Abstract
Local immunoregulatory processes during normal vascular biology or pathogenesis are mediated in part by the production of and response to cytokines by vessel wall cells. Among these cytokines interleukin (IL)-1 is considered to be of major importance. Although vascular smooth muscle (SMC) and endothelial cells (EC) expressed both IL-1α and IL-1β as cell-associated, 33-kilodalton (kD) precursors, SMC neither contained detectable mature IL-1β, nor processed recombinant IL-1β precursor into its mature 17-kD form. Thus, we investigated the expression and function of IL-1β–converting enzyme (ICE) in vascular cells. We demonstrate in processing experiments with recombinant IL-1 precursor molecules that EC processed IL-1β, in contrast to SMC. Despite the failure of SMC to process IL-1β, these cells expressed ICE mRNA, immunoreactive ICE protein, and the expected IL-1β nucleotide sequence. The lack of processing was explained by our finding that extracts of SMC specifically and concentration dependently blocked processing of IL-1β precursor by recombinant or native ICE. The initial biochemical characterization of the inhibitory activity showed that it is heat-labile, has a molecular size of 50–100 kD, and is associated to the cell membrane compartment. Inhibition of processing, i.e., activation of IL-1β precursor by SMC may constitute a novel regulatory mechanism during normal vascular biology or pathogenesis of vascular diseases.
IL-1 is a major mediator in the pathogenesis of chronic and acute inflammatory diseases, regulating multiple functions such as proliferation, differentiation, and activation of various cell types. Originally, IL-1 was considered to be released exclusively by activated monocytes (1). These cells express both the IL-1α and IL-1β isoform as 33-kD precursor proteins (2–4). The IL-1 precursor molecules do not contain a hydrophobic leader sequence as described for most secreted proteins and are not released through the established secretory pathways in monocytes. The IL-1 precursors are enzymatically processed into mature 17-kD IL-1. In contrast to the IL-1α precursor, the 33-kD form of IL-1β is biologically inactive, or at least much less active than mature IL-1β, since only processed IL-1β binds detectably to the IL-1 receptor (5). Two distinct IL-1 receptors have been characterized. The type I receptor was originally found on T cells and fibroblasts (6), whereas the type II receptor is the predominant form found on B cells, monocytes, and neutrophils (7). It has been reported that endothelial cells (EC)1 express IL-1 receptor type I (8) and that vascular smooth muscle cells (SMC) express specific IL-1 binding sites (9).
Proteolytic maturation of the IL-1β precursor into the active 17-kD form results from cleavage due to an endoproteinase denoted IL-1β–converting enzyme (ICE; 10, 11). ICE was isolated and cloned from cells of the monocytic lineage. Enzymatic and crystallographic studies have shown that ICE contains a cysteine at its active site (a QACRG motif ), which participates in substrate binding and catalysis (12, 13). ICE is synthesized as a precursor molecule of 45 kD and autocatalytically processed to form an active homodimeric enzyme of 20- and 10-kD subunits ([p20/ p10]2). Although both the 45-kD precursor protein and active ICE are detected in the cytoplasm, only active ICE is also localized on the cell surface membrane (14). The active cysteine protease specifically cleaves the 33-kD IL-1β precursor at the Asp116-Ala117 as well as the Asp27-Gly28 site to yield 17- and 28-kD proteins, respectively, but does not cleave the IL-1α precursor. Recently, additional substrates for ICE, the YAMA/CPP32 proteins, have been identified (15, 16). ICE and the serine protease granzyme B, which can also cleave YAMA/CPP32 (17), are the only known mammalian proteases cleaving after Asp.
Most of the studies investigating the expression of both IL-1 and ICE have focused on monocytes or monocytederived cell lines. However, recent research has indicated that active participation of vascular SMC and EC in local immune and inflammatory responses may also be mediated by IL-1. The responses of vascular cells to IL-1 include increased expression of adhesion molecules (18, 19) or induction of the proinflammatory cytokines IL-6 (20–23), IL-8 (24, 25), or IL-1 itself (26, 27). Furthermore, early atherosclerotic lesions have many characteristics in common with inflammatory lesions (28) and may, thus, also be influenced by IL-1. These findings suggest that during local regulatory processes under physiological or pathological conditions the cytokine IL-1 may be of major importance. We have previously shown that SMC produce IL-1 activity, but do not release this mediator (29). The cell-associated IL-1 activity mainly consists of IL-1α, which may serve as an activator of adjacent cells, as suggested before (29). In this report, we describe that SMC expressed IL-1β precursor protein. To contribute to regulatory mechanisms, the inactive IL-1β precursor produced by SMC has to be processed, i.e., activated by its specific processing enzyme ICE. However, it was unclear whether or not SMC express functionally active ICE. We, therefore, analyzed the expression and function of ICE in vascular SMC and EC in vitro. We report here that SMC and EC express ICE; SMC, however, fail to cleave recombinant IL-1β precursor, probably due to the expression of an ICE inhibitory activity.
Materials and Methods
Materials.
