Abstract
Cholera toxin (CT), the most commonly used mucosal adjuvant in experimental animals, is unsuitable for humans because of potent diarrhea-inducing properties. We have constructed two CT-A subunit mutants, e.g., serine→ phenylalanine at position 61 (S61F), and glutamic acid→ lysine at 112 (E112K) by site-directed mutagenesis. Neither mutant CT (mCT), in contrast to native CT (nCT), induced adenosine diphosphate-ribosylation, cyclic adenosine monophosphate formation, or fluid accumulation in ligated mouse ileal loops. Both mCTs retained adjuvant properties, since mice given ovalbumin (OVA) subcutaneously with mCTs or nCT, but not OVA alone developed high-titered serum anti-OVA immunoglobulin G (IgG) antibodies (Abs) which were largely of IgG1 and IgG2b subclasses. Although nCT induced brisk IgE Ab responses, both mCTs elicited lower anti-OVA IgE Abs. OVA-specific CD4+ T cells were induced by nCT and by mCTs, and quantitative analysis of secreted cytokines and mRNA revealed a T helper cell 2 (Th2)-type response. These results now show that the toxic properties of CT can be separated from adjuvanticity, and the mCTs induce Ab responses via a Th2 cell pathway.
The major enterotoxins produced by Vibrio cholerae and Escherichia coli, termed cholera toxin (CT)1 and heatlabile toxin (LT), respectively, are multisubunit macromolecules composed of two structurally, functionally, and immunologically separate A and B subunits (1–3). The B subunit of each toxin consists of five identical 11.6-kD peptides, but differ from each other in that the B subunit of CT (CT-B) only binds to GM1 ganglioside (4), whereas the B subunit of LT (LT-B) binds GM1 as well as asialo GM1 and GM2 (5). After the B subunit binds to epithelial cell GM1 or GM2 receptors, the A subunit reaches the cytosol, and after activation, binds to nicotinamide adenosine diphosphate and catalyzes ADP ribosylation of Gs-α (6). This GTP-binding protein activates adenyl cyclase with subsequent elevation of cAMP, which in epithelial cells results in secretion of water and chloride ions into the small intestine (7).
Both CT and LT are immunogenic and mucosal exposure results in secretory IgA (S-IgA) and serum Abs, which are almost entirely restricted to CT-B or LT-B (8, 9). Further, both toxins are also strong mucosal adjuvants for coadministered, unrelated protein Ags when given by oral, intranasal, or parenteral routes (8–10). We have shown that induction of maximal mucosal S-IgA and serum IgG Ab responses correlated directly with Ag-specific CD4+ Th cells secreting IL-4 and IL-5 in mice orally immunized with protein Ag and CT as adjuvant (10). Further, detailed analysis showed that CT elicits adjuvant responses by inducing Ag-specific CD4+ Th2-type cells which produce high levels of IL-4 and IL-5, responsible for supporting subsequent development of systemic IgG1 and IgG2b subclass, IgE and S-IgA Ab responses (11). On the other hand, oral immunization with LT promotes IgM, IgG1, IgG2a, IgG2b, and S-IgA Ab responses, which are supported by a mixed CD4+ Th1- and Th2-type response associated with IFN-γ, IL-4, IL-5, IL-6, and IL-10 production (12). Furthermore, when IL-4 levels produced by CD4+ T cells were compared when CT or LT were used as adjuvants, Ag-specific IL-4 production was lower when LT was used (11, 12).
Earlier studies have attempted to dissociate toxicity from adjuvanticity of CT and LT; however, it was shown that a mutant LT toxin, E112K, which had single amino acid substitution in the ADP-ribosyltransferase active center, was nontoxic and also lacked adjuvanticity (13). Recently, two groups have reported that single amino acid substitution mutants of LT, R7K, and R192G, which are outside of the ADP-ribosyltransferase cleft, were nontoxic, but retained adjuvant properties (14, 15). However, one of these LT mutants retained some ADP-ribosyltransferase activity and could potentially cause diarrhea in humans.
To develop an ideal nontoxic mucosal adjuvant, we constructed two CT mutants, S61F and E112K, by site-directed mutagenesis, which have single amino acid substitutions in the ADP-ribosyltransferase active center (16). Neither mutant showed toxic activity, and in the present study these mutants were used to assess adjuvant properties as well as signaling pathways involved in adjuvanticity.
Materials and Methods
Construction and Purification of CT Mutants.
A 3.1-kb EcoRI/ PstI DNA fragment including the CT gene (17) from V. cholerae O1 strain GP14 was cloned into phage M13mp19. Single strand DNA was prepared from a culture supernatant of E. coli CJ236 transfected with M13mp19 including the CT gene and was subjected to a site-directed mutagenesis system using Mutan K (Takara Biomedicals, Kyoto, Japan) as described previously (18). The sequences of oligonucleotides used for the serine to phenylalanine substitution at position 61 (S61F) and for the glutamate to lysine mutation at position 112 (E112K) were 5′-GGATATGTTTTTACCTCAATT-3′ and 5′-GATGAACAAAAAGTTTCTGCT-3′, respectively. The amino acid mutation sites (i.e., 61 and 112) are both in the CT-A subunit, located in the proposed ADP-ribosyltransferase active center of CT (16), and substitution of these amino acids in LT have been shown to completely inactivate ADP-ribosyltransferase activity and enterotoxicity (19, 20). After the DNA sequences were confirmed, pUC119 harboring the mutated CT genes at the EcoRI/PstI site were transformed into E. coli DH5-α. E. coli strains containing the plasmids for the mutant CT genes were grown in LB medium (10 g NaCl, 10 g tryptone, and 5 g yeast extract/liter) with 100 μg/ml of ampicillin, and CT mutants were purified using a D-galactose immobilized column (Pierce Chem. Co., Rockford, IL) from a cell suspension prepared by sonication. A plasmid containing the recombinant CT-B (rCT-B) gene (21) was provided by Dr. Charles O. Elson (University of Alabama at Birmingham, Birmingham, AL) and CT-B was also purified by use of a D-galactose immobilized column.
