Abstract
The 75-kD HS1 protein is highly tyrosine-phosphorylated during B cell antigen receptor (BCR)-mediated signaling. Owing to low expression of HS1, WEHI-231-derived M1 cells, unlike the parental cells, are insensitive to BCR-mediated apoptosis. Here, we show that BCR-associated tyrosine kinases Lyn and Syk synergistically phosphorylate HS1, and that Tyr378 and Tyr-397 of HS1 are the critical residues for its BCR-induced phosphorylation. In addition, unlike wild-type HS1, a mutant HS1 carrying the mutations Phe-378 and Phe-397 was unable to render M1 cells sensitive to apoptosis. Wild-type HS1, but not the mutant, localized to the nucleus under the synergy of Lyn and Syk. Thus, tyrosine phosphorylation of HS1 is required for BCR-induced apoptosis and nuclear translocation of HS1 may be a prerequisite for B cell apoptosis.
Stimulation of the antigen receptor on B lymphocytes (BCR) induces intracellular biochemical events that include rapid tyrosine phosphorylation of cellular proteins. Accumulating data reveal that cytoplasmic kinases such as the Syk kinase and Src-like kinases are associated with the BCR (1, 2) and play important roles in the signal transduction cascade through the BCR (3–5). We previously demonstrated that tyrosine phosphorylation of various cellular proteins was greatly enhanced in COS7 fibroblasts transfected with both Lyn and Syk expression plasmids as compared with those transfected with either the Lyn or Syk plasmid alone (6). Thus, these kinases may cooperate in phosphorylating substrates crucial for BCR-mediated B cell activation.
The 75-kD HS1 protein is highly tyrosine phosphorylated upon BCR cross-linking (7). Studies with HS1 −/− mice (8) and with a mutant WEHI-231 cell line that expresses very low level of HS1 (9) suggest that HS1 plays roles in not only B cell proliferation but also apoptosis upon BCR cross-linking. In this study, we addressed molecular mechanisms of HS1 phosphorylation and significance of HS1 phosphorylation in BCR-mediated apoptosis.
Materials and Methods
Cell Culture and Antibodies.
Retrovirus producer ψCRE cells, CV-1 monkey kidney fibroblasts, and COS7 cells, a derivative of CV-1 cells expressing SV40 large T antigen, were maintained in Dulbecco's modified Eagle medium containing 10% calf serum. WEHI-231 cells and its variant M1 cells (10) were maintained in RPMI-1640 as described (9). A mouse monoclonal antibody (mAb) specific to human HS1 was raised against 15 amino acids from Val-306 to Ser-320 of human HS1 (11). Specificity of the mAb was confirmed by immunoblotting the lysates of parental and human HS1-transfected WEHI-231 cells. Rabbit anti–human HS1 sera were as described (12). Affinity-purified goat Ab to mouse IgM F(ab′)2 was from Southern Biotechnology (Birmingham, AL), FITC-labeled goat Ab to mouse IgG was from PharMingen (San Diego, CA), and an anti-phosphotyrosine mAb PY-20 was from ICN (Irvine, CA).
Plasmid Construction and Preparation of Recombinant Viruses.
The cDNA fragments encoding human Lyn and porcine Syk were inserted into the expression vector pME-18S (13), to generate pME-Lyn and pME-Syk, respectively. The human HS1 cDNA and its mutant cDNA, encoding a product that lacks 23 amino acids from Tyr-378 to Val-400, were inserted into pME18S to generate pME-HS1 and pME-HS1-ΔYY, respectively. The cDNA fragments encoding human HS1 mutants, HS1-FY, HS1-YF, and HS1-FF, which carry Phe-378, Phe-397, and Phe378/Phe-397, were also inserted into pME-18S to generate pME-HS1-FY, pME-HS1-YF, and pME-HS1-FF, respectively. The site-directed mutagenesis was performed as described (14). The retroviral vectors were constructed by cloning the cDNAs of human HS1 and its mutants into the pM5-neo plasmid as described (9).
DNA Transfection and Viral Infection.
