Abstract
We have analyzed transgenic mice carrying versions of a human T cell receptor (TCR)-δ gene minilocus to study the developmental control of VDJ (variable/diversity/joining) recombination. Previous data indicated that a 1.4-kb DNA fragment carrying the TCR-δ enhancer (Eδ) efficiently activates minilocus VDJ recombination in vivo. We tested whether the transcription factor CBF/PEBP2 plays an important role in the ability of Eδ to activate VDJ recombination by analyzing VDJ recombination in mice carrying a minilocus in which the δE3 element of Eδ includes a mutated CBF/PEBP2 binding site. The enhancer-dependent VD to J step of minilocus rearrangement was dramatically inhibited in three of four transgenic lines, arguing that the binding of CBF/PEBP2 plays a role in modulating local accessibility to the VDJ recombinase in vivo. Because mutation of the δE3 binding site for the transcription factor c-Myb had previously established a similar role for c-Myb, and because a 60-bp fragment of Eδ carrying δE3 and δE4 binding sites for CBF/PEBP2, c-Myb, and GATA-3 displays significant enhancer activity in transient transfection experiments, we tested whether this fragment of Eδ is sufficient to activate VDJ recombination in vivo. This fragment failed to efficiently activate the enhancerdependent VD to J step of minilocus rearrangement in all three transgenic lines examined, indicating that the binding of CBF/PEBP2 and c-Myb to their cognate sites within Eδ, although necessary, is not sufficient for the activation of VDJ recombination by Eδ. These results imply that CBF/PEBP2 and c-Myb collaborate with additional factors that bind elsewhere within Eδ to modulate local accessibility to the VDJ recombinase in vivo.
The process of VDJ recombination assembles variable (V)1, diversity (D), and joining ( J) gene segments at TCR and Ig loci during lymphocyte development, generating the diverse antigen receptor repertoires that characterize mature T and B lymphocytes (1–5). VDJ recombination is under stringent developmental control, as it is activated at individual antigen receptor loci with unique cell lineage-specific and developmental stage-specific properties. Thus, fully rearranged TCR genes are only generated in developing T lymphocytes, and fully rearranged Ig genes are only generated in developing B lymphocytes. Further, TCR-β, -γ, and -δ rearrangement occurs earlier during thymocyte development than TCR-α rearrangement (6), and IgH rearrangement occurs earlier in B cell development than Igκ and λ rearrangement (1). Because all of these loci are thought to share both recombination signal sequences and the known components of the recombinase machinery, it is generally believed that these factors cannot account for locus-specific regulation of VDJ recombination. Rather, it is thought that locus-specific regulation is accomplished by modulating the accessibility of chromosomal recombination substrates to the recombinase (1–5).
The expression of fully rearranged TCR and Ig genes is controlled by a promoter flanking the V gene segment, and one or more transcriptional enhancers located adjacent to the C gene segment (7, 8). Recent studies have indicated that these cis-acting elements are also required for the developmental activation of VDJ recombination at individual antigen receptor loci. This has been accomplished by eliminating, mutating, or substituting enhancer or promoter elements within chromosomally integrated VDJ recombination substrates in transgenic mice (9–16), as well as by eliminating enhancer elements from endogenous antigen receptor loci by homologous recombination (17–23). These studies show that the efficiency of VDJ recombination is dramatically inhibited in the absence of a functional enhancer, and that the developmental activation of VDJ recombination can be modified by substitution of one enhancer for another.
We have previously studied the developmental control of VDJ recombination in transgenic mice carrying a human TCR-δ gene minilocus (13, 14). Efficient VDJ recombination of this minilocus requires the presence of Eδ within the Jδ3-Cδ intron. Interestingly, the first step of minilocus rearrangement, V to D, occurs even in the absence of Eδ, whereas the second step of minilocus rearrangement, VD to J, is Eδ dependent (13). Thus, a functional enhancer is critical for establishing J segment accessibility to the VDJ recombinase machinery. Furthermore, substitution of Eα for Eδ within the minilocus reveals that Eδ and Eα regulate both the T cell subset and developmental stage specificity of the VD to J step of minilocus rearrangement in a manner that mimics the developmental activation of VδDδJδ and VαJα rearrangement, respectively, at the endogenous TCR-α/δ locus (14). These results lead to the conclusion that Eδ and Eα are indeed responsible for the developmental regulation of VDJ recombination at the endogenous TCR-α/δ locus.
The developmental properties of Eδ and Eα presumably result from the binding of specific trans-acting factors to discrete sites within the enhancers. Therefore, to better understand the mechanism by which these enhancers control VDJ recombination, we have begun to introduce mutations into previously defined cis-acting enhancer elements within the context of the TCR-δ gene minilocus, and to measure the effects of these mutations on the process of VDJ recombination in vivo.
Eδ was initially defined and functionally dissected in transient transfection and in vitro protein binding studies (24– 28). These experiments identified an essential element of the enhancer, δE3, that contains adjacent binding sites for the transcription factors CBF/PEBP2 (29–31) and c-Myb (32). Intact binding sites for both CBF/PEBP2 (the “core” site) (25) and c-Myb (27) are required for transcriptional activation by Eδ. Because CBF/PEBP2 has been implicated in TCR-α, -β, and -γ enhancer activity as well (30, 33– 36), it appears to be a crucial and broadly important regulator of T cell development and T cell–specific gene expression. Mice carrying a homozygous mutation within the gene encoding one particular CBF/PEBP2 isoform (αB) display a very early defect in hematopoiesis and early embryonic lethality (37). Although these results clearly demonstrate an important role for CBF/PEBP2 in the development of early hematopoietic precursor cells, they do not provide information regarding subsequent molecular events that might be regulated by CBF/PEBP2 within developing thymocytes in vivo.
The present study was initiated to determine whether CBF/PEBP2 plays an important role in the developmental activation of TCR genes in vivo, by specifically testing its role in the activation of VDJ recombination by Eδ. We found that disruption of the δE3 core site significantly impairs the ability of Eδ to activate VDJ recombination within the TCR-δ gene minilocus, suggesting that CBF/PEBP2 is an important regulator of VDJ recombination in vivo. Since previous data had indicated an important role for c-Myb as well, we then asked whether a small fragment of Eδ carrying binding sites for these factors as well as GATA-3 was sufficient to activate VDJ recombination. We found that this was not the case, arguing that additional cis-acting elements of Eδ are also required to establish local accessibility to the VDJ recombinase.
Materials and Methods
Production of Transgenic Mice.
The CBF/PEBP2 binding site mutation was generated by PCR using as a template the 1.4-kb wild-type Eδ subcloned into the XbaI site of pBluescript KS+ (1.4EδBS). PCR overlap extension was performed as described (38) using mutagenic oligonucleotides ACOREM (AGCAATGCATGACCTTTCCAACCG) and BCOREM (CGGTTGGAAAGGTCATGCATTGCT) along with EDRA (CTTTTAAAATTCTAGCAAGC) and the reverse primer as outside primers. The final PCR product was digested with NsiI and BamHI to generate a 170-bp fragment of Eδ carrying the 3-bp change in δE3 that eliminates CBF/PEBP2 binding. The plasmid 1.4EδBS was also digested with PfiMI and NsiI to obtain an adjacent 590-bp fragment of Eδ. The two fragments were ligated together into PfiMI and BamHI cut 1.4EδBS. The structure of the resulting plasmid was determined by restriction mapping and dideoxynucleotide sequence analysis. The 1.4-kb EδmCore was excised from this plasmid with XbaI and cloned into XbaI-digested, phosphatase-treated pBluescript carrying the previously described enhancerless minilocus (13).
