Abstract
To address the possible role of replicative senescence in human immunodeficiency virus (HIV) infection, telomere length, telomerase activity, and in vitro replicative capacity were assessed in peripheral blood T cells from HIV+ and HIV− donors. Genetic and age-specific effects on these parameters were controlled by studying HIV-discordant pairs of monozygotic twins. Telomere terminal restriction fragment (TRF) lengths from CD4+ T cells of HIV+ donors were significantly greater than those from HIV− twins. In contrast, telomere lengths in CD8+ T cells from HIV+ donors were shorter than in HIV− donors. The in vitro replicative capacity of CD4+ cells from HIV+ donors was equivalent to that of HIV− donors in response to stimulation through T cell receptor CD3 and CD28. Little or no telomerase activity was detected in freshly isolated CD4+ or CD8+ lymphocytes from HIV+ or HIV− donors, but was induced by in vitro stimulation of both HIV+ and HIV− donor cells. These results suggest that HIV infection is associated with alterations in the population dynamics of both CD4+ and CD8+ T cells, but fail to provide evidence for clonal exhaustion or replicative senescence as a mechanism underlying the decline in CD4+ T cells of HIV-infected donors.
Aprogressive decrease in numbers of CD4+ T cells is a prominent feature of HIV infection and AIDS that correlates with progression of disease and susceptibility to infection. It appears that excessive destruction of CD4+ T cells occurs in HIV-1 (HIV) infection, and that an increased rate of generation of CD4+ cells occurs concurrently, perhaps reflecting compensatory mechanisms to maintain the population of CD4+ cells (1). CD4+ T cell counts decrease with progressive HIV infection, indicating that physiologic mechanisms of regeneration are insufficient to compensate adequately for cell loss. It has also been suggested that increased clonal expansion and possible clonal exhaustion of CD8+ T cells occur in response to HIV infection (2, 3).
Among the factors that can influence replicative potential and clonal expansion, one mechanism that has received considerable recent attention is the phenomenon of telomere shortening during cell division (4–8). Observations in somatic cells indicate that telomere shortening occurs with each cell division. When telomere shortening has proceeded to some critical minimal length, cell senescence or arrest of replication is observed, through mechanisms not yet elucidated. Thus, in the absence of a compensatory mechanism to prevent or reverse replication-associated loss of telomeric sequences, telomere shortening may contribute to the finite replicative lifespan of normal somatic cells. A ribonucleoprotein enzyme, telomerase, has the capacity to add hexanucleotide telomeric repeats to chromosomes, thus maintaining telomere length (9, 10). Therefore, it was of interest to determine whether HIV infection results in alterations of telomere length within T cell subpopulations as a reflection of the replicative history of these cells, and whether the residual replicative potential of T cells is altered in infected individuals.
Materials and Methods
Isolation and Fractionation of Peripheral Blood T Cells.
Subsets of peripheral blood T cells were isolated by immunomagnetic beading as previously described (11).
Assay of Telomere Length.
Genomic DNA was isolated from purified peripheral blood CD4+ and CD8+ T cells, digested with HinfI and RsaI (Boehringer Mannheim, Mannheim, Germany), and separated by electrophoresis on a 0.5% agarose gel. Gels were dried, denatured, hybridized with a 32P end-labeled oligonucleotide (CCCTAA)3 probe, and the gels analyzed by PhosphorImager (Molecular Dynamics, Sunnyvale, CA) as previously reported (11).
PCR-based Telomerase Assay.
The telomerase assay used here was modified from the telomeric repeats amplification protocol (TRAP)1 described previously (12). Cell extracts were prepared in CHAPS lysis buffer, and the telomerase assay and PCR amplification steps were carried out in separate tubes with different buffers to improve the telomerase efficiency. Telomerase products equivalent to the extracts from 104 cells were used for PCR with 27 cycles of amplification. The amplified products were separated on a 12% acrylamide gel (NOVEX, San Diego, CA) and the results analyzed on a PhosphorImager (Molecular Dynamics) or by exposure to radiographic film. To estimate the activity of telomerase, serial dilutions of cell extracts were employed, and an internal standard was included to allow quantitation of PCR by competition in selected experiments. Controls for the TRAP assay included RNase treatment of cell extracts, single primer (Ts or Cx alone), and water as template for PCR.