CV-1 cells were transfected with various combinations of pME-Lyn, pME-Syk, pMEHS1, and pME-HS1-ΔYY by the standard calcium–phosphate method. 2 d after transfection, cells were washed with serum-free medium and lysed with TNE (1% NP-40, 50 mM Tris, pH 8, 20 mM EDTA, 0.2 mM sodium orthovanadate with aprotinin at 10 μg/ml) buffer (7). To obtain high titer retroviruses carrying the sequences for the HS1 and mutant proteins, ψCRE helper cells (9) were transfected with the vector plasmids. The WEHI231 cells and M1 cells were infected by the recombinant viruses and infectants expressing the highest amount of exogenous HS1 or its mutants were cloned as described (9).
Immunoprecipitation and Immunoblotting.
The proteins in the cell lysates were subjected to immunoprecipitation with Abs to the human HS1 protein as described (7). The proteins in the lysates or the immunoprecipitates were resolved by 8.5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Bio Rad, Richmond, CA). Then, the blot was probed with mAb to phosphotyrosine, PY-20, or anti-human HS1 mAb.
Analysis for BCR-induced Tyrosine Phosphorylation and Apoptosis.
WEHI-231 cells or M1 cells were incubated with or without 20 μg/ml of anti-IgM Ab at 37°C for 1 min. Then, the cells were lysed and the proteins in the lysates were subjected to immunoprecipitation and immunoblotting. To examine the degree of the apoptotic death, cells were incubated with or without 4 μg/ml of anti-IgM for 48 h and the DNA content of the cells was measured as described (9).
Analysis of HS1 Subcellular Localization.
COS7 cells were transfected with various combinations of pME-Lyn, pME-Syk, pMEHS1, and pME-HS1-FF. The cells seeded onto cover slides were fixed and permeabilized as described (15). Then, the permeabilized cells were incubated for 1 h with anti-human HS1 mAb in PBS containing 1% BSA at room temperature. The cells on cover slides were incubated with FITC-labeled goat anti–mouse IgG and observed using fluorescence microscopy (Zeiss). To examine the amount of nuclear HS1 in the BCR-stimulated WEHI-231 cells, cells were incubated with 20 μg/ml of anti-IgM Ab for the indicated time. Then, nuclei were separated from the cell homogenates by centrifugation and solubilized as described (7).
Results and Discussion
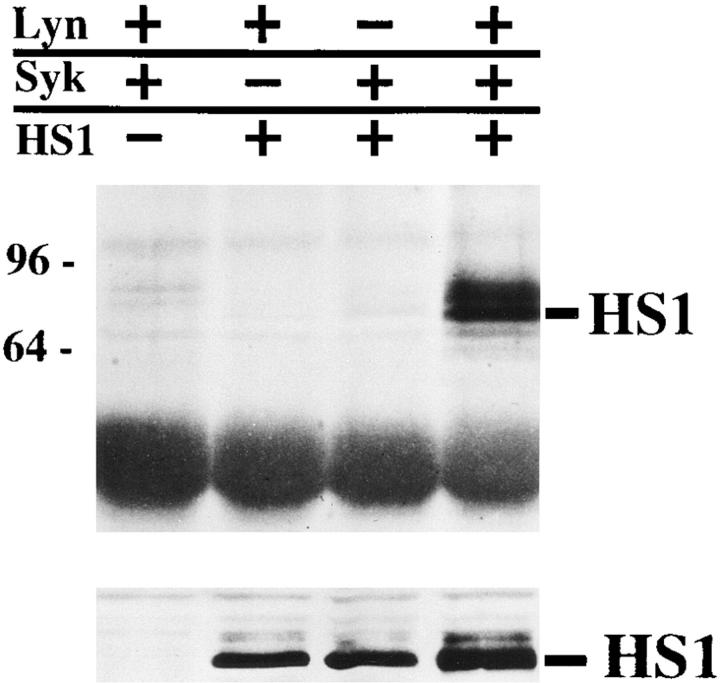
The HS1 protein is highly tyrosine-phosphorylated upon BCR cross-linking (7), probably by Lyn and/or Syk. Because tyrosine phosphorylation of various cellular proteins was greatly enhanced in fibroblasts transfected with both Lyn and Syk expression plasmids as compared with those transfected with either the Lyn or Syk plasmid alone (6), we examined whether Lyn and Syk can synergistically phosphorylate HS1 in the cells. The results showed that HS1 was highly tyrosine-phosphorylated only when both Lyn and Syk were coexpressed (Fig. 1). This is consistent with our previous observation that another Src family member, Fyn, cooperates with ZAP-70, an analogue of Syk, in phosphorylating HS1 in T cells (12). It has been shown that Lyn activates Syk when they are coexpressed in fibroblasts (6) and that BCR cross-linking induces little activation of Syk in the splenic B cells (Nishizumi, H., unpublished data) and mast cells (16) of lyn −/− mice. Accordingly, lyn −/− splenocytes failed to induce tyrosine phosphorylation of HS1 upon BCR cross-linking (4). In contrast, Lyn is not detectably activated by Syk (6). Therefore, our results suggest that Syk activated by Lyn phosphorylates HS1 directly in BCRmediated signaling.
