Abstract
The T cell response to the 65-kD mycobacterial heat-shock protein (Bhsp65) has been implicated in the pathogenesis of autoimmune arthritis. Adjuvant arthritis (AA) induced in the Lewis rat (RT-1l) by injection of Mycobacterium tuberculosis serves as an experimental model for human rheumatoid arthritis (RA). However, the immunological basis of regulation of acute AA, or of susceptibility/resistance to AA is not known. We have defined the specificity of the proliferative T cell responses to Bhsp65 during the course of AA in the Lewis rat. During the early phase of the disease (6–9 d after onset of AA), Lewis rats raised T cell responses to many determinants within Bhsp65, spread throughout the molecule. Importantly, in the late phase of the disease (8–10 wk after onset of AA), there was evidence for diversification of the T cell responses toward Bhsp65 carboxy-terminal determinants (BCTD) (namely, 417–431, 441–455, 465–479, 513–527, and 521–535). Moreover, arthritic rats in the late phase of AA also raised vigorous T cell responses to those carboxy-terminal determinants within self(rat) hsp65 (Rhsp65) that correspond in position to the above BCTD. These results suggest that the observed diversification is possibly triggered in vivo by induction of self(Rhsp65)-reactive T cells. Interestingly, another strain of rat, the Wistar Kyoto (WKY/NHsd) rat (RT-1l), with the same major histocompatibility complex class II molecules as the Lewis rat, was found to be resistant to AA. In WKY rats, vigorous responses to the BCTD, to which the Lewis rat responded only in the late phase of AA, were observed very early, 10 d after injection of M. tuberculosis. Strikingly, pretreatment with the peptides comprising the set of BCTD, but not its amino-terminal determinants, provided significant protection to naive Lewis rats from subsequent induction of AA. Thus, T cell responses to the BCTD are involved in regulating inflammatory arthritis in the Lewis rat and in conferring resistance to AA in the WKY rat. These results have important implications in understanding the pathogenesis of RA and in devising new immunotherapeutic strategies for this disease.
Rheumatoid arthritis (RA)1 is an autoimmune disease of unknown etiology (1, 2). In the past several years, considerable interest has been generated in the role of the 65-kD mycobacterial heat-shock protein (Bhsp65) in the pathogenesis of autoimmune arthritis both in experimental animals (3, 4) as well as in humans (5–7). In RA patients, an association between T cell responses to Bhsp65 and early stages of joint inflammation has been observed (8–10), suggesting that Bhsp65-reactive T cell responses are involved in the pathogenesis of this disease. Adjuvant arthritis (AA) can be induced in the inbred Lewis rat after immunization with Mycobacterium tuberculosis in oil (11–13). The disease can also be transferred to naive Lewis rats by T cell lines reactive to peptide 180–188 of Bhsp65 (3, 14). Although arthritic Lewis rats develop vigorous T cell responses to native Bhsp65 and to peptide 180–188 of Bhsp65, neither of these is arthritogenic when injected in protein or peptide form, respectively (15, 16). Interestingly, pretreatment with Bhsp65 can protect naive Lewis rats from development of arthritis upon subsequent immunization with M. tuberculosis (4, 17), suggesting that Bhsp65 contains protective as well as disease-inducing determinants. T cell lines specific for Bhsp65 have also been shown to protect against AA (14, 18). Similarly, Lewis rats afflicted with AA are resistant to reinduction of AA (13). Neither the mechanism of natural regulation of the acute inflammatory phase of AA nor the mechanism of protection from subsequent induction of AA is known. Likewise, the immunological basis of susceptibility or resistance to AA of different rat strains has not been revealed.
In the present study, we have defined the changing pattern of specificity of the T cell responses of Lewis rats to determinants within Bhsp65 during the course of AA. Arthritic Lewis rats in the early and late phase of the disease revealed distinct patterns of T cell responses to Bhsp65. Importantly, with progression of the disease, there was evidence for diversification of the T cell responses toward Bhsp65 carboxy-terminal determinants (BCTD). (The phenomenon of spreading of the T cell responses to new determinants within an antigen, after priming with a single determinant of the same antigen, has been previously described as determinant spreading [19]. On the other hand, we have termed the induction of T cell responses to new determinants after priming with the whole, multideterminant antigen as diversification [20, 21]). Moreover, arthritic Lewis rats in the late phase of AA also raised significant responses to certain carboxy-terminal determinants within self(rat) hsp65 (Rhsp65). (Rat hsp60 [22] has been referred to as rat hsp65 in this study to emphasize its relationship with Bhsp65.) These self-determinants correspond in position precisely to that of the BCTD, suggesting that diversification of response to Bhsp65 observed in vitro, might be triggered in vivo by self-hsp65. In contrast with AA-susceptible Lewis rats, MHC class II–identical, Wistar Kyoto (WKY/NHsd = WKY) rats (23, 24) were found to be resistant to induction of AA after immunization with M. tuberculosis. In fact, M. tuberculosis-immunized WKY rats raised early and vigorous responses to the BCTD to which the Lewis rats only respond during the late phase of the disease. Pertinently, pretreatment of naive Lewis rats with peptides comprising the BCTD, but not its amino-terminal determinants (BNTD), induced significant protection from AA. These results suggest that T cell responses to the BCTD are involved in regulation of acute inflammatory arthritis. Our study suggests one of the immunological bases for natural regulation of acute AA, and of protection (resistance) from development of AA.
Materials and Methods
Animals.
Inbred Lewis (RT-1l) and Wistar Kyoto (WKY/ NHsd) (=WKY) (RT-1l) rats were obtained from Harlan SpragueDawley (San Diego, CA). Male rats, 5–12-wk-old, were used for the experiments. The handling of animals and all procedures performed on them were done in compliance with the guidelines of the UCLA Chancellor's Animal Research Committee.
Antigens–Peptides.
Bhsp65 from Mycobacterium bovis (which is identical to hsp65 of M. tuberculosis) was obtained from the World Health Organization through Dr. R. van der Zee. The peptides containing amino acid sequences of the Bhsp65 or Rhsp65 (22, 25, 26; the National Biomedical Research Foundation data base) were prepared by three methods: (a) A complete series of overlapping peptides (15-mers with an overlap of 11 amino acid residues) spanning the entire sequence of Bhsp65 was obtained from Chiron Mimotopes (Clayton, Australia). The peptides were synthesized using the multi-pin peptide synthesis technique using repeated cycles of Fmoc deprotection and amino acid couplings (27). The procedure had been modified so that the peptides could be cleaved from the pins. The terminal amino group of each peptide was acetylated, whereas diketopiperazine was attached at the carboxy terminus. (b) Several peptides of Bhsp65 and Rhsp65 were synthesized in the UCLA Peptide Core Laboratory directed by Dr. J.R. Reeve, Jr., using a Multiple Peptide Synthesizer (Advanced Chem Tech, 396 MPS, Louisville, KY) as described elsewhere (28). The identity and purity of these peptides were determined by Fast Atom Bombardment Mass Spectrometry at the UCLA Center for Molecular and Medical Sciences Mass Spectrometry facility. (c) Some peptides were obtained from Macromolecular Resources (Colorado State University, Fort Collins, CO). These peptides were synthesized according to the method described elsewhere (29). Hen eggwhite lysozyme (HEL) peptide was synthesized according to the method previously described (29).
Induction of Adjuvant Arthritis.