Biologic, Enzymatic, and Toxicity Assays of Mutant CTs.
The ability of CT mutants (mCTs) and native CT (nCT) to induce toxic effects in cultured Chinese hamster ovary (CHO) cells was done as previously described (22). In brief, log10 dilutions of each toxin were added to CHO cell cultures (2 × 105 cells/0.5 ml of F10 medium containing 1% FCS) and cultured at 37°C in 5% CO2 for 24 h. Toxicicty was defined as spindle formation in >20% of cell cultures. For cAMP assessment, 106 CHO cells in F10 medium containing 1% FCS were cultured with 1 ng/ml of mCTs or nCT for 24 h as described above. The cellular protein precipitated with 5% trichloroacetic acid was dissolved in 0.2 N NaOH and the protein amount was determined (BioRad Labs., Hercules, CA). The supernatants were assessed for cAMP with an enzyme immunoassay system (Amersham Intl., Buckinghamshire, UK), and the levels of cAMP were expressed as pmol of cAMP/mg of protein.
ADP-ribosyltransferase Activity of mCTs.
The CT-A–catalyzed transfer of ADP-ribose from nicotinamide adenosine diphosphate to agmatine was done precisely as described previously (23). In brief, each assay tube contained 10 μg of mCTs or nCT in a total volume of 300 μl and a 50 μl aliquot of the assay mixture was assessed for radioactive ADP-ribosylated agmatine by liquid scintillation counting.
Assessment of Toxicity Using Mouse Ileal Loops.
The enterotoxicity of mCTs and nCT was examined using a mouse ileal loop test (24). Groups of mice were anesthetized, and 100 μl of PBS containing different doses of each toxin were injected into a 2-cm ileal loop, which was isolated by suture. The mice were killed 18 h after the injection, and the ratio of fluid to length was determined and defined as positive when the ratio was more than 40 μl/cm.
Mice and Their Immunization.
C57BL/6 mice were obtained from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, MD) at 5–6 wk of age, and were used at 8–12 wk of age. Mice were immunized subcutaneously with 100 μg of OVA (Sigma Chemical Co., St. Louis, MO) alone or together with 10 μg of mCTs (S61F or E112K), with 1 μg of nCT (List Biological Labs., Campbell, CA), or with 10 μg of rCT-B on days 0 and 14.
Detection of Ag-specific Abs by ELISA and Antibody-forming Cells by Enzyme-linked Immunospot (ELISPOT) Assay.
Ab titers in serum were determined by ELISA and splenic antibody-forming cells (AFCs) by enzyme-linked immunospot (ELISPOT) assay as described previously (10, 11). Endpoint titers determined by ELISA were expressed as the reciprocal log2 of the last dilution giving an OD450 of ⩾0.1 above unimmunized controls. In the ELISPOT assay, the AFCs were determined by direct counting of spots as described before (10, 11).
IgE Analysis.
Total IgE levels were determined by ELISA as described previously (11). Ag-specific IgE was detected by a modified IgE capture method (25). In brief, 96-well microplates (Dynatech Labs, Inc., Chantilly, VA) were coated with 1 μg/ml of rat anti–mouse IgE mAb (PharMingen, San Diego, CA) in 50 mM carbonate-bicarbonate buffer (pH 9.5). After blocking with 3% BSA-PBS, serial dilutions of serum were added. After incubation and washing, 2.5 μg/ml of biotinylated OVA or 1.5 μg/ml of CT-B (prepared as described previously [25]) were added in 3% BSA-PBS-Tween 20. The plates were then washed with 2 mM EGTA-PBS-Tween 20 and incubated with 10 ng/ml streptaequorin (SeaLite Sciences, Norcross, GA) in 2 mM EGTA-PBSTween 20. Light development was carried out in a luminometer (ML-3000; Dynatech Labs., Inc.) by injection of Ca2+ buffer (50 mM Tris, 20 mM calcium acetate, pH 7.5) (26). Endpoint titers were determined as the dilution of each sample showing a twofold higher level of luminometric units above background.
OVA- and CT-B–specific Splenic CD4+ T Cell Responses.
