Abstract
To determine the effect of a physiologically relevant elevation in the plasma concentrations of epinephrine on the activation of the hemostatic mechanism during endotoxemia, 17 healthy men were studied after intravenous injection of lipopolysaccharide (LPS, 2 ng/kg), while receiving a continuous infusion of epinephrine (30 ng/kg/min) started either 3 h (n = 5) or 24 h (n = 6) before LPS injection, or an infusion of normal saline (n = 6). Activation of the coagulation system (plasma concentrations of thrombin–antithrombin III complexes and prothrombin fragment F1+2) was significantly attenuated in the groups treated with epinephrine when compared with subjects injected with LPS only (P <0.05). Epinephrine enhanced LPS-induced activation of fibrinolysis (plasma levels of tissue-type plasminogen activator and plasmin-α2–antiplasmin complexes; P <0.05), but did not influence inhibition of fibrinolysis (plasminogen activator inhibitor type I). In subjects infused with epinephrine, the ratio of maximal activation of coagulation and maximal activation of fibrinolysis was reduced by >50%. Hence, epinephrine exerts antithrombotic effects during endotoxemia by concurrent inhibition of coagulation, and stimulation of fibrinolysis. Epinephrine, whether endogenously produced or administered as a component of treatment, may limit the development of disseminated intravascular coagulation during systemic infection.
Asystemic inflammatory response syndrome (SIRS) is frequently associated with disseminated intravascular coagulation, a serious complication that significantly contributes to organ injury and mortality (1). Enhanced release of epinephrine is part of the early host response to acute systemic infection and inflammation (2, 3). Although the role of epinephrine changes in substrate and energy metabolism, and cardiovascular control during SIRS has been widely recognized (2–4), knowledge of the in vivo effects of this catecholamine on activation of the hemostatic mechanism is limited.
It is conceivable that epinephrine influences coagulant and fibrinolytic pathways during SIRS for several reasons. Epinephrine has been reported to stimulate the release of tissue-type plasminogen activator (tPA) in humans in vivo (5, 6). Further, expression of tissue factor, the essential mediator of coagulation activation during SIRS (7, 8), can be inhibited in vitro by agents that elevate cellular cAMP (9, 10). Since β adrenergic stimulation results in an increase in cellular cAMP concentrations (11, 12), it can be anticipated that epinephrine is able to influence coagulation. Knowledge of the hemostatic effect of epinephrine is important not only for the understanding of influences of endogenous adrenergic responses on the regulation of coagulation during SIRS, but also for the therapeutic use of catecholamines in patients with septic shock. Therefore, in the present study we sought to determine the effect of a constant intravenous epinephrine infusion on the activation of the hemostatic mechanism during low dose endotoxemia in normal humans.
Materials and Methods
Study Design and Subjects.
This study was performed simultaneously with an investigation determining the effect of epinephrine on LPS-induced cytokine release (13). The study was approved by the Institutional Review Board, and written informed consent was obtained from all subjects before enrollment in the study. 19 male subjects, aged 28 ± 1 (mean ± SE) yr, were admitted to the Adult Clinical Research Center of the New York Hospital-Cornell University Medical Center for 4 d (day 0 to day 3). On day 1, 8 subjects were started on a constant intravenous infusion of epinephrine (30 ng/kg/min; Parke-Davis, Morris Plains, NJ), starting at 9:00 AM. On day 2, at 9:00 AM, 6 of these 8 subjects, were intravenously injected with a single dose of LPS (National Reference Endotoxin, Escherichia coli 0113 (lot EC-5), generously provided by Dr. H.D. Hochstein (Bureau of Biologics, Food and Drug Administration, Bethesda, MD) at a dose of 2 ng/ kg body weight (EPI-24 group). The infusion of epinephrine was continued until 6 h after LPS administration (3:00 PM). The 11 other subjects were randomized on day 2 to receive either a constant intravenous infusion of epinephrine (30 ng/kg/min; n = 5), starting at 6:00 AM (3 h before LPS administration) and continued until 3:00 PM (EPI-3 group), or an equivalent volume of normal saline (n = 6) (LPS group). These subjects also were injected with LPS (2 ng/kg) at 9:00 AM. Epinephrine was freshly prepared in normal saline every 8 h. 3 h before the administration of LPS a radial arterial catheter was placed in all subjects for blood sampling.
