B cells in a dose-dependent manner. sSLAM-induced B cell proliferation was in the same range as that induced by IL-2 (Fig. 3), which has been previously described to directly induce proliferation and differentiation of freshly isolated human B cells (35). IL-4 had no effect on B cell proliferation under similar culture conditions (Fig. 3). Optimal effects by sSLAM were observed at high concentrations of ∼20 μg/ml, but it has to be taken into account that gel filtration analysis has indicated that >99% of sSLAM is in dimeric form (data not shown), and that only monomers are available for binding to mSLAM.
Figure 3.
Effects of sSLAM on proliferation of freshly isolated splenic B cells. Purified B cells were cultured in the presence of increasing concentrations of recombinant sSLAM, IL-2 (100 U/ml) or IL-4 (100 U/ml), and proliferation was measured by [3H]thymidine incorporation during the last 16 h of a 4-d culture. Open symbols represents mean ± SD of triplicate cultures obtained in three separate experiments in the presence of sSLAM at concentrations indicated. Closed circle and square represent mean ± SD cpm obtained in response to IL-2 or IL-4, respectively, in triplicate cultures in the same three experiments as described for sSLAM.
The function of SLAM on B cells was also studied by adding anti-SLAM mAb A12 to B cell cultures stimulated with SAC or anti-CD40 in the presence or absence of IL-2, IL-4, or IL-10. This mAb was previously shown to induce proliferation of preactivated human T cells and T cell clones (29). Similar effects on T cells were observed irrespective whether mAb A12 or its F(ab′)2 or Fab fragments were used, suggesting that cross-linking does not play a major role in the signaling of SLAM. mAb A12 or its F(ab′)2 or Fab fragments at concentrations 0.01–100 μg/ml had no enhancing effects on spontaneous or anti-CD40 mAb and IL-4– induced B cell proliferation in six separate experiments (data not shown). In fact, anti-SLAM mAb slightly inhibited B cell proliferation induced by SAC or anti-CD40 mAbs plus IL-4 (data not shown). In addition, no stimulatory effects by mAb A12 were observed on B cells that had been preactivated with SAC or anti-CD40 mAbs and IL-4 or IL-10 for 3–5 d, further indicating that mAb A12 cannot replace sSLAM in induction of B cell proliferation.
Effects of sSLAM on B Cell Proliferation in the Presence of Polyclonal B Cell Activators.
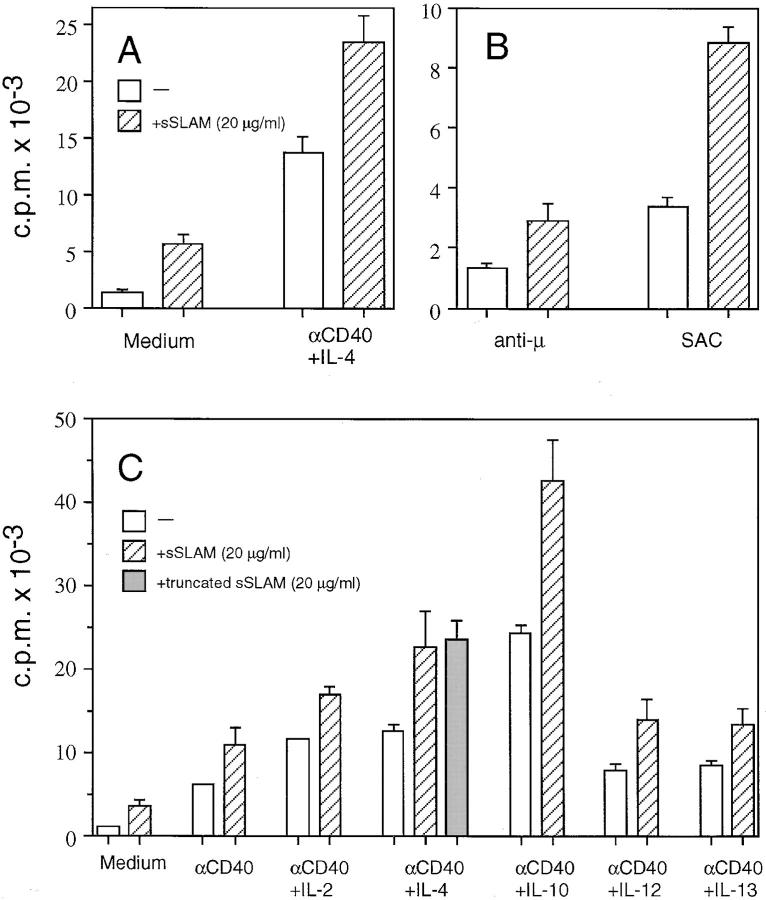
sSLAM also strongly enhanced proliferation of B cells cultured in the presence of other B cell growth promoting signals. sSLAM had additive or slightly synergistic effect on B cell proliferation induced by anti-CD40 mAbs and IL-4 (Fig. 4 A), and it also enhanced proliferation of B cells cultured in the presence of anti-μ mAbs or SAC (Fig. 4 B). These effects were observed irrespective of whether the cells were negatively selected using magnetic beads or positively sorted using cell sorting, suggesting that contaminating cells did not play a role in sSLAM- induced B cell proliferation (Fig. 4 B). Moreover, sSLAM had strong B cell growth promoting effects when saturating concentrations (100 U/ml) of IL-2, IL-4, IL-10, IL-12, or IL-13 were added to cultures stimulated with anti-CD40 mAbs, suggesting that the signaling pathway of sSLAM is independent of that of these cytokines (Fig. 4 C ). Importantly, the level of sSLAM-induced proliferation of CD40stimulated B cells was in the same range as that induced by IL-2, IL-4, IL-12, or IL-13 under the same conditions (Fig. 4 C ). In addition, although IL-10 was more potent than sSLAM in inducing B cell proliferation in the presence of anti-CD40 mAbs, sSLAM still enhanced IL-10–induced response in a synergistic fashion. The B cell growth-promoting effect of sSLAM was specific, because a control protein that binds the human obese gene product (36), produced and purified in a manner similar to sSLAM, failed to induce B cell proliferation when tested at concentrations up to 25 μg/ml (data not shown).