Recombinant human mature interleukin-1α, mature IL-1β, and TNF-α were a gift of Dr. H. Gallati (Hoffmann La-Roche Inc., Basel, Switzerland). Wild-type LPS of Salmonella friedenau was provided by Dr. H. Brade (Research Center Borstel, Borstel, Germany). Rabbit polyclonal antisera directed against IL-1α (L1), IL-1β (L4), or ICE (α-ICEP20) were generated by immunization with recombinant human mature IL-1α, mature IL-1β, or the ICE 20-kD subunit (ICEP20), respectively. The anti–IL-1β251-269 antibody (Fib 3) was raised as described elsewhere (30).
Isolation and Culture of Human Vascular Cells and Leukocytes.
Human vascular SMC and EC were isolated from saphenous veins and cultured as described previously (22–24). Both cell types were subcultured after trypsinization (0.05:0.02% trypsin/EDTA solution; Biochrom, Berlin, Germany) in 75-cm2 culture flasks (Dunn Labortechnik GmbH, Asbach, Germany) and used throughout passages 2–6. Culture media (M199, DMEM; both from Biochrom) contained <10 pg LPS/ml and FCS (Life Technologies Inc., Berlin, Germany) <50 pg LPS/ml as determined by the Limulus amoebocyte lysate assay (QLC-1000; BioWhittaker, Inc., Walkersville, MD). SMC were characterized by staining with anti–smooth muscle cell α-actin antibody (HHF35; Dako Diagnostika, Hamburg, Germany), and EC by staining with anti-vWF antibody (Dako). Monocytes were isolated from freshly prepared human PBMC by counterflow centrifugation. For this purpose, PBMC isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation were resuspended in HBSS plus 0.1% BSA and loaded into the standard chamber of the JE-6B elutriator system (Beckman Instrs. GmbH, München, Germany) at an initial flow rate of 25 ml/min, with the rotor speed being 1,720 g (12°C). Subsequently, the flow rate was increased stepwise to obtain elutriated monocyte fractions. Purity of the monocytes was ⩾98% as determined by FACS® analysis. Granulocytes were isolated and characterized as described previously (24).
Biochemical Methods.
To charactererize the ICE inhibitory activity of SMC supernatants were analyzed in ultrafiltration and centrifugation experiments. For ultrafiltration, supernatants were precleared by filtration through 0.2-μm filters. Subsequently, nitrogen-driven ultrafiltration of supernatants through YM membranes was performed in ultrafiltration devices as suggested by the manufacturer (Amicon GmbH, Witten, Germany). Aliquots of the filtrates or concentrates were collected and analyzed in the processing assay. To investigate the localization of the inhibitory activity, centrifugation experiments were performed. Supernatants or cells were obtained as described above. Part of the cells was incubated with digitonin (0.007%, 60 min; 31). Subsequently these preparations were centrifuged (10 min, 20,000 g) and the resultant pellet (Dig-Cell) or supernatant (Dig-SN) was analyzed in the processing assay.
Preparation of Recombinant Proteins.
Recombinant IL-1α and IL-1β precursor as well as ICE proteins were cloned into the pQE-30 vector, expressed in Escherichia coli, and purified by affinity chromatography using Ni-NTA-resin (Qiagen, Chatsworth, CA). For this purpose, mRNA isolation and reverse transcription (RT) were performed as described previously (24). Inserts were generated by RT-PCR using the primers described in Table 1. These primers introduced BamHI and PstI restriction sites into the PCR products to permit cloning into the pQE-30 vector. The resultant plasmids were transformed into the competent E. coli strain M15(pREP4) (GIBCO BRL, Gaithersburg, MD) by MgCl2 treatment. Plasmid sequences of transformants were verified by DNA sequencing. The recombinant proteins were isolated from the bacteria according to the procedure suggested by the manufacturer (Qiagen). The recombinant proteins were characterized in Western blot, processing assay, and NH2-terminal sequence analysis. The recombinant IL-1α and IL-1β precursor molecules showed the expected molecular mass of 33 kD (2–4) and contained the correct (10) NH2-terminal sequence. Recombinant ICEP20 (compare Fig. 2) and ICEP30 showed major bands of ∼20 or 30 kD, respectively, and also contained the correct NH2-terminal sequences (10). ICEP30 specifically processed the IL-1β, but not the IL-1α precursor, in a concentration-dependent manner. The apparent molecular weights of the processed IL-1β fragments corresponded to the data reported by other groups (10, 11).
Table 1.
Fluorescin-labeled Primers Used for RT-PCR and Sequencing
| Specificity | Sense* | Antisense* | Size‡ | |||
|---|---|---|---|---|---|---|
| bp | ||||||
| IL-1α | ATAT GGATCC GCCAAAGTTCCAGACAT | ATAT CTGCAG CTACGCCTGGTTTTCCAGTA | 810 | |||
| IL-1β | ATAT GGATCC ATGGCAGAAGTACCTGAGCTC | ATAT CTGCAG TTAGGAAGACACAAATTGCAT | 816 | |||
| ICEP30 | ATAT GGATCC GACAACCCAGCTATGCCC | ATAT CTGCAG ATGTCCTGGGAAGAGGTA | 350 | |||
| pQE-30 | GAATTCATTAAAGAGGAGAAA | ATCCAGATGGAGTTCTGAGG | 840 |
Sequences are given in 5′- to 3′-end orientation. The sequences for restriction sites are in italics.
Size of the expected PCR product in basepairs (bp).
Figure 2.