Sampling and Assays.
On day 1, venous blood was obtained before the start of infusion with epinephrine (t = 0 h), and at 1, 2, 4, and 8 h thereafter. On day 2, arterial blood was obtained at 6:00 AM (i.e., directly before the start of the infusion of epinephrine in the EPI-3 group or the start of saline infusion in the LPS group) (t = −3 h), directly before the injection of LPS (t = 0 h), and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 h thereafter. All blood samples were centrifuged at 4°C for 20 min at 1,600 g and plasma was stored at −70°C until assayed.
Activation of the coagulation system was determined in citrated plasma by measurements of prothrombin fragment F1+2 and thrombin–antithrombin III (TAT) complexes (ELISAs; Behringwerke AG, Marburg, Germany). Activation of fibrinolysis was determined in citrated plasma by measurements of tPA antigen and plasminogen activator inhibitor type I (PAI-1) antigen (ELISAs; Innogenetics, Nijmegen, The Netherlands), PAI-1 activity by amidolytic assay (Chromogenix, Mölndal, Sweden), and plasmin-α2–antiplasmin (PAP) complexes (8). Soluble E-selectin levels were measured in K2-EDTA anticoagulated plasma by ELISA (14).
Statistical Analysis.
All values are given as means ± SEM. Differences within groups were analyzed by one-way analysis of variance. Differences between groups were analyzed by two-way analysis of variance (interaction treatment and time). Two sample comparisons were performed by Wilcoxon test. P <0.05 was considered to represent a statistically significant difference.
Results
Activation of Coagulation.
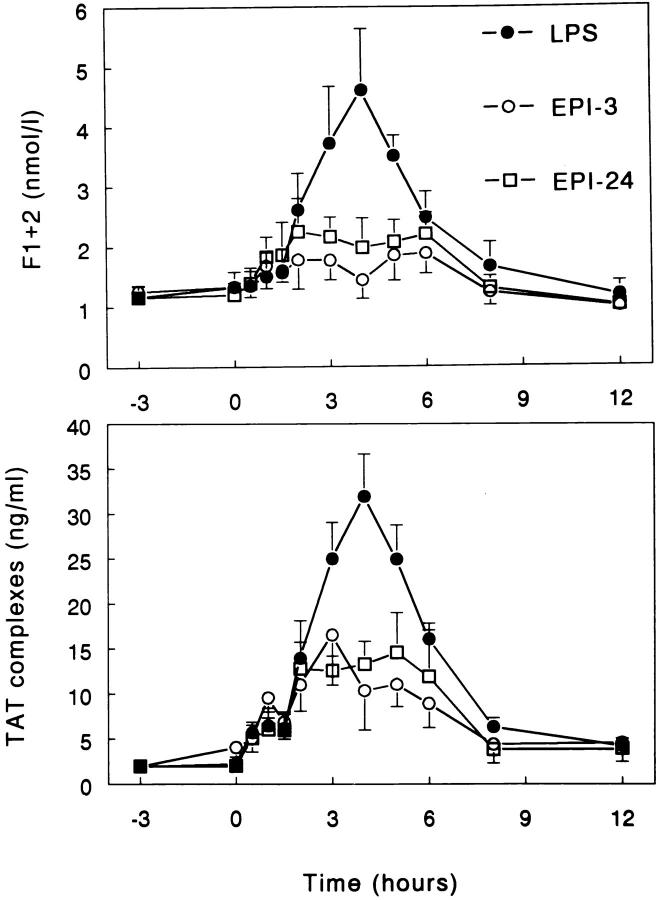
Infusion of epinephrine only (day 1) did not influence the plasma concentrations of F1+2 or TAT complexes (data not shown). Injection of LPS induced transient rises in plasma F1+2 and TAT complexes, peaking after 4 h (4.61 ± 1.03 nmol/l and 31.9 ± 4.7 ng/ml, respectively; both P <0.05 versus time). Both EPI-3 and EPI-24 strongly attenuated LPS-induced coagulation activation (Fig. 1). In the EPI-3 group, peak concentrations of F1+2 and TAT complexes were 1.88 ± 0.34 nmol/l and 16.5 ± 5.6 ng/ml, respectively, in the EPI-24 group, 2.25 ± 0.55 nmol/l and 14.6 ± 4.4 ng/ml, respectively (all P <0.05 versus LPS only).