Single spleen cell suspensions in complete RPMI 1640 medium were fractionated on a nylon wool column for 1 h at 37°C to remove adherent cells. The CD4+ T cell subset (>98% purity) was then obtained by positive sorting using a magnetic bead–activated cell separation system (Miltenyi Biotec Inc., Sunnyvale, CA) using biotinylated anti-CD4 mAb (GK1.5) and streptavidin-coated microbeads (Miltenyi Biotec Inc.). Purified splenic CD4+ T cells were cultured at a density of 2 × 106 cells/ml with 1 mg/ml of OVA or with 107 CT-B–coated beads/ml, T cell–depleted, irradiated (3,000 rads) splenic feeder cells (2.5 × 106 cells/ml), and IL-2 (10 U/ml; PharMingen) in complete medium (10, 11). As positive controls, CD4+ T cells from nonimmunized mice were stimulated with a solid-phase anti–mouse CD3 mAb (145-2C11). To measure cell proliferation, 0.5 μCi of [methyl-3H]thymidine (Du Pont New England Nuclear Products, Boston, MA) was added to individual culture wells 18 h before termination, the cells were harvested, and the radioactivity was assessed by liquid scintillation counting after 96 h of culture. To determine cytokine production by Ag-specific CD4+ T cells, the cells were harvested after 48 h of culture for quantitative reverse transcriptase (RT)–PCR analysis of cytokine-specific mRNA and the supernatants were collected after 96 h for evaluation of cytokines by ELISA. For IL-2, supernatants from 48-h cultures were used, since this represented the interval for maximal production of this cytokine.
Cytokine Analysis by ELISA.
Cytokines in culture supernatants were determined by a modified ELISA (11). Nunc-ImmunoMaxiSorpTM plates were coated with 2.5 μg/ml of anti–mouse IFN-γ, IL-2, IL-4, IL-5, IL-6, or IL-10 mAb (PharMingen). For secondary Abs and detection enzyme, 0.2 μg/ml of biotinylated rat anti–mouse cytokine mAb (PharMingen) and 1:4,000 diluted horseradish peroxidase–labeled antibiotin (Vector Labs., Inc., Burlingame, CA) were used, respectively.
Quantitative RT-PCR Analysis of Cytokine-specific mRNA.
Quantitative cytokine-specific RT-PCR using rRNA internal standards was conducted as described previously (11, 27, 28). Cytokinespecific rRNA for IFN-γ, IL-2, IL-4, IL-5, IL-6, or IL-10 were used as internal standards (28). For quantitation, aliquots of total RNA were added with a series of diluted rRNA internal standards (11, 28) and standard RT-PCR was performed as described elsewhere (27). Analysis of PCR products was conducted by capillary electrophoresis with a laser-induced fluorescence detection system (LIF-P/ACE; Beckman Instrs. Inc., Fullerton, CA) (27).
Statistical Analysis.
The results are expressed as the mean ± SEM. Statistical significance (P <0.05) was determined by Student's t test and by the Mann-Whitney U test of unpaired samples.
Results
Enzymatic Properties and Toxicity of mCTs.
A tandem of biologic functions of mCTs was done to compare their enzymatic properties with nCT. As expected, as little as 1.0 pg/ml of nCT induced spindle cell formation in CHO cell cultures, a response previously shown to be dependent upon adenyl cyclase–mediated increases in cAMP (22). However, neither mCT affected the appearance of CHO cells, even at levels of 1.0 μg/ml. These results were confirmed by direct measurement of intracellular cAMP levels in CHO cells, which were sharply increased in nCT-treated, but not in mCT-treated cultures (Table 1). Quantitative analysis of ADP-ribosyltransferase activity was assessed, and increased enzymatic activity was again associated with nCT but not with mCTs (Table 1). The toxicity of mCTs and nCT was also assessed in a mouse ileal loop assay, where as little as 100 ng of nCT induced significant fluid accumulation in ligated loops, while 1,000-fold higher levels (100 μg) of mCTs were nontoxic (Table 1).
Table 1.
Comparison of Biologic, Enzymatic, and Toxic Activity of mCTs and nCT
| Adjuvant assessed | CHO assay* | cAMP induction‡ | ADP- ribosyltransferase activity§ | Ileal loop test‖ | ||||
|---|---|---|---|---|---|---|---|---|
| pg/ml | pmol/mg | cpm | ng | |||||
| nCT | 1 | 739 ± 127 | 4,669 ± 256 | 100 | ||||
| S61F | >106 | 8.3 ± 1.8 | 93 ± 12 | >105 | ||||
| E112K | >106 | 6.2 ± 2.2 | 98 ± 15 | >105 | ||||
| PBS | — | 9.7 ± 2.2 | 98 ± 6 | — |
CHO cells were cultured in tissue culture chamber with log10 dilutions of each toxin for 24 h and the toxic effects were defined as spindle formation in >20% of cultured cells.
CHO cells were cultured with 1 ng/ml of each toxin for 24 h and cAMP assessed by an enzyme immunoassay system. The protein in 5% trichloroacetic acid pricipitates was determined and concentrations of cAMP were expressed as the mean pmol of cAMP per mg of protein ± SEM of three samples. The results are representative of three experiments.
The radioactivity of ADP-ribosylated agmatine induced by mCTs or nCT in a 50-μl aliquot of the assay mixture was expressed as the mean cpm ± SEM of six samples. The results are representative of three separate experiments.
The enterotoxicity of mCTs and nCT was examined using an ileal loop test, where mice were anesthetized, and 100 μl of PBS containing different levels of each toxin were injected into a 2-cm ileal loop. Loops were examined 18 h later and the ratio of fluid to length was defined as positive when the ratio was >40 μl/cm.