Inbred male Lewis rats were anesthetized using Halothane (Halocarbon Laboratories, River Edge, NJ), and then immunized subcutaneously in a hind footpad with 200 μl of M. tuberculosis H37Ra (Difco Laboratories, Detroit, MI) (10 mg/ml) suspended in IFA (Difco) or in mineral oil (Sigma Chemical Co., St Louis, MO). The bacteria were powdered in a mortar and pestle before suspension in oil. Beginning on day 7 after immunization, the rats were observed daily for clinical signs of arthritis in three of their limbs, excluding the limb in which immunization was performed. The severity of arthritis was evaluated on the basis of erythema, swelling, and deformity of the joint (30, 31), and graded on a scale of 0 to 4 as follows: 0 = no erythema or swelling, 1 = slight erythema or swelling of the ankle or wrist, 2 = moderate erythema and swelling at the wrist or ankle, 3 = moderate erythema and swelling at the wrist/ metacarpals or ankle/metatarsals, 4 = severe erythema and swelling of the forepaw or hindpaw (32, 33). Because only the three uninjected limbs were evaluated, the maximum arthritic score for any rat was 12.
Histopathological Examination of Arthritic Joints.
The experimental or age- and sex-matched control rats were sacrificed under anesthesia. A hind or front leg was cut above the ankle or the wrist, respectively, with the help of a bone cutter. For histopathological examination, the skin from the entire limb was removed and the limb was immersed in 10% buffered formalin phosphate (Fisher Scientific, Fair Lawn, NJ) for fixation for at least 2 d before decalcification. After decalcification, the sections were cut with a cryotome and then stained with hematoxylin and eosin. The stained sections were studied under the microscope for histopathological changes in the joints as previously described (32, 33).
Lymph Node and Splenic T Cell Proliferation Assay.
The draining lymph nodes of rats immunized subcutaneously with M. tuberculosis as described above, were removed and a single cell suspension prepared (29). The debris was allowed to settle, and the cells in the supernatant were washed twice with HBSS (GIBCO BRL, Gaithersburg, MD).These lymph node cells (LNC) were cultured in flat-bottomed 96-well plates at 5 × 105 cells/well in HL-1 serum-free medium (Ventrex Laboratories, Inc., Portland, ME) supplemented with 2 mM l-glutamine, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulphate, with or without antigen. (Splenic T cell proliferation assays were performed as described for LNC, except for plating SPC at a concentration of 6 × 105 cells/well). For the pin peptides, one or two wells were tested per peptide. Tuberculin purified protein derivative (PPD) (Evans Medical Limited, Horsham, England) was used at a final concentration of 2,000 U/well as a positive control. 1 μCi of [3H]thymidine (International Chemical and Nuclear, Irvine, CA) was added per well for the last 18 h of a 5 d culture. The cells were then harvested on a Printed Filtermat A glass fiber filter (Wallac Oy, Turku, Finland) using a Micro Cell Harvester (Skatron Instruments, Inc., Sterling, VA), and the incorporation of radioactivity was assayed by liquid scintillation counting, using the LKB 1205 Betaplate counter. The results were expressed as either cpm or stimulation index (S.I. = cpm with antigen/cpm with cells in medium alone). For some repeat experiments, HL-1 medium supplemented with 1% (vol/vol) heat-inactivated FCS (Gemini BioProducts, Inc., Calabasas, CA) or X-Vivo 10 serum-free medium (Bio-Whittaker, Walkersville, MD) supplemented with 2% FCS and/or 5 × 10−5 M 2-mercaptoethanol (Sigma) was used in place of HL-1 medium. In some of these assays, 2.5–4.5 × 105 cells/ well were used instead of 5–6 × 105 cells/well.
Results
Early and Late Arthritic Responses of Lewis Rats.
Lewis rats were immunized subcutaneously in a hind footpad with M. tuberculosis in oil, and from 7 d after immunization onwards, were observed daily for clinical signs of arthritis. The induction of arthritis was further confirmed by histopathological examination of joints as described in Materials and Methods.
Two timepoints were chosen for study of proliferative T cell responses to Bhsp65 of LNC of arthritic rats: 6–9 d (early phase of AA) or 8–10 wk (late phase of AA) after onset of clinical signs of arthritis (see Materials and Methods). The rationale for choosing days 6–9 was that once arthritis is evident in the hind and fore paws, it is anticipated that lymph nodes draining these limbs would contain Bhsp65sensitized T cells, which could be tested in a T cell proliferation assay (in a fashion similar to immunization of a naive rat subcutaneously with an antigen emulsified in an adjuvant, and then testing the draining LNC for proliferative responses to that antigen). The choice of 8–10 wk was made because rats usually successfully contain active inflammation in the joints by that time. The overall objective of testing LNC in the early and late phases of AA was to determine the dynamics of T cell responses to Bhsp65 during the course of disease.
Response of Lewis Rats to Bhsp65 in the Early Phase of AA.
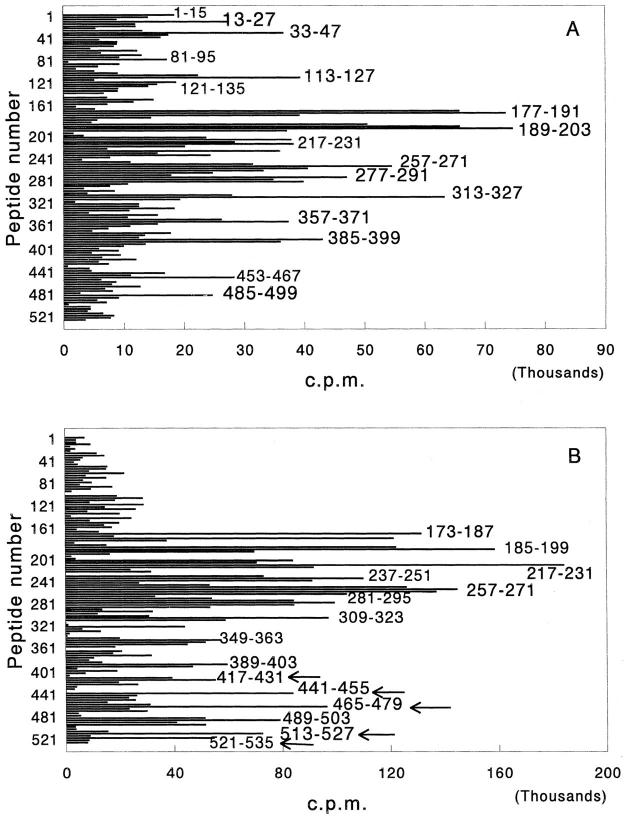
As evident from Fig. 1 A, during the early phase of AA, draining LNC of rats raised proliferative T cell responses to several determinants within Bhsp65: 13–27, 33– 47, 113–127, 177–191, 189–203, 217–231, 257–271, 277– 291, 313–327, 357–371, 385–399, 453–467, and 485–499. Because rats were immunized with M. tuberculosis, the immunogenic determinants shown in Fig. 1 A (and in Fig. 1 B) represent the dominant and subdominant determinants within Bhsp65. As discussed below, from the viewpoint of arthritis induction, the dominant and subdominant determinants within Bhsp65 are of utmost importance.
Figure 1.