In Vitro Stimulation.
CD4+ T cells were repeatedly stimulated with immobilized anti-CD3 and anti-CD28 as previously described (11). Population expansion was measured until cell cultures were unresponsive to further stimulation.
Results
Telomere Length in CD4+ and CD8+ T Cells from HIVinfected and Uninfected Twins.
To control for known genetic (13) and age-specific (4–8) effects on telomere length and replicative potential, peripheral blood T cells were analyzed from seven pairs of genetically identical monozygotic twins who were discordant for HIV infection (Table 1). Total CD4+ counts ranged from 92 to 562 cells/ml in HIV+ donors, and 500 to 1,516 in their HIV− twins. CD8 counts ranged from 501 to 1,131 in HIV+ donors, and from 378 to 786 in HIV− donors.
Table 1.
Donor Status
| Twin Pair | CD4 count* | %CD45RA/ CD4+‡ | CD8 count* | Age | HIV diagnosed | Current clinical status | Antiviral therapy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | |||||||||||||||
| yr | ||||||||||||||||||||
| 1 | 298 | 1516 | 31.7 | 24.6 | 1131 | 786 | 54 | 01/89 | Pres. PCp (1990)§ | AZT, ddI, D4T | ||||||||||
| 2 | 464 | 658 | 27.7 | 22.5 | 501 | 415 | 31 | 10/93 | Asymptomatic | AZT | ||||||||||
| 3 | 120 | 500 | 14.7 | 5.9 | 519 | 378 | 44 | 06/88 | Chronic Hepatitis B | AZT, 3TC | ||||||||||
| 4 | 124 | 712 | 42.5 | 24.7 | 591 | 642 | 30 | 05/90 | Asymptomatic | ddC, 3TC | ||||||||||
| 5 | 355 | 1037 | 51.9 | 57.3 | 709 | 499 | 29 | 12/92 | Asymptomatic | AZT | ||||||||||
| 6 | 92 | 605 | 51.2 | 26.9 | 771 | 546 | 54 | 1989 | Asymptomatic | None | ||||||||||
| 7 | 562 | 1344 | 57.7 | 52.2 | 1111 | 359 | 45 | 02/90 | Asymptomatic | AZT | ||||||||||
Number of cells/mm3 of peripheral blood.
Percent of CD4+ T cells which are CD5RA positive and CD45RO negative.
Presumed Pneumocystis corinii pneumonia noted in 1990, but asymptomatic at the time of apheresis.