Adjuvant Properties of mCTs.
To assess the immunologic properties of mCTs, groups of mice were immunized with OVA combined with each mCT or with nCT as a control. Immunization with OVA alone did not result in significant anti-OVA Ab responses, and admixture of OVA with rCT-B also failed to support anti-OVA Ab responses; however, both mCTs and nCT enhanced serum anti-OVA Abs and these responses were mainly of the IgG isotype (Fig. 1 A). Further, anti-OVA Abs were largely restricted to IgG1 with less IgG2b subclass response (Fig. 1 B). Significant numbers of splenic OVA-specific IgG AFCs were noted in mice given OVA combined with mCTs or with nCT, whereas low numbers of AFCs were observed in mice given OVA alone or OVA with rCT-B (Fig. 1 C). Thus, both mCTs induced an Ab pattern remarkably similar to the serum Ab responses which resulted from use of nCT as adjuvant. Mice immunized with OVA and mCTs or nCT as adjuvant also showed significant CT-B–specific IgG responses. Although IgG anti–CT-B responses were also seen in mice given rCT-B, the titers were ∼100-fold lower than seen when either mCTs or nCT were given (Fig. 1 A). In addition, mCTs and nCT induced high levels of CT-B–specific IgG1 and IgG2b Abs (Fig. 1 B). Large numbers of CT-B–specific IgG AFCs were present in splenic cells from mice immunized with mCTs or with nCT, whereas much lower numbers of AFCs were seen in mice immunized with rCT-B (Fig. 1 C).
Figure 1.
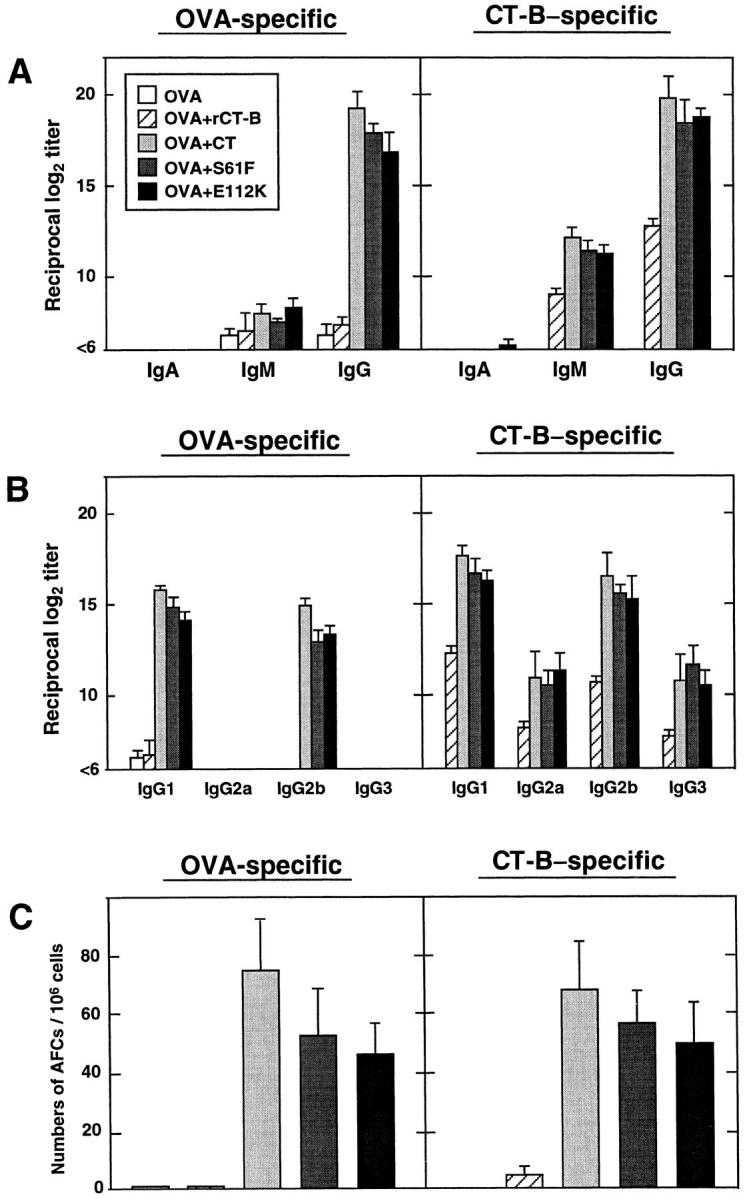
OVA- and CT-B–specific Ab responses after subcutaneous immunization with OVA combined with mCTs or nCT as adjuvants. Serum IgM, IgG, and IgA responses (A) and IgG subclass responses (B) were assessed by endpoint ELISA. Splenic Ag-specific AFCs (C) were determined by ELISPOT assay. Groups of C57BL/6 mice were immunized subcutaneously with 100 μg of OVA alone or together with 10 μg of rCT-B, 1 μg of nCT, or 10 μg of mCTs, S61F, or E112K, on days 0 and 14. All assays were performed on samples from mice taken 1 wk after the last immunization. Bars represent the mean Ab titer and mean number of AFCs ± SEM in each group of 10 mice and the data are representative of three separate experiments.