A minilocus construct containing the δE3,4 region was generated as follows. A 60-bp Nsi–AluI fragment of Eδ (δE3,4) had been previously subcloned into PstI and EcoRV cut pBluescript KS+. The insert was excised from this plasmid by digestion with BamHI and HindIII and the ends were blunted by treatment with the Klenow fragment of Escherichia coli DNA polymerase I. This fragment was then ligated into Xba I-digested, blunted, and phosphatase-treated pBluescript carrying the enhancerless minilocus. The structures of both minilocus constructs were confirmed by dideoxynucleotide sequence analysis.
Minilocus DNA was purified as described previously (13), and was microinjected into fertilized C57BL/6 × SJL F2 eggs by the Duke University Comprehensive Cancer Center Transgenic Mouse Shared Resource. Progeny tail DNA samples were analyzed on Southern blots as described previously (13). Transgenes were maintained on a mixed C57BL/6 × SJL background. Copy number was determined by analysis of tail DNA on a slot blot (Schleicher and Schuell, Keene, NH) using a radiolabeled Cδ cDNA probe. Hybridization signals were quantified relative to previously identified single copy integrants using a Betascope (Betagen, Waltham, MA).
Preparation and Analysis of Genomic DNA.
Genomic DNA preparation, PCR, gel electrophoresis, blotting onto nylon membranes (Hybond-N; Amersham, Arlington Heights, IL or MAGNA nylon; Micron Separations Inc., Westboro, MA), and hybridization with 32P-labeled probes were performed as described previously (13). The amount of DNA template used in PCR reactions (2–12 ng) was adjusted based on the results of amplification using Cδ primers to account for differences in transgene copy number and PCR efficiency between samples, so that all PCR signals were maintained in the linear range. TCR-δ minilocus PCR primers 1 (VD1), 2 (VD2), 3 (JD1), 4 (JD3), 5 (CDA), and 6 (CDB), as well as the Vδ1, Vδ2, and Cδ fragments used as probes to develop Southern blots of PCR products or genomic restriction digests, were described previously (13). These primers and probes allow detection of human TCR-δ minilocus sequences, but do not allow detection of endogenous murine TCR-δ locus sequences. Quantification of PCR signals was accomplished using either a Betascope or a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Results
An Intact CBF/PEBP2 Binding Site Is Necessary for Efficient TCR-δ Gene Rearrangement In Vivo.
The previously studied human TCR-δ gene minilocus is a 22.5-kb construct containing germline Vδ1, Vδ2, Dδ3, Jδ1, Jδ3, and Cδ gene segments, along with a wild-type version of Eδ within the Jδ3Cδ intron (13) (Fig. 1 A). The Vδ1 and Vδ2 coding segments carry mutations to prevent a rearranged transgene from encoding a TCR protein that might interfere with normal murine T cell development. Thus, the minilocus serves as a phenotypically neutral in vivo reporter of VDJ recombination.
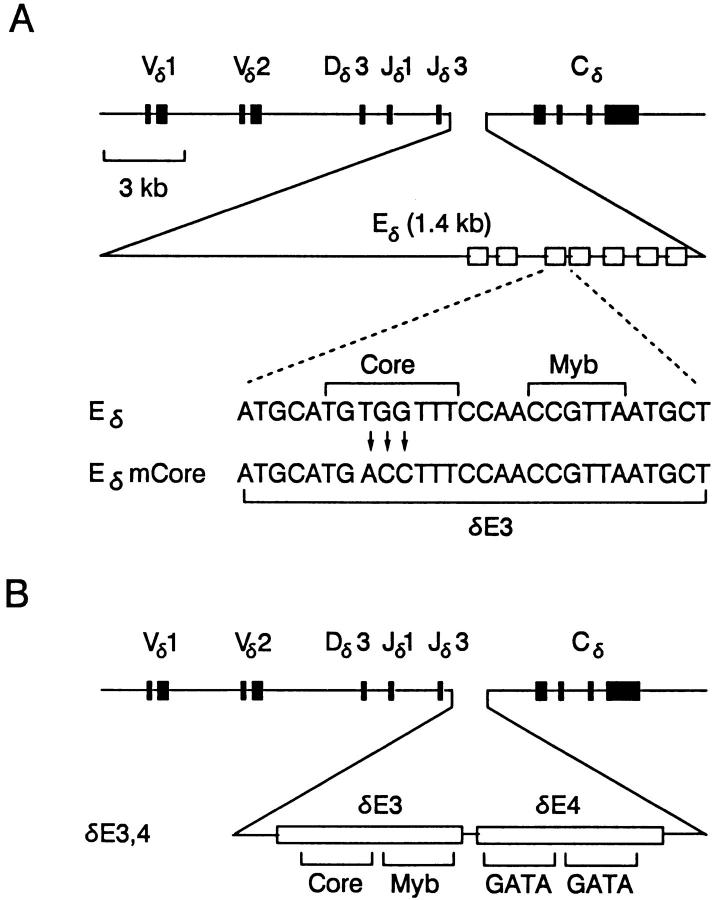
Figure 1.
Structure and analysis of the Eδ, EδmCore, and δE3,4 transgenes. (A) Schematic representations of the Eδ and EδmCore minilocus recombination substrates includes coding exons (filled boxes) and protein binding sites within Eδ (open boxes). The 3-bp substitution in the δE3 core site is shown. (B) Schematic representation of the δE3,4 minilocus recombination substrate. (C) Primers used for analysis of minilocus rearrangement by PCR from genomic DNA (arrowheads). (D) Depiction of PCR products generated from Vδ1 rearrangements using primers 1, 3, and 4. A similar set of PCR products are generated from Vδ2 rearrangements using primers 2, 3, and 4.
For this study, we initially constructed a new version of the minilocus, referred to as EδmCore, that carries a three– basepair change in the δE3 core sequence within Eδ (Fig. 1 A). The identical mutation was previously shown to eliminate CBF/PEBP2 binding to δE3 in vitro, and to eliminate transcriptional activation by Eδ in transient transfection experiments (25). Three transgenic founders, designated U, Y, and Z, were initially obtained. Breeding experiments indicated that in each of founders U and Z, the EδmCore minilocus was integrated at a single site in the mouse genome, whereas in founder Y there were two independently segregating transgene integration sites. As a result, four different EδmCore transgenic lines (U, Z, Y1, Y2) were established. As assessed by slot blot, the transgene copy numbers were: U, 8 copies; Z, 3 copies; Y1, 1 copy; and Y2, 2 copies. The single copy integrant in line Y1 was truncated so as to delete ∼4–5 kb at the 3′ end of the minilocus. This truncation leaves Vδ, Dδ, Jδ gene segments, Eδ and the first Cδ exon intact, and as such, is not expected to have a significant effect on minilocus VDJ recombination.