Figure 4.
Effects of sSLAM on proliferation of B cells activated with anti-μ mAbs, SAC, anti-CD40 mAbs and cytokines. (A) Negatively selected B cells were cultured in the presence or absence anti-CD40 mAbs (10 μg/ml), IL-4 (100 U/ml) and/or sSLAM (20 μg/ml) as indicated, and proliferation was measured by [3H]thymidine incorporation during the last 16 h of a 4-d culture. Mean ± SEM of triplicate cultures in nine separate experiments are shown. (B) Negatively selected B cells were stimulated with anti-μ mAbs (10 μg/ml), or sorted B cells were stimulated with SAC (0.005%) in the presence or absence of sSLAM (20 μg/ ml). Mean ± SD of triplicate cultures in a representative of three experiments are shown. (C) Negatively selected B cells were stimulated with anti-CD40 mAb in the presence or absence of IL-2, IL-4, IL-10, IL-12, or IL-13 (100 U/ml each) as indicated, and proliferation was measured by [3H]thymidine incorporation during the last 16 h of a 4-d culture. Mean ± SD of triplicate cultures in a representative of four experiments are shown. sSLAM, recombinant form of naturally occurring sSLAM; truncated sSLAM, truncated recombinant form of sSLAM comprising only the region corresponding to the extracellular portion of mSLAM.
Previously, mSLAM was shown to have two naturally occurring isoforms with different cytoplasmic tails (29). Myelin P0 is another homophilically interacting member of the Ig supergene family, and, interestingly, it was demonstrated that the cytoplasmic region of the molecule is required for optimal homophilic interactions of the extracellular portion (37). To study the role of the carboxy-terminal portion of sSLAM, which corresponds to the cytoplasmic tail of mSLAM, a truncated form of sSLAM lacking this region was generated. As shown in Fig. 4 C, the two forms of sSLAM had virtually identical effects on B cell proliferation, indicating that the carboxy-terminal tail of sSLAM is not required for the B cell proliferation inducing effect of the molecule.
Kinetics of sSLAM-induced B Cell Proliferation.
The time point of optimal effect of sSLAM on B cell proliferation was dependent on the costimulus used in the cultures. When B cells were activated by IL-10 and anti-μ mAbs, which typically induce rapid B cell activation (38), sSLAM induced its optimal effects already after a culture period of 2 d (Fig. 5 A). However, when B cells were activated by IL-10 and anti-CD40 mAbs, optimal effects were generally observed on day 4 and enhancing effects by sSLAM were still detectable after a culture period of 8 d (Fig. 5 A). The kinetics were primarily dependent on whether anti-μ or anti-CD40 mAbs were used to costimulate the cells, because the results were essentially the same irrespective of whether IL-2, IL-4, or IL-10 were included in these cultures (data not shown). IL-2 significantly enhanced sSLAMinduced B cell proliferation (Fig. 5 A), whereas IL-4 had no or minimal effects (data not shown), which is consistent with the fact that IL-2, but not IL-4, induces proliferation of freshly isolated B cells (Fig. 3). sSLAM alone or in combination with IL-2 induced its optimal effect after a culture period of 6 d (Fig. 5 A).
Figure 5.