IgE Responses by mCTs.
Past studies have shown that CT induces marked increases in both total and Ag-specific IgE Abs after mucosal immunization (11). We have used two sensitive assays to detect increased total serum IgE as well as Ag-specific IgE Abs in mice given OVA combined with mCTs or with nCT as adjuvant. In these studies, maximum IgE responses peaked by 3 wk, and although differences in total IgE levels were not significant in mice given mCTs or nCT (Table 2), anti-OVA IgE titers were lower in mice given mCT S61F (P <0.05), whereas CTB–specific IgE Abs were depressed in mice given mCTs S61F or E112K when compared with nCT (P <0.01) (Table 2). In mice given OVA alone or OVA plus rCT-B, neither total nor Ag-specific IgE responses were noted.
Table 2.
IgE Responses Induced by mCTs and nCT
| Treatment group* | Total IgE‡ | Ag specific-IgE (reciprocal log2 titer)‡ | ||||
|---|---|---|---|---|---|---|
| OVA | CT-B | |||||
| ng/ml | ||||||
| OVA alone | 128 ± 32 | 3.5 ± 0.9 | <3 | |||
| OVA + rCT-B | 134 ± 46 | 3.6 ± 1.1 | <3 | |||
| OVA + nCT | 1,408 ± 402 | 9.4 ± 0.7 | 5.5 ± 0.8 | |||
| OVA + S61F | 1,267 ± 416 | 7.3 ± 0.9§ | 3.1 ± 0.3‖ | |||
| OVA + E112K | 1,086 ± 313 | 7.8 ± 0.7 | <3‖ | |||
Mice were immunized subcutaneously with 100 μg of OVA alone or together with 10 μg of rCT-B, 1 μg of nCT, or 10 μg of mCTs, S61F, or E112K, on days 0 and 14. Each group contained 10 mice. The results are representative of three separate experiments.
IgE responses on day 21 were determined by ELISA ( total) and luminometric assay (Ag-specific).
Significantly lower (P <0.05 when compared with nCT).
Significantly lower (P <0.01 when compared with nCT).
Mutant CTs, like nCT, Induce CD4+ Th2 Responses.
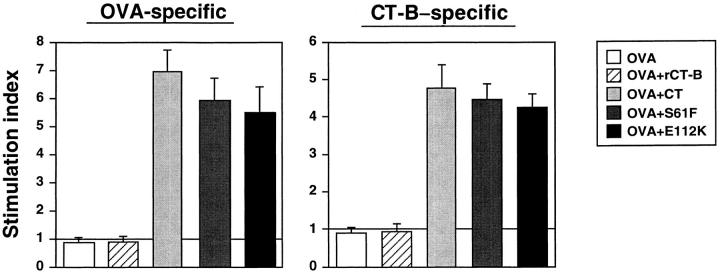
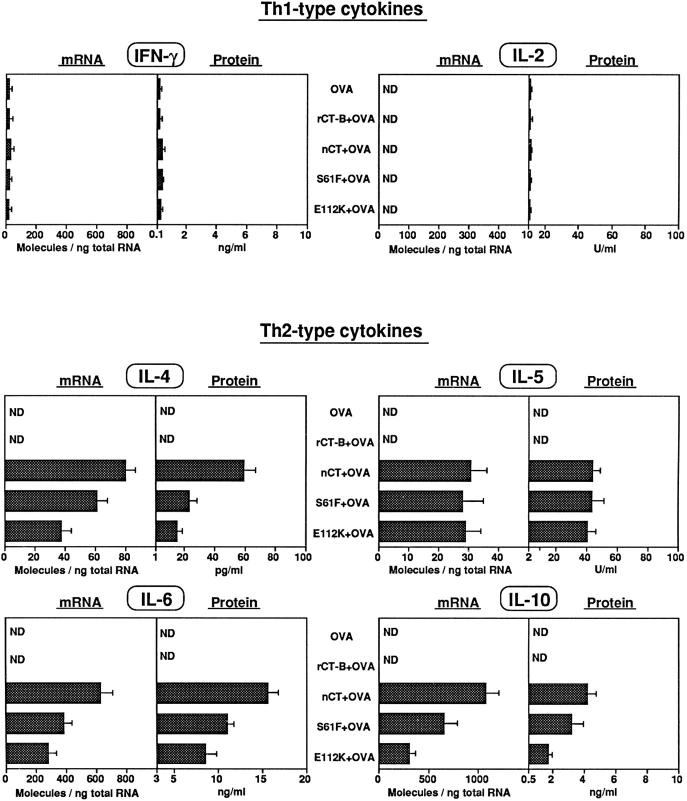
Mucosal administration of CT with protein Ags induces Ag-specific CD4+ T cell responses with an overall Th2 phenotype (10, 11). Of interest was our finding that both mCTs also induced OVA-specific CD4+ T cell proliferative responses, which were comparable to those seen in mice given nCT as adjuvant (Fig. 2). Moreover, splenic OVA-specific CD4+ T cells from mice given OVA together with mCTs produced high levels of Th2-type cytokines (IL-4, IL-5, IL-6, and IL-10), which were comparable to those seen when nCT was used as adjuvant (Fig. 3). On the other hand, CD4+ T cells from mice given OVA alone or OVA plus rCT-B did not produce detectable levels of cytokines other than IFN-γ when stimulated with OVA. Abundant Th2-type cytokine-specific mRNA was present in OVA-specific CD4+ T cell cultures taken from mice given OVA combined with mCTs or with nCT, but was not detected in CD4+ T cells from mice given OVA alone or OVA plus rCT-B (Fig. 3). Further, IFN-γ was detected at low levels in all cultures including OVA-stimulated controls from unimmunized mice. Splenic CT-B–specific CD4+ T cells from mice given mCTs or nCT also demonstrated significant proliferation (Fig. 2) and high levels of Th2-type with low but detectable levels of Th1-type cytokines (data not shown). The quantitative RT-PCR results together with levels of secreted cytokines were consistent with our previous studies (10) which showed that nCT induces CD4+ Th2-type responses.