Figure 1.
Epinephrine inhibits LPS-induced coagulation activation. Mean (± SE) plasma concentrations of prothrombin fragment F1+2 and TAT complexes after intravenous injection of LPS (2 ng/kg, lot EC-5) at t = 0. LPS = subjects injected with LPS only (n = 6); EPI-3 = subjects infused with epinephrine (30 ng/kg/min) from t = −3 h to 6 h (n = 5); EPI-24 = subjects infused with epinephrine (30 ng/kg/min) from t = −24 h to 6 h (n = 6). Both EPI-3 and EPI-24 attenuated LPS-induced increases in F1+2 and TAT complexes (P <0.05 versus LPS only).
Activation of Fibrinolysis.
Infusion of epinephrine only (day 1) modestly stimulated fibrinolysis, as reflected by transient rises in the plasma concentrations of tPA and PAP complexes. Epinephrine also caused a relatively late increase in the plasma levels of PAI-1 antigen (Table 1).
Table 1.
Effect of Epinephrine on Fibrinolysis
| Time | tPA antigen | PAP complexes | PAI-1 antigen | PAI-1 activity | ||||
|---|---|---|---|---|---|---|---|---|
| h | ng/ml | nmol/l | ng/ml | ng/ml | ||||
| 0 | 3.5 ± 0.6 | 5.9 ± 1.1 | 7.1 ± 1.0 | 4.0 ± 0.8 | ||||
| 1 | 11.6 ± 1.8 | 14.4 ± 2.3 | 7.3 ± 0.8 | 4.4 ± 0.9 | ||||
| 2 | 6.1 ± 1.7 | 9.9 ± 0.9 | 8.6 ± 0.6 | 4.9 ± 0.8 | ||||
| 4 | 4.6 ± 1.3 | 8.1 ± 0.8 | 5.6 ± 0.8 | 5.0 ± 0.8 | ||||
| 8 | 3.3 ± 0.6 | 7.5 ± 1.0 | 7.9 ± 0.9 | 7.1 ± 1.0 | ||||
| 21 | 3.6 ± 0.6 | 6.3 ± 1.1 | 10.6 ± 1.3 | 4.5 ± 0.7 | ||||
| 24 | 3.2 ± 0.6 | 6.1 ± 0.9 | 10.6 ± 1.4 | 5.3 ± 0.5 | ||||
| P vs. time | P <0.05 | P <0.05 | P <0.05 | NS |
Data are mean ± SE of eight subjects receiving a continuous 24-h intravenous infusion of epinephrine (30 ng/kg/min) starting at t = 0. NS, nonsignificant.
Administration of LPS resulted in a marked activation of fibrinolysis, followed by a strong inhibition coinciding with maximal coagulation activation (Fig. 2). Peak levels of indexes of fibrinolytic activation were found after 1.5 to 2 h, i.e., before maximal coagulation activation: tPA antigen (32.1 ± 2.0 ng/ml) and PAP complexes (33.9 ± 4.5 nmol/l) (both P <0.05 versus time). Peak levels of indexes of inhibition of fibrinolysis were found thereafter, i.e., after 4 h: PAI-1 antigen (140.7 ± 9.9 ng/ml) and PAI activity (42.0 ± 3.2 ng/ml) (both P <0.05 versus time). Both EPI-3 and EPI-24 enhanced LPS-induced activation of fibrinolysis (Fig. 2). In the EPI-3 group, peak levels of tPA antigen and PAP complexes were 47.8 ± 2.6 ng/ml and 50.2 ± 5.4 nmol/l (both P <0.05 versus LPS only), respectively. In the EPI-24 group, these levels were 44.2 ± 2.7 ng/ml and 50.6 ± 7.3 nmol/l (both P <0.05 versus LPS only), respectively. By contrast, neither EPI-3 nor EPI-24 influenced inhibition of fibrinolysis after injection of LPS, as reflected by unchanged increases in the plasma levels of PAI-1 antigen and PAI activity in the three study groups (Fig. 2).
Figure 2.