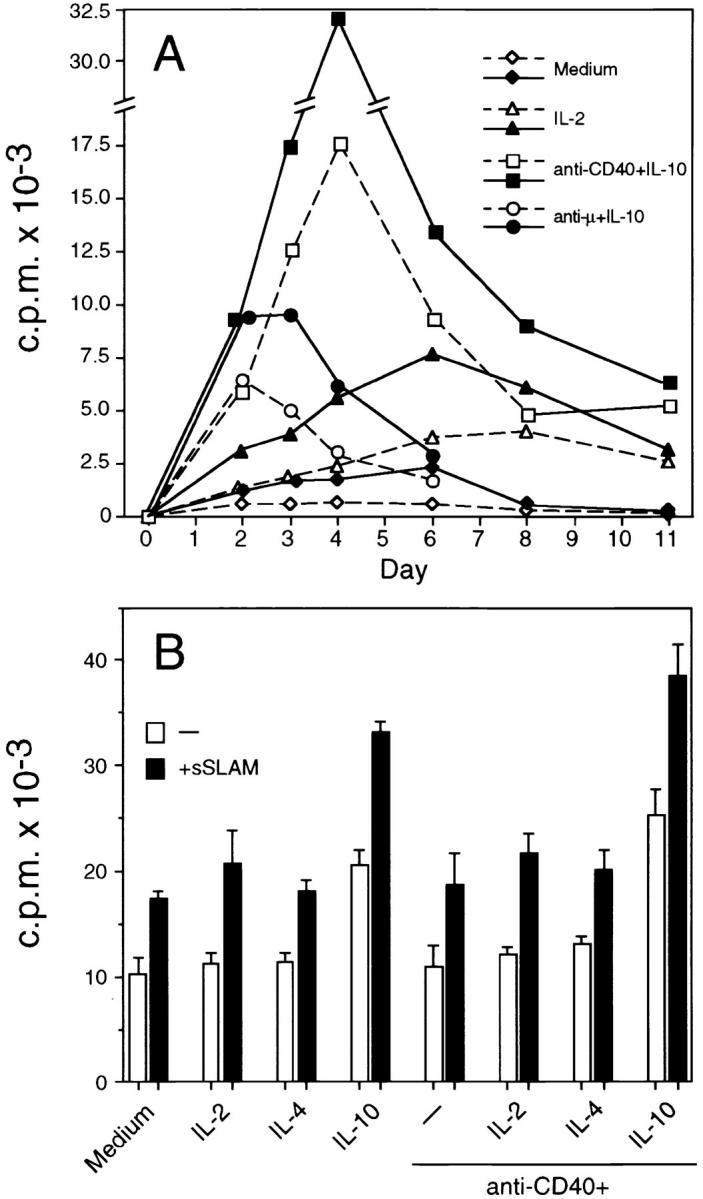
Kinetics of sSLAM-induced B cell proliferation, and effects of sSLAM on B cells derived from a long-term culture. (A) B cells were purified by negative selection, and subsequently cultured in the presence of medium only, IL-2 (100 U/ml), anti-μ mAbs (10 μg/ml) plus IL-10 (100 U/ml), or anti-CD40 mAbs (10 μg/ml) plus IL-10 (100 U/ml) as indicated. Open symbols with dashed lines represent data obtained in the absence of sSLAM and closed symbols with solid lines represent data obtained in the presence of sSLAM (20 μg/ml). Each value represents mean of triplicate cultures in a representative of two experiments. (B) B cells were cultured in the presence of anti-CD40 mAbs and IL-4 for 5 wk. Thereafter, the cells were harvested, washed, and recultured in the presence of medium only, IL-2, IL-4, IL-10, and/or anti-CD40 mAbs in the presence or absence of sSLAM. Each value represents mean ± SD of triplicate cultures. Proliferation was always measured by [3H]thymidine incorporation during the last 16 h of a 4-d culture.
Anti-CD40 mAbs and IL-4 have previously been shown to support long-term growth and survival of human B cells (32). To investigate the effects of sSLAM on B cells derived from long-term cultures, B cells were cultured for 5 wk in the presence of anti-CD40 mAbs and IL-4, whereafter the cells were recultured in the presence or absence of sSLAM. As shown in Fig. 5 B, sSLAM significantly enhanced proliferation of these B cells. sSLAM was also effective when IL-2, IL-4, IL-10, or anti-CD40 mAbs, or combinations of these were added to the cultures. Like sSLAM, IL-10 significantly enhanced proliferation of these B cells, but the effect of IL-10 could be further enhanced by sSLAM (Fig. 5 B). Anti-CD40 mAbs and IL-4 did not significantly affect B cell proliferation, which is likely to be due to their presence during the primary cultures. These results indicate that SLAM enhances B cell proliferation both during the initial phase of B cell activation as well as during expansion of preactivated B cells.
L Cells Transfected with mSLAM- and sSLAM-coated Polystyrene Latex Beads Induce B Cell Proliferation.
To study the effects of mSLAM on B cell proliferation murine L cells were transfected with cDNA encoding mSLAM. Importantly, the transfectants expressed mSLAM at levels comparable to those on activated T cells (29) and B cells (Fig. 2 A), whereas untransfected L cells were completely mSLAM negative (Fig. 6, A and B). Irradiated mSLAM+ L cells strongly enhanced B cell proliferation induced by anti-CD40 mAbs in the presence of IL-4, IL-10 (Fig. 6 C), IL-2, or IL-15 (data not shown). Like sSLAM, mSLAM transfectants also enhanced proliferation of anti-CD40–activated B cells in the absence of exogenous cytokines.
Figure 6.