Figure 2.
OVA- and CTB–specific CD4+ T cell proliferative responses. Groups of C57BL/6 mice were immunized subcutaneously with 100 μg of OVA alone or together with 10 μg of rCT-B, 1 μg of nCT, or 10 μg of mCTs, S61F, or E112K, on days 0 and 14. Purified splenic CD4+ T cells were cultured at a density of 2 × 106 cells/ml in the presence of 1 mg/ml of OVA or 107 CT-B–coated beads/ml, T cell–depleted, irradiated splenic feeder cells (2.5 × 106 cells/ml) and IL-2 (10 U/ml) in complete medium. Bars represent the mean stimulation index ± SEM in each group. Each group contained 10 mice and are representative of three separate experiments.
Figure 3.
Cytokine production from OVA-specific splenic CD4+ T cells. Molecules of cytokine-specific mRNA were determined by quantitative RT-PCR using rRNA internal standards. Cytokine production was determined by ELISA. The scale of each figure corresponds to mRNA molecules and protein levels produced by nonimmunized CD4+ T cells stimulated with anti-CD3 mAb. Bars represent the mean cytokine profile ± SEM in each group. ND indicates that the molecules were not detected. Each group contained five mice and are representative of three separate experiments.
Discussion
The present study shows that enzyme and toxic functions induced by CT could be eliminated through derivation of mutants with single amino acid substitutions, presumably in or closely associated with the ADP-ribosyltransferase cleft of the A subunit (16). In particular, two mutants S61F and E112K, were shown to (a) be devoid of ADP-ribosyltransferase activity, (b) be unable to induce increases in intracellular cAMP, and (c) fail to elicit fluid accumulation in mouse-ligated ileal loops. Even more importantly, the mCTs retained their ability to boost Ab responses to the coadministered protein OVA as well as enhance Ab responses to the molecule itself, e.g., to CT-B. These adjuvant effects of mCTs were due to the A subunit portion of the molecule, since coadministration of rCT-B at much higher concentrations with OVA did not enhance antiOVA Ab responses. Furthermore, both mCTs induced significant OVA-specific CD4+ T cell proliferative responses, with subsequent production of IL-4, IL-5, IL-6, and IL-10 (Th2-type) that were comparable to a Th2-type response seen when nCT was used as adjuvant. These findings have important implications, since we have shown that CT elicits adjuvanticity by induction of Ag-specific CD4+ Th cells secreting Th2-type cytokines, which in turn provide B cell help for serum IgG1, IgG2b, IgA, and IgE and mucosal S-IgA Ab responses (10, 11). Thus, we conclude from these results that both mCTs and nCT act as adjuvants by the same mechanism to enhance the immunogenicity of a coadministered, unrelated protein, and that this pathway is independent of ADP-ribosyltransferase activity.
Several groups have studied potential mechanisms for the adjuvant effects induced by CT and LT, and studies have also focused on separating the ADP-ribosyltransferase function from adjuvanticity, e.g., removal of toxic effects while retaining the beneficial effects of the molecule. Since the A subunit is responsible for toxicity, several studies have suggested that CT-B can be used as adjuvant. Although CT-B alone appeared to have adjuvant properties in some studies (29, 30), CT-B prepared from holotoxin generally contains a small amount of CT-A, which is sufficient to enhance the immune responses (31). Others have assessed the adjuvanticity of an LT mutant without ADP-ribosyltransferase activity (E112K) and rCT-B (13), and their results showed that neither mutant nor rCT-B could enhance the immune response to a coadministered protein. This led to the conclusion that ADP-ribosyltransferase activity was essential for adjuvanticity of both LT and CT (13).
In vitro studies in a variety of cells, e.g., B cells (32), T cell lines (33, 34), macrophages (35), and epithelial cells (36), were used to assess the potential mechanism whereby CT and LT enhance the immune response. In most of these studies, it was concluded that adjuvanticity of CT resulted from the ADP-ribosyltransferase activity, i.e., induction of increased intracellular cAMP formation. In one study, CT as well as forskolin was shown to inhibit T cell receptor– mediated IL-2 production and proliferation in cloned Th1 cells, but not IL-4 production and proliferation in a clone of Th2 cells, indicating that Th1 and Th2 cells differ in their sensitivity to increases in intracellular cAMP (34). However, based on our results, mCTs retained adjuvanticity despite a lack of ADP-ribosyltransferase activity. Furthermore, the IgG subclass Ab responses induced by mCTs were largely IgG1 and IgG2b, and OVA-specific CD4+ T cells from mice given mCT were of the Th2 type, a pattern essentially identical to those results obtained with nCT. Thus, in marked contrast to previous observations, our findings indicate that adjuvanticity of CT can be dissociated from ADP-ribosyltransferase activity and enterotoxicity, and the CD4+ Th2-type T cell responses induced by CT are due to a pathway separate from the adenyl cyclase system.