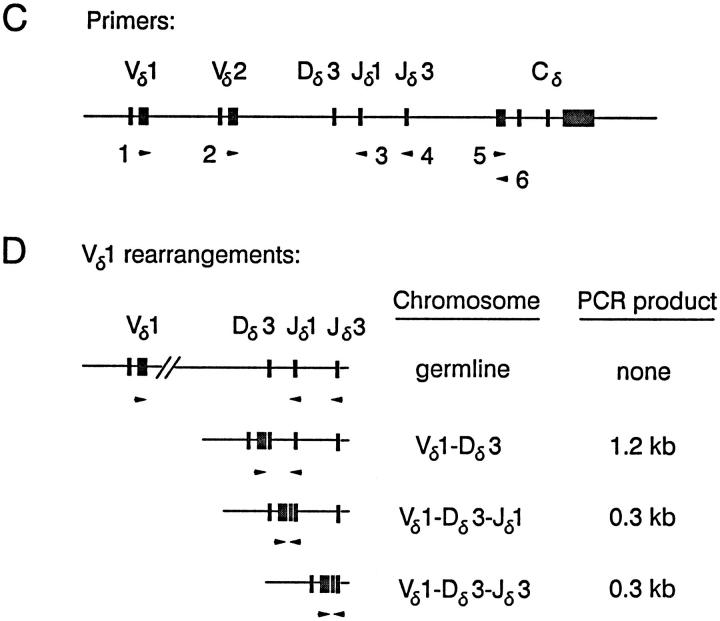
Analysis of wild-type and EδmCore minilocus VDJ recombination was performed by PCR from thymus genomic DNA templates, using specific Vδ1, Vδ2, Jδ1, and Jδ3 primers (primers 1, 2, 3 and 4; Fig. 1 C) as described previously (13). Previous studies have shown the wild-type minilocus to rearrange stepwise, first V to D, and then VD to J (13). Amplification with Vδ and Jδ1 primers yields 0.3kb fragments that represent complete VDJ rearrangements, and in addition, 1.2-kb fragments that represent the VD rearrangement intermediates (Fig. 1 D). Amplification with Vδ and Jδ3 primers yields 0.3-kb VDJ products only. PCR reactions were also performed with a pair of Cδ primers (primers 5 and 6; Fig. 1 C), to control for differences in transgene copy number and PCR efficiency between samples; the amount of genomic DNA template used in PCR reactions was typically adjusted to obtain similar Cδ amplification signals in each sample. PCR products were detected and quantified by agarose gel electrophoresis followed by blotting and hybridization with appropriate 32P-labeled probes. In agreement with our previous studies (13), quantification of PCR signals revealed amplification to be linear over a broad range of template concentrations (Fig. 2).
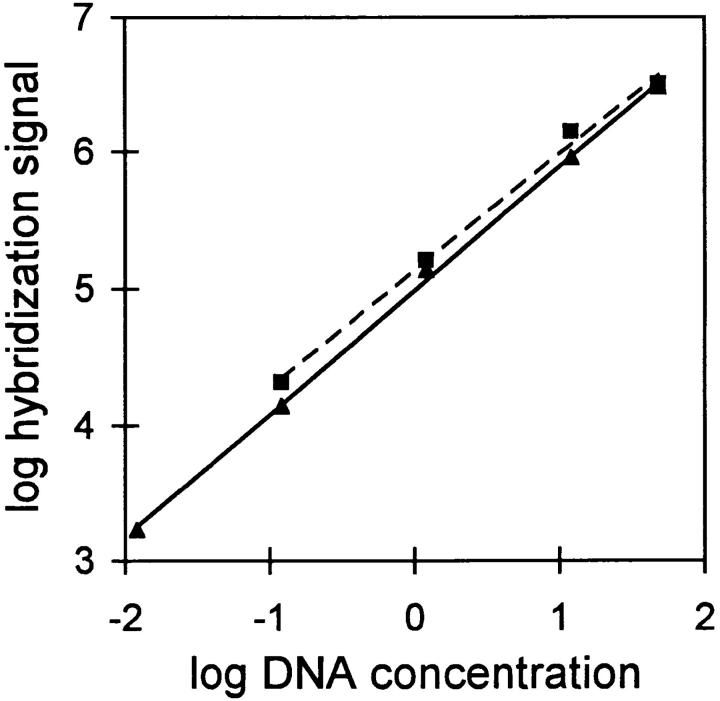
Figure 2.
Quantification of minilocus VDJ rearrangement. Serially diluted samples of thymus genomic DNA from Eδ line A were amplified by PCR using primers 1 and 3 or primers 5 and 6, and Southern blots were developed using radiolabeled Vδ1 and Cδ cDNA probes. The Vδ1Dδ3-Jδ1 (solid line) and Cδ (dotted line) hybridization signals were quantified using a PhosphorImager. The results are expressed log hybridization signal in arbitrary units versus log DNA concentration in μg/ml. The second most concentrated DNA sample in this experiment corresponds to the amount of DNA used for analysis of single copy integrants in all subsequent experiments. Lower amounts of DNA were used for multicopy integrants.
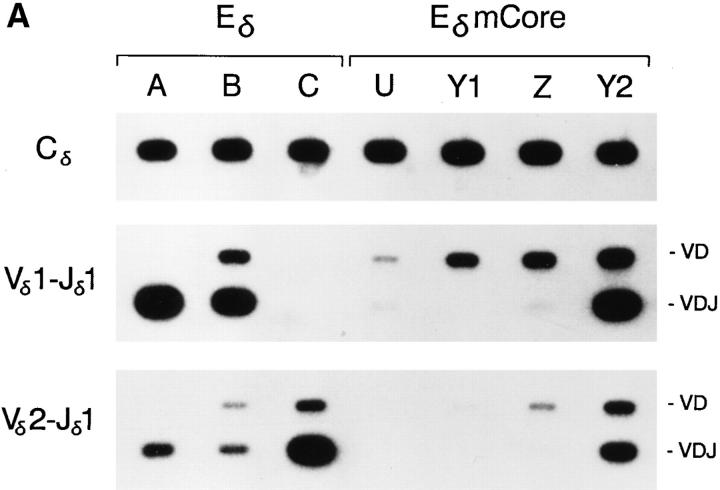
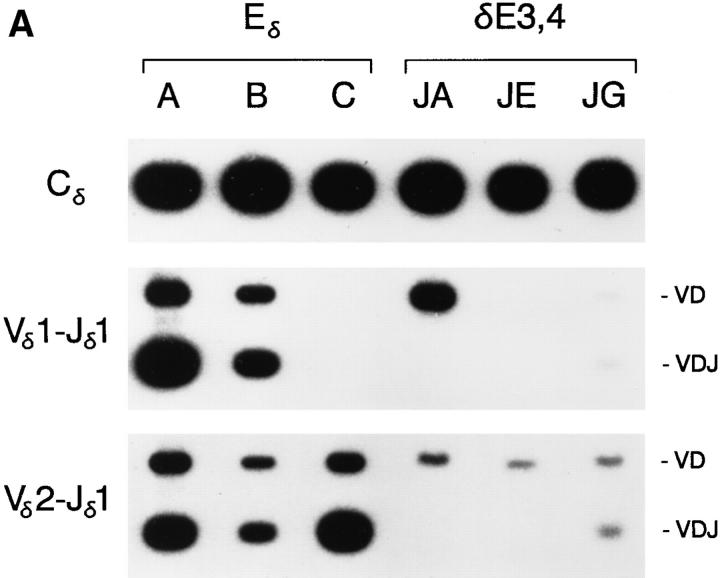
We analyzed the effect of the core site mutation on minilocus VDJ recombination by comparing VD and VDJ rearrangement levels in thymocytes from three previously characterized lines of mice carrying a minilocus with a wild-type Eδ (A, B, C) (13) to thymocytes in the four lines of mice carrying the EδmCore minilocus (Fig. 3, A and B). All Eδ lines of mice carry single copy integrations of the minilocus. Whereas the minilocus integrations in lines A and B include all relevant gene segments, the integration in line C is truncated at the 5′ end and is missing the Vδ1 gene segment. Thus, lines A and B are informative for both Vδ1 and Vδ2 rearrangement, whereas line C is informative for Vδ2 rearrangement only. As in previous studies, PCR analysis of VDJ recombination in the Eδ lines revealed high levels of 0.3-kb VDJ rearrangement products in each case (Fig. 3, A and B). Levels of 1.2-kb VD rearrangement products were lower and more variable (Fig. 3 A). Thus, as observed previously (13), the enhancer-dependent VD to J step of transgene rearrangement occurs efficiently in each of the Eδ lines.