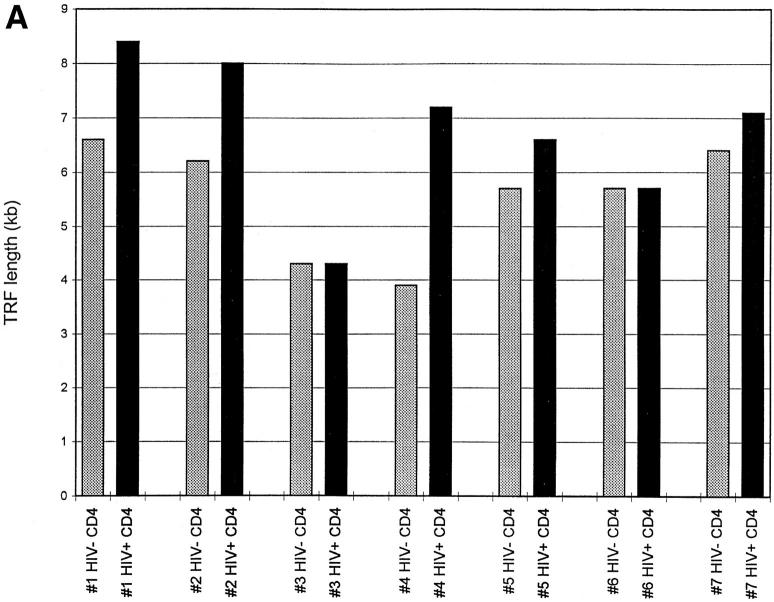
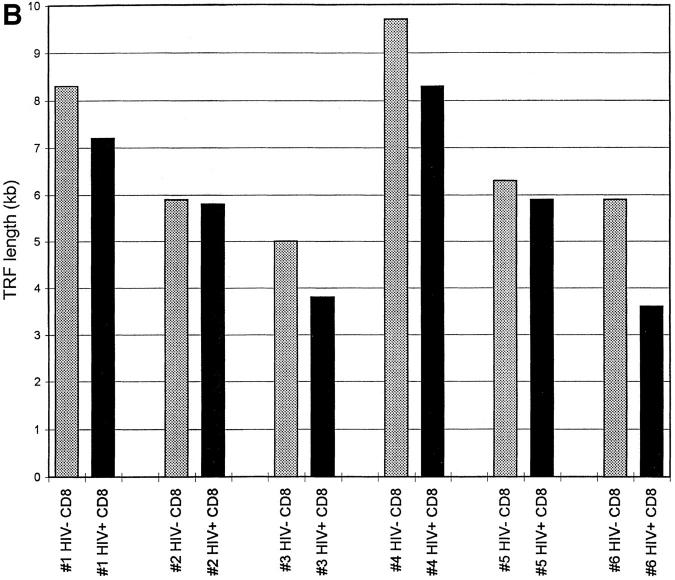
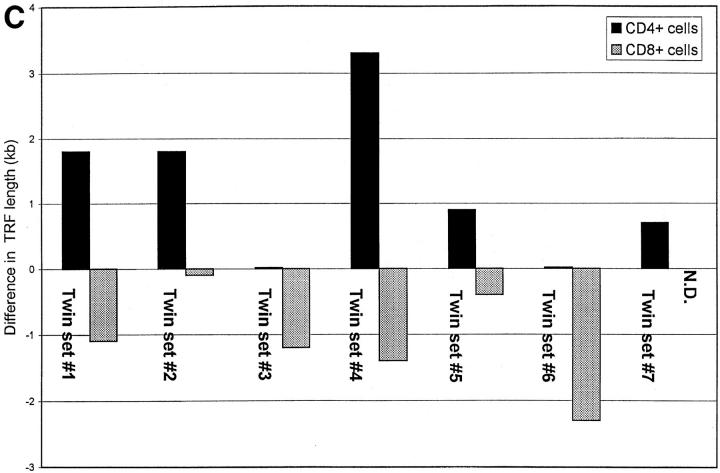
If, as has been suggested (14, 15), peripheral blood CD4+ cells from HIV-positive donors undergo increased cell division, this difference might be reflected in shortening of telomeres from infected donors when compared with uninfected twins. Representative telomere terminal restriction fragment (TRF) length analysis of CD4+ and CD8+ T cells from HIV-positive and negative monozygotic twins is presented in Fig. 1. When comparisons of mean TRF length were made within each twin pair, it was observed that TRF length in CD4+ cells on average was significantly greater in the infected than in the uninfected twins (mean difference 1.2 ± 0.4 kb) (Fig. 2, A and C). When TRF length was analyzed in CD8+ populations from HIV-discordant twins, the opposite pattern was observed, with TRF length in CD8+ cells shorter in HIV-infected twins than in uninfected twins (mean difference 1.1 ± 0.3 kb) (Fig. 2, B and C). In HIV-uninfected donors, TRF length in CD8+ T cells tended to be longer than in CD4+ cells from the same individual (mean difference 1.2 ± 0.7 kb); whereas in HIV-infected twins, TRF length was greater in CD4+ cells (mean difference 0.9 ± 0.4 kb).