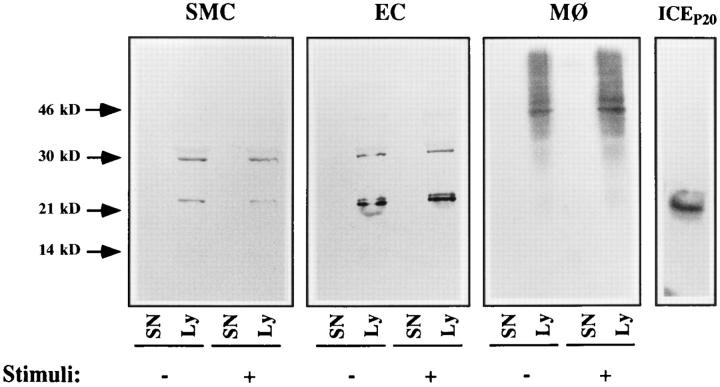
Expression of immunoreactive ICE by human vascular SMC and EC. Supernatants (SN) or lysates (Ly; 2.5 × 104 cells/μl) of unstimulated (−) or TNF-α–stimulated (+; 50 ng/ml) human vascular SMC or EC, as well as unstimulated (−) or LPS-stimulated (+; 1 μg/ml) monocytes (MØ) were separated by SDS-PAGE and analyzed by Western blot for ICE expression (α-ICEP20, 1:300). The right lane shows the recombinant ICEP20 protein used for immunization. Similar results were obtained in five independent experiments.
Processing Assay and Western Blot.
For processing, 15 μl of recombinant ICEP30 (0.4 μg/ml) were incubated with 15 μl of the respective precursor protein (10 μg/ml) in processing buffer (final concentrations: 10 mM Hepes, 1 mM dl-dithiotreitol, 10% glycerol; all from Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany). For inhibition studies, cell lysates or culture supernatants (15 μl) were preincubated (37°C for 10 min) with the IL-1β precursor before the addition of recombinant ICEP30, as well as granulocyte or monocyte extracts. All assays were performed in a final volume of 50 μl. After 15 min of incubation (37°C), processing was stopped by addition of SDS-PAGE sample buffer (10 μl; 1 M Tris, 25% glycerol, 0.5% SDS, 15% 2-mercaptoethanol, 0.1 mg/ ml bromphenol blue) and heating the samples (70°C for 30 min). Finally, the samples were analyzed in Western blot. For this purpose, samples were separated by standard SDS-PAGE under reducing conditions and blotted to polyvinylidene difluoride membranes (0.6 mA for 15 h; Immobilon™-P; Millipore GmbH, Eschborn, Germany). The blots were blocked with 0.05% Tween 20 and 0.01% merthiolate in PBS (30 min) and subsequently incubated in PBS containing 0.05% Tween 20, 15% bovine serum (Sigma-Aldrich Chemie GmbH), and the respective monoclonal or polyclonal antibody. After 1 h, peroxidase-conjugated goat anti–mouse or goat anti–rabbit antibody (both 1:2,000; Dianova GmbH, Hamburg, Germany) was added for another hour. The blots were washed three times with PBS/0.05% Tween 20 after each incubation step. Finally, diaminobenzidine (50 μg/ml; Sigma Chemical Co.) in substrate buffer (17 mM citric acid, 65 mM NaH2PO4, 0.1% H2O2, 0.01% [wt/vol] thimerosal) was added.
NH2-terminal Amino Acid Sequencing.
Amino terminal sequence analysis was performed on a pulsed liquid sequencer (model 473A; Applied Biosystems Inc., Foster City, CA; 32). In brief, after electroblotting, the membrane was washed in double-distilled water (30 min), stained by 0.1% (wt/vol) Coomassie R-250 (Serva Feinbiochemica GmbH & Co., KG, Heidelberg, Germany) in 50% (vol/vol) methanol (10 min), destained in methanol (50%), and air dried. Protein spots containing the proteins to be analyzed were cut out. Microsequencing was performed by Edman degradation using a protein sequencer (473 A) with an online phenylthiohydantoin amino acid analyzer (both from Applied Biosystems Inc.).
Results
Human Vascular SMC Express but Do Not Process the IL-1β Precursor.