Figure 3.
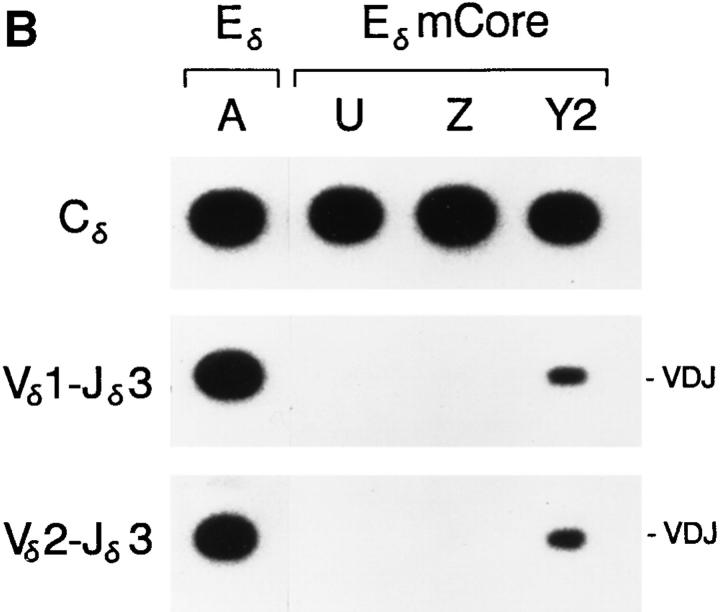
PCR analysis of Eδ and EδmCore minilocus rearrangement. (A) Genomic DNA from thymi of wild-type Eδ mice from lines A, B, and C, and from EδmCore mice from lines U, Y1, Z, and Y2 were amplified by PCR using primers 5 and 6 (top), primers 1 and 3 (middle) and primers 2 and 3 (bottom). Southern blots were developed using radiolabeled Cδ, Vδ1 or Vδ2 cDNA probes. The mice analyzed were A-26 (3 wk old), B-29 (2 wk old), C-306 (6 wk old), U-19 (5 wk old), Y1-83 (2 wk old), Z-92 (3 wk old), and Y287 (2 wk old). (B) Genomic DNA from the thymi of mice from lines A, U, Z, and Y2 were amplified by PCR using primers 5 and 6 (top), primers 1 and 4 (middle) and primers 2 and 4 (bottom). The mice analyzed were A-866, U-339, Z-419 and Y2-280 (all 4 wk old).
Analysis of VDJ recombination in the four EδmCore lines revealed quite different results. In three of the lines (U, Y1, and Z), 0.3-kb products representing VDJ rearrangement were barely detectable, even though 1.2-kb VD rearrangement products were readily apparent (Fig 3, A and B). In the fourth line (Y2), VD and VDJ rearrangement products were both detected at levels that were comparable to their representation in Eδ lines. The analysis of a second animal in each line (data not shown) yielded quite similar results, arguing that these VDJ recombination phenotypes are stable and reproducible characteristics of the individual transgenic lines. Because PCR amplifications with the Cδ primer pair and with the Vδ1-Jδ1 primer pair were shown to be linear over several orders of magnitude (Fig. 2) we were justified in quantifying the level of VDJ recombination in the different lines by normalizing the Vδ1-Jδ1 PCR signal to the Cδ PCR signal in each line. The levels of VDJ recombination in lines U, Y1 and Z, each calculated as the mean of three independent determinations, were found to be 0.8, 1.3, and 0.3%, respectively, of the level in Eδ line A, and 3.1, 4.9, and 1.2%, respectively, of the level in Eδ line B (Table 1). Similar quantification revealed VDJ recombination in line Y2 to be 41.7% of the level in line A, and 159.8% of the level in line B (Table 1).
Table 1.
VDJ Rearrangement in Eδ, EδmCore, and δE3,4 Transgenic Lines
| Construct | Line | Vδ1-Dδ3-Jδ1/Cδ | ||
|---|---|---|---|---|
| Eδ | A | (100) | ||
| B | 26.1 ± 11.1 | |||
| EδmCore | U | 0.8 ± 0.4 | ||
| Y1 | 0.3 ± 0.3 | |||
| Z | 1.3 ± 0.8 | |||
| Y2 | 41.7 ± 7.8 | |||
| δE3,4 | JA | 1.4 ± 0.6 | ||
| JG | 4.7 ± 2.6 |
The Vδ1-Dδ3-Jδ1 and Cδ hybridization signals were measured in three independent experiments. In each experiment, the Vδ1-Dδ3-Jδ1 signal was normalized to the Cδ signal, and the results are reported as the mean ± SD. VDJ recombination expressed as a percentage of VDJ recombination in line A.