An interesting aspect of this study was that the mCT E112K exhibited significant adjuvant activity, whereas an LT mutant E112K was not effective (13). Although CT and LT share a significant degree of homology (∼80% amino acid sequence homology; 1) and some antibodies induced to CT-B cross-react with LT-B and vice versa, significant differences exist. Although CT and LT are both potent adjuvants, the molecules differ in terms of the nature of CD4+ Th subsets induced and the profile, isotype, and subclass of Abs induced. For example, CT elicits adjuvanticity by promoting Ag-specific CD4+ Th2-type responses associated with high levels of IL-4 and IL-5 production with provision of help for IgG1 subclass, IgE and S-IgA responses (10, 11), whereas LT promotes both Th1- and Th2-type responses with high levels of IFN-γ and IL-5 production and subsequent IgG1, IgG2a, IgG2b subclass and S-IgA Ab responses (12). Furthermore, oral administration of CT as adjuvant failed to enhance Ag-specific S-IgA responses in IL-4 gene disrupted (IL-4−/−) mice (37), whereas LT was able to induce Ag-specific mucosal S-IgA as well as serum IgG responses in both IL-4−/− and IL-4+/+ mice (our unpublished data). These differences cannot be ascribed to ADP-ribosyltransferase activity which both molecules share.
The finding that 10-fold higher doses of mCTs were required to elicit comparable adjuvant responses as nCT also merits discussion. Two broad possibilities could explain these differences. First, the site-directed mutagenesis step may have caused a conformational change in the actual site responsible for adjuvanticity of the CT molecule. If this is correct, then the responsible site for adjuvanticity may be located close to the ADP-ribosyltransferase active center (16). The second possibility is that adjuvanticity of CT may be derived from two (or more) mechanisms, which include ADPribosyltransferase activity, since several reports have shown that intracellular cAMP accumulation after ADP-ribosylation activates T cell (33) and epithelial cell lines (36). Of importance to this point is that in most in vitro studies, CT has been shown to inhibit mitogen-, Ag-, or anti-CD3– induced T cell proliferative responses (38), whereas in vivo studies have shown that CT induces strong Ag-specific CD4+ T cell responses which provide effective help for Ab responses (10, 11). Thus, in vitro studies may result in direct inhibition of signal transduction pathways for T cells, whereas CT in vivo may transduce activation pathways that include the induction of CD4+ Th2-type responses.
In summary, this study has shown that the newly developed mCTs, S61F, and E112K, retain adjuvanticity despite a complete lack of ADP-ribosyltransferase activity and subsequent enterotoxicity. Further, mCTs induced CD4+ Th2-type responses, while Ag-specific IgE Abs in the serum of mice immunized with OVA plus mCTs were lower than those induced by nCT. These findings indicate that these mCTs may have significant advantages when used as adjuvants. Our results not only show that adjuvanticity of CT can be separated from ADP-ribosytransferase activity and enterotoxicity, but that these mCTs could be ideal candidates for a nontoxic mucosal adjuvant. We are currently assessing the mucosal adjuvanticity of these mCTs.
Acknowledgments
We thank Drs. Masami Miyake and Daisuke Matsuura for the assays of ADP-ribosyltransferase activity, Dr. Prosper Boyaka for help with the ileal loop test, and Ms. Sheila D. Turner for preparation of the manuscript and figures.
This study was supported by National Institutes of Health (NIH) grants AI 18958, DK 44240, DE 04217, AI 35932, DE 09837, and AI 35544, NIH contracts N01 AI 65298 and N01 AI 65299, as well as by grants from Japanese Ministry of Education, Science, Sports and Culture, and the Ministry of Health and Welfare.
Footnotes
1 Abbreviations used in this paper: AFC, antibody-forming cell; CHO, Chinese hamster ovary; CT, cholera toxin; ELISPOT, enzyme-linked immunospot; LT, heat-labile toxin; m, mutant; n, native; r, recombinant; RT, reverse transcriptase; S, secretory.