Response of arthritic Lewis rats to Bhsp65 in the early (A) and late (B) phases of AA. Inbred Lewis rats were immunized subcutaneously with M. tuberculosis in a hind footpad, and then observed for signs of arthritis. 6–9 d (early phase; A) or 8–10 wk (late phase; B) after the appearance of clinical AA, rats were killed and their popliteal, inguinal, and axillary LNC pooled together were tested in a proliferation assay. In each group, LNC from four rats were pooled for testing with peptides. Overlapping pin peptides spanning the entire length of the Bhsp65 were used for the in vitro recall response in the assay. Each peptide is identified by its first amino acid residue. The results are expressed as cpm. The results of a representative experiment using pooled LNC from four rats are shown in each section of the figure. Similar results were obtained in repeat experiments with pooled LNC or upon testing cells from individual rats with selected Bhsp65 peptides (data not shown). In B, the T cell responses to new, unique Bhsp65 carboxy-terminal determinants (BCTD) are indicated by arrows.
Response of Arthritic Lewis Rats to Bhsp65 in the Late Phase of AA.
In another series of experiments, the LNC of arthritic Lewis rats in the late phase of the disease were tested in a proliferation assay using pin peptides of Bhsp65. The results of one representative experiment using pooled LNC from four rats are given in Fig. 1 B. The predominant peaks representing the dominant and subdominant determinants within Bhsp65 were found to correspond to the following peptides: 173–187, 179–193, 185–199, 217–231, 237–251, 257–271, 281–295, 309–323, 349–363, 389–403, 417–431, 441–455, 465–479, 489–503, 513–527, and 521–535.
Upon detailed comparison of the profiles of proliferative T cell responses of LNC of arthritic Lewis rats in the early and late phases of the disease (Fig. 1, Table 1), three noteworthy findings were observed. First, Lewis rats in the late phase of AA gave strong proliferative responses to several new BCTD; namely, 417–431, 441–455, 465–479, 513– 527, and 521–535, compared with Lewis rats in the early phase of AA. These BCTD had an average relative strength (see Table 1 for description of this index) of 47.8% in the late response in comparison to 13.0% in the early response. Second, rats in the late phase of AA gave significantly decreased responses to certain BNTD; namely, 1–15, 13–27, 33–47, 113–127, and 121–135 compared with the pattern of responses of rats in the early phase of the disease. The average relative strength of these amino-terminal determinants was 52% in the early response in comparison to 10% in the late response (data not shown). Third, in general, the level of the proliferative response to the middle region determinants of Bhsp65 was much higher in the late phase of the disease but essentially unchanged in pattern.
Table 1.
T Cell Responses of Arthritic Lewis Rat to the COOH-terminal Determinants of Bhsp65
| Peptide | Early phase of AA | Late phase of AA | ||||||
|---|---|---|---|---|---|---|---|---|
| cpm × 10−3 (S.I.) | Relative strength | cpm × 10−3 (S.I.) | Relative strength | |||||
| % | % | |||||||
| 417–431 | 6.4 (2.2) | 11.9 | 55.9 (16.2) | 36.8 | ||||
| 441–455 | 4.6 (1.6) | 8.6 | 84.0 (24.3) | 55.3 | ||||
| 465–479 | 8.0 (2.7) | 14.8 | 97.3 (28.2) | 64.2 | ||||
| 513–527 | 3.9 (1.3) | 7.3 | 72.4 (21.0) | 47.7 | ||||
| 521–535 | 12.1 (4.1) | 22.6 | 53.5 (15.5) | 35.3 | ||||
| Mean value | 7.0 (2.4) | 13.0 | 72.6 (21.0) | 47.8 | ||||
The T cell responses of arthritic Lewis rats to certain selected peptides of Bhsp65 shown in Fig. 1 are expressed above in three ways: cpm (in a β-scintillation counter), stimulation index (S.I. = cpm with antigen/ cpm without antigen [cells in medium alone]) and relative strength (%) (= cpm with the particular peptide relative to the mean cpm of the other four peaks in the pepscan of both the early and the late phase of AA; the value was expressed as a percentage. To assure equivalent comparisons, the same four peaks were chosen as reference [100%] in the early and late phases of AA). To attempt to express an overall index, the average RS value for BCTD in early and late AA were calculated.
Lewis Rats in the Late Phase of AA Raise Significant T Cell Responses to Carboxy-terminal Determinants within Self(rat) hsp65 (Rhsp65).
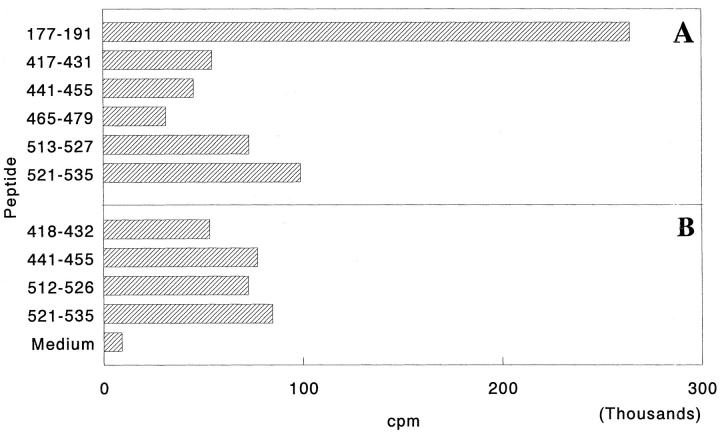
To determine whether T cell responses to new carboxy-terminal determinants observed in the late phase of AA are restricted to Bhsp65 or also involve self(rat) hsp65 (Rhsp65), spleen cells (SPC) of arthritic Lewis rats (4 wk after onset of arthritis) were tested in a proliferation assay using peptides containing those carboxy-terminal determinants of Rhsp65 that correspond in position to the BCTD to which T cell responses are diversified during the late phase of AA (Table 2). The results given in Fig. 2 demonstrate that arthritic Lewis rats raise vigorous T cell responses to the carboxy-terminal determinants of both Bhsp65 and Rhsp65. These results suggest that there is induction of Rhsp65-reactive T cells in vivo during the course of AA.
Table 2.
The Amino Acid Sequences of the Peptides Comprising the COOH-terminal Determinants of Bhsp65 and Rhsp65
| Peptides | Amino acid sequences | |
|---|---|---|
| Bhsp 417–431 | LLQAAPTLDELKLEG | |
| Rhsp 418–432 | --RCI-A--S--PAN | |
| Bhsp 441–455 | KVALEAPLKQIAFNS | |
| Rhsp 441–455 | II-R--KI-AMT--K | |
| Bhsp 465–479 | KVRNLPAGHGLNAQT | |
| Rhsp 465–479 | VE-ILQSSSEV-YD- | |
| Bhsp 513–527 | TT*EAVVADKPEKEKA | |
| Rhsp 512–526 | LL--A----TEI-*-- | |
| Bhsp 521–535 | KPEKEKASV*PGGGDM | |
| Rhsp 521–535 | T-IP-EEKD--M-A- |
− = identical residue;
= gap introduced for best alignment.
Figure 2.
Response of arthritic Lewis rats to peptides containing the COOH-terminal determinants within Bhsp65 (A) or Rhsp65 (B). Arthritis was induced as described in Materials and Methods. 4 wk after the appearance of clinical AA, rats were killed and their spleen cells (SPC) tested in a proliferation assay. The results are expressed as cpm. The results of a representative experiment are shown here. Similar results were obtained in repeat experiments (data not shown). The amino acid sequences of the five peptides comprising the BCTD and of the corresponding Rhsp65 peptides are given in Table 2. Response to peptide 177–191 is shown as a positive control. Rhsp65 peptide 465–479 could not be tested in this series of experiments.