Figure 1.
Cooperation between Lyn and Syk in HS1 phosphorylation. CV-1 cells (1.5 × 106) were transfected with the expression plasmids (5 μg), pMELyn (+), pME-Syk (+), pMEHS1 (+), or with H2O in place of DNA solution (−), and lysed with TNE buffer. The lysates were subjected to immunoprecipitation with anti-serum to HS1. The immunoprecipitates were immunoblotted with PY20 (top). Aliquots (1/50) of the lysates were immunoblotted with anti-HS1 Ab (bottom). Positions of HS1 and standard protein markers (kD) are indicated.
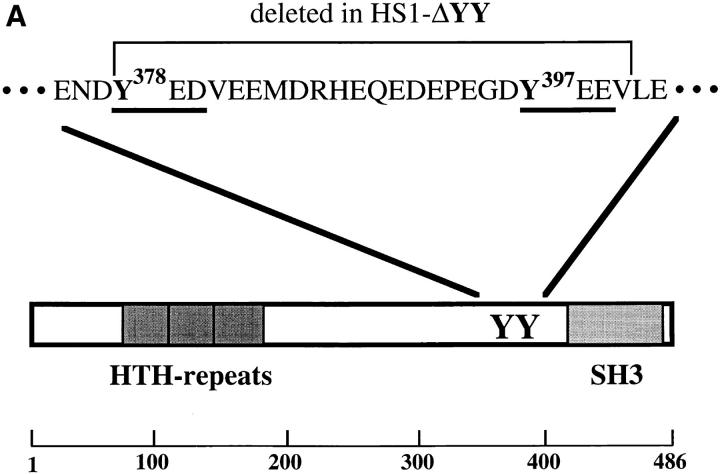
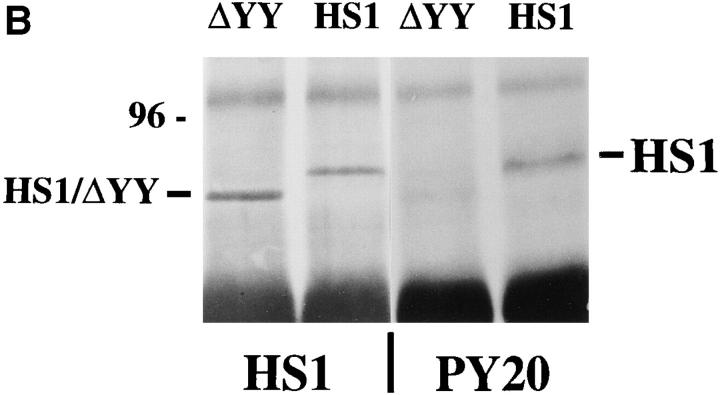
Of the 17 tyrosine residues in human HS1, Tyr-378 and Tyr-397 are preceded by acidic residues (ENDY378 and EGDY397), which are characteristic of many tyrosine phosphorylation sites (17, 18). In fact, the EGDY397EEV sequence of HS1 is the best known substrate for Syk kinase (19). Because tyrosine-phosphorylated HS1 interacts with the SH2 domains of Src family kinases (7), and because a phosphorylated pYED/E sequence shows the highest affinity for these domains (20), we predicted that the relevant phosphorylation sites on HS1 would be followed by two acidic residues. Of all the tyrosine residues in HS1, only Tyr-378 and Tyr-397 match the consensus (Y378ED and Y397EE) (Fig. 2 A). These amino acids are also conserved in mouse HS1 (21). Thus, Tyr-378 and Tyr-397 of human HS1 are likely phosphorylated by the BCR-associated kinases. Indeed, when coexpressed with Lyn and Syk in CV-1 cells, a deletion mutant HS1-ΔYY, lacking 23 amino acids from Tyr-378 to Val-400, was tyrosine phosphorylated at a greatly reduced level compared with wild-type HS1 (Fig. 2 B).