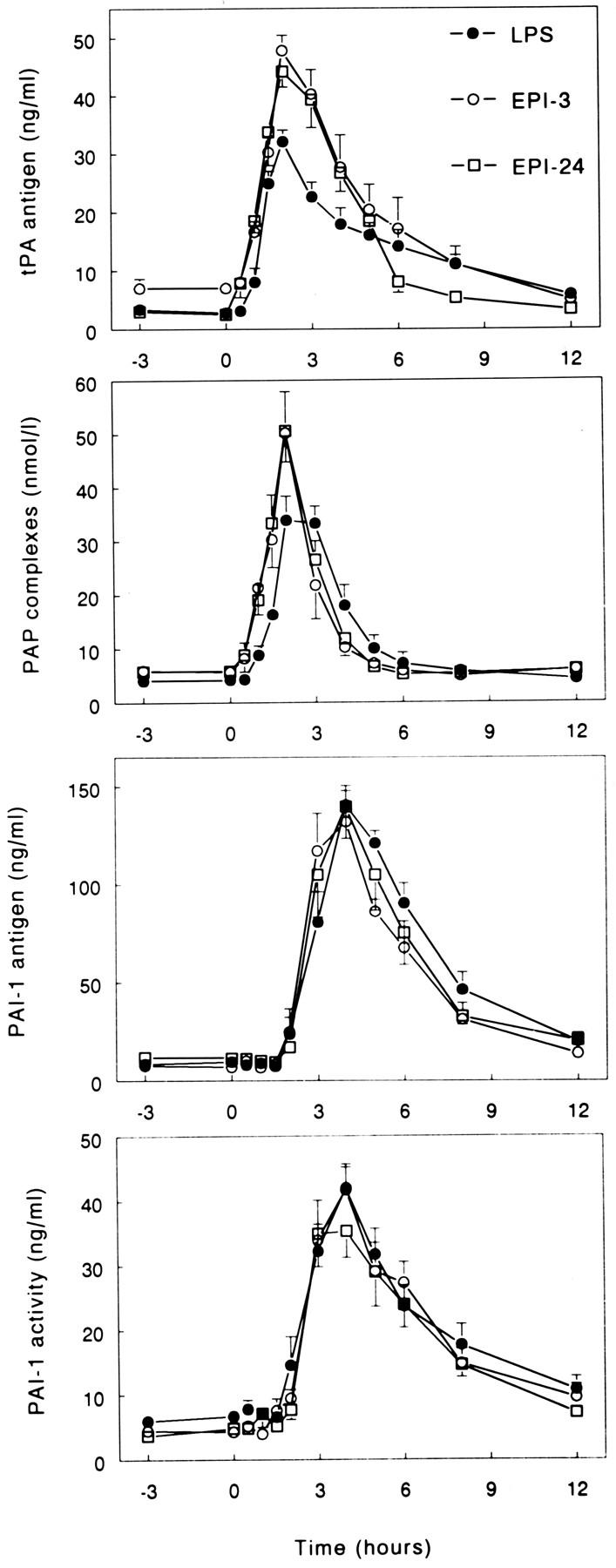
Epinephrine enhances LPS-induced fibrinolytic activation, while not influencing inhibition of fibrinolysis. Mean (± SE) plasma concentrations of tPA antigen, PAP complexes, PAI-1 antigen, and activity after intravenous injection of LPS (2 ng/kg, lot EC-5) at t = 0. LPS = subjects injected with LPS only (n = 6); EPI-3 = subjects infused with epinephrine (30 ng/kg/min) from t =−3 h to 6 h (n = 5); EPI-24 = subjects infused with epinephrine (30 ng/kg/min) from t = −24 h to 6 h (n = 6). EPI-3 and EPI-24 enhanced LPS-induced increases in tPA and PAP complexes (P <0.05 versus LPS only), while not significantly changing PAI-1 antigen or activity.
Ratio of Coagulation and Fibrinolysis.
Since epinephrine appeared to inhibit coagulation and enhance fibrinolysis, we were interested in the effect of epinephrine on the balance between coagulation and fibrinolysis. For this purpose we calculated the ratio of the peak plasma levels of TAT complexes and PAP complexes. Both EPI-3 and EPI-24 reduced this ratio to <50% of the ratio found after administration of LPS only (P <0.05; Table 2).
Table 2.