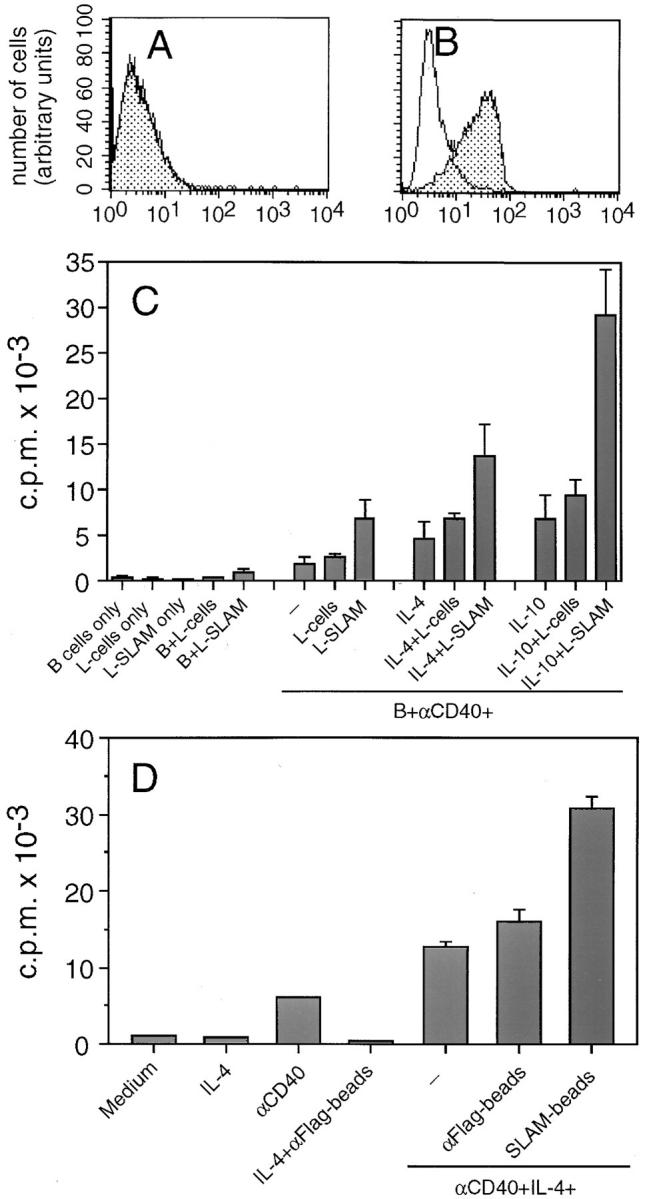
Expression of mSLAM on normal and mSLAM transfected L cells, and the effects of mSLAM transfectants and sSLAM cross-linked to polystyrene latex beads on B cell proliferation. Normal L cells (A) and L cells transfected with cDNA encoding mSLAM (B) were stained with PE-conjugated SLAM-specific mAb A12 (stippled histograms) or a PE-conjugated, isotype-matched control Ab with irrelevant specificity (open histograms), and the cells were analyzed by flow cytometry. (C ) Negatively selected B cells were cocultured in the presence or absence of irradiated (7,300 rad) normal or SLAM transfected L cells (L-SLAM), anti-CD40 mAbs (10 μg/ml), IL-4 (100 U/ml), and/or IL-10 (100 U/ml) as indicated. Data represent mean ± SD of triplicate cultures in three separate experiments. (D) Polystyrene latex beads coated with sSLAM by using anti-Flag mAb M2 (SLAM-beads) were cocultured with purified B cells under culture conditions indicated. Polystyrene latex beads coated with only anti-Flag mAb (αFlag-beads) were used as controls. Proliferation was measured by [3H]thymidine incorporation during the last 16 h of a 4-d culture. Data represent mean ± SD of triplicate cultures.
Because normal L cells slightly enhanced B cell responses in some experiments, we examined whether the B cell growth promoting effects of mSLAM transfectants were due to B cell growth factors released by L cells activated through SLAM–SLAM interactions. Supernatants from both untransfected L cells and SLAM transfectants did have B cell growth promoting activities, but the responses were always <15% of those induced by SLAM transfectants, and no significant difference between supernatants derived from normal and transfected L cells could be observed (data not shown). In addition, supernatants from irradiated L cells, irrespective of whether they were transfected or not, had no effect on B cell proliferation induced by anti-CD40 mAbs and IL-4 (data not shown). To further investigate the effects of surface-bound SLAM, we also studied proliferation of B cells cultured in the presence of polystyrene latex beads coated with sSLAM using mAb specific for the Flagsequence that was attached to the carboxy-terminus of sSLAM. Similar to mSLAM transfectants, sSLAM-coated beads enhanced B cell proliferation induced by anti-CD40 mAbs and IL-4 (Fig. 6 D), supporting the conclusion that SLAM activates B cells not only in its soluble form, but also in its surface-bound form. These data also further indicate that SLAM–SLAM interactions between the L cells did not play a role in the capacity of SLAM transfectants to induce B cell proliferation.
Effects of sSLAM on Ig Synthesis by B Cells.
Finally, we studied the effects of sSLAM on Ig-synthesis by purified B cells cultured in the presence or absence of anti-CD40 mAbs. sSLAM significantly enhanced spontaneous IgM, IgG and IgA synthesis produced by highly purified total B cells, but the effects of sSLAM were generally more potent when anti-CD40 mAbs were added to these cultures (Fig. 7 A). The concentrations required for optimal induction of Ig synthesis were comparable to those required for induction of optimal B cell proliferation (∼20 μg/ml). No IgE synthesis was observed in these cultures (data not shown). These results indicate that SLAM–SLAM interactions enhance not only B cell proliferation, but also Ig synthesis by B cells.
Figure 7.