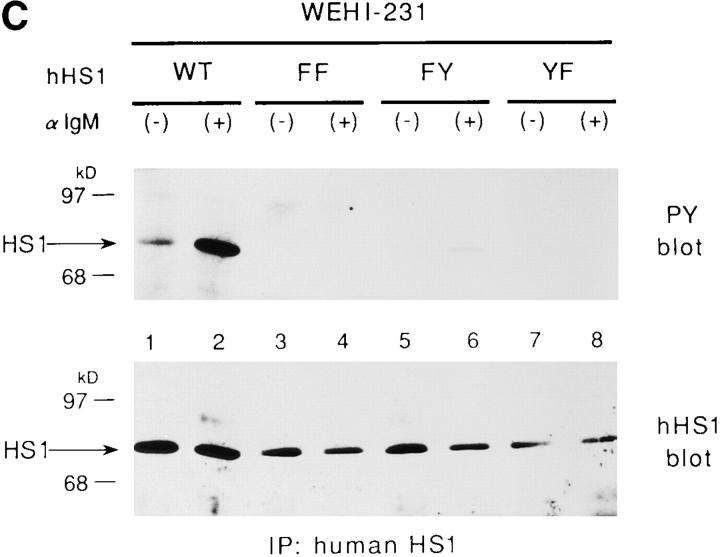
Figure 2.
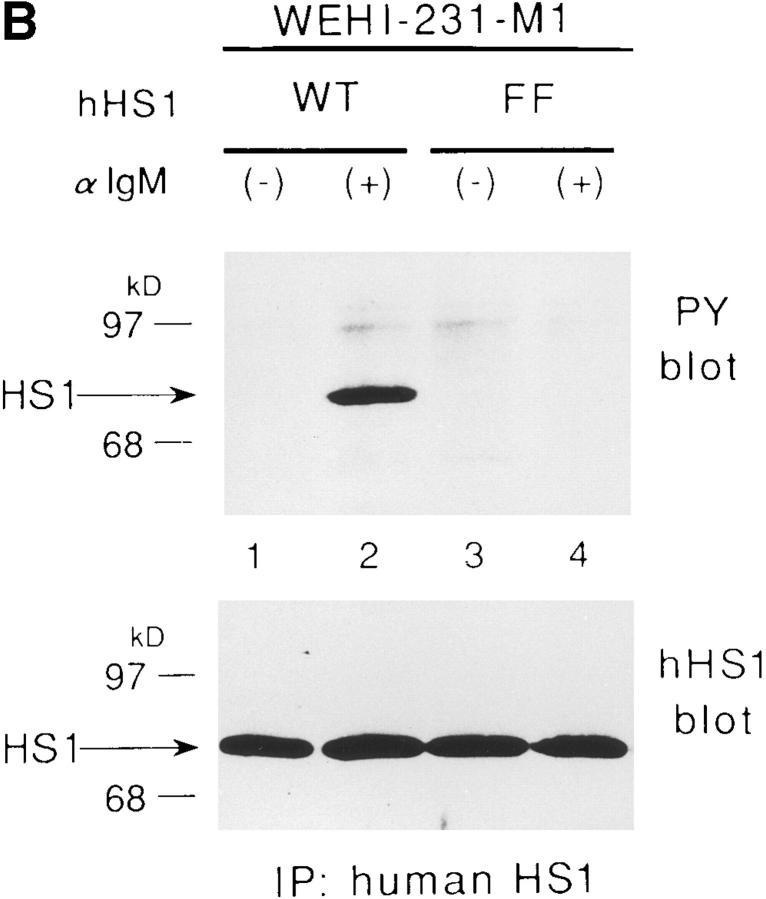
Requirement of Tyr-378 and Tyr-397 in tyrosine phosphorylation of HS1 upon BCR stimulation. (A) Schematic structure of the human HS1 protein. The 23 amino acids sequence of HS1, Tyr-378 to Val-400, deleted to generate HS1-ΔYY, is shown on the top. Possible target sites of the SH2 domain of the Src-like kinase are underlined. Two tyrosine residues, Tyr-378 and Tyr-397, in the sequence are indicated in bold. The positions of the two tyrosine residues (YY), three helix–turn– helix repeats (HTH repeats) and the SH3 domain (SH3) are indicated in the schematic drawing of HS1. The amino acid numbers (from Met-1 to Glu-486) are shown on the bottom. (B) Tyrosine phosphorylation of HS1 and HS1-ΔYY in CV-1 cells. CV-1 cells (1.5 × 106) were transfected with the expression plasmids (5 μg), pME-Lyn, pME-Syk, and pMEHS1-ΔYY (ΔYY) or pME-HS1 (HS1), then lysed with TNE buffer. The lysates were equally divided into two samples and subjected to immunoprecipitation with antiserum to HS1. The immunoprecipitates were analyzed by anti-HS1 (HS1) or by PY-20 immunoblotting. The levels of Lyn and/or Syk-mediated tyrosine phosphorylation of the other cellular proteins were virtually the same between the cells expressing wild-type HS1 and those expressing HS1-ΔYY (data not shown). Positions of HS1, HS1-ΔYY, and a 96-kD standard protein marker are indicated. (C) Tyrosine phosphorylation of HS1 mutants in WEHI-231 cells. WEHI-231derived cells (1 × 107), which express human HS1 (lanes 1 and 2), HS1FF (lanes 3 and 4), HS1-FY (lanes 5 and 6), or HS1-YF (lanes 7 and 8), were incubated with (lanes 2, 4, 6, and 8) or without (lanes 1, 3, 5, and 7) anti-IgM Ab. The cell lysates were subjected to immunoprecipitation with the human HS1-specific Ab and the immunoprecipitates probed by anti-phosphotyrosine (PY blot) or anti-human HS1 (hHS1 blot) immunoblotting. BCRmediated tyrosine phosphorylation of the other proteins was not affected by expressing exogenous HS1 or its mutants (data not shown). Positions of HS1 and standard protein markers are indicated.