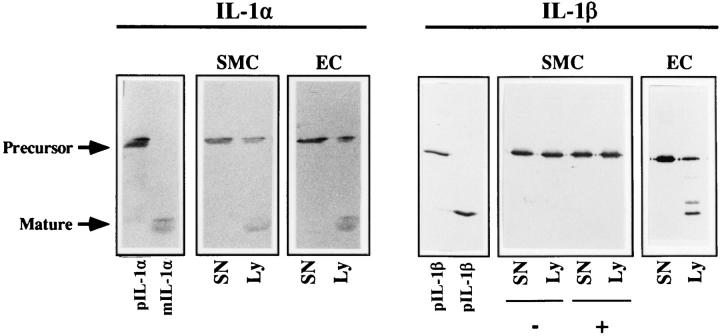
It has been previously described that human vascular SMC and EC express IL-1 activity. However, this IL-1 activity has not been characterized biochemically. In Western blot analysis we found that stimulated (IL-1/TNF) human SMC and EC express both IL-1 isoforms (IL-1α and IL-1β) cell associated as 33-kD precursor molecules. IL-1β, however, is only active as the processed mature 17-kD form. Thus, we investigated whether cell preparations or culture supernatants of SMC or EC can convert the recombinant IL-1β precursor into the mature form. Culture supernatants of SMC or EC did not cleave the recombinant IL-1α or IL-1β precursor into detectable products (Fig. 1). However, incubation of the IL-1α precursor with lysates of SMC or EC resulted in the detection of a cleavage product with an apparent molecular mass of 17 kD, as expected for mature IL-1α. In contrast, the IL-1β precursor was solely converted by EC, but not by SMC lysates. Incubation of the recombinant IL-1β precursor with EC lysates resulted in cleavage products comparable in molecular weight to the IL-1 proteins detected upon processing by monocytes, which are known to express active ICE (data not shown). NH2-terminal sequencing of the larger cleavage product revealed the expected amino acid sequence (Ala-Pro-ValArg-Ser-Leu-Asn-?-Thr-Leu-Arg-Asp-Ser) identical to the NH2 terminus of mature IL-1β expressed in monocytes (3). The NH2-terminal sequence of the smaller product corresponded to the mature sequence lacking the first eight amino acids. In contrast to EC, granulocytes, and monocytes, SMC did not process the recombinant IL-1β precursor under any condition tested, i.e., at a lysate concentration of up to 250,000 cells/μl or further stimulation (10 ng/ml IL-1α, 1 μg/ml LPS).
Figure 1.
Human vascular SMC, in contrast to EC, do not process recombinant IL-1β precursor. Recombinant IL-1α or IL-1β precursor (10 μg/ml, 15 μl) was incubated (37°C, 15 min) with processing buffer alone (pIL-1α/pIL-1β), culture supernatants (SN; 15 μl), or cell lysates (Ly; 2.5 × 104 cells/μl, 15 μl) of unstimulated (−) or TNF-α–stimulated (+; 50 ng/ml) vascular SMC or unstimulated EC. The products were separated by SDS-PAGE and analyzed by Western blot with the respective antibody (IL-1β: Fib 3, 1:1,000; IL-1α: L4, 1:500). For control, the recombinant mature IL-1 isoforms (mIL-1α/ mIL-1β) were included. Similar results were obtained in four independent experiments.
Human Vascular Cells Express ICE.
As shown above, SMC cannot process the recombinant IL-1β precursor. Thus, we investigated potential reasons for the lack of IL-1β processing, including sequence analysis of SMC-derived IL-1β, as well as expression of ICE. With regard to the cDNA and amino acid sequence analysis, it was unlikely that an altered sequence of the IL-1β precursor expressed in SMC was responsible for the lack of processing, since its cDNA sequence was identical to the sequence published for monocyte-derived IL-1β (data not shown). In addition, NH2 terminus sequencing of the native 33-kD IL-1β protein obtained from vascular SMC revealed the same amino acid sequence as described for the IL-1β precursor produced by monocytes (3).
Since the failure of SMC to process recombinant IL-1β precursor could also be due to the absence of ICE in these cells, we investigated ICE expression in SMC and EC. RTPCR experiments provided evidence that vascular cells express ICE mRNA constitutively (data not shown). Furthermore, cycle sequencing demonstrated that the sequence of SMC- or EC-derived ICE cDNA was identical to the sequence of monocytic ICE cDNA (data not shown). In line with the expression of ICE mRNA, Western blot experiments showed that SMC and EC express immunoreactive ICE protein constitutively (Fig. 2). Stimulation of the cells with TNF-α (50 ng/ml; +) did not detectably alter the number or intensity of the stained bands. In further experiments, stimulation of the cells with IL-1α, IL-1β, or LPS did not alter these findings (data not shown). Vascular cells expressed only cell-associated ICE, similar to monocytes. However, vascular cells expressed a different pattern of immunoreactive ICE proteins than monocytes. Cell preparations of SMC or EC contained bands with an apparent molecular mass of 22 and 30 kD, as well as a faint band possessing an apparent molecular mass of 45 kD. EC expressed the 22-kD protein as a double band. In contrast, control lysates of monocytes expressed a prominent band with an apparent molecular mass of 45 kD.
Human Vascular SMC Inhibit Processing of IL-1β Precursor by ICE.