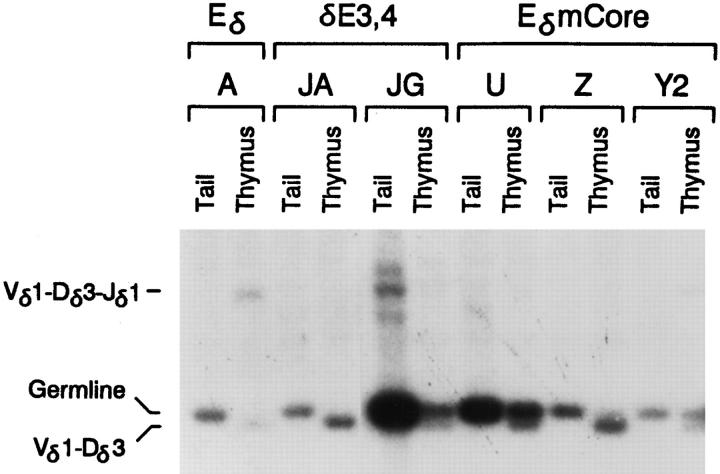
The conclusions drawn from PCR analysis were confirmed through analysis of Vδ1-Dδ3 and Vδ1-Dδ3-Jδ1 rearrangements by genomic Southern blot (Fig. 4). Eδ line A thymocytes displayed nearly undetectable germline Vδ1 (1.0 kb), moderate Vδ1-Dδ3 rearrangement (0.9 kb), and substantial Vδ1-Dδ3-Jδ1 rearrangement (1.7 kb). In accord with the PCR data, Vδ1-Dδ3-Jδ1 rearrangement was not detected in lines U and Z, even though transgene copy was higher in these lines than in Eδ line A. Importantly, Vδ1Dδ3 rearrangement was readily detected in both lines, and accounted for almost all of the Vδ1 signal in line Z. Although the reduced sensitivity of genomic Southern blot analysis as compared to PCR analysis does not allow an independent evaluation of the extent to which VDJ recombination is reduced in lines U and Z, the readily detectable Vδ1-Dδ3 rearrangement demonstrates that the ratio of VD to VDJ rearrangement has been dramatically perturbed in these lines. As these results argue that the VD to J step of transgene rearrangement has been specifically inhibited, they provide strong support for the PCR data. Also in accord with the PCR data, Vδ1-Dδ3 and Vδ1-Dδ3-Jδ1 rearrangements were both detected in line Y2. Thus, on the basis of both PCR and genomic Southern blot analyses, we conclude that the enhancer-dependent step of minilocus rearrangement is dramatically and preferentially impaired in three of the four EδmCore transgenic lines, but occurs quite normally in the fourth line.
Figure 4.
Eδ, δE3,4, and EδmCore minilocus rearrangement analyzed by genomic Southern blot. A Southern blot carrying PstI plus EcoRI digested tail (germline control) and thymus DNA samples from Eδ line A, δE3,4 lines JA and JG, and EδmCore lines U, Z, and Y2 was developed with a radiolabeled 1.0 kb Vδ1 genomic PstI fragment as a probe. The mice analyzed were A-866, JA-81, JG-42, U-339, Z-352, and Y2-280 (all 4 wk old).
These results are reminiscent of our previous work analyzing VDJ recombination within a TCR-δ minilocus lacking Eδ (13) or containing a disrupted binding site for c-Myb (39). In four of five transgenic lines carrying the minilocus construct lacking Eδ, and in three of four transgenic lines carrying the minilocus construct with a disrupted binding site for c-Myb, inhibition of the VD to J rearrangement step was almost complete. However, in each case one of the transgenic lines displayed higher levels of VD to J rearrangement. Such heterogeneity in TCR-δ minilocus transgenic lines in which Eα has been eliminated or inactivated probably reflects the distinct properties of the different transgene integration sites. We propose that in E−, EδmMyb, and EδmCore lines in which the VD to J step still occurs, the minilocus has integrated adjacent to active regulatory elements that partially or completely supplant the need for Eδ. Heterogeneity of this magnitude has not been observed in transgenic lines carrying an intact enhancer, as VD to J rearrangement is efficient in three of three Eδ lines (Fig. 3) and four of four Eα lines (14). On the basis of the phenotype displayed by the majority of EδmCore transgenic lines, we conclude that elimination of a functional CBF/PEBP2 binding site within Eδ has a dramatic effect on the ability of Eδ to provide the regional accessibility to the VDJ recombinase that is required for efficient VDJ recombination in vivo.
Intact Binding Sites for CBF/PEBP2, c-Myb, and GATA-3 Are not Sufficient for Efficient TCR-δ Gene Rearrangement In Vivo.
Previous in vitro binding and transient transfection studies identified a functionally important binding site for c-Myb that is within the δE3 element and adjacent to the CBF/PEBP2 binding site (27), as well as two functionally important binding sites for GATA-3 within the adjacent δE4 element (40, 41). A 60-bp δE3,4 fragment of Eδ containing only these binding sites displays 20–50% of the activity of the intact 1.4-kb Eδ in transient transfection experiments (25). Since CBF/PEBP2 (this study) and c-Myb (39) were implicated as important regulators of VDJ recombination in vivo, we asked whether the combination of CBF/ PEBP2, c-Myb and GATA-3 binding sites within δE3 and δE4 was sufficient to activate this process.
Therefore, the 60-bp δE3,4 fragment of Eδ was introduced into the TCR-δ gene minilocus in place of Eδ (Fig. 1 B). Three transgenic founders were obtained and were used to generate independent lines of transgenic mice designated JA, JE, and JG. Slot blot analysis indicated transgene copy numbers of 2, 1, and 24 for JA, JE, and JG, respectively. As in Eδ line C, the single copy δE3,4 line JE is truncated such that it lacks Vδ1 but retains Vδ2. Hence, this line is informative for Vδ2 rearrangement only.
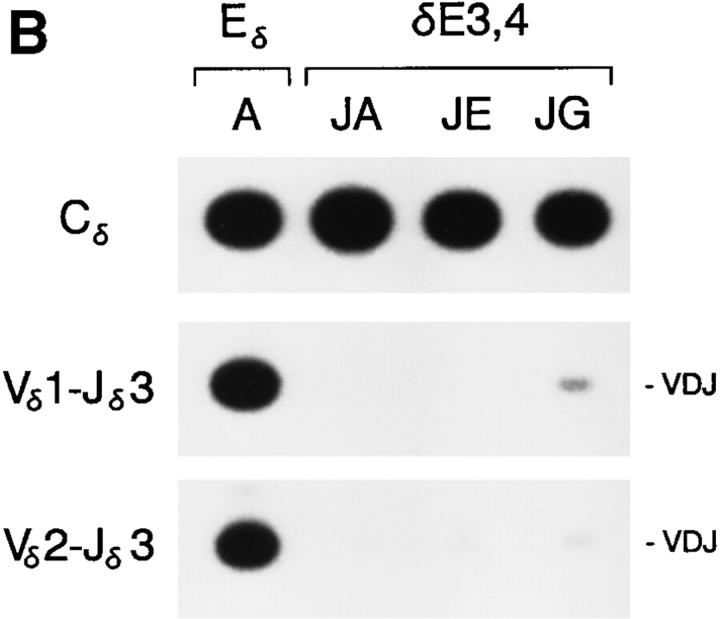
Analysis of wild-type Eδ and δE3,4 minilocus VDJ recombination was performed by PCR as described above. All three δE3,4 lines revealed dramatically reduced levels of VDJ rearrangement as compared to Eδ lines A, B, and C (Fig. 5, A and B). In lines JA and JE, VDJ rearrangement was essentially undetectable, whereas in line JG, VDJ rearrangement was detectable at reduced levels. Nevertheless, VD rearrangement signals were readily detected by PCR in all three lines, and were detected at particularly high levels in line JA. Quantification of Vδ1-Jδ1 and Cδ PCR signals indicated that VDJ rearrangement in lines JA and JG were reduced to 1.4 and 4.7%, respectively, of the level in Eδ line A, and to 5.2 and 17.9%, respectively, of the level in Eδ line B (Table 1). Analysis of Vδ1-Dδ3 and Vδ1-Dδ3-Jδ1 rearrangements by genomic Southern blot confirmed that essentially all copies of Vδ1 were rearranged to Dδ3 in line JA, but that rearrangement was blocked at this stage (Fig. 4). Although the low level of VDJ rearrangement detected in line JG could not be confirmed due to both the limited sensitivity of detection and the presence of comigrating germline fragments in the tail DNA control, the results for JG did suggest that PCR may have underestimated the level of Vδ1-Dδ3 rearrangement in this line. Also of note is the significant reduction in transgene copy number in thymus relative to tail in this line (Fig. 4 and data not shown). This most likely results from the rearrangement of Vδ1 in one copy of the tandemly arrayed transgene to Dδ3 in another copy, with deletion of intervening copies. In summary, on the basis of the dramatic inhibition of VD to J recombination detected in both PCR and genomic Southern blot analyses, we conclude that the combination of CBF/ PEBP2, Myb, and GATA-3 binding sites contained within δE3 and δE4 is not by itself capable of promoting the accessible chromatin configuration required for efficient minilocus VDJ recombination.