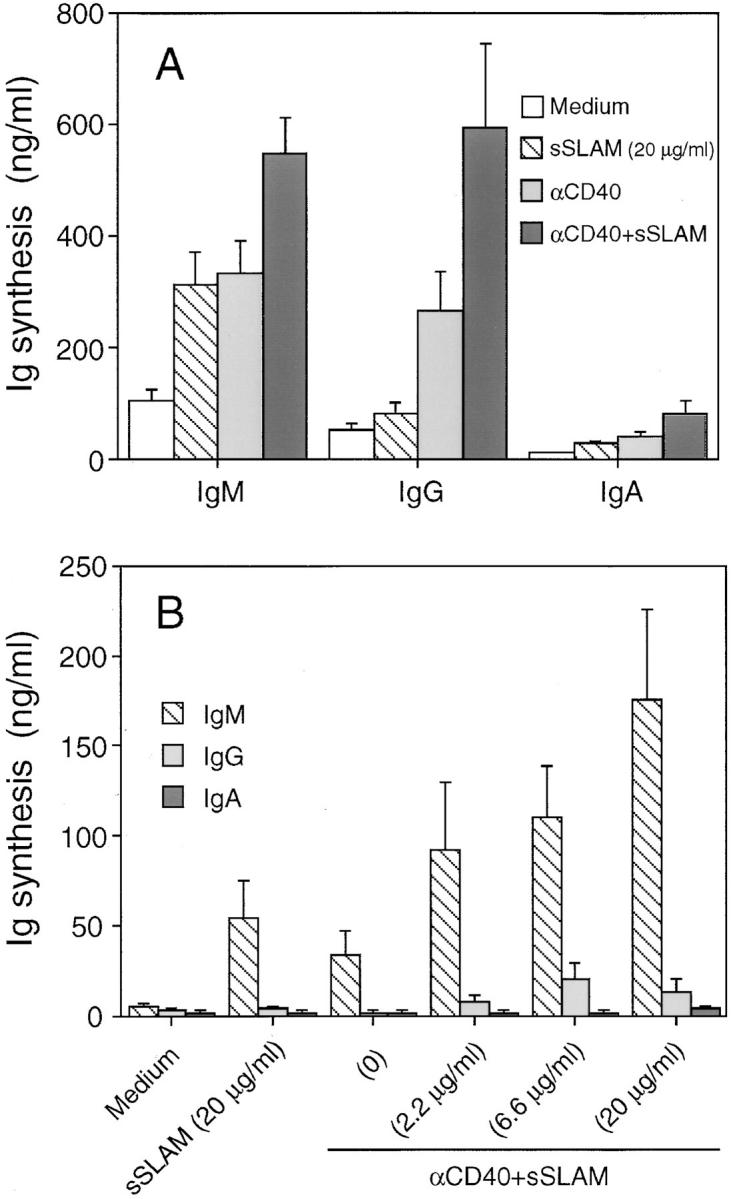
Effects of sSLAM on Ig synthesis by purified B cells. (A) negatively selected total B cells or (B) sorted sIgD+ B cells were cultured in the presence or absence of anti-CD40 mAbs (10 μg/ml) and sSLAM at concentrations indicated for 12 d. Ig levels in the culture supernatants were measured by isotype-specific ELISA. The data represent mean ± SEM of quadruplicate cultures in four experiments (A) or triplicate cultures in two experiments (B).
To investigate whether sSLAM induces Ig isotype switching, sorted sIgD+ naive B cells were cultured in the presence of sSLAM. As shown in Fig. 7 B, sSLAM induced in a dose-dependent manner IgM synthesis by sIgD+ B cells cultured in the presence of anti-CD40 mAbs. However, no IgG, IgA, or IgE was produced by these cells, indicating that sSLAM induces human B cell differentiation, but fails to induce Ig isotype switching.
Discussion
In the present study it is demonstrated that sSLAM and mSLAM transfectants promote growth and differentiation of human B cells. These effects were partially or completely mediated by homophilic SLAM–SLAM interactions, because mSLAM was induced or upregulated on virtually all B cells after activation in vitro, and because SLAM was recently shown to be a high-affinity self-ligand (Cocks, B.G., G. Aversa, S. Balasubramanian, C.-C.J. Chang, B. Bennett, D. Peterson, J.M. Carballido, J. Punnonen, and J.E. de Vreis, manuscript submitted for publication). Thus, SLAM is an unusual molecule in that it acts both as a signaling membrane receptor and as a soluble and membrane-bound growth promoting molecule. Other members of the CD2 family of cell surface molecules have previously been shown to mediate both B and T cell activation. Engagement of CD2 by a pair of agonistic mAbs induces T cell proliferation in the absence of T cell receptor signaling (39,40), and CD58, the natural ligand for CD2 (41), on antigen-presenting cells reduces the concentration of antigen required for T cell activation (42). CD2+ human B progenitor cell lines also proliferate in response to agonistic mAbs specific for CD2 (43), which is expressed on a subset of CD20+ cells in human fetal thymus and bone marrow (43, 44). Furthermore, blocking of CD2 on the surface of T cells inhibits the capacity of these cells to mediate productive T-B cell interactions (45), and mAbs specific for CD58 induce IgE isotype switching and IgE synthesis in a CD40-independent manner by purified B cells cultured in the presence of IL-4 (46). Recombinant sSLAM and murine L cells transfected with cDNA encoding mSLAM induced proliferation and IgM, IgG, and IgA production by unfractionated B cells, but only IgM production by sorted sIgD+ B cells was observed, indicating that SLAM acts as a B cell growth and differentiation promoting molecule, but not as an Ig isotype switch factor.