To verify BCR-mediated phosphorylation on Tyr-378 and Tyr-397 of HS1, we generated three HS1 mutants, HS1-FY, HS1-YF, and HS1-FF, in which Tyr-378, Tyr397, and both have been substituted by phenylalanine, respectively. These mutants and wild-type HS1 were expressed in WEHI-231 cells by retroviral infection. By probing the anti-human HS1 immunoprecipitates with the antiphosphotyrosine antibody, we showed that HS1-FF was not detectably tyrosine-phosphorylated upon BCR crosslinking, whereas wild-type HS1 was highly and rapidly tyrosine-phosphorylated (Fig. 2 C). Both HS1-FY and HS1-YF were phosphorylated at a very low level. Thus, Tyr-378 and Tyr-397 are important for tyrosine phosphorylation of HS1 upon BCR stimulation.
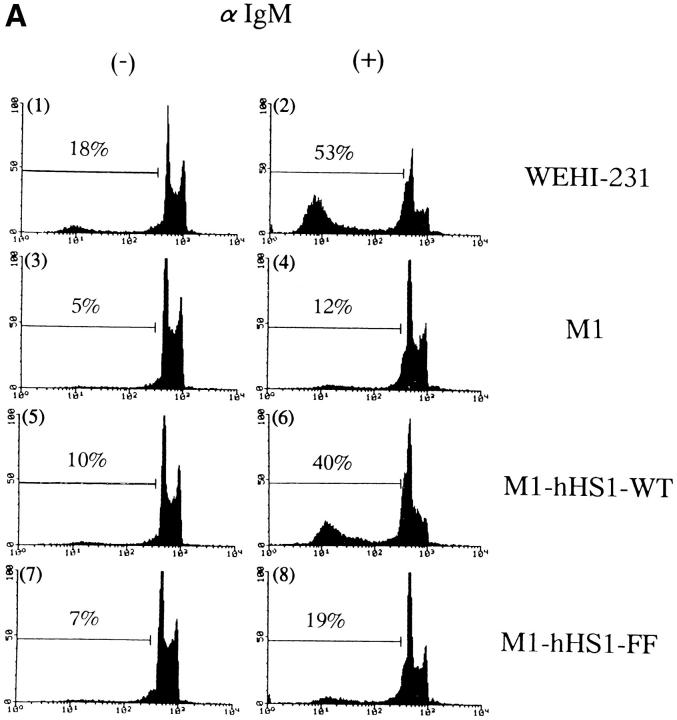
WEHI-231–derived M1 cells are resistant to mIgMinduced apoptosis, unlike their parental cells. This resistibility is due to very low expression of HS1 in the cells (9). M1 cells are rendered sensitive to BCR-mediated apoptosis by the exogenous expression of wild-type HS1 (9). Furthermore, peritoneal B cells from HS1 −/− mice do not undergo apoptosis upon BCR cross-linking (8). These data indicate that HS1 is a critical molecule for BCR-mediated apoptosis. However, unlike wild-type HS1, the exogenously introduced HS1-FF protein failed to restore the sensitivity of M1 cells to BCR-mediated apoptosis (Fig. 3 A). This was not due to differences in the expression levels of these proteins. In addition, the HS1-FF protein in M1 cells, as in WEHI-231 cells, was not tyrosine-phosphorylated by BCR cross-linking (Fig. 3 B). Therefore, tyrosine phosphorylation of HS1 is essential for BCR-mediated apoptosis.