Figure 5.
PCR analysis of Eδ and δE3,4 minilocus rearrangement. Genomic DNA from thymi of wild-type Eδ mice from lines A, B, and C, and from δE3,4 mice from lines JA, JE, and JG were amplified by PCR using primers 5 and 6 (top), primers 1 and 3 (middle) and primers 2 and 3 (bottom). Southern blots were developed using radiolabeled Cδ, Vδ1, or Vδ2 cDNA probes. The mice analyzed were A-765 (4 wk old), B-31 (8 wk old), C-114 (4 wk old), JA-81 (4 wk old), JE101 (4 wk old), and JG-42 (4 wk old). (B) Genomic DNA from the thymi of mice from lines A, JA, JE, and JG were amplified by PCR using primers 5 and 6 (top), primers 1 and 4 (middle) and primers 2 and 4 (bottom). The mice analyzed were A-866, JA-81, JE-101, and JG-42 (all 4 wk old).
Discussion
We previously documented enhancer-dependent VDJ recombination within a human TCR-δ gene minilocus construct in transgenic mice (13, 14), and have now begun to address the mechanisms by which Eδ exerts its effects on VDJ recombination in this system. The data presented here indicates that a mutation that destroys the binding site for the transcription factor CBF/PEBP2 within the δE3 element of Eδ seriously compromises the ability of Eδ to activate the VD to J step of minilocus rearrangement. Hence, by binding to Eδ, this or a very closely related factor plays a crucial role in the developmental activation of minilocus rearrangement in vivo. We interpret the pattern of transgene rearrangement in the presence or absence of a functional Eδ to indicate that a functional Eδ is required to promote the accessibility of Jδ gene segments to the VDJ recombinase within the transgenic minilocus. Although not directly proven, we infer that a functional Eδ is also required to promote Jδ gene segment accessibility, and hence VDJ recombination, within the endogenous TCR-δ locus. Our data therefore suggest strongly that CBF/PEBP2 family transcription factors are likely to be important regulators of TCR-δ gene rearrangement at the endogenous TCR-δ locus in vivo. Nevertheless, formal proof for this notion would require elimination of the Eδ CBF/PEBP2 binding site from the endogenous locus by homologous recombination.
CBF/PEBP2 was initially identified by virtue of its ability to bind to and activate transcription from polyoma virus enhancer and the long terminal repeats of murine T lymphotropic retroviruses (42, 43). Functional CBF/PEBP2 binding sites have been identified in the regulatory elements of several cellular genes expressed in either the T lymphoid or myeloid cell lineages (44–48), including the enhancers of all four TCR genes (30, 33–36). CBF/PEBP2 is actually a complex family of transcription factors, each of which is composed of a DNA-binding α subunit and an associated β subunit (29–31). Three distinct genes (αA, αB, and αC) encode related α subunits (30, 49–52), a separate gene encodes a shared β subunit (29, 31), and additional complexity is introduced by production of multiple α and β isoforms from the individual genes (29–31, 53). Thus, any one of a number of CBF/PEBP2 species might be the crucial regulator of TCR-δ gene rearrangement in vivo. Although recent analysis of CBF/PEBP2 αB null mice emphasizes a crucial role for this particular factor in hematopoiesis, the early lethality and pleiotropic effects of this mutation preclude any specific conclusions regarding a role in TCR-δ gene rearrangement (37). A candidate regulator of TCR-δ gene rearrangement would have to be expressed as early as the CD4−CD8− stage of thymocyte development, since the endogenous TCR-δ locus (54, 55) and our transgenic minilocus (14) are both activated during this stage. Notably, CBF/PEBP2 αA and αB are both expressed at highest levels in the thymus and are both expressed in CD4−CD8− thymocytes (56). Thus, both of these factors have expression patterns that would be consistent with a role in the activation TCR-δ gene rearrangement and expression in vivo.
Related studies using the transgenic minilocus approach have also implicated the transcription factor c-Myb in the activation of TCR-δ gene rearrangement in vivo (39). Hence CBF/PEBP2 and c-Myb appear to synergize to activate TCR-δ gene VDJ recombination in vivo, much as they were found to synergistically activate TCR-δ gene transcription in transient transfection studies (27, 28). Nevertheless, our results indicate that the combination of CBF/PEBP2, Myb, and GATA-3 binding sites within δE3 and δE4 is not, by itself, sufficient to promote the accessibility required for efficient activation of VDJ recombination within the TCR-δ gene minilocus. This suggests that additional cis-acting elements contained within the 1.4-kb Eδ are crucial for this process. Such elements may be contained within the 370-bp fragment of Eδ found to contain maximal enhancer activity in transient transfection experiments. Analyses of truncated forms of the enhancer suggest δE2, δE5, and δE6 as candidate cis-acting elements of Eδ that might individually or in combination increase the activity of the minimal enhancer by two- to threefold (24, 25). Little is known about the identities of the factors that interact with these elements. Nevertheless, it may be misleading to identify candidate determinants of Eδ recombinational enhancer activity in a chromosomally integrated context in transgenic mice by extrapolating from those required for transcriptional enhancer activity in transient transfection experiments. For example, nuclear matrix attachment sites that flank Eμ are irrelevant for transcriptional activity as measured in transient transfection experiments or in chromosomally integrated substrates in stably transfected cells, but are important for the induction of transcriptional activity and general sensitivity to DNase I digestion in a chromosomally integrated substrate in transgenic mice (57). Similarly sequences that flank the human adenosine deaminase gene enhancer are irrelevant for transcriptional activity in transient transfection experiments, but are required for high level expression and the establishment of enhancer DNase I hypersensitivity in transgenic mice (58, 59). It is therefore quite possible that cis-elements contained within the 1.4-kb Eδ might not appear relevant for gene expression on the basis of transient transfection experiments, but might be critical for Eδ induced accessibility and VDJ recombination in transgenic mice. These additional cis-acting elements could be required for the stable assembly of CBF/PEBP2, c-Myb, and GATA-3 onto their δE3 and δE4 binding sites in a chromatin context. Alternatively, δE3 and δE4 might by themselves be able to support the assembly of a stable nucleoprotein complex, but additional cis-acting enhancer elements might contribute independently to accessibility and VDJ recombination. The hierarchy of assembly of nucleoprotein complexes at Eδ, and the mechanisms by which assembled nucleoprotein complexes modulate regional chromatin accessibility and VDJ recombination, will be important issues to address in future studies.