The Effect of Epinephrine on the Coagulation/ Fibrinolysis Balance
| Subject | LPS | EPI-3 | EPI-24 | |||
|---|---|---|---|---|---|---|
| 1 | 0.61 | 0.69 | 0.54 | |||
| 2 | 0.83 | 0.49 | 0.51 | |||
| 3 | 1.17 | 0.18 | 0.14 | |||
| 4 | 0.89 | 0.42 | 0.64 | |||
| 5 | 1.07 | 0.38 | 0.29 | |||
| 6 | 1.08 | − | 0.25 | |||
| Mean ± SE | 0.94 ± 0.08 | 0.43 ± 0.08* | 0.40 ± 0.08* |
Data are ratios of peak plasma levels of TAT complexes and PAP complexes, reflecting activation of coagulation and fibrinolysis respectively. LPS, subjects injected with LPS only; EPI-3, subjects infused with epinephrine (30 ng/kg/min) from t = −3 h to 6 h; EPI-24, subjects infused with epinephrine (30 ng/kg/min) from t = −24 h to 6 h.
P <0.05 versus LPS.
Soluble E-Selectin.
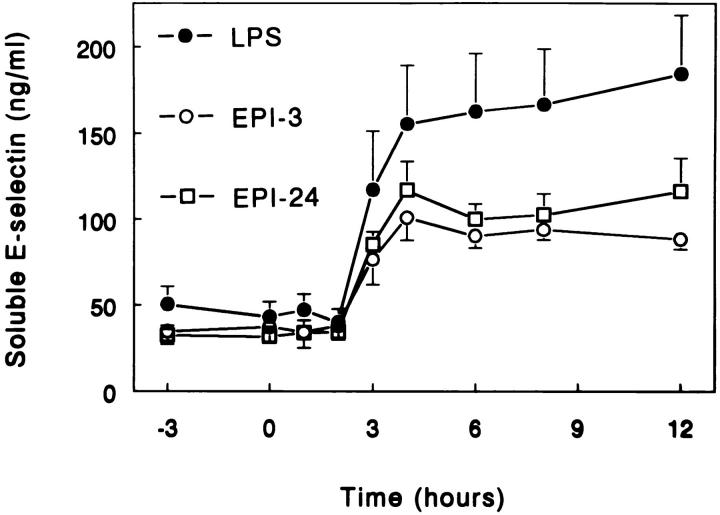
LPS injection elicited a sustained increase in the plasma levels of soluble E-selectin (P <0.05 versus time), which was attenuated by EPI-3 and EPI-24 (P <0.05 versus LPS only; Fig. 3). Soluble E-selectin levels were not influenced by infusion of epinephrine only (day 1) (data not shown).
Figure 3.
Epinephrine inhibits LPS-induced soluble E-selectin release. Mean (± SE) plasma concentrations of soluble E-selectin after intravenous injection of LPS (2 ng/kg, lot EC-5) at t = 0. LPS = subjects injected with LPS only (n = 6); EPI-3 = subjects infused with epinephrine (30 ng/kg/min) from t = −3 h to 6 h (n = 5); EPI-24 = subjects infused with epinephrine (30 ng/kg/min) from t = −24 h to 6 h (n = 6). EPI-3 and EPI-24 reduced LPS-induced increases in soluble E-selectin levels (P <0.05 versus LPS only).
Discussion
This study demonstrates for the first time that epinephrine exerts a strong anticoagulant effect during endotoxemia. Epinephrine attenuated activation of the coagulation system induced by intravenous injection of LPS, and concurrently enhanced activation of the fibrinolytic system. The anticoagulant properties of epinephrine were further demonstrated by a >50% reduction in the ratio of maximal coagulation activation and maximal fibrinolytic activation. These effects of epinephrine on the hemostatic mechanism were found at physiologically relevant plasma concentrations (1,000–1,200 pg/ml) (13). Indeed, the infusion of epinephrine sought to mimic two clinically relevant situations, i.e. epinephrine concentrations achieved were in the same range as those found in patients with SIRS, and the rate and dose at which epinephrine was infused resembled the rate and dose at which the hormone is started as part of supportive treatment of SIRS (13).