References
- 1.Spangler DB. Structure and function of cholera toxin and related Escherichia coliheat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill DM. The arrangement of subunits in cholera toxin. Biochemistry. 1976;15:1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- 3.Gill DM, Clements JD, Robertson DC, Finkelstein RA. Subunit number and arrangement in Escherichia coliheat-labile enterotoxin. Infect Immun. 1981;33:677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Heyningen S. Cholera toxin: interaction of subunits with ganglioside GM1. Science (Wash DC) 1974;183:656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- 5.Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coliheat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill DM, King CA. The mechanisms of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975;250:6424–6432. [PubMed] [Google Scholar]
- 7.Field M, Rao MC, Chang EB. Intestinal electrolyte transport and diarrheal disease I. N Engl J Med. 1989;321:800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- 8.Elson CO, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2744. [PubMed] [Google Scholar]
- 9.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coliheat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 10.Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR. Helper T cell subsets for IgA responses. Oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa-associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 12.Takahashi I, Kiyono H, Marinaro M, Jackson RJ, Nakagawa I, Fujihashi K, Hamada S, Clements JD, Bost KL, McGhee JR. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia colilabile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 13.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coliheat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 14.Douce G, Turcottee C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coliheat-labile toxin lacking ADP-ribosyltransferase activity acts as non-toxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickinson BL, Clements JD. Dissociation of Escherichia coliheat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sixma TK, Pronk SE, Kalk KW, Wartna ES, van Zanten BAM, Witholt B, Hol WGJ. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. . Nature (Lond) 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 17.Mekalanos JJ, Swarts DJ, Pearson GDN, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature (Lond) 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 19.Harford S, Dykes CW, Hobden AN, Read MJ, Halliday IJ. Inactivation of the Escherichia coli heat-labile enterotoxin by in vitromutagenesis of the A-subunit gene. Eur J Biochem. 1989;183:311–316. doi: 10.1111/j.1432-1033.1989.tb14930.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji T, Inoue T, Miyama A, Okamoto K, Honda T, Miwatani T. A single amino acid substitution in the A subunit of Escherichia colienterotoxin results in a loss of its toxic activity. J Biol Chem. 1990;265:22520–22525. [PubMed] [Google Scholar]
- 21.Dertzbaugh MT, Macrina FL. Plasmid vectors for constructing translational fusions to the B subunit of cholera toxin. Gene (Amst) 1989;82:335–342. doi: 10.1016/0378-1119(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 22.Guerrant RL, Brunto LL, Schnaitman TC, Rebhun LI, Gilman AG. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxin of Vibrio cholerae and Escherichia coli. . Infect Immun. 1974;10:320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noda M, Tsai S, Adamik R, Bobak DA, Moss J, Vaughan M. Activation of immobilized, biotinylated choleragen A1 protein by a 19-kilodalton guanine nucleotidebinding protein. Biochemistry. 1989;28:7936–7940. doi: 10.1021/bi00445a057. [DOI] [PubMed] [Google Scholar]
- 24.Fujita K, Finkelstein RA. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972;125:647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi M, Inoue S, Miyazawa H, Tamura S. Measurement of antigen-specific mouse IgE by a fluorometric reverse (IgE-capture) ELISA. J Immunol Methods. 1989;190:189–197. doi: 10.1016/0022-1759(89)90202-0. [DOI] [PubMed] [Google Scholar]
- 26.Jackson RJ, Fujihashi K, Kiyono H, McGhee JR. Luminometry: a novel bioluminescent immunoassay enhances the quantitation of mucosal and systemic antibody responses. J Immunol Methods. 1996;190:189–197. doi: 10.1016/0022-1759(95)00276-6. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Kawabata K, Fujihashi K, McGhee JR, Van Dyke TE, Bamberg TV, Hiroi T, Kiyono H. Absence of exogenous interleukin-4–induced apoptosis of gingival macrophages may contribute to chronic inflammation in periodontal diseases. Am J Pathol. 1996;148:331–339. [PMC free article] [PubMed] [Google Scholar]
- 28.Hiroi T, Fujihashi K, McGhee JR, Kiyono H. Polarized Th2 cytokine expression by both mucosal γδ and αβ T cells. Eur J Immunol. 1995;25:2743–2751. doi: 10.1002/eji.1830251005. [DOI] [PubMed] [Google Scholar]
- 29.Tamura S, Samegai Y, Kurata H, Ngamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee A, Chen M. Successful immunization against gastric infection with Helicobacterspecies: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegel S. Cautionary note on the use of the B subunit of cholera toxin as a ganglioside GM1 probe: detection of cholera toxin A subunit in B subunit preparations by a sensitive adenylate cyclase assay. J Cell Biochem. 1990;42:143–152. doi: 10.1002/jcb.240420305. [DOI] [PubMed] [Google Scholar]
- 32.Lycke N, Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989;142:3781–3787. [PubMed] [Google Scholar]
- 33.Lee HJ, Koyono-Nakagawa N, Naito Y, Nishida J, Arai N, Arai K, Yokota T. cAMP activates the IL-5 promoter synergistically with phorbol ester through the signaling pathway involving protein kinase A in mouse thymoma line EL-4. J Immunol. 1993;151:6135–6142. [PubMed] [Google Scholar]
- 34.Munoz E, Zubiaga M, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. . J Immunol. 1991;146:2908–2914. [PubMed] [Google Scholar]
- 36.McGee DW, Elson CO, McGhee JR. Enhancing effect of cholera toxin on interleukin-6 secretion by IEC-6 intestinal epithelial cells: mode of action and augmenting effect of inflammatory cytokines. Infect Immun. 1993;61:4637–4644. doi: 10.1128/iai.61.11.4637-4644.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vajdy M, Kosco-Vilbois M, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin-4–targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 38.Imboden JB, Shoback DM, Pattison G, Stobo JD. Cholera toxin inhibits the T cell antigen receptor– mediated increases in inositol triphosphate and cytoplasmic free calcium. Proc Natl Acad Sci USA. 1986;83:5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]