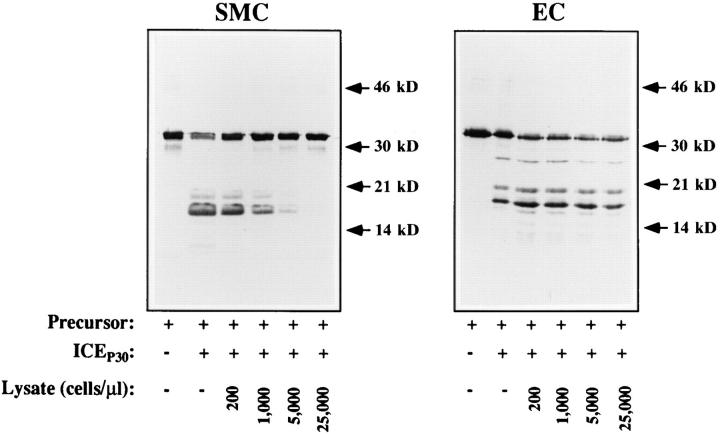
The findings discussed and presented above demonstrate that SMC express the IL-1β precursor and ICE, but do not process recombinant IL-1β precursor. Therefore, we investigated whether vascular SMC may inhibit ICE activity. We report here that supernatants or lysates of vascular SMC block the enzymatic activity of recombinant or native ICE. Preincubation of the recombinant IL-1β precursor with extracts of unstimulated SMC before the processing assay resulted in a concentration-dependent inhibition of processing (Fig. 3). Lysates containing ⩽200 SMC/μl did not block the processing of the precursor, whereas cell concentrations of ⩾25,000 SMC/μl completely blocked processing. In addition, prolonged incubation with ICEP30 (up to 30 min) did not lead to appearance of processed bands. In contrast, simultaneous addition of IL-1β precursor and lysates to the recombinant ICEP30 resulted in detection of faint bands of cleavage products. Further experiments showed that supernatants of SMC also contained ICE inhibitory activity (compare Fig. 4). SMC preparations did not only interfere with processing by the recombinant ICEP30, they also inhibited processing of the recombinant IL-1β precursor by native ICE as present in monocyte or granulocyte lysates (data not shown). In contrast to SMC lysates, preincubation of IL-1β precursor with lysates of EC (or fibroblasts, data not shown) did not inhibit the ICEP30 activity (Fig. 3), indicating the specificity of the inhibition.
Figure 3.
Processing of the recombinant IL-1β precursor by ICEP30 is specifically inhibited by human vascular SMC. Recombinant IL-1β precursor (10 μg/ml, 15 μl) was preincubated for 10 min (37°C) with 15 μl of processing buffer alone (Lysate, −) or cell lysates of vascular SMC or EC at the designated concentrations. Subsequently, the preparations were incubated (37°C, 15 min) with (+) or without (−) recombinant ICEP30 (0.4 μg/ml, 15 μl). The products were separated by SDS-PAGE and analyzed by Western blot with the IL-1β– specific antibody (Fib 3, 1:1,000). Similar results were obtained in seven independent experiments.
Figure 4.
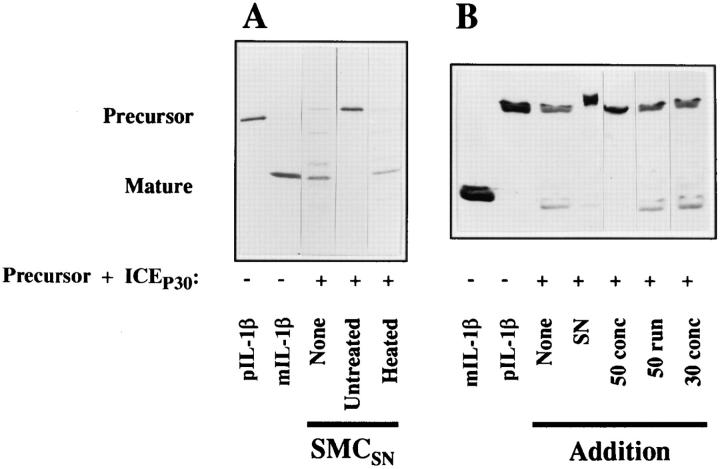
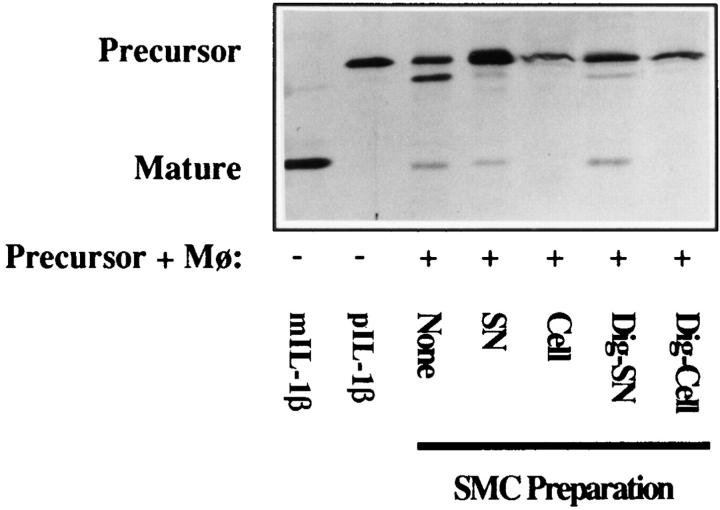
The ICE inhibitory activity of human vascular SMC is heatlabile and expresses a molecular size of 50–100 kD. (A) Heat treatment reverses the inhibitory activity of the ICE inhibitor. Recombinant IL-1β precursor (10 μg/ml, 15 μl) was incubated (37°C, 15 min) with heattreated (Heated; 70°C, 30 min) or untreated supernatants (15 μl). Immediately thereafter, recombinant ICEP30 (0.4 μg/ml, 15 μl) was added. All samples were separated by SDS-PAGE and analyzed by Western blot with the IL-1β–specific antibody (Fib 3, 1:1,000). Similar results were obtained in two independent experiments. (B) Ultrafiltration of inhibitory SMC supernatants. The supernatant of SMC was filtered through 0.2 μm filters and a 100-kD cutoff filter. This preparation was applied to 50-kD cutoff ultrafiltration (50 conc). The run-through (50 run) of this preparation was then filtered through a 30-kD cutoff filter and the concentrate was collected (30 conc). These samples (15 μl) were tested in the processing assay as described above. Three similar experiments were performed.