We chose to start the infusion of epinephrine at 3 or 24 h before LPS administration because in vitro studies had suggested that the effect of epinephrine on intracellular cAMP levels is dependent on the duration of exposure of cells to this hormone (12). Thus, incubation of mononuclear cells with epinephrine for 2 h increased cellular cAMP concentrations, while incubation for 24 h was associated with a decrease in cAMP levels (12). cAMP plays a pivotal role in many inflammatory reactions, including expression of tissue factor on mononuclear cells (9, 10), a process mediating activation of the common pathway of the coagulation system during SIRS (1, 7, 8). We hypothesized that β adrenergic stimulation would inhibit LPS-induced tissue factor expression as a consequence of a direct increase in cAMP levels. Considering the biphasic effect of epinephrine on cellular cAMP concentrations in vitro (12), we considered it important to determine the influence of epinephrine infusions of various durations on the coagulation system. However, both EPI-3 and EPI-24 inhibited LPS-induced coagulation activation. The release of TNF, a cytokine of which the production is negatively regulated by cAMP (12, 15), was also reduced in both the EPI-3 and EPI-24 groups (13), albeit to a lesser extent in the latter group. However, the circulating levels of IL-10, the production of which is positively regulated by cAMP (16), were increased only in the EPI-3 group (13). Hence, the duration of the effect of epinephrine may vary from response to response, i.e., brief (IL-10), intermediate (TNF), or long (coagulation). We did not directly measure cAMP concentrations within circulating mononuclear cells. It should be noted, however, that we were previously unable to demonstrate enhanced tissue factor expression on circulating monocytes in this model of low grade endotoxemia (17), suggesting that cells not present in the circulation are involved in initiation of coagulation. Nonetheless, our data do not firmly establish the duration of the in vivo effect of epinephrine on intracellular cAMP, or the mechanism underlying the variable duration of epinephrine effects considered to be mediated by elevated cAMP levels.
It can be argued that decreased TNF and increased IL-10 concentrations (13) might have contributed to the anticoagulant effect of epinephrine, since TNF stimulates and IL-10 inhibits tissue factor expression in vitro (18, 19). However, it is unlikely that the inhibition of coagulation activation by epinephrine was mediated via an effect on the cytokine system. Indeed, TNF is not necessary for activation of coagulation during low grade endotoxemia (20), and IL-10 levels were unaltered in the EPI-24 group, whereas this group showed a similar inhibition of coagulation as the EPI-3 group (13). Further, neither epinephrine infusion influenced LPS-induced release of IL-6 (13), a cytokine that appears to play an important role in LPS-induced coagulation activation (21).
Continuous infusion of epinephrine per se (day 1) was associated with a transient activation of the fibrinolytic system. Earlier studies demonstrated tPA release after shortterm exposure of humans to epinephrine (5, 6), which likely reflects a direct effect of epinephrine on the vascular endothelium (22). Additionally, epinephrine potentiated the release of tPA in response to injection of LPS. It should be noted that epinephrine did not cause a general endothelial cell activation, since the release of soluble E-selectin, a molecule that is shed by the vascular endothelium upon activation (14, 23), was inhibited in the EPI-3 and EPI-24 groups. Epinephrine did not influence LPS-induced release of the main inhibitor of plasminogen activation, PAI-1. The gradual increase in PAI-1 antigen during infusion of epinephrine only (day 1) likely reflects a modest acute phase protein response induced by this hormone, considering that PAI-1 behaves as an acute phase protein during inflammation (24), and that other acute phase proteins also gradually increased during epinephrine infusion (data not shown).
Systemic infection and acute injury lead to the activation of multiple host mediator systems. We demonstrate here that a physiologically relevant elevation in the plasma levels of epinephrine markedly influences the activation of hemostatic mechanism during endotoxemia in humans. The anticoagulant effect of epinephrine represents a property of this catecholamine not previously recognized that may be important for the understanding of the complex agonistic and antagonistic interactions between various mediator systems involved in the pathogenesis of disseminated intravascular coagulation in acutely ill patients.
Acknowledgments
Supported by RO1 GM 34695, and RR0047 from the U.S. Public Health Service. Tom van der Poll is a fellow of the Royal Netherlands Academy of Arts and Sciences.