Figure 1.
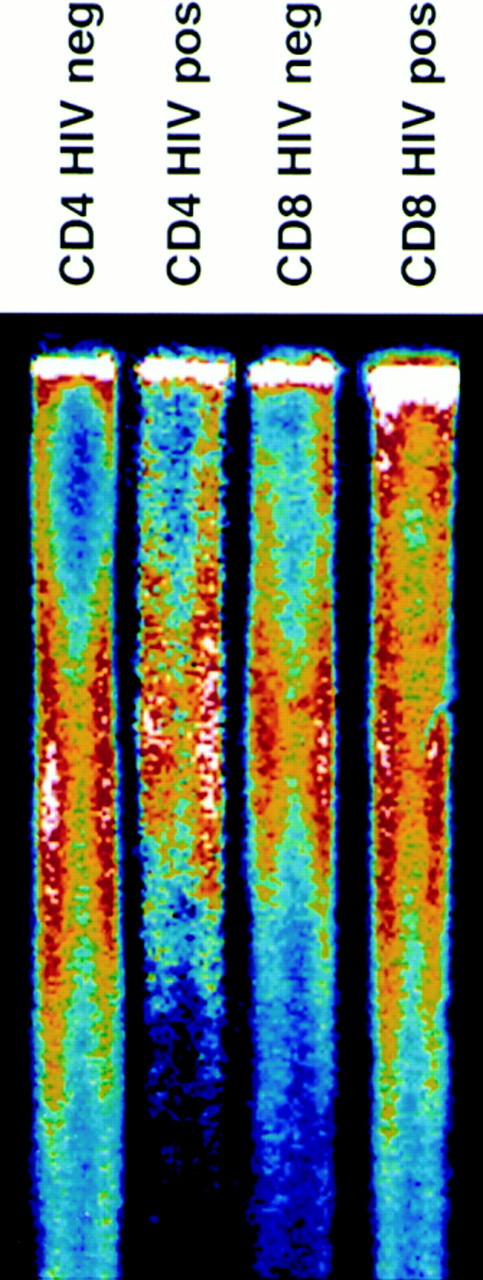
Telomere length in CD4+ and CD8+ T cells. Genomic DNA was isolated from purified peripheral blood CD4+ and CD8+ T cells, digested, separated by electrophoresis, and the dried and denatured gel was hybridized with a 32P end-labeled oligonucleotide (CCCTAA)3 probe. Hybridized gels were subjected to PhosphorImager analysis.
Figure 2.
Telomere length in CD4+ and CD8+ T cells from HIV-positive and -negative twins. TRF analysis was carried out as described in Fig. 1, and mean TRF length calculated. (A) TRF length is shown for CD4+ T cells from HIV-negative (stippled bars) and HIV-positive (closed bars) twin donors. (B) TRF length is shown for CD8+ T cells from HIVnegative (stippled bars) and HIV-positive (closed bars) twin donors. (C) The difference in TRF length for HIV-positive and HIV-negative twin donors is shown for CD4+ (closed bar) and CD8+ (stippled bar) T cells. Positive values indicate TRF length that is greater in the HIV-positive twin, and negative values indicate TRF length that is greater in the HIV-negative twin.
Telomerase Activity in CD4+ and CD8+ T Cells from HIVinfected and Uninfected Twins.