Acknowledgments
We thank Cristina Hernandez-Munain for helpful comments on the manuscript, and Cheryl Bock and Wendy Callahan of the Duke University Comprehensive Cancer Center Transgenic Mouse Shared Resource for production of transgenic mice.
This work was supported by Public Health Service grant GM41052. M.S. Krangel is the recipient of American Cancer Society Faculty Research Award FRA-414. J.L. Roberts was supported in part by Public Health Service training grant CA09058.
Footnotes
1 Abbreviations used in this paper: D, diversity; J, joining; V, variable.
References
- 1.Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science (Wash DC) 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- 2.Alt FW, Oltz EM, Young F, Gorman J, Taccioli G, Chen J. VDJ recombination. Immunol Today. 1992;13:306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- 3.Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 4.Lewis SM. The mechanism of V(D)J joining: lessons from molecular, immunological and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 5.Sleckman BP, Gorman JR, Alt FW. Accessibility control of antigen receptor variable region gene assembly: role of cis-acting elements. Annu Rev Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]
- 6.Fowlkes BJ, Pardoll DM. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- 7.Staudt LM, Lenardo MJ. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- 8.Leiden JM. Transcriptional regulation of T cell receptor genes. Annu Rev Immunol. 1993;11:539–570. doi: 10.1146/annurev.iy.11.040193.002543. [DOI] [PubMed] [Google Scholar]
- 9.Ferrier P, Krippl B, Blackwell TK, Furley AJW, Suh H, Winoto A, Cook WD, Hood L, Costantini F, Alt FW. Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO (Eur Mol Biol Organ) J. 1990;9:117–125. doi: 10.1002/j.1460-2075.1990.tb08087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauster R, Reynaud C-A, Martensson I-L, Peter A, Bucchini D, Jami J, Weill J-C. Promoter, enhancer and silencer elements regulate rearrangement of an immunoglobulin transgene. EMBO (Eur Mol Biol Organ) J. 1993;12:4615–4623. doi: 10.1002/j.1460-2075.1993.tb06150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallenbach S, Babinet C, Pournin S, Cavelier P, Goodhardt M, Rougeon F. The intronic immunoglobulin κ gene enhancer acts independently on rearrangement and on transcription. Eur J Immunol. 1993;23:1917–1921. doi: 10.1002/eji.1830230828. [DOI] [PubMed] [Google Scholar]
- 12.Capone M, Watrin F, Fernex C, Horvat B, Krippl B, Wu L, Scollay R, Ferrier P. TCRβ and TCRα gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO (Eur Mol Biol Organ) J. 1993;12:4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauzurica P, Krangel MS. Enhancer-dependent and -independent steps in the rearrangement of a human T cell receptor δ transgene. J Exp Med. 1994;179:43–55. doi: 10.1084/jem.179.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauzurica P, Krangel MS. Temporal and lineage-specific control of T cell receptor α/δ gene rearrangement by T cell receptor α and δ enhancers. J Exp Med. 1994;179:1913–1921. doi: 10.1084/jem.179.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada A, Mendelsohn M, Alt F. Differential activation of transcription versus recombination of transgenic T cell receptor β variable region gene segments in B and T lineage cells. J Exp Med. 1994;180:261–272. doi: 10.1084/jem.180.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernex C, Capone M, Ferrier P. The V(D)J recombinational and transcriptional activities of the immunoglobulin heavy-chain intronic enhancer can be mediated through distinct protein-binding sites in a transgenic substrate. Mol Cell Biol. 1995;15:3217–3226. doi: 10.1128/mcb.15.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Young F, Bottaro A, Stewart V, Smith RK, Alt FW. Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO (Eur Mol Biol Organ) J. 1993;12:4635–4645. doi: 10.1002/j.1460-2075.1993.tb06152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda S, Zou Y-R, Bluethmann H, Kitamura D, Muller U, Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO (Eur Mol Biol Organ) J. 1993;12:2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serwe M, Sablitzky F. V(D)J recombination in B cells is impaired but not blocked by targeted deletion of the immunoglobulin heavy chain intron enhancer. EMBO (Eur Mol Biol Organ) J. 1993;12:2321–2327. doi: 10.1002/j.1460-2075.1993.tb05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman JR, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt FW. The Igκ 3′ enhancer influences the ration of Igκ versus Igλ B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Davidson L, Alt FW, Baltimore D. Deletion of the Igκ light chain intronic enhancer/matrix attachment region impairs but does not abolish VκJκ rearrangement. Immunity. 1996;4:377–385. doi: 10.1016/s1074-7613(00)80251-4. [DOI] [PubMed] [Google Scholar]
- 22.Bories J-C, Demengeot J, Davidson L, Alt FW. Gene-targeted deletion and replacement of the T-cell receptor β-chain enhancer: the role of enhancer elements in controlling V(D)J recombination accessibility. Proc Natl Acad Sci USA. 1996;93:7871–7876. doi: 10.1073/pnas.93.15.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouvier G, Watrin F, Naspetti M, Verthuy C, Naquet P, Ferrier P. Deletion of the mouse T-cell receptor β gene enhancer blocks αβ T-cell development. Proc Natl Acad Sci USA. 1996;93:7877–7881. doi: 10.1073/pnas.93.15.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redondo JM, Hata S, Brocklehurst C, Krangel MS. A T cell specific transcriptional enhancer within the human T cell receptor δ locus. Science (Wash DC) 1990;247:1225–1229. doi: 10.1126/science.2156339. [DOI] [PubMed] [Google Scholar]
- 25.Redondo JM, Pfohl JL, Krangel MS. Identification of an essential site for transcriptional activation within the human T cell receptor δ enhancer. Mol Cell Biol. 1991;11:5671–5680. doi: 10.1128/mcb.11.11.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redondo JM, Pfohl JL, Hernandez-Munain C, Wang S, Speck NA, Krangel MS. Indistinguishable nuclear factor binding to functional core sites of the T-cell receptor δ and murine leukemia virus enhancers. Mol Cell Biol. 1992;12:4817–4823. doi: 10.1128/mcb.12.11.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez-Munain C, Krangel MS. Regulation of the T-cell receptor δ enhancer by functional cooperation between c-Myb and core-binding factors. Mol Cell Biol. 1994;14:473–483. doi: 10.1128/mcb.14.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Munain C, Krangel MS. c-Myb and core-binding factor (CBF/PEBP2) display functional synergy but bind independently to adjacent sites in the TCR δ enhancer. Mol Cell Biol. 1995;15:3090–3099. doi: 10.1128/mcb.15.6.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Wang Q, Crute BE, Melnikova IN, Keller SR, Speck NA. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993;13:3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa E, Maruyama M, Kagoshima H, Inuzuka I, Lu J, Satake M, Shigesada K, Ito Y. PEBP2/PEA2 represents a new family of transcription factor homologous to the products of the Drosophila runt and the human AML1. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa E, Inuzuka M, Maruyama M, Satake M, Naito-Fujimoto M, Ito Y, Shigesada K. Molecular cloning and characterization of PEBP2β, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2α. Virol. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 32.Shen-Ong GLC. The myb oncogene. Biochim Biophys Acta. 1990;1032:39–52. doi: 10.1016/0304-419x(90)90011-o. [DOI] [PubMed] [Google Scholar]