Figure 3.
HS1 tyrosine phosphorylation is required for BCR-mediated apoptosis. (A) Restoration of BCR-mediated apoptosis by exogenous HS1 but not by HS1-FF in M1 cells. WEHI-231 cells (1 and 2), M1 cells (3 and 4), M1-derived cells expressing wild-type HS1 (5 and 6), and M1-derived cells expressing the HS1-FF protein (7 and 8) (1–2 × 105) were incubated for 48 h with (2, 4, 6, and 8) or without (1, 3, 5, and 7) anti-IgM Ab, stained with propidium iodide, and analyzed by flow cytometry. The DNA content of the cells (non-gated) was expressed as the histogram of propidium iodide fluorescence intensity. The percentage of apoptotic cells is denoted in each histogram (above horizontal bars). The results are representative of three independent experiments. Cells containing subdiploid DNA represent apoptotic cells. (B) Expression and tyrosine phosphorylation of wild-type HS1 and HS1-FF in M1 cells. M1-derived cells (1 × 107), which express the human HS1 (lanes 1 and 2) or HS1-FF (lanes 3 and 4), were incubated with (lanes 2 and 4) or without (lanes 1 and 3) anti-IgM Ab. The cells were then lysed in TNE buffer and the lysate were analyzed by immunoprecipitation with the anti-HS1 mAb and by anti-phosphotyrosine (PY blot) or antihuman HS1 (hHS1 blot) immunoblotting of the immunoprecipitates. Positions of HS1 and standard protein markers are indicated.
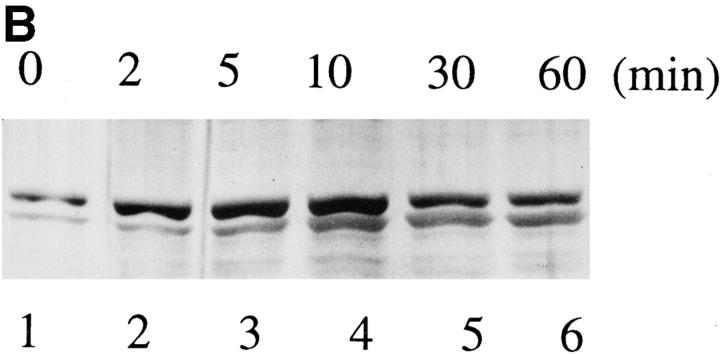
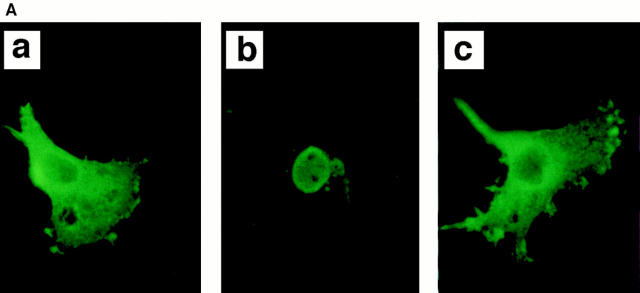
Despite the presence of a putative nuclear localization signal, HS1 localizes mainly in the cytoplasm of resting B cells (7). Consistently, HS1 expressed in COS7 cells was present in the cytoplasm. However, HS1 coexpressed together with Lyn and Syk was mostly in the nucleus. In contrast, HS1-FF remained in the cytoplasm in the presence of Lyn and Syk (Fig. 4 A). Thus, tyrosine phosphorylation of HS1 appears to be required for its own translocation from the cytoplasm to the nucleus. These data suggest that BCR cross-linking causes a significant fraction of tyrosine-phosphorylated HS1 to localize to the nucleus. Consistently, subcellular localization experiments showed that the amount of HS1 in the nucleus is increased after BCR cross-linking (Fig. 4 B). Similarly, the amount of wild-type human HS1 but not HS1-FF mutant expressed in M1 cells was increased in the nuclei upon BCR stimulation (Nishizumi, H., unpublished data). Tyrosine phosphorylation on HS1 may trigger its conformational alteration that allows its nuclear translocation and thereby signaling to its downstream targets.