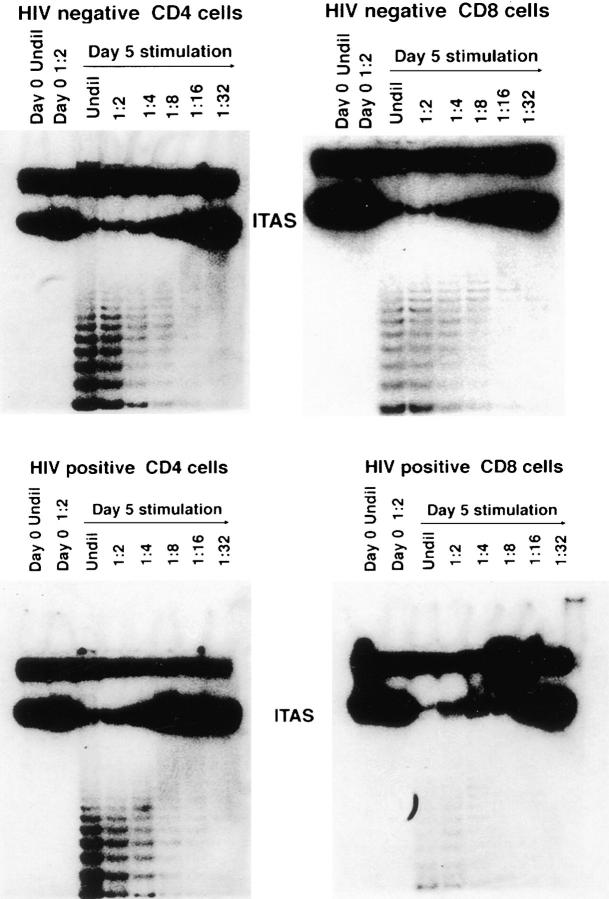
Two opposing factors are known to contribute to telomere length: the shortening of telomeres that occurs with cell division and chromosomal replication, and the extension of telomeres mediated by telomerase. To determine whether the TRF length of CD4+ T cells from HIV-infected donors might be influenced by telomerase activity, telomerase was measured using the PCR-amplified TRAP assay. Telomerase activity was low or undetectable in ex vivo isolated CD4+ or CD8+ cells from a separate panel of 15 (non-twin) HIV-positive donors analyzed or from HIV-negative controls (Fig. 3). Stimulation of CD4+ T cells from HIV-negative donors with antiCD3 and anti-CD28 induced vigorous telomerase activity, consistent with previous studies (12, 16); and T cells from HIV-positive donors exhibited similar levels of telomerase activity after stimulation (Fig. 3).
Figure 3.
Telomerase activity in T cells from HIV-positive and HIVnegative donors. CD4+ and CD8+ T cells were isolated from HIV-positive and HIV-negative donors by negative selection. Telomerase activity was assayed in freshly isolated cells and after in vitro stimulation with antiCD3 and anti-CD28. T cells were stimulated for 5 d with a combination of bead-bound anti-CD3 and CD28 mAbs. Results shown are representative of 15 HIV+ donors and their HIV− controls. 293 is a transformed human kidney cell line that expresses high levels of telomerase and is used here as a positive control. HUVEC are normal epithelial cells isolated from human umbilical vein used here as a negative control.
Replicative Potential of CD4+ T Cells from HIV-infected and Uninfected Twins.
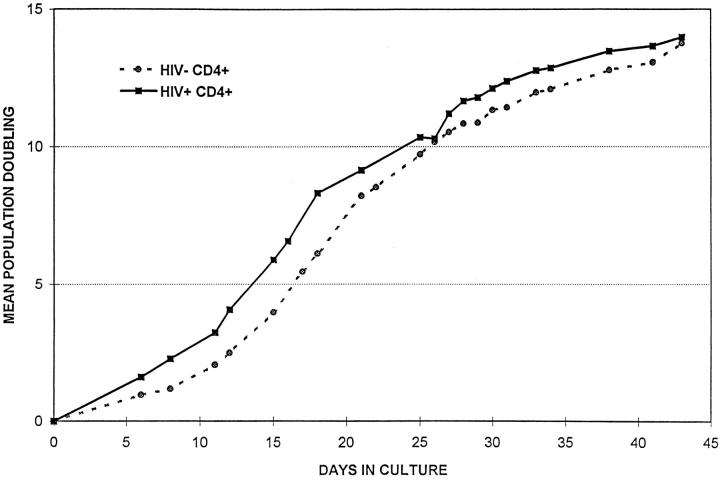
To determine more directly whether the progressive decrease in CD4+ cell counts during HIV infection reflects replicative senescence, CD4+ T cells were stimulated repeatedly with immobilized anti-CD3 and antiCD28 antibodies, and replicative capacity was measured as the mean number of population doublings (mpd) achieved before proliferative response ceased (Fig. 4). When CD4+ cells from five sets of discordant twins were compared, there was no significant difference in the capacity for in vitro cell division exhibited by HIV-positive and negative donors (16.6 ± 2.2 mpd for HIV-positive donors and 18.2 ± 2.1 mpd for HIV-negative donors).