WKY Rats, with the Same MHC Class II Haplotype as the Lewis Rat, Are Resistant to AA.
To determine the role of MHC versus non-MHC genes in determining susceptibility or resistance to AA, we tested the WKY rats, which are of the same MHC class II haplotype as the Lewis rat (23, 24). Strikingly, under the same conditions used for induction of AA in the Lewis rat, age- and sex-matched WKY rats were found to be resistant to AA. Only 3 out of 51 (5.9%) WKY rats developed mild (grade 1) AA. The remaining WKY rats, observed for more than 18 mo, did not manifest any clinical or histopathological signs of AA (data not shown).
Early and Vigorous Response to the Carboxy-terminal Determinants of Bhsp65 in AA-resistant WKY Rats after Immunization with M. tuberculosis.
To determine the immunological basis of susceptibility/resistance to AA, we tested the T cell responses of WKY rats to peptides of Bhsp65 following injection of M. tuberculosis. Based on the results shown in Fig. 1, we reasoned that susceptibility or resistance of rat strains of the same MHC haplotype to AA might relate primarily to differences in processing and presentation of Bhsp65: efficient and earlier display of the BCTD would ensure protection from AA, whereas lack of or inefficient presentation of these determinants would result in active disease. Alternatively, the resistance to AA of the WKY rat strain could be owing to its inability to process and present the potentially arthritogenic determinant 180–188 within Bhsp65.
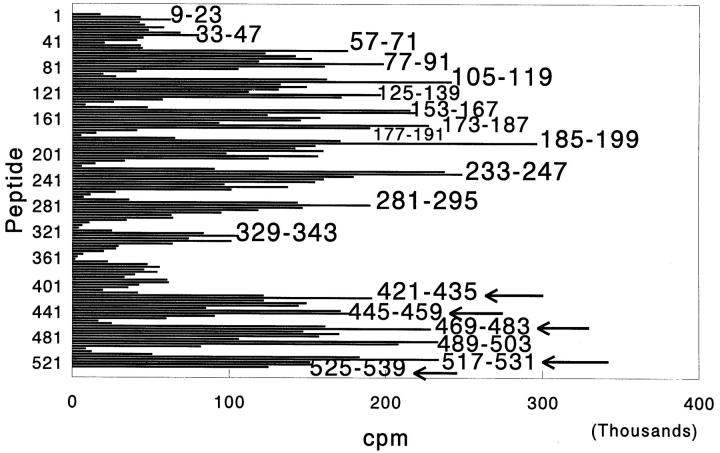
The results given in Fig. 3 clearly demonstrate that 10 d after immunization with M. tuberculosis, WKY rats raised T cell responses to several determinants within Bhsp65. Interestingly, unlike Lewis rats in the early phase of AA (see Fig. 1 A), WKY rats could raise significant T cell responses to the BCTD; Lewis rats raised T cell responses to the BCTD only in the late phase of AA (see Fig. 1 B). Although the highest proliferative responses to Bhsp65 peptides in the carboxy-terminal region correspond to peptides 421– 435, 445–459, 469–483, 517–531, and 525–539, WKY rats also raised significant responses to the overlapping peptides immediately preceding these peptides in the pepscan, namely, 417–431, 441–455, 465–479, 513–527, and 521–535 (Fig. 3). In repeat experiments, the proliferative responses to the adjacent overlapping peptides comprising each of these five pairs of peptides either was comparable or the pattern was reversed compared with the above pattern (data not shown). Importantly, WKY rats were not deficient in raising responses to peptide 177–191, which contains the minimal arthritogenic determinant, 180–188, described for Lewis rats (4), and is cross-reactive with it. These results suggest that despite reactivity to a known (established) arthritogenic determinant within Bhsp65, efficient T cell responses to carboxy-terminal determinants of the same protein were successful in affording protection to WKY rats from development of AA.
Figure 3.
Response of AA-resistant Wistar Kyoto (WKY) rats to Bhsp65 peptides after injection of M. tuberculosis. Rats were immunized with M. tuberculosis subcutaneously and after 10 d, the draining LNC from four rats were pooled and tested in a proliferation assay as described in Fig. 1. The results are expressed as cpm. The T cell responses to the unique carboxy-terminal determinants of Bhsp65 (to which arthritic Lewis rats respond only in the late phase of AA; shown in Fig. 1 B) are indicated by arrows. Although the highest proliferative responses correspond to the peptides marked by arrows, comparable or even higher responses also were raised to the adjacent overlapping peptides; namely, 417–431, 441– 455, 465–479, 513–527, and 521–535 in repeat experiments (data not shown). Responses to the BCTD were observed in repeat experiments in WKY rats tested 10–13 d after M. tuberculosis injection (data not shown).
Pretreatment of Naive Lewis Rats with Peptides Containing the BCTD Afford Protection from AA.
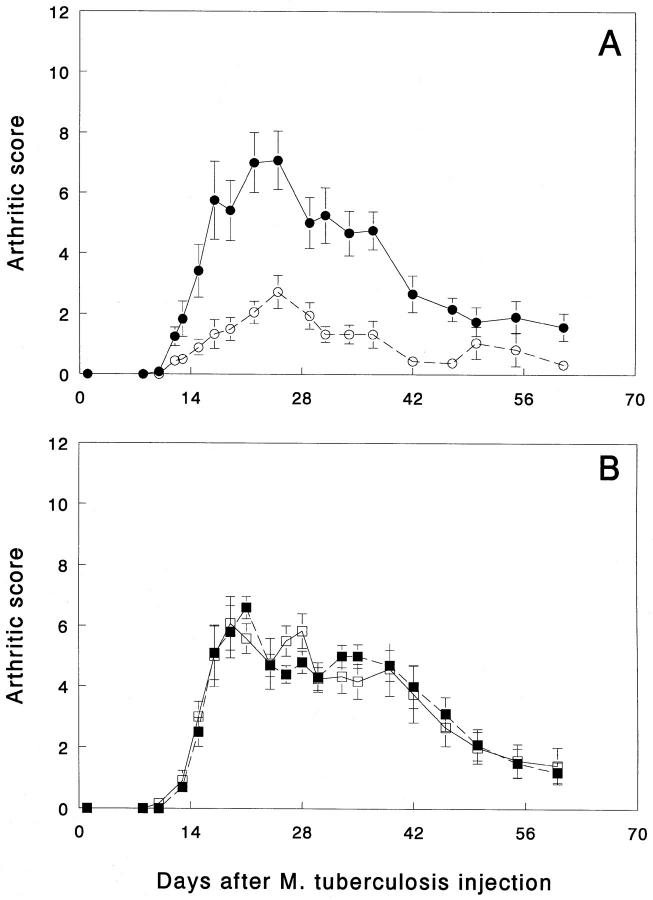
Finally, to determine the physiologic role of diversification of T cell responses to Bhsp65 in AA, we treated naive Lewis rats with an immunogenic combination of five peptides containing the sequence of the BCTD. 5–6 wk later, these rats were then challenged with M. tuberculosis in an attempt to induce AA, and thereafter these rats were observed regularly for clinical signs of AA. The results are given in Fig. 4 A. Strikingly, pretreatment with the BCTD afforded significant protection to naive Lewis rats from subsequent induction of AA, whereas age- and sex-matched control Lewis rats treated with an irrelevant HEL peptide exhibited the usual course of the disease. In comparison to control rats, BCTDtreated rats developed much milder arthritis or not at all and, eventually, did not reveal permanent joint deformities. Similar results were obtained in two other similar experiments (data not shown). On the contrary, in another series of experiments in age- and sex-matched Lewis rats using the same protocol except for injection of M. tuberculosis 2 wk (instead of 5–6 wk) after pretreatment with the BCTD, there was no evidence of protection from AA (data not shown). Thus, the duration of the period after pretreatment with the BCTD is the most crucial factor in determining the outcome of the interplay between the BCTD-specific regulatory T cells and the arthritogenic T cells.