Figure 4.
Subcellular localization of HS1. (A) Nuclear localization of HS1 coexpressed with Lyn and Syk. COS7 cells (1.5 × 106) were transfected with the expression plasmids (5 μg), pME-Lyn, pME-Syk, and either pME-HS1 (b), or pME-HS1-FF (c). As a control, cells were transfected with pME-HS1 alone (a). Subcellular localization of the HS1 protein was investigated by indirect immunofluorescence microscopy using antiHS1 mAb and FITC-labeled second Ab. No significant signal was detectable in the absence of primary Ab and in untransfected COS7 cells. (B) Increment of nuclear HS1 in BCR-stimulated WEHI-231 cells. WEHI-231 cells were incubated with anti-IgM Ab for the time indicated above. Then the cells were subjected to subcellular fractionation. Samples of nuclear fraction from 2 × 105 cells per lane were tested for HS1 by immunoblotting.
Because de novo protein synthesis is required for BCRmediated apoptosis of WEHI-231 cells (22), the transcriptional and/or translational regulation of an as yet unidentified gene(s) is involved in the process. HS1 possesses motifs characteristic of transcription factors (11). Therefore, it may regulate gene expression as a transcription factor following translocation into the nucleus, as is proposed for the STAT family of proteins. Alternatively, HS1 may interact with the other transcription factors or may transport a protein(s) critical for apoptosis into the nucleus. Molecules that may interact with the other motifs, such as the SH3 domain, of HS1 have yet to be determined.
Basing on the present data we propose a model that, upon BCR cross-linking, Syk becomes fully activated by Lyn and phosphorylates HS1 on Tyr-378 and Tyr-397. Because the Lyn SH2 domain binds tyrosine-phosphorylated HS1 (7), phosphorylated Y378ED and Y397EE could be the binding sites. This interaction would then allow Lyn to phosphorylate HS1 on other tyrosine residues. The model is consistent with the sequential phosphorylation model in which the primary kinase phosphorylates a residue that is directly recognized by the secondary phosphate-directed kinase. The secondary kinase then phosphorylates another residue nearby (23). A similar mechanism is proposed for tyrosine phosphorylation of p130CAS by Abl (24). Accordingly, not only wild-type HS1 but also HS1-YF and HS1FY would be phosphorylated upon BCR stimulation on Tyr-378 and Tyr-397, respectively, and thereby would interact with Lyn, allowing their further phosphorylation. However, HS1-YF and HS1-FY were much less tyrosine phoshorylated than wild-type HS1 (Fig. 2 C), as though no secondary kinases were available. It should be noted that Syk can interact with unphosphorylated HS1 and that the interaction terminates once the HS1 protein becomes tyrosine-phosphorylated at appropriate sites (Fukuda, T., unpublished data). This allows us to speculate that Lyn interacts with HS1 only when the two residues, Tyr-378 and Tyr-397, become phosphorylated and HS1 is dissociated from Syk. It is likely that HS1-YF and HS1-FY are still associated with Syk even after BCR stimulation, which prevents Lyn from interacting with HS1. Once the two tyrosine residues are both phosphorylated, processive phosphorylation of HS1 by Lyn and the other Src family kinases would take place, producing hyperphosphorylated form of HS1. Finally, it is this hyperphosphorylated form of HS1 that translocates to the nucleus and activates B cell apoptosis.
Acknowledgments
We thank A. Tanaka, B. Chen, C. Roman, S. Cherry, and R. Gandi for critical reading of this manuscript. Y. Yamanashi is grateful to people at the Yamamoto and Baltimore laboratories for generous help and encouragement.
This work was supported by grants from the Ministry of Education, Sciences, Sports, and Culture of Japan and a grant from the Human Frontier Scientific Program.