Experiments were performed to initially characterize the ICE inhibitory activity. Fig. 4 A shows that ICE processes the recombinant precursor molecule (None) and that an untreated supernatant of SMC was able to block this activity. However, the inhibition of the processing activity by the supernatant of SMC was reversed by prior heat treatment of the SMC supernatant. Similar results were obtained when cell preparations of SMC were subjected to heat treatment (data not shown). We also characterized the molecular size of this inhibitory activity in ultrafiltration experiments with filters of 100-, 50-, and 30-kD cutoff (Fig. 4 B). The SMC supernatant used (SN) in these experiments blocked IL-1β processing by ICE. The filtrate of a 100-kD cutoff filter was still inhibitory (data not shown). This filtrate was then applied to a 50-kD cutoff ultrafiltration. The figure shows that the obtained 50-kD concentrate (50 conc) potently inhibited the ICE processing. In contrast, the 50-kD filtrate (50 run) or the subsequent 30-kD concentrate (30 conc) did not inhibit the ICE activity, suggesting that the inhibitory activity is larger than 50 kD, but smaller than 100 kD. To investigate the localization of the putative inhibitor in the cell preparations, the cells were applied to digitonin treatment. Cytosolic proteins are expected in the supernatant of digitonin-treated cells, whereas membrane-associated molecules are expected in the resulting pellet. The presented experiment (Fig. 5) shows that a supernatant of SMC inhibited the processing to some degree, and that the corresponding cell extract completely blocked processing. An aliquot of the cells was treated by digitonin, which permeabilizes cells and releases cytosolic proteins from the cells. However, the supernatant (Dig-SN) obtained after centrifugation of the digitonin-treated cells did not contain inhibitory activity. In contrast, the pellet of the so-treated cells (Dig-Cell) inhibited the IL-1β processing as potently as cell preparations of the untreated SMC. In additional experiments, centrifugation supernatants of sonified cells also did not block processing (data not shown). These data suggest that the putative inhibitor is localized in cell membranes rather than in the cytoplasm.
Figure 5.
ICE inhibitory activity of SMC is membrane associated. The supernatant (SN) of SMC was collected, the cells were trypsinized, washed, and half of the cells was lysed by freeze–thaw cycles (Cell). The other part of the cells was treated by digitonin (0.007%) and centrifuged (20 min, 20,000 g). The supernatant (Dig-SN) and the remaining pellet (Dig-Cell) of this centrifugation were collected. For inhibition assay, these samples were incubated with recombinant IL-1β precursor (10 μg/ml, 15 μl), and subsequently with recombinant ICEP30 (0.4 μg/ml, 15 μl). The products were separated by SDS-PAGE and analyzed by Western blot with the IL-1β–specific antibody (Fib 3, 1:1,000). Similar results were obtained in two independent experiments.
Discussion
This report provides evidence that human vascular SMC express IL-1β precursor and ICE, but lack the capacity to process the IL-1β precursor, probably due to the presence of an ICE-inhibitory activity. The IL-1α precursor is thought to be the major cell-associated IL-1 isoform and, therefore, mediates activation of neighboring SMC (29). In contrast, activation of IL-1β precursor requires enzymatic processing of the precursor molecule. As shown in this report, vascular SMC, in contrast to EC, do not process the recombinant IL-1β precursor and therefore, likewise do not process the native protein. Under the conditions used, we did not detect any mature IL-1β in SMC. The detection limit of the Western blot may have contributed to this result, but even in sensitive biological test systems (data not shown), we could not detect IL-1β activity in the supernatants of SMC. Nevertheless, the processing studies with recombinant IL-1β precursor indicated that the lack of mature IL-1β in SMC probably results from the lack of processing activity in these cells and is not simply a failure to detect the mature protein.
To study the lack of mature IL-1β in more detail we raised three questions. (a) Is the lack of processing of IL-1β due to a sequence that cannot be cleaved by ICE? This explanation does not pertain. SMC, like EC, express the IL-1β precursor identical in its cDNA sequence, molecular weight, and NH2-terminal amino acid sequence to monocyte-derived IL-1β. (b) Do SMC lack ICE? This report demonstrates that SMC and EC express the ICE, identical in its cDNA sequence to the sequence published for monocyte-derived ICE. Furthermore, there is no evidence that SMC express a nonfunctional ICE mRNA, since its in vitro expression, as shown in this report, resulted in a specifically active enzyme (ICEP30). Under the conditions tested, neither mRNA, nor protein expression of the enzyme, is detectably altered in stimulated SMC or EC. This finding is in line with the report of an unchanged amount of ICEP45 in monocytes after LPS stimulation (33). However, the immunoreactive forms of IL-1β convertase detected in SMC and EC show a different expression pattern than monocyte-derived ICE. In our tests, monocytes expressed ICE as a major protein band with an apparent molecular mass of 45 kD. A similar form of ICE is described by Ayala et al. (33). In contrast, vascular SMC or EC expressed two detectable immunoreactive ICE proteins of lower molecular mass and a weak band at 45 kD that migrates at the same size as the one found in monocytes. The ICE protein of SMC expressing an apparent molecular mass of 30 kD may result from incomplete autocatalysis, whereas the 22-kD immunoreactive ICE protein may represent the ICEP20 subunit of the homodimeric enzyme. The used ICE antibody did not detect the 10-kD subunit, since it was raised against the ICEP20 subunit and, therefore, does not bind to the ICEP10 subunit.