Figure 4.
Pretreatment of naive Lewis rats with peptides comprising the BCTD afford protection from subsequent induction of AA. One group of 5–6-wk-old Lewis rats (n = 9) was immunized subcutaneously with a combination of five Bhsp65 peptides (namely, 417–431, 441–455, 465–479, 513–527, and 521–535) (A) mixed in N,N-Dimethyl-N,Ndioctadecyl ammonium chloride (DDA) (GERBU adjuvant; GERBU Biotechnik GmbH, Gaiberg, Germany) (17, 55). Each rat received 100 μg of each of the five peptides mixed together in the same suspension. Another group of age- and sex-matched control Lewis rats (n = 6) was immunized with HEL peptide 85–96/DDA. After 5 wk, these rats were immunized subcutaneously with M. tuberculosis for induction of AA. From 7 d onwards, rats were examined daily or on alternate days for signs of arthritis. The severity of arthritis in each of the three uninjected paws was graded on a scale from 0 to 4 as described in Materials and Methods, and the highest score achievable in any rat was 12 (32, 33). Rats were observed for up to 61 d after injection of M. tuberculosis. The difference between the arthritic score (mean ± SEM) of experimental (○) and control rats (•) from day 10 through day 47 (after M. tuberculosis injection) was found to be statistically significant (e.g., day 12, P <0.05; day 17, P <0.01; day 25, P <0.01; day 34, P <0.01, and day 47, P <0.001, all by Student's t test. The results of the two groups of rat were also statistically significant when analyzed by nonparametric, Wilcoxon-ranked sum test). Another group of Lewis rats (n = 6) (□) was immunized subcutaneously with a combination of three Bhsp65 amino-terminal peptides (namely, 13–27, 33–47, and 121–135) (B) mixed in DDA. The control group (n = 5) (▪) was immunized with HEL peptide 85–96/DDA. The conditions of the experiment were similar to those of the experiment shown in A. Rats were examined for up to 60 d after injection of M. tuberculosis. The difference between the two groups of rats was not statistically significant at any of the timepoints tested.
In another experiment, pretreatment of naive Lewis rats with peptides comprising certain Bhsp65 amino-terminal determinants (BNTD); namely, 13–27, 33–47, and 121–135, was performed to determine whether peptides from a region other than the carboxy-terminal region of Bhsp65 have any effect on the outcome of AA. The conditions of this experiment were similar to that of the above experiment using pretreatment with BCTD. The control HEL peptide used in the two experiments also was the same. The results of the experiment given in Fig. 4 B show that the course of AA in BNTD-treated rats was similar to that of the age- and sex-matched HEL peptide-treated rats. Furthermore, both these groups of rats exhibited disease characteristics similar to that of the control group of HEL peptide-treated rats shown in Fig. 4 A. These results demonstrate that the protective effect of Bhsp65 peptides against AA is simply not a general property of just any region of Bhsp65; as shown above, the carboxy-terminal, but not the aminoterminal peptides of Bhsp65 were protective against AA.
In summary, the above results demonstrate that induction of T cell responses to the BCTD are involved in providing protection from subsequent induction of AA and, thereby, are also capable of inducing natural regression of acute inflammatory arthritis in vivo.
Discussion
The phenomenon of broadening of the T cell response to other determinants within a particular native antigen, after induction of disease with only a single determinant of the same antigen has previously been described as determinant spreading (19). In this study, we observed a shift in the specificity of T cell responses to new determinants within Bhsp65 after priming with the whole multideterminant antigen (native Bhsp65 as a component of M. tuberculosis), and have termed this diversification of the T cell response (20, 21). Determinant spreading has previously been reported in the diseases murine experimental autoimmune encephalomyelitis (EAE) and insulin-dependent diabetes mellitus (IDDM) (19, 34). In the case of EAE, the disease was induced by a self-peptide, Ac1-11, of myelin basic protein (MBP), but during the course of the disease, the T cell response spread to other determinants within MBP, and in other studies (35, 36), to determinants on proteolipid protein (PLP) by intermolecular spreading. Likewise, in the case of spontaneously developed IDDM, there was evidence for both intramolecular (within glutamic acid decarboxylase; GAD65) and intermolecular (to determinants of hsp65, carboxypeptidase H, and insulin) determinant spreading (34). The novel features of our study on diversification of the T cell response in AA compared with the above two examples are the following: first, the observed diversification involved determinants within a foreign (mycobacterial) antigen, Bhsp65; second, both in EAE and IDDM, determinant spreading was implicated in perpetuation of the autoimmune response, whereas in our study we demonstrate that diversification of the T cell response to the carboxy-terminal determinants of Bhsp65 is involved in inducing regulation of inflammatory arthritis or protection from arthritis (regulatory diversification) in the AA-susceptible Lewis or the AA-resistant WKY rat strains, respectively. Furthermore, our results suggest that this diversification might be accentuated by determinants within self(rat) hsp65. In this regard, our study extends a novel dimension to the functional significance of diversification of T cell response in autoimmunity. Here, we describe diversification of T cell responses during the course of an autoimmune disease induced by a foreign antigen. In addition, this model offers us a unique opportunity to analyze relationships between the T cell repertoires directed against pathogenic foreign antigen and the homologous self-antigen. We believe that the principles elucidated in this study are widely applicable to several autoimmune situations in which autoreactivity is induced by molecular mimicry (29, 37, 37a) between a foreign and a selfantigen. Immediate and direct application of this knowledge would be in those autoimmune diseases (e.g., diabetes, Behcet's disease, multiple sclerosis) that might be induced/perpetuated by heat-shock proteins.