sSLAM had B cell growth promoting effects even in the absence of other stimuli, but the most potent effects were observed when B cells were cultured in the presence of polyclonal B cell stimuli and cytokines. Importantly, the levels of [3H]thymidine incorporation induced by sSLAM were comparable to those induced by IL-2, IL-4, IL-12, or IL-13 in B cell cultures costimulated by anti-CD40 mAbs. In addition, although sSLAM was less potent than IL-10 in inducing B cell proliferation, it also strongly enhanced IL-10– induced proliferative responses. Both sSLAM and murine L cells transfected with cDNA encoding mSLAM had growth promoting effects irrespective whether IL-2, IL-4, IL-10, IL-12, IL-13, IL-15, anti-μ mAbs, anti-CD40 mAbs, or SAC were added to the cultures, suggesting that the signaling pathway of SLAM is independent of that of these B cell activators. The exact mechanisms of SLAM signaling remain to be studied, but it seems that tyrosine kinases that contain Src homology 2 (SH2) domains are involved, because the intracellular portion of mSLAM has four tyrosine residues (29), three of which conform to the consensus sequence phosphotyrosine-hydrophobic-X-hydrophobic, determined for binding to SH2 domains (47). Because sIgM and CD40 mediate activation signals at different stages of B cell responses, SLAM appears to costimulate B cells not only during the initial antigen-induced activation phase, but also during T cell–dependent, antigen-independent expansion of preactivated B cells. This notion is supported by our kinetics studies, which indicated that sSLAM enhances B cell proliferation at all time points during culture periods of 2–8 d. Moreover, sSLAM enhanced proliferation of B cells, which had been maintained in culture with antiCD40 mAbs and IL-4 for 5 wk.
It is well established that recognition of a specific antigen results in vigorous B cell proliferation giving rise to the dark zones of germinal centers in the lymph nodes. Despite these regions are relatively devoid of T cells, recognition of antigen by a single B cell may give rise to 104 antigen-specific B cells in 3 d, suggesting that the expansion of B cells in germinal centers predominantly occurs in a T cell– independent manner (17). The fact that SLAM is rapidly induced on B cells after activation, and that the high expression levels are sustained for several days in vitro suggest that SLAM–SLAM interactions may play a major role in mediating homotypic B cell contacts in germinal centers resulting in expansion of antigen-specific B cells.
SLAM was previously shown to preferentially stimulate production of Th1-type cytokines. Engagement of SLAM by the specific mAb A12 during antigen-specific T cell activation induced IFN-γ production even in Th2-like T cell clones (29), suggesting that SLAM is primarily involved in delayed-type hypersensitivity responses. However, despite that Th2-type cytokines are generally considered to be the main factors involved in B cell activation and Ig synthesis, Th1 cytokines IL-2 and IFN-γ have also been shown to have direct effects on B cell function. IL-2 directly enhances proliferation and Ig synthesis of in vitro and in vivo preactivated B cells (35, 48), and IFN-γ has been shown to induce Ig isotype switching in murine B cells (49). IL-2 also significantly enhanced sSLAM-induced proliferation of freshly isolated B cells, whereas IL-4 was ineffective. Organ specific and systemic autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, are characterized by both increased production of Th1-type cytokines, including IL-2, and hyperactivity of B cells with elevated production of autoantibodies (50–53). In addition, patients with rheumatoid arthritis (RA) have elevated levels of CD45RO+ T cells, which were shown to coexpress SLAM in healthy individuals (29), suggesting that expression of SLAM may be elevated in T cells from patients with RA. Indeed, we have recently demonstrated that synovial fluid CD4+ T cells from patients with rheumatoid arthritis express significantly higher levels of SLAM than PB CD4+ T cells (Isomäki, P., G. Aversa, B.G. Cocks, R. Luukkainen, P. Toivanen, J.E. de Vries, and J. P., manuscript submitted for publication). These data, together with the observations that SLAM interacts homophilically, suggest that SLAM may contribute to the productive T–B cell interactions and B cell hyperactivity observed in patients with autoimmune diseases.
Although our results clearly indicate that both mSLAM and sSLAM are capable of inducing B cell proliferation, the relative contribution of these two forms of SLAM in the regulation of B cell function remains to be determined. The fact that the expression level of mSLAM on L cells transfected with cDNA encoding mSLAM was comparable to that on activated B or T cells strongly suggests that mSLAM acts as a B cell costimulatory molecule under physiological conditions in vivo. Whether SLAM is in solution or expressed on cell surface does not appear to affect the function of the molecule, because mSLAM transfectants and sSLAM had essentially similar effects on B cell proliferation. In addition, the region that corresponds to the cytoplasmic portion of mSLAM was not required for the function of sSLAM, because the effects of a truncated sSLAM lacking this region were virtually identical to those of the naturally occurring sSLAM. However, the high concentrations of sSLAM required for induction of B cell proliferation suggest that mSLAM may be the dominant active form in physiological situations. On the other hand, because SLAM is a self-ligand, sSLAM primarily exists in a dimeric form in solution. Based on gel filtration analysis, the proportion of monomeric sSLAM is <1% of the total concentration of sSLAM (data not shown), and, therefore, the concentrations of monomeric sSLAM available for homophilic binding to mSLAM are far less than the total concentration of sSLAM.
Like activated T cells (29), activated B cells expressed sSLAM and the potential cytoplasmic form, which lacks the leader sequence. The relative contributions of these, and the two different alternatively spliced isoforms of mSLAM, in the regulation of B cell function remain to be determined. Nevertheless, sSLAM had potent growth promoting effects on B cells also when cross-linked to polystyrene latex beads using mAb specific for the Flag sequence attached to the molecule. These data are in line with the notion that sSLAM primarily acts in its monomeric form through binding to mSLAM, because the Flag sequence was attached to the carboxy-terminal end of sSLAM, and, therefore, the region corresponding to the extracellular portion of mSLAM was available for homophilic binding. Accordingly, transfection of mSLAM into murine pre-B cell line Baf/3 triggered aggregation of these cells, indicating that mSLAM also mediates cell adhesion (Cocks, B.G., G. Averso, S. Balasubramanian, C.-C.J. Chang, B. Bennett, D. Peterson, J.M. Carballido, J. Punnonen, and J.E. de Vries, manuscript submitted for publication). Consequently, it may also be possible that sSLAM, through binding to mSLAM, functions as an inhibitor of cell adhesion mediated by mSLAM– mSLAM interactions. However, this does not seem to be the case, because sSLAM did not block B cell proliferation induced by SLAM transfectants (unpublished observation).