These investigations raised the third question: Do SMC express an ICE inhibitory activity? We found that cultured SMC constitutively expressed an inhibitory activity, blocking the processing of recombinant IL-1β precursor by recombinant ICE, as well as granulocyte or monocyte lysates used as a source of native ICE. As far as we are aware, this is the first report describing a physiological cell-derived ICE inhibitor. Previously, other groups have shown inhibition by synthetic peptides (i.e., YVAD; reference 10) as well as viral inhibitors (crmA or baculovirus p35; references 34 and 35), and discussed them as potential therapeutic agents. Furthermore, Boudreau et al. (36) reported a correlation between ICE activity and expression of extracellular matrix in epithelial cells. Other authors demonstrated the expression of enzymatically inactive homologues of ICE in insect cells, resulting from alternatively spliced mRNA forms as derived from monocytic cell lines (37). One of these homologues, a 10-kD fragment, competed with the ICEP10 subunit for binding to ICEP20, thereby inhibiting processing of the IL-1β precursor by ICE. Bump et al. (35) demonstrated that equimolar amounts of a recombinant baculovirus protein inhibited the activity of purified ICE, and that inhibition is accompanied by a cleavage of the ICEP30 subunit to 22- and 10-kD fragments, which build stable complexes with ICE, thereby inactivating it. At present, we cannot rule out such possibilities; however, our data show that the inhibitory activity found in SMC expresses a molecular mass of 50–100 kD, and fractions containing proteins smaller than 30 kD, as those suggested by Bump et al. (35) and Alnemri et al. (37), were not inhibitory. The initial biochemical characterization also indicated that the inhibitory activity resides in the membrane compartment of the cells. It has been shown previously that active ICE is located in the membranes (14). Thus, a colocalization with an inhibitory molecule at the membrane could explain the lack of processing in SMC. The heat sensitivity of the inhibitory activity provided further evidence that it may be a protein.
Although ICE was characterized as the protease that cleaves the inactive precursor of IL-1β to yield the active cytokine, its possible connection to cell death only emerged from cloning of the Caenorhabditis elegans death gene ced-3 (38). Striking evidence that ICE family proteases are part of the apoptotic pathway is coming from studies demonstrating that inhibitors of these proteases block some of the apoptotic pathways (39), although ICE probably is not the only protein involved. However, the finding that certain cells of the vascular vessel wall express an inhibitor for a member of the apoptotic pathway may be the beginning of further studies investigating the regulation of apoptotic events in the vessel wall, i.e., during pathological processes such as atherosclerosis (40, 41).
Reporting here on the expression of immunoreactive ICE protein in human vascular SMC and EC as well as the expression of an ICE inhibitory activity in SMC, we provide novel information regarding the capacity of vascular cells to control and regulate normal vascular biology as well as vascular pathogenesis. Both expression of IL-1β precursor, probably serving as a reservoir to be activated by processing capacities of infiltrating leukocytes, and expression of ICE in SMC or EC might be of major importance for the regulation of vascular diseases.
Acknowledgments
We thank Drs. A. Haverich and K. Hirt (Kiel University, Kiel, Germany) for providing saphenous vein specimens, and Dr. M. Ernst, D. Heinrich, and E. Kaltenhäuser for providing us with elutriated monocytes. The kind gift of S. friedenau LPS by Dr. H. Brade and of recombinant mature IL-1α, IL-1β, and TNF-α by Dr. H. Gallati (Hoffmann La-Roche Inc., Basel, Switzerland) is gratefully acknowledged. We are grateful to G. Tillmann and S. Bark for their expert technical assistance.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to Dr. H. Loppnow (Lo 385/ 4-1) and to Dr. U. Schönbeck (Scho 614/1-1).
Footnotes
1 Abbreviations used in this paper: EC, endothelial cells; ICE, IL-1β–converting enzyme; RT, reverse transcription; SMC, smooth muscle cells.