References
- 1.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science (Wash DC) 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 2.Bolen JB. Protein tyrosine kinases in the initiation of antigen receptor signaling. Curr Opin Immunol. 1995;7:306–311. doi: 10.1016/0952-7915(95)80103-0. [DOI] [PubMed] [Google Scholar]
- 3.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 4.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 5.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinase Lyn and Syk regulates B cell receptor-coupled Ca2+mobilization through distinct pathways. EMBO (Eur Mol Biol Organ) J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanashi Y, Okada M, Senba T, Yamori T, Umemori H, Tsunasawa S, Toyoshima K, Kitamura D, Watanabe T, Yamamoto T. Identification of HS1 proteins a major substrate of protein–tyrosine kinase(s) upon B cell antigen receptor–mediated signaling. Proc Natl Acad Sci USA. 1993;90:3631–3635. doi: 10.1073/pnas.90.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniuchi I, Kitamura D, Maekawa Y, Fukuda T, Kishi H, Watanabe T. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinase. EMBO (Eur Mol Biol Organ) J. 1995;14:3664–3678. doi: 10.1002/j.1460-2075.1995.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda T, Kitamura D, Taniuchi I, Maekawa Y, Benhamou LE, Sarthou P, Watanabe T. Restoration of surface IgM-mediated apoptosis in an anti-IgM-resistant variant of WEHI-231 lymphoma cells by HS1, a protein– tyrosine kinase substrate. Proc Natl Acad Sci USA. 1995;92:7302–7306. doi: 10.1073/pnas.92.16.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibner U, Benhamou LE, Haury M, Cazenave P-A, Sarthou P. Signaling of programmed cell death induction in WEHI-231 B lymphoma cells. Eur J Immunol. 1993;23:2821–2825. doi: 10.1002/eji.1830231115. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura D, Kaneko H, Miyagoe Y, Ariyasu T, Watanabe T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucl Acids Res. 1989;17:9367–9369. [PMC free article] [PubMed] [Google Scholar]
- 12.Fusaki N, Matsuda S, Nishizumi H, Umemori H, Yamamoto T. Physical and functional interactions of protein tyrosine kinases, p59fyn and ZAP-70, in T cell signaling. J Immunol. 1996;156:1369–1377. [PubMed] [Google Scholar]
- 13.Takeuchi M, Kuramochi S, Fusaki N, Nada S, Kawamura-Tsuzuku J, Matsuda S, Semba K, Toyoshima K, Okada M, Yamamoto T. Functional and physical interaction of protein–tyrosine kinases Fyn and Csk in the T-cell signaling system. J Biol Chem. 1993;268:27413–27419. [PubMed] [Google Scholar]
- 14.Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokai N, Nishiyama AF, Toyoshima Y, Yonemura S, Tsukita S, Inoue J, Yamamoto T. Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO (Eur Mol Biol Organ) J. 1996;15:457–467. [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in Lyn-deficient bone marrow–derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 17.Patschinsky T, Hunter T, Esch FS, Cooper JA, Sefton B. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci USA. 1982;79:973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter T. Synthetic peptides substrates for a tyrosine protein kinase. J Biol Chem. 1982;257:4843–4848. [PubMed] [Google Scholar]
- 19.Brunati AM, Donella-Deana A, Ruzzene M, Marin O, Pinna LA. Site specificity of p72sykprotein tyrosine kinase: efficient phosphorylation of motifs recognized by Src homology 2 domains of the Src family. FEBS Lett. 1995;367:149–152. doi: 10.1016/0014-5793(95)00555-n. [DOI] [PubMed] [Google Scholar]
- 20.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura D, Kaneko H, Taniuchi I, Akagi K, Yamamura K, Watanabe T. Molecular cloning and characterization of mouse HS1. Biochem Biophys Res Commun. 1995;208:1137–1146. doi: 10.1006/bbrc.1995.1452. [DOI] [PubMed] [Google Scholar]
- 22.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene family, upon programmed cell death. EMBO (Eur Mol Biol Organ) J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruzzene M, Brunati AM, Marin O, Donella-Deana A, Pinna LA. SH2 domains mediate the sequential phosphorylation of HS1 protein by p72sykand Src-related protein–tyrosine kinases. Biochemistry. 1996;35:5327–5332. doi: 10.1021/bi9528614. [DOI] [PubMed] [Google Scholar]
- 24.Pawson T. Protein–tyrosine kinases: getting down to specifics. Nature (Lond) 1995;373:477–478. doi: 10.1038/373477a0. [DOI] [PubMed] [Google Scholar]