In this study, we observed that in the late phase of arthritis in the Lewis rat there was diversification of the T cell responses to include new determinants within the Bhsp65. T cell responses to the BCTD were evident even at 4 wk after onset of AA. Moreover, results of the experiments employing pretreatment with peptides comprising the BCTD clearly demonstrate that BCTD-reactive T cells are indeed responsible for inducing significant regression of acute inflammatory arthritis in the Lewis rat, presumably by downregulating the activity of disease-inducing effector T cells. Furthermore, the protective effect against AA is not simply a property of peptides derived from any region of Bhsp65; in this study, pretreatment with BCTD but not BNTD brought about protection from subsequent induction of AA. However, the timing of pretreatment with BCTD is a critical factor in determining the final outcome; significant protection from AA in Lewis rats is only achieved if pretreatment is done 5–6 wk before injection of M. tuberculosis; pretreatment 2 wk before induction of AA has no effect on the course of the disease. These results suggest that the appearance of, and manifestation of the effect of, regulatory T cells either during the course of AA or after pretreatment of naive Lewis rats with the BCTD apparently requires 4–6 wk of time. Interestingly, it had been reported earlier that pretreatment of naive Lewis rats with native Bhsp65 or with peptide 180–188 of Bhsp65 induced protection from subsequent induction of AA; again, the protective effect of Bhsp65 or of peptide 180–188 was observed when these antigens were administered 5 wk before induction of AA (4, 16). At this time, we do not know how pretreatment with the BCTD affords protection from AA. It is conceivable that the activity of arthritogenic T cells can be controlled either by cytokines (e.g., by induction of Th2 cells) or through a TCR-centered idiotypic circuit (38). Considering that peptide 180–188 (16) or peptide 256–270 (39) of Bhsp65 also can afford protection from AA, we suggest that protection against AA induced by pretreatment either with peptide 180–188 or 256–270, or by the BCTD might be mediated through parallel or, conceivably, a final common pathway of regulation. The precise mechanisms underlying this regulation are currently under investigation. Considering the size of Bhsp65, it is feasible that different regions of the same molecule are protective, and that this degeneracy in regulation (or multilayered regulation) might be of evolutionary advantage. Additional support for similar redundant regulatory pathways derives from studies using the nonobese diabetic (NOD) mouse: diabetes in this mouse strain can be modulated by administration of any one of several defined antigens (reviewed in reference 40).
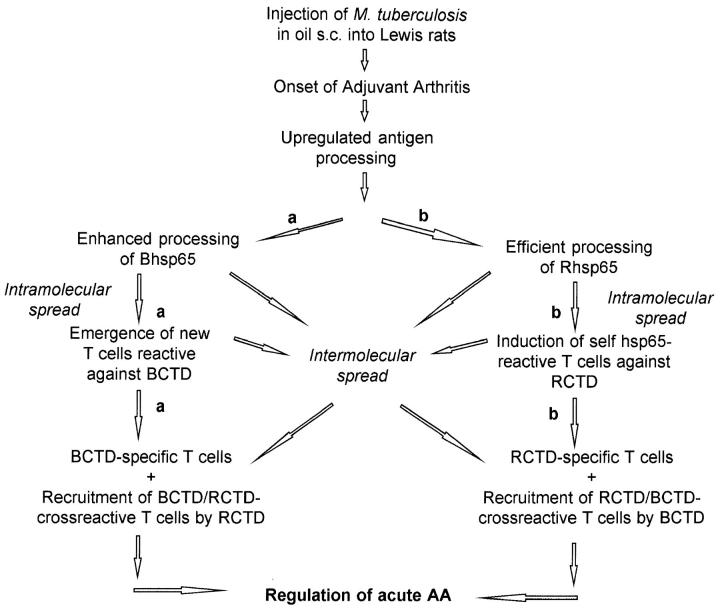
The diversification of T cell responses to the carboxyterminal determinants of hsp65 during the course of AA could be attributed to enhanced processing and presentation of Bhsp65 and/or Rhsp65 under the inflammatory conditions (41–43) prevalent during acute AA (Fig. 5). Our results shown in Figs. 2 and 4 suggest that the T cell repertoire shared between Bhsp65 and Rhsp65 is involved in diversification of the response as well as in regulation of AA. Thus, the induction of RCTD-reactive T cells in vivo, and their recruitment by cross-reactive BCTD might be a major mechanism underlying the observed diversification of the T cell response to the COOH-terminal determinants of Bhsp65 (Fig. 5, scheme b). However, it is possible that both mechanisms shown in Fig. 5 might operate simultaneously, and that T cell responses to BCTD as well as RCTD could arise even in the absence of significant cross-reactivity between BCTD and RCTD. Which of the above mechanisms is (are) actually involved in the diversification of the T cell response to BCTD and in natural regulation of AA is the subject of current investigations.
Figure 5.
A schematic representation of the proposed mechanisms of diversification of T cell responses to the COOH-terminal determinants of hsp65: (a) The Bhsp65 diversification pathway: enhanced processing of Bhsp65 under the inflammatory milieu raised in acute AA, leading to induction of a diversified T cell response to BCTD. These BCTD-reactive T cells, which include BCTD-specific and BCTD/RCTD-cross-reactive T cells, then regulate the activity of arthritogenic T cells. (b) The Rhsp65 intermolecular spreading pathway: upregulation of antigen processing during acute AA induced by M. tuberculosis results in induction of T cell responses to the COOH-terminal determinants of Rhsp65 (RCTD) through mechanisms of intermolecular and intramolecular determinant spreading (34, 41). Some of these self-hsp65 responses are regulatory from both RCTDspecific as well as RCTD/BCTD-cross-reactive T cells and bring about natural remission from acute AA. Induction of response to Rhsp65 in turn can initiate intermolecular spreading to determinants within Bhsp65.
In comparison to our results shown in Fig. 1, Anderton et al. (17) (a) observed proliferative responses to relatively fewer dominant/subdominant determinants of Bhsp65 after immunization of Lewis rat with Bhsp65. We believe that these differences are primarily owing to lower sensitivity under their immunization and assay conditions, as well as to possibly subtle differences in the inherent responsiveness of the rat strains used from different sources, as has been reported in the case of EAE (44); (b) did not find any change in the pattern of reactivity at different timepoints after immunization with native Bhsp65. Our interpretation of these results is that because immunization with Bhsp65 (mixed in the adjuvant, DDA) did not cause AA, the requisite conditions for diversification of T cell responses to Bhsp65 simply did not exist.
We observed that coupled with the diversification of T cell response during the course of AA in the Lewis rat, the proliferative T cell responses to certain BNTD were significantly downmodulated in the late phase of the disease. At this time, we do not know whether downmodulation of responses to BNTD has any physiologic significance. One possibility is that besides the arthritogenic determinant 180–188, the BNTD also might be contributing to induction/perpetuation of arthritis in the Lewis rat and, therefore, to contain the autoimmune reactivity, the responses to these determinants must be downregulated.
We have observed that the WKY rat, with the same MHC class II molecules as the Lewis rat, is resistant to AA. In WKY rats, vigorous responses to the BCTD, to which the Lewis rat responded only in the late phase of AA, were observed very early, 10 d after injection of M. tuberculosis. These results suggest that T cell responses to the BCTD are involved in the downregulation of the acute inflammatory arthritis in the late phase of the disease in AA-susceptible Lewis rat; similarly, T cell responses to the same determinants initiated soon after injection of M. tuberculosis induce effective protection against arthritis in WKY rats. The early and efficient display of the BCTD by M. tuberculosis–immunized WKY rats but not by Lewis rats could be owing to subtle differences in the processing and presentation of Bhsp65 by the APC of WKY and Lewis rats, which in turn could be attributable to the difference in the non-MHC genes of these two rat strains. However, our results do not rule out other factors that also might contribute to the AA resistance of WKY rats, e.g., (a) hormonal and other neurophysiological parameters (45, 46), (b) difference in the RT.6 locus of WKY and Lewis rats (23, 47, 48), and (c) a change in the balance of Th1/Th2 induction (49). In most of the earlier reports on AA, the Fisher F344 rat (RT.1lvl), which is resistant to AA, has been extensively studied and used as a control strain for the Lewis rat (14). It has been reported that the Fisher rat is deficient in generating T cell responses to the arthritogenic determinant, 180–188, of Bhsp65 after injection of M. tuberculosis, and thereby is protected from AA (14). On the contrary, our results show that AA-resistant WKY rats raise vigorous responses to determinant 180–188 (within peptide 177–191) of Bhsp65 upon challenge with M. tuberculosis. Thus, clearly the immunological mechanisms underlying resistance to AA of Fisher and WKY rats are different. In this regard, study of AA in WKY rats would offer novel insights into the pathogenesis and treatment of this disease. Moreover, this rat strain might also be valuable in studying other animal models of autoimmune disease like EAE (49a).