- 33.Prosser HM, Wotton D, Gegonne A, Ghysdael J, Wang S, Speck NA, Owen MJ. A phorbol ester response element within the human T-cell receptor β-chain enhancer. Proc Natl Acad Sci USA. 1992;89:9934–9938. doi: 10.1073/pnas.89.20.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiang YH, Spencer D, Wang S, Speck NA, Raulet DH. The role of viral enhancer “core” motif-related sequences in regulating T cell receptor-γ and -δ gene expression. J Immunol. 1993;150:3905–3916. [PubMed] [Google Scholar]
- 35.Wotton D, Ghysdael J, Wang S, Speck NA, Owen MJ. Cooperative binding of Ets-1 and core binding factor to DNA. Mol Cell Biol. 1994;14:840–850. doi: 10.1128/mcb.14.1.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giese K, Kingsley C, Kirshner JR, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and mutliple protein-protein interactions. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 37.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 38.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene (Amst) 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Munain C, Lauzurica P, Krangel MS. Regulation of T cell receptor δ gene rearrangement by c-Myb. J Exp Med. 1996;183:289–293. doi: 10.1084/jem.183.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joulin V, Bories D, Eleouet J-F, Labasteie M-C, Chretien S, Mattei M-G, Romeo PH. A T-cell specific TCR δ DNA binding protein is a member of the human GATA family. EMBO (Eur Mol Biol Organ) J. 1991;10:1809–1816. doi: 10.1002/j.1460-2075.1991.tb07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marine J, Winoto A. The human enhancerbinding protein Gata3 binds to several T-cell receptor regulatory elements. Proc Natl Acad Sci USA. 1991;88:7284–7288. doi: 10.1073/pnas.88.16.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990;64:4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Speck NA. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992;12:89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzow J, Friedman AD. The murine myeloperoxidase promoter contains several functional elements, one of which binds a cell type-restricted transcription factor, myeloid nuclear factor 1 (MyNF1) Mol Cell Biol. 1993;13:2141–2151. doi: 10.1128/mcb.13.4.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuchprayoon I, Meyers S, Scott LM, Suzow J, Hiebert S, Friedman AD. PEBP2/CBF, the murine homolog of the human myeloid AML1 and PEBP2β/CBFβ proto-oncoproteins, regulates the murine myeloperoxidase and neutrophil elastase genes in immature myeloid cells. Mol Cell Biol. 1994;14:5558–5568. doi: 10.1128/mcb.14.8.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D-E, Fujioka K-I, Hetherington CJ, Shapiro LH, Chen H-M, Look AT, Tenen GT. Identification of a region which directs the monocytic activity of the colony-stimulating factor 1 (macrophage colony-stimulating factor) receptor promoter and binds PEBP2/CBF (AML1) Mol Cell Biol. 1994;14:8085–8095. doi: 10.1128/mcb.14.12.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron S, Taylor DS, TePas EC, Speck NA, Mathey-Prevot B. Identification of a critical regulatory site in the human interleukin-3 promoter by in vivo footprinting. Blood. 1994;83:2851–2859. [PubMed] [Google Scholar]
- 48.Takahashi A, Satake M, Yamaguchi-Iwai Y, Bae S-C, Lu J, Maruyama M, Zhang YW, Oka H, Arai N, Aria K, et al. Positive and negative regulation of granulocytemacrophage colony-stimulating factor promoter activity by AML1-related transcription factor, PEBP2. Blood. 1995;86:607–616. [PubMed] [Google Scholar]
- 49.Bae SC, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Isolation of PEBP2αB cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993;8:809–814. [PubMed] [Google Scholar]
- 50.Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. AML1, AML2, and AML3, the human members of the runt domain gene family: cDNA structure, expression, and chromosomal localization. Genomics. 1994;23:425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- 51.Bae S-C, Takahashi E, Zhang YW, Ogawa E, Shigesada K, Namba Y, Satake M, Ito Y. Cloning, mapping and expression of PEBP2αC, a third gene encoding the mammalian Runt domain. Gene (Amst) 1995;159:245–248. doi: 10.1016/0378-1119(95)00060-j. [DOI] [PubMed] [Google Scholar]
- 52.Wijmenga C, Speck NA, Dracopoli NC, Hofker MH, Liu P, Collins FS. Identification of a new murine runt domain-containing gene, Cbfa3, and localization of the human homolog, CBFA3, to chromosome 1p35-pter. Genomics. 1995;26:611–614. doi: 10.1016/0888-7543(95)80185-o. [DOI] [PubMed] [Google Scholar]
- 53.Bae S-C, Ogawa E, Maruyama M, Oka H, Satake M, Shigesada K, Jenkins NA, Gilbert DJ, Copeland NG, Ito Y. PEBP2 B/mouse AML1 consist of multiple isoforms that possess differential transactivation potentials. Mol Cell Biol. 1994;14:3242–3252. doi: 10.1128/mcb.14.5.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chien Y-H, Iwashima M, Wettstein DA, Kaplan KB, Elliot JF, Born W, Davis MM. T-cell receptor δ gene rearrangements in early thymocytes. Nature (Lond) 1987;330:722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- 55.Wilson A, Held W, MacDonald HR. Two waves of recombinase gene expression in developing thymocytes. J Exp Med. 1994;179:1355–1360. doi: 10.1084/jem.179.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satake M, Nomura S, Yamaguchi-Iwai Y, Takahama Y, Hashimoto Y, Niki M, Kitamura Y, Ito Y. Expression of the runt domain-encoding PEBP2α genes in T cells during thymic development. Mol Cell Biol. 1995;15:1662–1670. doi: 10.1128/mcb.15.3.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forrester WC, van Genderen C, Jenuwein T, Grosschedl R. Dependence of enhancer-mediated transcription of the immunoglobulin μ gene on nuclear matrix attachment regions. Science (Wash DC) 1994;265:1221–1225. doi: 10.1126/science.8066460. [DOI] [PubMed] [Google Scholar]
- 58.Aronow BJ, Silbiger RN, Dusing MR, Stock JL, Yager KL, Potter SS, Hutton JJ, Wiginton DA. Functional analysis of the human adenosine deaminase gene thymic regulatory region and its ability to generate positionindependent transgene expression. Mol Cell Biol. 1992;12:4170–4185. doi: 10.1128/mcb.12.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aronow BJ, Ebert CA, Valerius MT, Potter SS, Wiginton DA, Witte DP, Hutton JJ. Dissecting a locus control region: facilitation of enhancer function by extended enhancer-flanking sequences. Mol Cell Biol. 1995;15:1123–1135. doi: 10.1128/mcb.15.2.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]