The SLAM-specific mAb 12 did not induce or enhance B cell proliferation or Ig synthesis, indicating that it does not act as a surrogate SLAM ligand on B cells. These data are partially different from the data obtained on T cells, because both the mAb A12 (29) and sSLAM (Cocks, B.G., G. Averso, S. Balasubramanian, C.-C.J. Chang, B. Bennett, D. Peterson, J.M. Carballido, J. Punnonen, and J.E. de Vries, manuscript submitted for publication) induced proliferation of preactivated human T cells. Therefore, the functions of SLAM on activated B and T cells may not be completely comparable. However, because the mAb A12 recognizes both isoforms of mSLAM with different cytoplasmic tails, it remains possible that differential expression of these isoforms on the surface of B and T cells results in distinct signaling events after SLAM engagement. Moreover, there may be differences in B and T cells in the molecules that associate with the cytoplasmic tails of mSLAM isoforms. In addition, we cannot rule out the possibility that SLAM may also signal through molecule(s) other than SLAM on B cells, which might explain the differential effects of the mAb A12 on B and T cells. Unfortunately, preincubation of SLAM+ cells in the presence of mAb A12 did not block the binding of sSLAM to mSLAM (data not shown), indicating that this mAb cannot be used in blocking experiments to address this question.
Taken together, the present study indicates that activated B cells, like T cells, express four alternatively spliced isoforms of SLAM, and that both the soluble and the membrane-bound forms of SLAM induce proliferation and Ig synthesis by highly purified B cells, suggesting an important role for this molecule in the regulation of T–B cell interactions. In addition, because B cells are in close contact to each other in germinal centers, homophilic SLAM interactions during homotypic B cell contacts are likely to contribute to the rapid antigen-driven expansion of B cells in vivo, and may play a role in hyperactivity of B cells in disease situations.
Acknowledgments
We thank Jim Cupp, Eleni Callas, and Josephine “Dixie” Polakoff for their expert FACS® assistance, and JoAnn Katheiser for her careful secretarial help.
Footnotes
DNAX Research Institute is supported by Schering-Plough, Inc.
1 Abbreviations used in this paper: aa, amino acid; CD40L, CD40 ligand; MC, mononuclear cells; HPRT, hypoxanthine phosphoribosyltransferase; PB, peripheral blood; PI, propidium iodide; RA, rheumatoid arthritis; SAC, Staphylococcus aureus Cowan I; SH2, Src homology 2; SLAM, signaling lymphocytic activation molecule.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 3.De Vries, J. E. and J. Punnonen. 1996. Interleukin-4 and interleukin-13. In Cytokine Regulation of Humoral Immunity: Basic and Clinical Aspects. C.M. Snapper, editor. John Wiley & Sons, Ltd., West Sussex, UK. 195–215.
- 4.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature (Lond) 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 5.Kimata H, Yoshida A, Ishioka C, Lindley I, Mikawa H. Interleukin 8 (IL-8) selectively inhibits immunoglobulin E production induced by IL-4 in human B cells. J Exp Med. 1992;176:1227–1231. doi: 10.1084/jem.176.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu D H, Kastelein R, Moore K W, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakasugi H, Rimsky L, Mahe Y, Kamel A M, Fradelizi D, Tursz T, Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci USA. 1987;84:804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieckmann P, D'Alessandro F, Nordan R P, Fauci A S, Kehrl J H. IL-6 and tumor necrosis factor-alpha. Autocrine and paracrine cytokines involved in B cell function. J Immunol. 1991;146:3462–3468. [PubMed] [Google Scholar]
- 9.Burdin N, Peronne C, Banchereau J, Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 1993;177:295–304. doi: 10.1084/jem.177.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boussiotis VA, Nadler LM, Strominger JL, Goldfeld AE. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc Natl Acad Sci USA. 1994;91:7007–7011. doi: 10.1073/pnas.91.15.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrov Z, Kurzrock R, Pocsik E, Pathak S, Kantarjian HM, Zipf TF, Harris D, Talpaz M, Aggarwal BB. Lymphotoxin is an autocrine growth factor for EpsteinBarr virus-infected B cell lines. J Exp Med. 1993;177:763–774. doi: 10.1084/jem.177.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci USA. 1986;83:4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature (Lond) 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbaugh D, Grosmaire LS, Kullas CD, Chalupny NJ, Braesch-Andersen S, Noelle RJ, Stamenkovic I, Ledbetter JA, Aruffo A. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO (Eur Mol Biol Organ) J. 1992;11:4313–4321. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocks BG, de Waal R, Malefyt, Galizzi JP, De Vries JE, Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5:657–663. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 16.Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature (Lond) 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 18.Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature (Lond) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 19.Kobata T, Jacquot S, Agematsu K, Schlossman SF, Morimoto C. CD27-CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci USA. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aversa G, Punnonen J, De Vries JE. The 26kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J Exp Med. 1993;177:1575–1585. doi: 10.1084/jem.177.