References
- 1.Levi M, ten Cate H, van der Poll T, van Deventer SJH. New insights in the pathogenesis of disseminated intravascular coagulation in sepsis. JAMA (J Am Med Assoc) 1993;270:975–979. [PubMed] [Google Scholar]
- 2.Frayn KN. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol. 1986;24:577–599. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ, Lowry SF, Fahey TJ, III, Albert J, Fong Y, Hesse D, Beutler B, Manogue KR, Calvano S, Wei H, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164:415–422. [PubMed] [Google Scholar]
- 4.Fellows IW, Bennett T, MacDonald LA. The effect of adrenaline upon cardiovascular and metabolic functions in man. Clin Sci. 1985;69:215–222. doi: 10.1042/cs0690215. [DOI] [PubMed] [Google Scholar]
- 5.Chandler WI, Veith RC, Fellingham GW, Levy WC, Schwartz RS, Cerquera MD, Kahn SF, Larson VG, Cain KC, Beard JC, et al. Fibrinolytic response during exercise and epinephrine infusion in the same subjects. J Am Coll Cardiol. 1992;19:1412–1420. doi: 10.1016/0735-1097(92)90596-f. [DOI] [PubMed] [Google Scholar]
- 6.Chandler WI, Levy WC, Veith RC, Stratton JS. A kinetic model of the circulatory regulation of tissue plasminogen activator during exercise, epinephrine infusion, and endurance training. Blood. 1993;81:3293–3302. [PubMed] [Google Scholar]
- 7.Taylor Jr., F.B., A. Chang, W. Ruf, J.H. Morrissey, L. Hinshaw, R. Catlett, K. Blick, and T.S. Edgington. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 8.Levi M, ten Cate H, Bauer KA, van der Poll T, Edgington TS, Büller HR, van Deventer SJH, Hack CE, ten Cate JW, Rosenberg RD. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994;93:114–120. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crutchley DJ, Hirsch MJ. The stable prostacyclin analog, Iloprost, and prostaglandin E1, inhibit monocyte procoagulant activity in vitro. Blood. 1991;78:382–386. [PubMed] [Google Scholar]
- 10.Ollivier V, Houssaye S, Ternisien C, Leon A, de Verneuil H, Elbim C, Mackman N, Edgington TS, de Prost D. Endotoxin-induced tissue factor messenger RNA in human monocytes is negatively regulated by a cyclic AMPdependent mechanism. Blood. 1993;81:973–979. [PubMed] [Google Scholar]
- 11.Verghese MW, Snyderman R. Hormonal activation of adenylate cyclase in macrophage membranes is regulated by guanine nucleotides. J Immunol. 1983;130:869–873. [PubMed] [Google Scholar]
- 12.Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and ß-adrenergic agonists. J Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- 13.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-α and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 15.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJH. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2 . J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poll T, Coyle SM, Levi M, Boermeester MA, Braxton CC, Jansen PM, Hack CE, Lowry SF. Fat emulsion infusion potentiates coagulation activation during human endotoxemia. Thromb Haemostas. 1996;75:83–86. [PubMed] [Google Scholar]
- 18.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradier O, Gérard C, Delvaux A, Lybin M, Abramowicz D, Capel P, Velu T, Goldman M. Interleukin-10 inhibits the induction of monocyte procoagulant activity by bacterial lipopolysaccharide. Eur J Immunol. 1993;23:2700–2703. doi: 10.1002/eji.1830231048. [DOI] [PubMed] [Google Scholar]
- 20.van der Poll T, Levi M, van Deventer SJH, ten Cate H, Haagmans BL, Biemond BJ, Büller HR, Hack CE, ten Cate JW. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood. 1994;83:446–451. [PubMed] [Google Scholar]
- 21.van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJH, Eerenberg AJM, de Groot ER, Jansen J, Gallati H, Büller HR, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjaelgaard A, Kjaelgaard M. In vitro stimulation of plasminogen activator release from vein walls by adrenaline. J Clin Pathol. 1986;39:1241–1244. doi: 10.1136/jcp.39.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman W, Beall LD, Carson CW, Hunder GG, Graben N, Randhawa ZI, Gopal TV, Wiener-Kronisch J, Matthay MA. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in the serum of patients with septic shock. J Immunol. 1993;150:644–654. [PubMed] [Google Scholar]
- 24.De Boer JP, Abbink JJ, Brouwer MC, Meijer C, Roem D, Voorn GP, Lambers JWJ, van Mourik JA, Hack CE. PAI-1 synthesis in the human hepatoma cell line Hep G2 is increased by cytokines. Evidence that the liver contributes to acute phase behaviour of PAI-1. Thromb Haemostas. 1991;65:181–185. [PubMed] [Google Scholar]