References
- 1.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lomedico PT, Gubler U, Hellmann CP, Dukovitch M, Giri JG, Pan Y-CE, Collier K, Semionow R, Chua AO, Mizel SB. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. . Nature (Lond) 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 3.Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin-1 precursor cDNA. Proc Natl Acad Sci USA. 1984;81:7907–7911. [Google Scholar]
- 4.March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature (Lond) 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 5.Mosley BD, Dower SK, Gillis S, Cosman D. Determination of the minimum polypeptide lengths of the functionally active sites of human interleukins-1α and-1β. Proc Natl Acad Sci USA. 1987;84:4572–4576. doi: 10.1073/pnas.84.13.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dower SK, Kronheim SR, Hopp TP, Cantrell M, Deeley M, Gillis S, Henney CS, Urdal CS. The cell surface receptors for interleukin-1α und interleukin-1β are identical. Nature (Lond) 1986;324:266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- 7.MacMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, Grubin CE, Wignall JM, Jenkins NA, Brannan CI, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO (Eur Mol Biol Organ) J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akeson AL, Mosher LB, Woods CW, Schröder KK, Bowlin TL. Human aortic endothelial cells express the type I but not the type II receptor for interleukin-1 (IL-1) J Cell Physiol. 1992;153:583–588. doi: 10.1002/jcp.1041530320. [DOI] [PubMed] [Google Scholar]
- 9.French JF, Schröder KK, Akeson AL, Dage RC, Bowlin TL. Identification of a specific receptor for interleukin-1 in vascular smooth muscle cells: regulation by IL-1 and IL-6. Eur J Pharmacol. 1993;233:109–112. doi: 10.1016/0014-2999(93)90355-l. [DOI] [PubMed] [Google Scholar]
- 10.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature (Lond) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 11.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, van Ness K, Greenstreet TA, March CC, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1β converting enzyme. Science (Wash DC) 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 12.Walker NPC, Talanian RV, Brady KD, Dang LC, Bump NJ, Ferenz CR, Franklin S, Ghayur T, Hackett MC, Hammill LD, et al. Crystal structure of the cysteine protease interleukin-1β–converting enzyme: a (p20/ p10)2homodimer. Cell. 1994;78:343–352. doi: 10.1016/0092-8674(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Wilson KP, Black J-AF, Thomson JA, Kim EE, Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, et al. Structure and mechanism of interleukin-1β– converting enzyme. Nature (Lond) 1994;370:270–274. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 14.Singer II, Scott S, Chin J, Bayne EK, Limjuco G, Weidner J, Miller DK, Capman K, Kostura MJ. The interleukin-1β–converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmatic ground substance of human monocytes by immuno-electron microscopy. J Exp Med. 1995;182:1447–1459. doi: 10.1084/jem.182.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32β, a mammalian homolog of CED-3, is a crmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature (Lond) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 17.Quan LT, Tewari M, O'Rourke K, Dixit V, Snipas SJ, Poirier GG, Ray C, Pickup DJ, Salvesen GS. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc Natl Acad Sci USA. 1996;93:1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferongamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 19.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA., Jr Interleukin-1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jirik FR, Podor TJ, Hirano T, Kishimoto T, Loskutoff DJ, Carson DA, Lotz M. Bacterial lipopolysaccharides and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989;142:144–147. [PubMed] [Google Scholar]
- 21.Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142:549–553. [PubMed] [Google Scholar]
- 22.Loppnow H, Libby P. Adult human vascular endothelial cells express the IL-6 gene differentially in response to LPS or IL-1. Cell Immunol. 1989;122:493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 23.Loppnow H, Libby P. Proliferating or interleukin 1–activated human vascular smooth muscle cells secrete copious interleukin-6. J Clin Invest. 1990;85:731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönbeck U, Brandt E, Petersen F, Flad H-D, Loppnow H. Interleukin-8 specifically binds to endothelial cells but not to smooth muscle cells. J Immunol. 1995;154:2375–2383. [PubMed] [Google Scholar]
- 25.Wang JM, Sica A, Peri G, Walter S, Padura IM, Libby P, Ceska M, Colotta F, Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1166–1174. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Ordovas JM, Birinyi LK, Auger KR, Dinarello CA. Inducible interleukin-1 expression in human vascular smooth muscle cells. J Clin Invest. 1986;78:1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner SJC, Auger KR, Libby P. Human interleukin-1 induces interleukin-1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987;165:1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science (Wash DC) 1990;248:1009–1011. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- 29.Loppnow H, Libby P. Functional significance of human vascular smooth muscle cell-derived interleukin-1 in paracrine and autocrine regulation pathways. Exp Cell Res. 1992;198:283–290. doi: 10.1016/0014-4827(92)90381-h. [DOI] [PubMed] [Google Scholar]
- 30.Herzbeck H, Blum B, Rönspeck W, Frenzel B, Brandt E, Ulmer AJ, Flad H-D. Functional and molecular characterization of a monoclonal antibody against the 165-186 peptide of human IL-1β. Scand J Immunol. 1989;30:549–562. doi: 10.1111/j.1365-3083.1989.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 31.Fiskum G, Craig SW, Decker GL, Lehninger AL. The cytoskeleton of digitonin-treated rat hepatocytes. Proc Natl Acad Sci USA. 1980;77:3430–3434. doi: 10.1073/pnas.77.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 33.Ayala JM, Yamin T-T, Egger LA, Chin J, Kostura MJ, Miller DK. IL-1–β converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. J Immunol. 1994;153:2592–2599. [PubMed] [Google Scholar]
- 34.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β–converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 35.Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science (Wash DC) 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 36.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science (Wash DC) 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alnemri ES, Fernandes-Alnemri T, Litwack G. Cloning and expression of four novel isoforms of human IL-1β– converting enzyme with different apoptotic activities. J Biol Chem. 1995;270:4312–4317. doi: 10.1074/jbc.270.9.4312. [DOI] [PubMed] [Google Scholar]
- 38.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. eleganscell death gene ced-3 encodes a protein similar to mammalian interleukin-1β–converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 39.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature (Lond) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 40.Geng Y-J, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with ICE. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 41.Han DKM, Haudenschild CC, Hong MK, Tinkle BT, Leon MB, Liau G. Evidence for apoptosis in human atherogenesis and in rat vascular injury model. Am J Pathol. 1995;147:267–277. [PMC free article] [PubMed] [Google Scholar]