AA can be induced in the Lewis rat by injection of M. tuberculosis; however, the precise autoantigen(s) responsible for AA are not known. Several candidate autoantigens have been proposed (13, 50, 51), but there is no direct evidence so far that any one of these antigens is arthritogenic in Lewis rats. We (29) and others (52–54) have suggested that self-hsp65 (Rhsp65) may be a target of T cells primed by Bhsp65. Based on our earlier study on mouse lysozyme as a model self-protein (29), we suggested that newly displayed cryptic determinants within Rhsp65 could serve as targets of autoimmune reactivity for T cells primed with the corresponding dominant determinants within Bhsp65. In addition, any self-antigenic determinants within the tissue components of the joints, with the appropriate cross-reactive homology with determinants within self-hsp65, could also serve as targets for the pathogenic T cells. We are currently characterizing the T cell responses to self-hsp65 to evaluate their direct role in the pathogenesis of arthritis.
Acknowledgments
We wish to thank Drs. M. Kronenberg, D. Yu, N.K. Nanda, and B.H. Hahn for critical reading of the manuscript; Drs. D. Stevens, A. Miller, and Ms. M.L. Banquerigo for helpful discussions; O.J. Yun for excellent technical assistance; Dr. T.S. Ferguson for help and guidance in statistical analysis of the data; J.R. Guadron for taking excellent care of our rats; Ms. S. Shapourifar-Tehrani and Ms. A.M. Zaragoza for preparing histopathological specimens of joints from arthritic rats; and Drs. J. Baumer and Tom Dulaney for their help in accessing the National Biomedical Research Foundation data base.
This work was supported by grants from the National Institutes of Health (AR 3683406, AR 42200, AI11183), and the Bertram A. Maltz Laboratory of Molecular Rheumatology.
Footnotes
1 Abbreviations used in this paper: AA, adjuvant arthritis; BCTD, Bhsp65 carboxy-terminal determinants; Bhsp65, 65-kD mycobacterial heat-shock protein; BNTD, Bhsp65 amino-terminal determinants; EAE, experimental autoimmune encephalomyelitis; HEL, hen eggwhite lysozyme; IDDM, insulin-dependent diabetes mellitus; LNC, lymph node cells; MBP, myelin basic protein; NOD, nonobese diabetic; PLP, proteolipid protein; PPD, purified protein derivative; RA, rheumatoid arthritis; RCTD, carboxy-terminal determinants of Rhsp65; Rhsp65, rat hsp65; S.I., stimulation index; SPC, spleen cells.
References
- 1.Nepom GT, Hansen JA, Nepom BS. The molecular basis for HLA class II associations with rheumatoid arthritis. J Clin Immunol. 1987;7:1–7. doi: 10.1007/BF00915418. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky, P.E. 1991. Rheumatoid arthritis. In Harrison's Principles of Internal Medicine. J.D. Wilson, E. Braunwald, K.J. Isselbacher, R.G. Petersdorf, J.B. Martin, A.S. Fauci, and R.K. Root, editors. 12th edition. McGraw-Hill, Inc., New York. 1437–1443.
- 3.Holoshitz J, Naparstek Y, Ben-Nun A, Cohen IR. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science (Wash DC) 1983;219:56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- 4.Van Eden W, Thole JER, Van der Zee R, Noordzij A, Van Embden JDA, Hensen EJ, Cohen IR. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature (Lond) 1988;331:171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- 5.Holoshitz J, Klajman A, Drucker I, Lapidot Z, Yaretzky A, Frenkel A, van Eden W, Cohen IR. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986;2:305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 6.Res PCM, Schaar CG, Breedveld FC, Van Eden W, Van Embden JDA, Cohen IR, De Vries RRP. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988;2:478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 7.Gaston JSH, Life PF, Bailey LC, Bacon PA. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989;143:2494–2500. [PubMed] [Google Scholar]
- 8.Gaston JSH, Life PF, Jenner PJ, Colston MJ, Bacon PA. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990;171:831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quayle AJ, Wilson KB, Li SG, Kjeldsen-Kragh J, Oftung F, Shinnick T, Sioud M, Forre O, Capra JD, Natvig JB. Peptide recognition, T cell receptor usage and HLA restriction elements of human heat-shock protein (hsp) 60 and mycobacterial 65-kDa hsp-reactive T cell clones from rheumatoid synovial fluid. Eur J Immunol. 1992;22:1315–1322. doi: 10.1002/eji.1830220529. [DOI] [PubMed] [Google Scholar]
- 10.Henwood J, Loveridge J, Bell JI, Gaston JSH. Restricted T cell receptor expression by human T cell clones specific for mycobacterial 65-kDa heat-shock protein: selective in vivoexpansion of T cells bearing defined receptors. Eur J Immunol. 1993;23:1256–1265. doi: 10.1002/eji.1830230610. [DOI] [PubMed] [Google Scholar]
- 11.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956;91:95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 12.Pearson CM, Wood FD. Studies of polyarthritis and other lesions induced in rats by injection of mycobacterial adjuvant. I. General clinical and pathologic characteristics and some modifying factors. Arthritis Rheum. 1959;2:440–459. [Google Scholar]
- 13.Van Eden W, Hogervorst EJM, Hensen EJ, Van der Zee R, Van Embden JDA, Cohen IR. A cartilage-mimicking T-cell epitope on a 65K mycobacterial heatshock protein: adjuvant arthritis as a model for human rheumatoid arthritis. Curr Topics Microbiol Immunol. 1989;145:27–43. doi: 10.1007/978-3-642-74594-2_3. [DOI] [PubMed] [Google Scholar]
- 14.Hogervorst EJM, Boog CJP, Wagenaar JPA, Wauben MH, van der Zee R, van Eden W. T cell reactivity to an epitope of the mycobacterial 65-kDa heatshock protein (hsp65) corresponds with arthritis susceptibility in rats and is regulated by hsp65-specific cellular responses. Eur J Immunol. 1991;21:1289–1296. doi: 10.1002/eji.1830210529. [DOI] [PubMed] [Google Scholar]
- 15.Billingham MEJ, Carney S, Butler R, Colston MJ. A mycobacterial 65-kD heat-shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990;171:339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XD, Gasser J, Feige U. Prevention of adjuvant arthritis in rats by a nonapeptide from the 65-kD mycobacterial heat-shock protein. Clin Exp Immunol. 1990;81:189–194. doi: 10.1111/j.1365-2249.1990.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderton SM, van der Zee R, Noordzij A, van Eden W. Differential mycobacterial 65-kDa heat shock protein T cell epitope recognition after adjuvant arthritisinducing or protective immunization protocols. J Immunol. 1994;152:3656–3664. [PubMed] [Google Scholar]
- 18.Lider O, Karin N, Shinitzky M, Cohen IR. Therapeutic vaccination against adjuvant arthritis using autoimmune T cells treated with hydrostatic pressure. Proc Natl Acad Sci USA. 1987;84:4577–4580. doi: 10.1073/pnas.84.13.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature (Lond) 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 20.Moudgil, K.D., T. Chang, H. Eradat, and E.E. Sercarz. 1994. Identification of determinants within mycobacterial hsp65 involved in induction, perpetuation and recovery from autoimmune arthritis in the Lewis rat. 12th European Immunology Meeting, June 14–17, Barcelona, Spain. Abstract no. W42/1.