Figure 4.
Replicative potential of CD4+ T cells from HIV-positive and HIV-negative donors. CD4+ T cells were repeatedly stimulated with immobilized anti-CD3 and anti-CD28. Population expansion was measured until cell cultures were unresponsive to further stimulation. The data shown are representative of five twin pairs studied.
Discussion
The population dynamics of T cell subsets from HIVinfected patients are of considerable interest in efforts to understand the pathogenesis and natural history of AIDS and to design therapeutic intervention. A consistent feature of HIV infection is the progressive decrease in CD4+ T cells that appears to correlate strongly with immune deficiency and susceptibility to opportunistic infection (1). Several lines of experimental evidence have suggested that there is increased destruction of CD4+ T cells in HIV infection, and that there is an increased production rate of CD4+ T cells in HIV-infected individuals, possibly in response to this increased destruction (14, 15). These previous reports suggested that the ultimate decrease in CD4+ T cell levels observed in AIDS might result, at least in part, from exhaustion of the capacity to generate CD4+ cells (17). Since the demonstration 35 years ago that human fibroblasts, a model of normal somatic cells, have a finite capacity for cell division measured in vitro (18), numerous studies have confirmed this observation for cell populations including human T cells. Such findings are consistent with the possibility that a form of clonal exhaustion or senescence might contribute to the immune deficiency seen in AIDS.
The studies reported here have assessed the replicative history and potential of T cells from HIV-infected donors by evaluating several parameters. Telomere length in normal human somatic cells, including T lymphocytes, decreases with cell replication in vitro, on average by 50–200 bp per population doubling (4–8, 11). A mechanistic explanation for this shortening has been proposed, based on the primer requirement for DNA polymerases and the consequent inability to completely replicate chromosomal termini during cellular S phase. In vivo, telomere length decreases with age of the donor, suggesting that a similar process occurs with aging. These observations have led to a model in which telomere shortening contributes to the finite replicative lifespan of normal somatic cells, with senescence occurring when telomere length reaches some critical minimum that is required for cell replication. Measurement of telomere length has been used to analyze the replicative history of cell populations, an approach that has been applied to analysis of human T cells from healthy uninfected donors. The finding that TRF from CD4+ cells of memory (CD45RO) phenotype are on average 1.4 kb shorter than TRF from naive (CD45RA) CD4+ cells, and that memory cells have less residual replicative potential than naive cells under defined conditions, has been used as the basis for speculation concerning the dynamics of clonal expansion relating these two populations (11).
In the studies reported here, it was found that CD8+ T cells from infected donors had TRF that were shorter than those of uninfected donors. Analysis of telomere length has recently been applied to subsets of human CD8+ T cells. The CD28− subset of CD8+ T cells from healthy uninfected donors was characterized as a clonally expanded subset and was reported to have TRF that were significantly shorter than TRF from CD28+CD8+ cells of the same donor (19). Consistent with this finding, it was recently reported that telomere length was reduced in the expanded CD28− subset of CD8+ cells from HIV-infected patients (20). The possibility that this difference is related to a functional compromise of CD8+ cells in HIV patients deserves further attention.