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature (Lond) 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 22.Fuleihan R, Ramesh N, Loh R, Jabara H, Rosen RS, Chatila T, Fu SM, Stamenkovic I, Geha RS. Defective expression of the CD40 ligand in X chromosomelinked immunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Santo JP, Bonnefoy JY, Gauchat JF, Fischer A, De Saint G, Basile CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature (Lond) 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 24.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, Bedell MA, Edelhoff S, Disteche CM, Simoneaux D K, Fanslow BC, Belmont J, Spriggs MK. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science (Wash DC) 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 25.Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 26.Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 28.Life P, Gauchat JF, Schnuriger V, Estoppey S, Mazzei G, Durandy A, Fischer A, Bonnefoy JY. T cell clones from an X-linked hyper-immunoglobulin (IgM) patient induce IgE synthesis in vitro despite expression of nonfunctional CD40 ligand. J Exp Med. 1994;180:1775–1784. doi: 10.1084/jem.180.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cocks BG, Chang C-CJ, Carballido JM, Yssel H, De Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature (Lond) 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 30.Davis S J, van der Merwe P A. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today. 1996;17:177–187. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 31.Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- 32.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science (Wash DC) 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian S, Chernov-Rogan T, Davis AM, Whitehorn E, Tate E, Bell MP, Zurawski G, Barrett RW. Ligand binding kinetics of IL-2 and IL-15 to heteromers formed by extracellular domains of the three IL-2 receptor subunits. Int Immunol. 1995;7:1839–1849. doi: 10.1093/intimm/7.11.1839. [DOI] [PubMed] [Google Scholar]
- 34.Punnonen J, Aversa G, Cocks BG, McKenzie ANJ, Menon S, Zurawski G, de Waal R, Malefyt, De Vries JE. Interleukin 13 induces Interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.thi Bich-Thuy L, Fauci AS. Direct effect of interleukin 2 on the differentiation of human B cells which have not been preactivated in vitro. Eur J Immunol. 1985;15:1075–1079. doi: 10.1002/eji.1830151102. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature (Lond) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 37.Wong MH, Filbin MT. The cytoplasmic domain of the myelin P0 protein influences the adhesive interactions of its extracellular domain. J Cell Biol. 1994;126:1089–1097. doi: 10.1083/jcb.126.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proust JJ, Chrest FJ, Buchholz MA, Nordin AA. G0 B cells activated by anti-m acquire the ability to proliferate in response to B cell-activating factors independently from entry into G1 phase. J Immunol. 1985;135:3056–3061. [PubMed] [Google Scholar]
- 39.Meuer SC, Hussey RE, Fabbi M, Fox D, Acuto O, Fitzgerald KA, Hodgdon JC, Protentis JP, Schlossman SF, Reinherz EL. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984;36:897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 40.Siliciano RF, Pratt JC, Schmidt RE, Ritz J, Reinherz EL. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature (Lond) 1985;317:428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- 41.Selvaraj P, Plunkett ML, Dustin M, Sanders ME, Shaw S, Springer TA. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature (Lond) 1987;326:400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- 42.Koyasu S, Lawton T, Novick D, Recny MA, Siliciano RF, Wallner BP, Reinherz EL. Role of interaction of CD2 molecules with lymphocyte function-associated antigen 3 in T-cell recognition of nominal antigen. Proc Natl Acad Sci USA. 1990;87:2603–2607. doi: 10.1073/pnas.87.7.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muraguchi A, Kawamura N, Hori A, Horii Y, Ichigi Y, Kimoto M, Kishimoto T. Expression of the CD2 molecule on human B lymphoid progenitors. Int Immunol. 1992;8:841–849. doi: 10.1093/intimm/4.8.841. [DOI] [PubMed] [Google Scholar]
- 44.Punnonen J, De Vries JE. Characterization of a novel CD2+human thymic B cell subset. J Immunol. 1993;151:100–110. [PubMed] [Google Scholar]
- 45.Sen J, Bossu P, Burakoff SJ, Abbas AK. T cell surface molecules regulating noncognate B lymphocyte activation: role of CD2 and LFA-1. J Immunol. 1992;148:1037–1042. [PubMed] [Google Scholar]
- 46.Diaz-Sanchez D, Chegini S, Zhang K, Saxon A. CD58 (LFA-3) stimulation provides a signal for human isotype switching and IgE production distinct from CD40. J Immunol. 1994;153:10–20. [PubMed] [Google Scholar]
- 47.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 48.Muraguchi A, Kehrl JH, Longo DL, Volkman DJ, Smith KA, Fauci AS. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985;161:181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science (Wash DC) 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz DA, Wang H, Gray JD. Cytokine gene profile in circulating blood mononuclear cells from patients with systemic lupus erythematosus: increased interleukin-2 but not interleukin-4 mRNA. Lupus. 1994;3:423–428. doi: 10.1177/096120339400300511. [DOI] [PubMed] [Google Scholar]
- 51.Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis. J Immunol. 1995;155:5029–5037. [PubMed] [Google Scholar]
- 52.Mills JA. Systemic lupus erythematosus. N Engl J Med. 1994;330:1871–1879. doi: 10.1056/NEJM199406303302608. [DOI] [PubMed] [Google Scholar]
- 53.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]