- 21.Moudgil, K.D., T. Chang, H. Eradat, A. Chen, O. Yun, R.S. Gupta, and E.E. Sercarz. 1995. Involvement of diversification of T cell responses to mycobacterial hsp65 (Bhsp65) in inducing remission or protection from adjuvant-induced arthritis. The 9th Int'l. Congress of Immunology, San Francisco, July 23–29. Abstract no. 2367.
- 22.Venner TJ, Gupta RS. Nucleotide sequence of rat hsp60 (chaperonin, GroEL homolog) cDNA. Nucleic Acids Res. 1990;18:5309. doi: 10.1093/nar/18.17.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunz, H.W., and T.J. Gill, III. 1996. Commonly used rat strains. Curr. Prot. Immunol. 3(Suppl. 13):A.1H.1–A.1H.2. [DOI] [PubMed]
- 24.H'Doubler PB, Jr, Petersen M, Shek W, Auchincloss H, Abbott WM, Orkin RW. Spontaneously hypertensive and Wistar Kyoto rats are genetically disparate. Lab Animal Sci. 1991;41:471–473. [PubMed] [Google Scholar]
- 25.Shinnick TM. The 65-kilodalton antigen of Mycobacterium tuberculosis. . J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thole JER, Keulen WJ, De Bruyn J, Kolk AHJ, Groothuis DG, Berwald LG, Tiesjema RH, van Embden JDA. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in Escherichia coliK-12. Infect Immun. 1987;55:1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeji NJ, Bray AM, Geysen HM. Multi-pin peptide synthesis strategy for T cell determinant analysis. J Immunol Meth. 1990;134:23–33. doi: 10.1016/0022-1759(90)90108-8. [DOI] [PubMed] [Google Scholar]
- 28.Moudgil KD, Grewal IS, Jensen PE, Sercarz EE. Unresponsiveness to a self-peptide of mouse lysozyme owing to hindrance of T cell receptor–major histocompatibility complex/peptide interaction caused by flanking epitopic residues. J Exp Med. 1996;183:535–546. doi: 10.1084/jem.183.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moudgil KD, Sercarz EE. Dominant determinants in hen eggwhite lysozyme correspond to the cryptic determinants within its self-homologue, mouse lysozyme: implications in shaping of the T cell repertoire and autoimmunity. J Exp Med. 1993;178:2131–2138. doi: 10.1084/jem.178.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood FD, Pearson CM, Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35:456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- 32.Peacock DJ, Ku G, Banquerigo ML, Brahn E. Suppression of collagen arthritis with antibodies to an arthritogenic, oligoclonal T cell line. Cell Immunol. 1992;140:444–452. doi: 10.1016/0008-8749(92)90210-g. [DOI] [PubMed] [Google Scholar]
- 33.Ku G, Kronenberg M, Peacock DJ, Tempst P, Banquerigo ML, Braun BS, Reeve JR, Brahn E. Prevention of experimental autoimmune arthritis with a peptide fragment of type II collagen. Eur J Immunol. 1993;23:591–599. doi: 10.1002/eji.1830230302. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GSP, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulindependent diabetes. Nature (Lond) 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McRae BL, Vanderlugt CL, Dal MC, Canto, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross AH, Tuohy VK, Raine CS. Development of reactivity to new myelin antigens during chronic relapsing autoimmune demyelination. Cell Immunol. 1993;146:261–269. doi: 10.1006/cimm.1993.1025. [DOI] [PubMed] [Google Scholar]
- 37.Oldstone MBA. Molecular mimicry as a mechanism for the cause and a probe uncovering etiologic agent(s) of autoimmune disease. Curr Topics Microbiol Immunol. 1989;145:127–135. doi: 10.1007/978-3-642-74594-2_11. [DOI] [PubMed] [Google Scholar]
- 37a.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen IR. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- 39.Anderton SM, van der Zee R, Prakken B, Noordzij A, van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995;181:943–952. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegmann DR. The immune response to islets in experimental diabetes and insulin-dependent diabetes mellitus. Curr Opin Immunol. 1996;8:860–864. doi: 10.1016/s0952-7915(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 42.Opdenakker G, Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol Today. 1994;15:103–107. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 43.Moudgil KD, Sercarz EE. The T cell repertoire against cryptic self determinants and its involvement in autoimmunity and cancer. Clin Immunol Immunopathol. 1994;73:283–289. doi: 10.1006/clin.1994.1200. [DOI] [PubMed] [Google Scholar]
- 44.Gould KE, Stepaniak JA, Swanborg RH. Variable susceptibility of Lewis rats to experimental autoimmune encephalomyelitis. J Neuroimmunol. 1994;54:145–146. doi: 10.1016/0165-5728(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 45.Cools AR, Rots NY, Ellenbroek B, de Kloet ER. Bimodal shape of individual variation in behaviour of Wistar rats: the overall outcome of a fundamentally different make-up and reactivity of the brain, the endocrinological and the immunological system. Neuropsychobiol. 1993;28:100–105. doi: 10.1159/000119009. [DOI] [PubMed] [Google Scholar]
- 46.Sternberg EM, Young WS, III, Bernardini R, Calogero AE, Chrousos GP, Gold PW, Wilder RL. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall–induced arthritis in Lewis rats. Proc Natl Acad Sci USA. 1989;86:4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes: characterization of the CD4+T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greiner DL, Mordes JP, Handler ES, Angelillo M, Nakamura N, Rossini AA. Depletion of RT6.1+T lymphocytes induces diabetes in resistant Biobreeding/ Worcester (BB/W) rats. J Exp Med. 1987;166:461–475. doi: 10.1084/jem.166.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 49a.Stevens, D.B., E.E. Sercarz, and K.D. Moudgil.1997. Biphasic EAE, rather than protection against EAE, in Lewis rat recipients of RT1l congenic Wistar Kyoto rat spleen cells. J. Allergy Clin. Immunol. 99:S262 (Abstr.).
- 50.Feige U, Schulmeister A, Mollenhauer J, Brune K, Bang H. A constitutive 65 kDa chondrocyte protein as a target antigen in adjuvant arthritis in Lewis rats. Autoimmunity. 1994;17:233–239. doi: 10.3109/08916939409010659. [DOI] [PubMed] [Google Scholar]
- 51.van den Broek MF, van den Berg WB, Arntz OJ, van de Putte LBA. Reaction of bacterium-primed murine T cells to cartilage components: a clue for the pathogenesis of arthritis? . Clin Exp Immunol. 1988;72:9–14. [PMC free article] [PubMed] [Google Scholar]
- 52.Koga T, Wand-Wurttenberger A, DeBruyn J, Munk ME, Schoel B, Kaufmann SHE. T cells against a bacterial heat shock protein recognize stressed macrophages. Science (Wash DC) 1989;245:1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- 53.van Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 54.Anderton SM, van der Zee R, Goodacre JA. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993;23:33–38. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- 55.Snippe, H., and C.H. Kraaijeveld. 1989. The immunoadjuvant dimethyldioctadecylammonium bromide. In Immunological Adjuvants and Vaccines. G. Gregoriadis, A.C. Allison, and G. Poste, editors. Plenum Press, New York. 47–59.