Previous reports have suggested that in HIV-positive donors, increased proliferation of CD4+ T cells may occur in vivo (14). Were this the case, it might have been expected that CD4+ cells from HIV-positive twins would, as a correlate of increased clonal expansion, exhibit shorter telomeres than cells from uninfected genetically identical twins. However, the studies reported here demonstrated that, in contrast with what was observed for CD8+ cells, CD4+ cells from HIV-positive donors in fact had TRF significantly longer than those of uninfected twins. Limitations in the amount of blood that was available from donors in the present study prevented direct analysis of telomere length in fractionated CD45RA+ and CD45RO+ subsets of CD4+ cells. However, there was no preferential decrease in the fraction of CD45RA+ (naive) CD4+ T cells in HIV-infected twins in the present study, and, therefore, the differences in telomere length observed between HIV+ and HIV− twins did not correlate with differences in the proportions of these subpopulations. These observations might be interpreted to indicate that, in HIV-infected donors, CD4+ cells were on average derived from stem cells by a path that involved fewer cell divisions than occur in uninfected individuals, resulting in telomere preservation. For example, this might occur if destruction of CD4+ cells resulted in regeneration from a pool of stem cells which themselves had retained longer telomeres. Alternatively, telomere shortening may be uncoupled from cell division in CD4+ cells from HIV-infected donors, for example, by a mechanism such as the induction of telomerase. The present study provided no evidence that in vivo telomerase activity contributes to telomere length differences associated with HIV infection, although it cannot be excluded that telomerase activity in sites other than peripheral blood, or at levels not detected by the assay employed, plays a role. Finally, the possibility that HIV infection selectively destroys CD4+ T cells with shorter telomeres cannot be excluded. These findings, together with the demonstration that the in vitro replicative potential of CD4+ cells from AIDS patients was not diminished relative to that of uninfected twins, suggest that HIV infection may be associated with alterations in the population dynamics of both CD4+ and CD8+ T cells, but that clonal exhaustion or senscence is not a dominant factor in the progressive loss of CD4+ cells in patients with AIDS.
Acknowledgments
The authors wish to thank the patients who donated cells for these studies and the physicians who referred those patients to us. Additionally, we thank J. Metcalf and C. Bechtel for facilitating leukopheresis and gathering patient demographics. The authors also wish to thank Dr. R. Walker for his advice and Dr. G. Shearer for his thoughtful comments and review of this manuscript.
This work was supported in part by Army contract DAMD17-93-V-3004, by the Navy Medical Research and Development Command, and by the Henry M. Jackson Foundation. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Navy, Department of Defense, nor the United States Government.
Footnotes
1 Abbreviations used in this paper: mpd, mean population doublings; TRAP, telomeric repeats amplification protocol; TRF, terminal restriction fragment.
References
- 1.Lane HC, Depper JM, Greene WC, Whalen G, Waldmann TA, Fauci AS. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- 2.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 3.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Viral persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature (Lond) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allsopp RC, Harley CB. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 8.Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 9.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymenaextracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science (Wash DC) 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 11.Weng N-P, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci USA. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng N, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 14.Ho D, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature (Lond) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 15.Mahalingham M, Pozniak A, McManus TJ, Vergani D, Peakman M. Cell cycling in HIV infection: analysis of in vitro activated lymphocytes. Clin Exp Immunol. 1996;102:481–486. doi: 10.1111/j.1365-2249.1995.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broccoli D, Young JW, De Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miedema F, Klein MR. AIDS pathogenesis: A finite immune response to blame? . Science (Wash DC) 1996;272:505–506. doi: 10.1126/science.272.5261.505. [DOI] [PubMed] [Google Scholar]
- 18.Hayflick L, Moorhead P. The serial cultivation of human diploid strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 19.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 20.Effros RB, Allsopp R, Chiu C-P, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28−CD8+cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]